Abstract
BACKGROUND:
Therapeutic plasma exchange (TPE) removes coagulation proteins, but its impact on therapeutic anticoagulation is unknown. We performed a systematic review of the literature to determine the coagulation effects of TPE in patients receiving systemic anticoagulation.
STUDY DESIGN AND METHODS:
We searched MEDLINE, CINAHL, EMBASE, and Web of Science until June 2018 for studies combining controlled vocabulary and keywords related to: therapeutic plasma exchange, plasmapheresis, anticoagulants, and therapy. The primary outcome was the effect of TPE on anti-Xa activity, activated partial thromboplastin time (aPTT), or International Normalized Ratio (INR). The secondary outcome was reports of post-TPE bleeding or thrombosis.
RESULTS:
1830 references were screened and a total of eight studies identified. Our selected studies (five case reports and three case series) involved 23 patients and evaluated the effects of seven anticoagulants. Six studies of unfractionated heparin (UFH), low molecular weight heparins (LMWH), and direct oral anticoagulants (DOAC) demonstrated anti-Xa level decline. Two studies of UFH and LMWH showed aPTT increase. One study of warfarin showed a post-TPE INR increase. Reports of post-TPE bleeding occurred in two patients and thrombosis in one.
CONCLUSION:
In patients receiving therapeutic anticoagulation, TPE is associated with anti-Xa activity decline and aPTT and INR increase. These coagulation changes do not appear to significantly increase bleeding or thrombotic risk. Our data suggest the need for prospective studies to investigate the true clinical impact of TPE on therapeutic anticoagulation.
INTRODUCTION
In therapeutic plasma exchange (TPE), the process of plasma removal and replacement affects blood coagulation proteins.1 TPE-mediated coagulation protein removal, and its effects on fibrin clot formation, alters the hemostatic profile.2–5 The coagulation profile is further modified by TPE-related factors such as procedure frequency and duration, procedural plasma volume, and replacement fluid.6 Despite TPE’s effects on coagulation, studies have consistently demonstrated that TPE-associated bleeding risk is low.2,3,7–11
TPE’s hemostatic effects are likely to be amplified by the addition of therapeutic anticoagulation. Due to their known effects on coagulation proteins, therapeutic anticoagulants increase bleeding risk.12,13 However, studies suggest that, depending on pharmacokinetic profile and timing of administration relative to the TPE procedure, anticoagulants are removed by TPE.14–18 Therefore, the combined hemostatic impact of both TPE and systemic anticoagulation is unknown.
Given the poor understanding of TPE’s effects on the coagulation profile in patients on systemic anticoagulation, we conducted a systematic review on the effects of TPE on coagulation parameters in patients receiving therapeutic anticoagulation.
STUDY DESIGN AND METHODS
The study was a systematic review of the effects of TPE on coagulation parameters in patients receiving therapeutic anticoagulation for any indication. The primary outcome was post-TPE change in anticoagulant-specific coagulation parameters, including anti-Xa activity, activated partial thromboplastin time (aPTT), and International Normalized Ratio (INR). The secondary outcome was report of bleeding or thrombosis.
This systematic review followed the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). The PRISMA statement includes a 27-item checklist that is used as a basis for reporting systematic review of randomized trials. The study protocol was registered with PROSPERO Trial Registration (#CRD42018097244). Covidence,19 a web-based software platform for systematic review production, was used to manage all stages of the review process.
Search Strategy and Selection
An electronic search of the literature was conducted in Medline (PubMed), CINAHL Complete (EBSCOhost), Embase (Elsevier), and Web of Science (Core Collection) from inception to December 12, 2017. An updated search was conducted June 11, 2018. A sensitive search strategy was used, combining controlled vocabulary and keywords related to: therapeutic plasma exchange, plasmapheresis, anticoagulants and therapy. A Duke modified Cochrane All Studies filter was applied to the results. The literature search included publications, posters, abstracts and conference proceedings. The detailed search strategy can be found in the appendix.
The reference lists of all selected publications were further reviewed to retrieve relevant publications that were not identified in the computerized search. To identify relevant articles, two reviewers independently screened titles and abstracts of all citations identified through the search. Where there was a conflict, a third reviewer made the final selection decision.
Study Inclusion Criteria
All study designs and publications were eligible for inclusion in our review if they addressed use of therapeutic anticoagulation and monitoring of at least one appropriate anticoagulant-specific coagulation parameter in the setting of TPE. Eligible studies involved human studies of any age. All TPE and therapeutic anticoagulation indications were included.
Study Exclusion Criteria
Articles were excluded if therapeutic anticoagulation was used only during the TPE procedure, and if coagulation parameters were not measured or reported. Discrepancies were resolved by discussion or via the involvement of a third reviewer when necessary.
Data Abstraction
Data were extracted using a standardized data collection table. We collected study design, patient demographics, TPE indication, anticoagulant therapy indication, and data on bleeding and thrombotic outcomes. For each study, we retrieved the following variables: TPE indication, TPE fluid replacement, anticoagulant type, anticoagulant indication, bleeding or thrombotic complications, and pre-and post-TPE aPTT and AT (for unfractionated heparin), anti-Xa (for low molecular weight heparins and direct oral anticoagulants) and INR, factor II, and fibrinogen (for warfarin). Where coagulation parameters were measured but not reported in the published manuscript, we contacted the corresponding authors to request the missing data.
Study Quality
Study quality was assessed using the Joanna Briggs Institute critical appraisal tools.20 Any disagreements were resolved by third party adjudication.
Statistical Analysis
We planned to perform a meta-analysis to evaluate the impact of TPE on anticoagulant therapy; however, the selected studies did not report outcomes uniformly. Therefore, since a true meta-analysis could not be done, we outlined the available literature descriptively.
RESULTS
Study Selection
Our initial literature search identified 1830 articles (for details of search strategy, see Appendix). As detailed in the Figure, after duplicates were removed and titles and abstracts were screened, 51 full text articles were reviewed, and eight studies met our inclusion criteria (N = 23 patients). Of the included studies, there were three case series and five case reports. Two manuscripts included five patients receiving unfractionated heparin (UFH); three included seven patients receiving low molecular weight heparin (LMWH; enoxaparin, dalteparin, and nadroparin); and two articles included two patients receiving direct oral anticoagulant (DOAC) therapy (see Table 1). One article included nine patients receiving warfarin therapy (Table 2). The findings of each study are summarized in Tables 1 and 2 and also detailed below.
Figure.
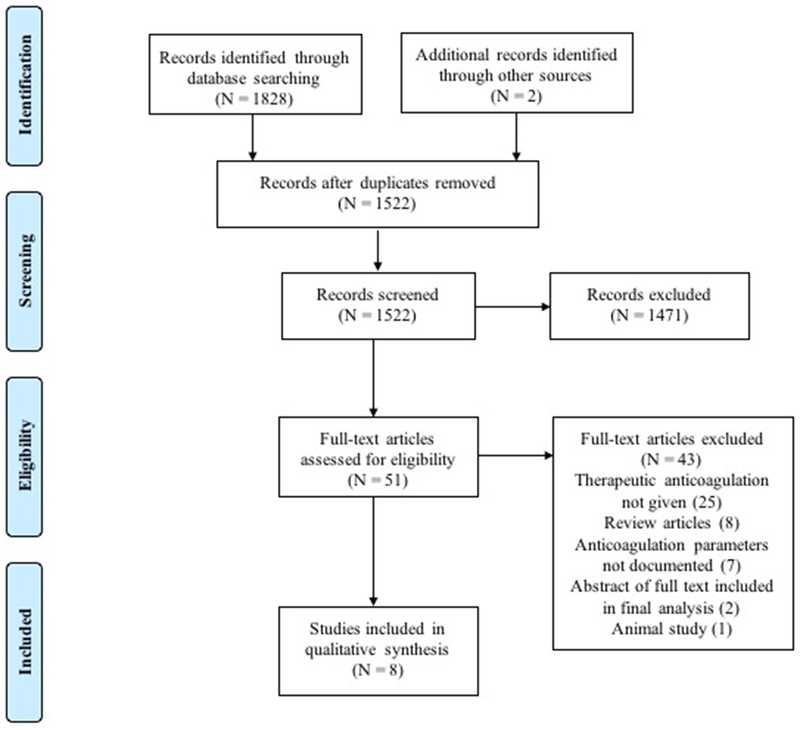
1830 references were identified through database searching and other sources. After duplicates were removed, 1522 studies were title and abstract screened and 1471 studies were excluded. Fifty-one studies were assessed for full-text eligibility with 43 studies excluded: 25 for therapeutic anticoagulation not given; 8 due to being review articles; 7 due to anticoagulation parameters not documented; one meeting abstract was excluded in favor of the published manuscript; and 1 was excluded for being an animal study. The final tally of studies included for extraction was 8.
Table I.
Studies Showing TPE-Related Anticoagulation Parameter Changes for Unfractionated Heparin, Low Molecular Weight Heparin and Direct Oral Anticoagulants
| Author/Year N |
Age/Sex | TPE Indication (#) Fluid replacement | AC | Indication | Infusion Δ | Anti Xa (IU/mL) (range) | aPTT (sec) | AT% | Comments | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Δ | Pre | Post | Δ | Pre | Post | Δ | |||||||
|
Unfractionated Heparin Studies | |||||||||||||||
| Kaplan 201621 4 |
21M | CAPS (25) Plasma |
Heparin | DVT | Unchanged | 0.54 (0.52-0.55) |
0.29 (0.26-0.31) |
↓ | No bleeding reported. 3 patients had warfarin reversed prior to starting heparin. One patient stopped fondaparinux 3 days prior to heparin initiation. Aggregate Anti-Xa data is shown for 1st 3 patients. | ||||||
| 52 F | CAPS (3) Plasma |
Heparin | DVT | Stopped | 0.45 | <0.10 | ↓ | ||||||||
| 26 F | CAPS (17) Plasma |
Heparin | DVT | ↑65% | 1.14 (0.98-1.29) |
0.42 (0.39-0.53) |
↑ | ||||||||
| 0.31* (0.17-0.58) |
0.37* (0.14-0.59) |
||||||||||||||
| 52 F | Desensitization (5) 5% Albumin |
Heparin | VAD thrombosis | Unchanged | 0.16 | 0.10 | ↓ | 29 | 41 | ↑ | 92 | 34 | ↓ | ||
| ↑ 30% | 0.31 | 0.12 | ↓ | 45 | 127 | ↑ | 83 | 26 | ↓ | ||||||
| ↑ 69% | 0.21 (0.18-0.28) |
0.25 (0.19-0.36) |
↑ | 41 | >240 | ↑ | 95 | 35 | ↓ | ||||||
| 37 | 139 | ↑ | 94 | 34 | ↓ | ||||||||||
| – | 158 | – | – | 28 | – | ||||||||||
| Usami 200922 1 |
34 F | Thyrotoxicosis (3) Plasma |
Heparin | Cerebral thrombosis | Unchanged | – | – | – | NR | NR | ↑ | – | – | – | aPTT data shown in a graph but actual values not reported |
| Low Molecular Weight Heparin Studies | |||||||||||||||
| Rahawi 201718 1 |
13 F | Encephalitis (3) 5% Albumin |
Enoxaparin | PE | – | 1.06 | 0.90 | ↓ | Last 2 TPE only. Hematuria and CVC insertion site bleeding. Anti-Xa decay rate with and without TPE: 0.28 vs. 0.088 IU/mL/hr. | ||||||
| Sabloff 200015 1 |
70 F | Cold agglutinin (5) 5% Albumin |
Dalteparin | PE | – | 0.25 0.41 |
0.24 0.39 |
↓ | Anti-Xa decay rate with and without TPE: 0.29 vs. 0.06 IU/mL/hr | ||||||
| Koessler 201523 | 55 M | NMO HES & 5% albumin |
Nadroparin | PE | – | 0.29 0.27 0.33 |
0 0 0.01 |
↓ | 33.7 30.0 28.3 |
50.7 54.2 58.2 |
↑ | 106 118 107 |
48 46 41 |
↓ | The average rate of anti-Xa activity decrease was 0.22 IU/ml/hr compared to 0.02 IU/ml/hr for immunoadsorption. |
| 55 M | Cervical myelitis HES & 5% albumin |
Nadroparin | AFib | – | 0.84 0.68 |
0.26 0.21 |
↓ | 42.8 33.4 |
72.5 63.4 |
↑ | 97 107 |
42 50 |
↓ | ||
| 71 M | Transverse myelitis HES & 5% albumin |
Nadroparin | AFib | – | 0.45 0.60 |
0.09 0.09 |
↓ | 45.7 42.4 |
96.8 82.6 |
↑ | – 99 |
– – |
|||
| 85 M | Myasthenia gravis HES & 5% albumin |
Nadroparin | AFib | – | 0.36 0.71 0.27 |
0 0.20 0.04 |
↓ | 44.8 60 49.9 |
83.5 109.3 112.3 |
↑ | 103 105 74 |
33 42 30 |
↓ | ||
| 71 M | Myasthenia gravis HES & 5% albumin |
Nadroparin | Mechanical valve, AFib | – | >1.10 (1.42)** |
0.56 | ↓ | 44.0 | 86.9 | ↑ | – | – | |||
| Direct Oral Anticoagulant Studies | |||||||||||||||
| Lam 201514 1 |
82 M | AC reversal (1) Plasma |
Apixaban | A Fib | -- | 0.76 (U) | 0.22 (U) | ↓ | Patient presented with hemorrhagic pericardial effusion. Waste fluid anti Xa level: 0.82 IU/mL (L) | ||||||
| 0.84 (L) | 0.35 (L) | ||||||||||||||
| Kumar 201824 1 |
65 M | AC reversal (1) Plasma |
Rivaroxaban | AFib | -- | 0.40 | 0.22 | ↓ | Waste fluid anti Xa level: 0.22 IU/mL | ||||||
N = number; TPE = Therapeutic plasma exchange; CAPS = Catastrophic APS; DVT = Deep vein thrombosis; PE = Pulmonary embolism; NMO = Neuromyelitis Optica; NR = Not reported; M = Male; F = Female; IU = International Units; mL/hr = milliliters per hour; Afib = Atrial fibrillation; U = Unfractionated heparin anti-Xa level; L = Low molecular weight heparin anti-Xa level;
1.5 plasma volume;
value above upper detection limit (measured in diluted sample).
Table II.
Data from Single Prospective Study Showing TPE-Related Anticoagulation Parameter Changes for Warfarin
| Author/Y N |
Age/Sex | TPE Indication | TPE # | Fluid Replacement | AC Indication | INR (Mean, SD) Range |
Factor II | Fibrinogen | Comments | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Δ | Pre | Post | Δ | Pre | Post | Δ | |||||||
| Zantek 201417 8 |
34 F | Myasthenia gravis | 30 | 5% Albumin | NR | 2.09,0.58 0.99-3.65 |
4.12,1.44 1.73-8.05 |
↑ | 29,7 10-86 |
11,7 4-40 |
↓ | 263, 76 147-666 |
105, 31 <61-255 |
↓ | One patient developed mild bleeding at catheter site that resolved with dressing change; one patient developed AV fistula thrombosis |
| 53 M | ABOi renal transplant | 8 | |||||||||||||
| 61 M | Myasthenia gravis | 10 | |||||||||||||
| 61 M | Multiple sclerosis | 7 | |||||||||||||
| 55 F | Hypertriglyceridemia | 58 | |||||||||||||
| 47 M | Hypertriglyceridemia | 17 | |||||||||||||
| 43 F | Heart transplant rejection | 3 | |||||||||||||
| 62 F | Hypertriglyceridemia | 21 | |||||||||||||
| 41 F | Myasthenia gravis | 30 | |||||||||||||
Y = Year; N = Number; TPE = Therapeutic plasma exchange; AC = Anticoagulation; SD = Standard Deviation;
Unfractionated Heparin Studies
Two studies, Kaplan et al21 and Usami et al22 addressed the effects of TPE in patients receiving unfractionated heparin (Table 1).
Kaplan’s study was a retrospective case series of four patients. One patient received intravenous, low-dose heparin, while the other three were treated therapeutically. The therapeutically-treated patients had a goal anti-Xa activity of 0.3-0.7 IU/mL with levels measured before and after the TPE procedure. All three patients received plasma replacement and underwent a total of 15 TPE sessions while receiving heparin. For eight TPE sessions, heparin infusion rates were increased empirically based on theoretical removal of 65% of heparin during TPE. When adjusted, an average increase in anti-Xa of 0.02 (10%) was noted. Without empiric adjustment, a considerable average decrease in anti-Xa of 47% was noted. For the one patient who was not anticoagulated therapeutically (anti-Xa goal 0.15-0.35 IU/mL), the replacement fluid was 5% albumin and the patient underwent a total of 6 TPE sessions. For 2 TPE sessions, heparin doses were not adjusted and anti-Xa measures decreased from 0.16 to 0.10 IU/mL (38% change). For one TPE session, heparin dose was increased by 30% and anti-Xa decreased from 0.31 to 0.12 IU/mL (61% change). For three sessions, heparin was empirically increased by ~69% and anti-Xa activity increased from 0.21 to 0.25 IU/mL (15% change).
In the above study, aPTT levels were not measured consistently but were measured in the 1 patient who was maintained on low-dose heparin with albumin replacement therapy. APTT levels increased as follows: 41% with no change in UFH rate, 182% when the UFH rate was increased by 30%, and an average of 380% when the UFH rate was increased by 69%.
Usami’s study was a case report of one patient who underwent 2 TPE sessions using plasma as the replacement fluid. In this study, the aPTT target was 1.5X normal aPTT and each TPE session was 80 minutes long followed by slow, continuous TPE for 12 hours and eight hours after the first and second session respectively. During both TPE sessions, aPTT measures are noted to have increased (reported in a graph and estimated at 10-25 s).
Taken together, these studies demonstrate that, when the unfractionated heparin infusion is left unchanged, anti-Xa levels uniformly decline, while aPTT increases.
Low Molecular Weight Heparin (LMWH) Studies
Three studies (two case reports and one case series) detail the effect of TPE on enoxaparin,18 dalteparin,15 and nadroparin.23
Enoxaparin:
The study by Rahawi et al is a case report of TPE in 1 patient receiving therapeutic enoxaparin.18 The patient underwent 5 total sessions of TPE with 5% albumin replacement. However, therapeutic enoxaparin was only initiated during the final 2 TPE sessions. Anti-Xa measures without TPE revealed a 4-hour rate of decay of 0.088 IU/mL while pre and post TPE revealed a 4-hour rate of decay of 0.28 IU/mL.
Dalteparin:
The case report by Sabloff and Wells evaluated the effects of TPE on therapeutic dalteparin.15 The patient underwent a total of five sessions of TPE with dalteparin use during the last three TPE sessions. The highest anti-Xa level decrease was from 0.41 to 0.39 IU/mL. When compared to anti-Xa levels without TPE, results showed that TPE was associated with a dalteparin anti-Xa level decay of 483%.
Nadroparin:
A case series by Koessler et al reported the effects of immunoadsorption (IA) and TPE on anti-Xa activity in five patients receiving nadroparin.23 Anti-Xa activity was measured before and after five IA and eleven TPE procedures performed with plasma and 5% albumin replacement. Average time for TPE was approximately two hours. With TPE, anti-Xa levels declined from 0.57 ± 0.10 to 0.13 ± 0.05 IU/mL. When compared to IA, post-TPE anti-Xa levels declined ten-fold.
Taken together, all three LMWH studies demonstrated that, following TPE, anti-Xa activity levels decrease (Table 1).
Direct Oral Anticoagulant (DOAC) Studies
Two studies addressed the effects of TPE on the DOACs, apixaban and rivaroxaban.14,24
Apixaban:
A case report by Lam et al demonstrated the effect of TPE on apixaban.14 Anti-Xa activity calibrated for LMWH was elevated at 0.84 IU/mL prior to TPE and decreased to 0.35 IU/mL following TPE. Measurement of LMWH anti-Xa activity of the plasma waste was 0.82 IU/mL, indicating drug removal during TPE.
Rivaroxaban:
A case report by Kumar et al reviewed the effects of TPE in a patient who required urgent reversal of rivaroxaban.24 An anti-Xa assay calibrated for LMWH measured prior to TPE was 0.4 IU/mL. Following a 90-minute TPE with fresh frozen plasma replacement, the anti-Xa measure was 0.21 IU/mL with an anti-Xa measure of plasma waste of 0.22 IU/mL, indicative of rivaroxaban removal.
Both case reports showed post-TPE anti-Xa level decrease as well as anticoagulant removal in waste plasma.
Vitamin K Antagonist Study
We identified one study evaluating the effects of TPE on the vitamin K antagonist, warfarin.17 Zantek et al published a prospective case series of eight patients who underwent 123 TPE sessions with 5% albumin replacement while on therapeutic warfarin. Following TPE, INR measures increased approximately two-fold from an average pre-procedure INR of 2.09 ± 0.58 (range 0.99-3.65) to a post-procedure INR of 4.12 ± 1.44 (range, 1.73-8.05). Factor II and fibrinogen measures decreased by 60% and 65% respectively.
Bleeding and Thrombotic Complications
Post-TPE bleeding was documented in two studies.17,18 One patient on warfarin developed post-procedural tunneled catheter site bleeding that stopped after a dressing change17 and one patient on enoxaparin developed central line insertion site bleeding and hematuria.18
Thrombosis was reported in one study.17 One patient with a prior history of fistula thrombosis developed a fistula occlusion on warfarin. At the time of diagnosis, INR was subtherapeutic (1.44).
DISCUSSION
Our systematic review demonstrated effects of TPE on coagulation parameters in patients receiving therapeutic anticoagulation. Anti-Xa levels decreased and both aPTT and INR levels increased. Mild bleeding was reported in two patients and thrombosis in one.
For patients receiving albumin replacement, TPE alone, without the added impact of anticoagulant therapy, has been shown to have an effect on both coagulation proteins, including aPTT and INR levels (see Table III).4,25,26 In a prospective study of 10 patients who underwent albumin TPE, TPE prolonged aPTT (28 ± 3 vs. 45 ± 8 s) and INR (0.95 ± 0.06 vs. 1.25 ± 0.16).4 Another study of 35 patients showed aPTT increase from 41.6 to 49.3 s and INR increase from 1.28 to 1.74.25 In this second study, patients who received plasma alone or a combination of albumin and plasma showed a decline in aPTT and no significant change or minimal increase in INR (aPTT: 46.9 vs. 39.5 s and INR: 1.20 vs. 1.18 in patients receiving plasma alone and aPTT 45.0 vs. 42.3 s and INR 1.20 vs. 1.32 in combination plasma and albumin). One additional study that presented aggregate data for nine patients treated with albumin, plasma, or a combination of both, showed post-procedure aPTT increase (26.04 vs. 29.76 s) and INR increase (1.09 vs. 1.31).26 Together, these studies suggest that TPE increases aPTT and INR independently of the addition of systematic anticoagulation. The higher aPTTs and INRs obtained from the selected studies, however, suggest that the addition of anticoagulant therapy causes these effects to be more pronounced. Nevertheless, prospective studies are needed to elucidate the aPTT or INR increase that is attributable to either TPE or anticoagulant therapy alone.
Table III.
Known independent effects of TPE on commonly assayed coagulation parameters
| Author Year N |
Exchange Fluid | Fibrinogen (mg/dL) | AT (%) | INR | Prothrombin Time (s) Or Index (%) |
aPTT (s) | TT (s) |
|---|---|---|---|---|---|---|---|
| Tholking 20154 10 |
Albumin | 482 vs. 223 −54%; |
103 vs. 54 −48% |
0.95 vs. 1.25 +32% |
108 vs. 68% −37% |
28 vs. 45 +60% | 18 vs. 20 +11% |
| Blasi 20165 6 |
Albumin | 140 vs. 70 −67% |
– | – | 86 vs. 49%; −49% | – | – |
| Witt 201725 35 (peds) |
S/D plasma S/D plasma + albumin Albumin |
322 vs. 263; −18% 297 vs. 210; −29% 204 vs. 112; −45% |
89 vs. 80; −9% 90 vs. 68; −24% 83 versus 49; −42% |
1.20 vs 1.18; −2% 1.20 vs 1.32; +9% 1.28 vs 1.74; +36% |
– – – |
47 vs. 39; −16 % 45 vs. 42; +6% 42 vs, 49; +19% |
– – – |
| Pearlman 199727 10 |
Albumin | Median −61% | Median −59% | – | – | – | – |
| Flaum 197928 3 |
Albumin or plasma protein fraction | – | −58% | – | – | – | – |
| Keller 19798 18 |
Colloid (PPS and/or Haemaccel) | −25% ±1.2% | – | – | – | – | – |
| Yamada 201626 9 |
Albumin, plasma, or plasma + albumin | 412 versus 187 | – | 1.09 vs 1.31 | 11.4 vs 13.53s | 26.04 vs 29.76 | – |
| Wood 19861 7 |
Albumin (Median) | 360 vs. 86 | 130 vs. 39 | 88 vs. 35% | 36 vs. 59 | – | |
| Overall Effect | N/A | ↓ | ↓ | ↑ | PT ↑ PI↓ | ↑ | ↑ |
AT = antithrombin; INR = international normalized ratio; aPTT = activated partial thromboplastin time; TT = thrombin time.
Anti-Xa levels are typically measured in the context of anticoagulant therapy. Therefore, data that demonstrates TPE’s effects on anti-Xa activity in the absence of anticoagulant therapy are not readily available. However, because antithrombin (AT) is a substrate for many anti-Xa assays, it is pertinent to understand TPE’s effects on AT. As noted in Table III, AT levels uniformly decline with TPE.4,5 When compared to plasma-only replacement (−9%)25 or a combination of plasma and albumin replacement (−24%),25 AT decline is more pronounced with albumin-only replacement (42 to 70% decline).1,4,25,27,28 Therefore, in chromogenic anti-Xa assays for unfractionated heparin and LMWH, decline in AT levels should result in excess anticoagulant in the sample and lead to an artificially high anti-Xa level. Nevertheless, our data suggest that this artificial increase in anti-Xa levels does not occur. Rather, anti-Xa levels are consistently shown to decline. This decline may be explained by a concurrent decrease in the anticoagulant being measured as studies suggest that TPE removes anticoagulants.14,24 Therefore, a decrease in the levels of the anticoagulant being measured in the assay likely contributes to the post-TPE decline in anti-Xa levels. This finding may suggest that, in patients receiving albumin TPE, anti-Xa activity is not a reliable measure of anticoagulant activity.
If anti-Xa levels are not a reliable measure of anticoagulant activity, then, for similar reasons, during TPE, aPTT is also not likely to be a reliable measure of unfractionated heparin therapy. Removal of AT and UFH during the TPE procedure also contributes to a skewed result that likely over-estimates the aPTT. This point is nicely illustrated in a study by Usami et al, where the UFH infusion is adjusted in response to changes in aPTT levels during TPE.22 Following TPE, the UFH infusion returns to its pre-TPE rate, suggesting that adjustment of the heparin infusion during TPE did not lead to any meaningful change in the patient’s heparin requirement. Based on this one study and the confusion inherent in interpreting either anti-Xa levels or aPTT during TPE, it may be best to simply leave the heparin infusion unchanged. Future studies may be needed to determine if this is the best approach.
Similar to the concerns raised with measuring aPTT and anti-Xa levels, INR levels are affected by periprocedural removal of factor II and fibrinogen. Both factor II (29 ± 7 vs. 11 ± 7%) and fibrinogen levels (263 ± 76 vs. 105 ± 31) decline and skew INR levels upwards (2.09 ± 0.58 vs. 4.12 ± 1.44).17 As noted above, patients may be better served by continuing on their dose of warfarin unchanged, unless clinically-significant bleeding ensues. This recommendation notwithstanding, prospective studies are needed to help clarify bleeding risk when post-TPE supratherapeutic INRs develop.
A more appropriate assessment of the post-TPE coagulation profile may be whole blood clotting assays. In fact, prospective whole blood clotting assay studies suggest that albumin-only TPE alters fibrin clot formation.4,5 As measured by thromboelastography, TPE with albumin fluid replacement both alters clot structure and decreases clot formation and firmness. In a prospective study of 10 patients who underwent albumin TPE, pre- and post-TPE whole blood rotational thromboelastometry (ROTEM; Pentapharm, Munich, Germany) was performed.4 On INTEM analysis, clot formation time (CFT) was prolonged (68 ± 26 vs. 97 ± 50 s; +43%, p = 0.01) and maximum clot firmness (MCF) decreased (66 ± 8 vs. 58 ± 11 mm; −12%, P = 0.006). Similar trends occurred with EXTEM analysis: CFT (79 ± 28 vs. 134 ± 55 s; +70%, p = 0.002) and MCF (67 ± 8 vs. 57 ± 10 mm; −15%, p = 0.006). Despite these differences, clotting time (CT) was unchanged (168 vs. 172s; +2%, p = 0.1; and 49 vs. 52s; +6%, p = 0.6) for both INTEM and EXTEM analysis, respectively. CFT and MCF are both affected by platelet count and fibrinogen level while CT reflects the activity of all clotting factors. These findings suggest that although decreased fibrinogen levels may affect both CFT and MCF, overall clotting time is not affected.
While whole blood clotting assays may better contribute to our understanding of a patient’s post-TPE hemostatic profile while on therapeutic anticoagulation, of more pertinent clinical concern is TPE’s ultimate effect on bleeding or thrombosis. In our selected studies, thrombosis rates were low; however, the studies did not explicitly state how long this outcome was assessed. Similarly, with regards to bleeding, though better documented than thrombosis outcomes, it is not clear how long after TPE bleeding complications were evaluated. Consistent documentation of bleeding concerns across studies leads us to believe that our finding of 9% bleeding rates is a fair estimate; nevertheless, prospective studies would better evaluate the true prevalence of TPE and anticoagulation-associated bleeding.
Finally, in addition to medication characteristics, variability in the TPE procedure may also impact anticoagulant removal. Frequency and duration of TPE will affect amount of anticoagulant removed. Additionally, variation in plasma volume removed during TPE sessions will impact degree of anticoagulant removal.6 Lastly, if medication targets, such as coagulant proteins, are removed with TPE, the anticoagulant will be rendered less effective. The effect of TPE on anticoagulant therapy is difficult to characterize due to variation in the TPE procedures and differences in drug pharmacokinetics.
Our study is limited by the small numbers and quality of the studies included in our final analysis. Therefore, broad generalizations of our findings cannot be recommended. Additionally, the absence of a rigorous assessment of bleeding and thrombotic complications affects our ability to understand the true clinical impact of TPE in patients receiving therapeutic anticoagulation. Nevertheless, our comprehensive search strategy and careful analysis on a per-study basis is a strength and the best available data today. In addition to understanding impact on coagulation parameters, future studies can build on our findings to develop prospective analyses of clinically-relevant endpoints.
CONCLUSIONS
In patients receiving therapeutic anticoagulation and undergoing TPE, TPE-mediated removal of coagulation proteins is associated with decrease in measured anti-Xa activity levels and increase in aPTT and INR levels. Beyond the impact of TPE alone, the extent to which anticoagulants contribute to TPE’s effects on coagulation parameters are unknown. Prospective studies are needed to understand the effects of TPE on coagulation proteins and anticoagulants, to elucidate optimal measures of anticoagulation in the setting of TPE, and to assess its true impact on both bleeding and thrombosis risk.
Appendix
Search Strategy Report:
Topic: In patients on therapeutic anticoagulation receiving therapeutic plasma exchange (TPE), is there increased bleeding or thrombosis risk?
Database: PubMed (MEDLINE)
| Set # | Results | |
|---|---|---|
| 1 | “plasmapheresis”[MeSH Terms] OR “plasmapheresis”[tiab] OR “plasma exchange”[tiab] OR “plasma exchange”[MeSH Terms] | 19453 |
| 2 | “Anticoagulants”[Pharmacological Action] OR “Anticoagulants”[Mesh] OR anticoagulant[tiab] OR anticoagulants[tiab] OR “apixaban”[Supplementary Concept] OR “apixaban”[tiab] OR “rivaroxaban”[MeSH Terms] OR “rivaroxaban”[tiab] OR “edoxaban”[Supplementary Concept] OR “edoxaban”[tiab] OR “dabigatran”[MeSH Terms] OR “dabigatran”[tiab] OR “warfarin”[MeSH Terms] OR “warfarin”[tiab] OR “enoxaparin”[MeSH Terms] OR “enoxaparin”[tiab] OR “dalteparin”[MeSH Terms] OR “dalteparin”[tiab] OR “heparin”[MeSH Terms] OR “heparin”[tiab] OR “tinzaparin”[Supplementary Concept] OR “tinzaparin”[tiab] OR “argatroban”[Supplementary Concept] OR “argatroban”[tiab] OR “bivalirudin”[Supplementary Concept] OR “bivalirudin”[tiab] OR “betrixaban”[Supplementary Concept] OR “betrixaban”[tiab] OR “ximelagatran”[Supplementary Concept] OR “ximelagatran”[tiab] | 261674 |
| 3 | therapy[tiab] OR therapies[tiab] OR “therapy”[Subheading] OR “therapeutic use”[Subheading] OR therapeutic[tiab] | 8587273 |
| 4 | #1 AND #2 AND #3 | 970 |
| 5 | #4 AND (randomized controlled trial[pt] OR controlled clinical trial[pt] OR randomized[tiab] OR randomised[tiab] OR randomization[tiab] OR randomisation[tiab] OR placebo[tiab] OR drug therapy[sh] OR randomly[tiab] OR trial[tiab] OR groups[tiab] OR Clinical trial[pt] OR “clinical trial”[tiab] OR “clinical trials”[tiab] OR “evaluation studies”[Publication Type] OR “evaluation studies as topic”[MeSH Terms] OR “evaluation study”[tiab] OR evaluation studies[tiab] OR “intervention studies”[tiab] OR “intervention study”[tiab] OR “intervention studies”[tiab] OR “case-control studies”[MeSH Terms] OR “case-control”[tiab] OR “cohort studies”[MeSH Terms] OR cohort[tiab] OR “longitudinal studies”[MeSH Terms] OR “longitudinal”[tiab] OR longitudinally[tiab] OR “prospective”[tiab] OR prospectively[tiab] OR “retrospective studies”[MeSH Terms] OR “retrospective”[tiab] OR “follow up”[tiab] OR “comparative study”[Publication Type] OR “comparative study”[tiab] OR systematic[subset] OR “meta-analysis”[Publication Type] OR “meta-analysis as topic”[MeSH Terms] OR “meta-analysis”[tiab] OR “meta-analyses”[tiab]) | 425 |
| 6 | #5 NOT (Editorial[ptyp] OR Letter[ptyp] OR Comment[ptyp]) | 418 |
| 7 | #6 NOT (animals[mh] NOT humans[mh]) | 409 |
| 8 | #7 AND (“2017/12/21”[PDAT] : “3000/12/31”[PDAT]) | 4 |
Database: CINAHL
| Set # | Results | |
|---|---|---|
| 1 | (MH “Plasmapheresis”) OR (MM “Plasma Exchange”) OR TI (plasmapheresis OR “plasma exchange”) OR AB (plasmapheresis OR “plasma exchange”) | 1845 |
| 2 | (MH “Anticoagulants+”) OR TI (anticoagulant OR anticoagulants OR apixaban OR rivaroxaban OR edoxaban OR dabigatran OR warfarin OR enoxaparin OR dalteparin OR heparin OR tinzaparin OR argatroban OR bivalirudin OR betrixaban OR ximelagatran) OR AB (anticoagulant OR anticoagulants OR apixaban OR rivaroxaban OR edoxaban OR dabigatran OR warfarin OR enoxaparin OR dalteparin OR heparin OR tinzaparin OR argatroban OR bivalirudin OR betrixaban OR ximelagatran) | 31196 |
| 3 | TI (therapy OR therapies OR therapeutic OR therapeutics) OR AB (therapy OR therapies OR therapeutic OR therapeutics) | 414438 |
| 4 | #1 AND #2 AND #3 | 46 |
| 5 | #4 AND (TI (“randomized controlled trial” OR “controlled clinical trial” OR “randomized” OR “randomized” OR “randomization” OR “randomization” OR “placebo” OR “randomly” OR “trial” OR “groups” OR “systematic review” OR “meta-analysis” OR “meta-analyses” OR AB (“randomized controlled trial” OR “controlled clinical trial” OR “randomized” OR “randomized” OR “randomization” OR “randomization” OR “placebo” OR “randomly” OR “trial” OR “groups” OR “systematic review” OR “meta-analysis” OR “meta-analyses”) OR (MH “Randomized Controlled Trials” OR MH “Systematic Review” OR MH “Meta Analysis”)) | 4 |
| 8 | #5 AND Limiters - Published Date: 20171201- | 0 |
Database: Embase
| Set # | Results | |
|---|---|---|
| 1 | ‘plasmapheresis’/exp OR plasmapheresis:ab,ti OR ‘plasma exchange’/exp OR ‘plasma exchange’:ab,ti | 38854 |
| 2 | ‘anticoagulant agent’/exp OR anticoagulant:ab,ti OR anticoagulants:ab,ti OR ‘apixaban’/exp OR ‘apixaban’:ab,ti OR ‘rivaroxaban’/exp OR ‘rivaroxaban’:ab,ti OR ‘edoxaban’/exp OR ‘edoxaban’:ab,ti OR ‘dabigatran’/exp OR ‘dabigatran’:ab,ti OR ‘warfarin’/exp OR ‘warfarin’:ab,ti OR ‘enoxaparin’/exp OR ‘enoxaparin’:ab,ti OR ‘dalteparin’/exp OR ‘dalteparin’:ab,ti OR ‘heparin’/exp OR ‘heparin’:ab,ti OR ‘tinzaparin’/exp OR ‘tinzaparin’:ab,ti OR ‘argatroban’/exp OR ‘argatroban’:ab,ti OR ‘bivalirudin’/exp OR ‘bivalirudin’:ab,ti OR ‘betrixaban’/exp OR ‘betrixaban’:ab,ti OR ‘ximelagatran’/exp OR ‘ximelagatran’:ab,ti | 663610 |
| 3 | ‘drug therapy’/exp OR therapy:ab,ti OR therapies:ab,ti OR therapeutic:ab,ti | 5022871 |
| 4 | #1 AND #2 AND #3 | 3113 |
| 5 | #4 AND (‘randomized controlled trial’/exp OR ‘crossover procedure’/exp OR ‘double blind procedure’/exp OR ‘single blind procedure’/exp OR random*:ab,ti OR factorial*:ab,ti OR crossover*:ab,ti OR (cross NEAR/1 over*):ab,ti OR placebo*:ab,ti OR (doubl* NEAR/1 blind*):ab,ti OR (singl* NEAR/1 blind*):ab,ti OR assign*:ab,ti OR allocat*:ab,ti OR volunteer*:ab,ti OR ‘clinical study’/exp OR ‘clinical trial’:ti,ab OR ‘clinical trials’:ti,ab OR ‘controlled study’/exp OR ‘evaluation’/exp OR ‘evaluation study’:ab,ti OR ‘evaluation studies’:ab,ti OR ‘intervention study’:ab,ti OR ‘intervention studies’:ab,ti OR ‘case control’:ab,ti OR ‘cohort analysis’/exp OR cohort:ab,ti OR longitudinal*:ab,ti OR prospective:ab,ti OR prospectively:ab,ti OR retrospective:ab,ti OR ‘follow up’/exp OR ‘follow up’:ab,ti OR ‘comparative effectiveness’/exp OR ‘comparative study’/exp OR ‘comparative study’:ab,ti OR ‘comparative studies’:ab,ti OR ‘evidence based medicine’/exp OR ‘systematic review’:ab,ti OR ‘meta-analysis’:ab,ti OR ‘meta-analyses’:ab,ti) NOT (‘case report’/exp OR ‘editorial’/exp OR ‘letter’/exp OR ‘note’/exp) NOT (‘editorial’/exp OR ‘letter’/exp OR ‘note’/exp) | 1094 |
| 6 | #5 AND [humans]/lim | 1036 |
| 7 | #6 AND 2018:py | 23 |
Database: Web of Science
| Set # | Results | |
|---|---|---|
| 1 | TS=(plasmapheresis OR plasma exchange) OR TI=(plasmapheresis OR plasma exchange) | 38700 |
| 2 | TS=(anticoagulant OR anticoagulants OR apixaban OR rivaroxaban OR edoxaban OR dabigatran OR warfarin OR enoxaparin OR dalteparin OR heparin OR tinzaparin OR argatroban OR bivalirudin OR betrixaban OR ximelagatran) OR TI=(anticoagulant OR anticoagulants OR apixaban OR rivaroxaban OR edoxaban OR dabigatran OR warfarin OR enoxaparin OR dalteparin OR heparin OR tinzaparin OR argatroban OR bivalirudin OR betrixaban OR ximelagatran) | 152321 |
| 3 | TS=(therapy OR therapies OR therapeutic OR therapeutics) OR TI=(therapy OR therapies OR therapeutic OR therapeutics) | 2576500 |
| 4 | #1 AND #2 AND #3 | 405 |
| 5 | #4 [excluding] DOCUMENT TYPES: ( NOTE OR EDITORIAL MATERIAL OR LETTER ) | 392 |
| 6 | #5 AND Publication Date: 12/2017- | 13 |
Footnotes
The authors report no conflict of interest.
REFERENCES
- 1.Wood L, Jacobs P. The effect of serial therapeutic plasmapheresis on platelet count, coagulation factors, plasma immunoglobulin, and complement levels. Journal of clinical apheresis. 1986;3(2):124–128. [DOI] [PubMed] [Google Scholar]
- 2.Cid J, Carbasse G, Andreu B, Baltanas A, Garcia-Carulla A, Lozano M. Efficacy and safety of plasma exchange: ann 11-year single-center experience of 2730 procedures in 317 patients. Transfusion and apheresis science : official journal of the World Apheresis Association : official journal of the European Society for Haemapheresis. 2014;51(2):209–214. [DOI] [PubMed] [Google Scholar]
- 3.Shemin D, Briggs D, Greenan M. Complications of therapeutic plasma exchange: a prospective study of 1,727 procedures. Journal of clinical apheresis. 2007;22(5):270–276. [DOI] [PubMed] [Google Scholar]
- 4.Thölking G, Mesters R, Dittrich R, Pavenstädt H, Kümpers P, Reuter S. Assessment of Hemostasis after Plasma Exchange Using Rotational Thrombelastometry (ROTEM). PloS one. 2015;10(6):e0130402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blasi A, Cid J, Beltran J, Taura P, Balust J, Lozano M. Coagulation profile after plasma exchange using albumin as a replacement solution measured by thromboelastometry. Vox sanguinis. 2016;110(2):159–165. [DOI] [PubMed] [Google Scholar]
- 6.Kaplan AA. Therapeutic plasma exchange: a technical and operational review. Journal of clinical apheresis. 2013;28(1):3–10. [DOI] [PubMed] [Google Scholar]
- 7.Couriel D, Weinstein R. Complications of therapeutic plasma exchange: A recent assessment. Journal of clinical apheresis. 1994;9(1):1–5. [DOI] [PubMed] [Google Scholar]
- 8.Keller AJ, Chirnside A, Urbaniak SJ. Coagulation abnormalities produced by plasma exchange on the cell separator with special reference to fibrinogen and platelet levels. British journal of haematology. 1979;42(4):593–603. [DOI] [PubMed] [Google Scholar]
- 9.Sengul Samanci N, Ayer M, Gursu M, et al. Patients treated with therapeutic plasma exchange: a single center experience. Transfusion and apheresis science : official journal of the World Apheresis Association : official journal of the European Society for Haemapheresis. 2014;51(3):83–89. [DOI] [PubMed] [Google Scholar]
- 10.Sutton DM, Nair RC, Rock G. Complications of plasma exchange. Transfusion. 1989;29(2):124–127. [DOI] [PubMed] [Google Scholar]
- 11.Yeo FE, Bohen EM, Yuan CM, Sawyers ES, Abbott KC. Therapeutic plasma exchange as a nephrological procedure: a single-center experience. Journal of clinical apheresis. 2005;20(4):208–216. [DOI] [PubMed] [Google Scholar]
- 12.Crowther MA, Warkentin TE. Bleeding risk and the management of bleeding complications in patients undergoing anticoagulant therapy: focus on new anticoagulant agents. Blood. 2008;111(10):4871–4879. [DOI] [PubMed] [Google Scholar]
- 13.Shoeb M, Fang MC. Assessing bleeding risk in patients taking anticoagulants. Journal of thrombosis and thrombolysis. 2013;35(3):312–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lam WW, Reyes MA, Seger JJ. Plasma Exchange for Urgent Apixaban Reversal in a Case of Hemorrhagic Tamponade after Pacemaker Implantation. Texas Heart Institute journal. 2015;42(4):377–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sabloff M, Wells PS. The effect of plasmapheresis on the serum activity level of dalteparin: a case report. Blood coagulation & fibrinolysis : an international journal in haemostasis and thrombosis. 2000;11(4):395–400. [DOI] [PubMed] [Google Scholar]
- 16.Schinzel H, Berghoff K, Beuermann I, Sauer O, von Mach MA, Weilemann LS. Anticoagulation with low-molecular-weight heparin (dalteparin) in plasmapheresis therapy: initial experience. Transfusion. 2006;46(4):624–629. [DOI] [PubMed] [Google Scholar]
- 17.Zantek ND, Morgan S, Zantek PF, Mair DC, Bowman RJ, Aysola A. Effect of therapeutic plasma exchange on coagulation parameters in patients on warfarin. Journal of clinical apheresis. 2014;29(2):75–82. [DOI] [PubMed] [Google Scholar]
- 18.Rahawi KW, Higgins KL, Noda C, Stultz JS. Effect of Plasmapheresis on the Anti-Factor Xa Activity of Enoxaparin in an Obese Adolescent Patient. Pharmacotherapy. 2017;37(4):e16–e20. [DOI] [PubMed] [Google Scholar]
- 19.Covidence systematic review software, Veritas Health Innovation, Melbourne, Australia: Available at www.covidence.org. [Google Scholar]
- 20.Joanna Briggs Institute. Checklists for Case Reports and Case Series. Url: http://joannabriggs.org/research/critical-appraisal-tools.html last accessed 8/27/18.
- 21.Kaplan A, Raut P, Totoe G, Morgan S, Zantek ND. Management of systemic unfractionated heparin anticoagulation during therapeutic plasma exchange. Journal of clinical apheresis. 2016;31(6):507–515. [DOI] [PubMed] [Google Scholar]
- 22.Usami K, Kinoshita T, Tokumoto K, et al. Successful treatment of plasma exchange for severe cerebral venous thrombosis with thyrotoxicosis. Journal of stroke and cerebrovascular diseases : the official journal of National Stroke Association. 2009;18(3):239–243. [DOI] [PubMed] [Google Scholar]
- 23.Koessler J, Kobsar A, Kuhn S, et al. The effect of immunoadsorption with the Immusorba TR-350 column on coagulation compared to plasma exchange. Vox sanguinis. 2015;108(1):46–51. [DOI] [PubMed] [Google Scholar]
- 24.Kumar V, Allencherril J, Bracey A, Chen AJ, Lam WW. Therapeutic Plasma Exchange for Urgent Rivaroxaban Reversal. Texas Heart Institute journal. 2018;45(2):96–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Witt V, Pichler H, Beiglboeck E, Kursten F, Weidner L. Changes in hemostasis caused by different replacement fluids and outcome in therapeutic plasma exchange in pediatric patients in a retrospective single center study. Transfusion and apheresis science : official journal of the World Apheresis Association : official journal of the European Society for Haemapheresis. 2017;56(1):59–65. [DOI] [PubMed] [Google Scholar]
- 26.Yamada C, Pipe SW, Zhao L, et al. Coagulation status after therapeutic plasma exchange using citrate in kidney transplant recipients. Transfusion. 2016;56(12):3073–3080. [DOI] [PubMed] [Google Scholar]
- 27.Pearlman ES, Litty CA, Edger N, Ballas SK. Effect of therapeutic plasmapheresis on coagulation parameters. Journal of clinical apheresis. 1994;9(3):202–203. [DOI] [PubMed] [Google Scholar]
- 28.Flaum MA, Cuneo RA, Appelbaum FR, Deisseroth AB, Engel WK, Gralnick HR. The hemostatic imbalance of plasma-exchange transfusion. Blood. 1979;54(3):694–702. [PubMed] [Google Scholar]


