Abstract
The plant extract “total glucosides of peony” (TGP) constitutes a mixture of glycosides that is isolated from the roots of the well-known traditional Chinese herb Paeonia lactiflora Pall. Paeoniflorin (Pae) is the most abundant component and the main biologically active ingredient of TGP. Pharmacologically, Pae exhibits powerful anti-inflammatory and immune regulatory effects in some animal models of autoimmune diseases including Rheumatoid Arthritis (RA) and Systemic Lupus Erythematosus (SLE). Recently, we modified Pae with an addition of benzene sulfonate to achieve better bioavailability and higher anti-inflammatory immune regulatory effects. This review summarizes the pharmacological activities of Pae and the novel anti-inflammatory and immunomodulatory agent Paeoniflorin-6′-O-benzenesulfonate (CP-25) in various chronic inflammatory and autoimmune disorders. The regulatory effects of Pae and CP-25 make them promising agents for other related diseases, which require extensive investigation in the future.
Keywords: Pae, CP-25, inflammation, arthritis, treatment
Introduction
The Developmental Process of TGP-Pae-CP-25
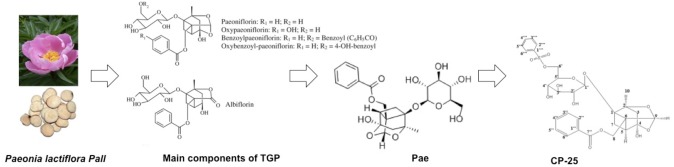
The glycoside mixture TGP is an active plant extract that is isolated from the roots of Paeonia lactiflora Pall, a traditional Chinese medicine (TCM). In 1998, TGP was approved by the National Medical Products Administration (NMPA) for anti-inflammatory and immunomodulatory therapy in China. It has been used in the treatment of Rheumatoid Arthritis (RA) and Systemic Lupus Erythematosus (SLE) (Chen et al., 2013; Wang et al., 2014). Meanwhile a series of further studies has demonstrated that TGP also has therapeutic value in the treatment of chronic nephritis (Zhang et al., 2014), atherosclerosis (Li et al., 2011), and Sjögren’s syndrome (Li et al., 2013). Although TGP is effective without toxicity or off-target effects being evident, it has been shown to have a slow onset time by oral administration (Wang C. et al., 2012).
The main ingredients of TGP include Paeoniflorin (Pae), hydroxy-paeoniflorin, albiflorin, and benzoylpaeoniflorin (Absolute et al., 1972). Among them, Pae is the most abundant component (> 40%) and the main biologically active ingredient (Su et al., 2010). Recent investigations of Pae exhibited anti-inflammatory (Chang et al., 2011; Wang et al., 2013), anti-neoplastic (Sun, 2013), anti-hyperglycemia (Yang et al., 2004), and neuroprotective effects (Liu et al., 2005). Since Pae is characterized as a monoterpene, water-soluble glucoside with a low lipophilicity, bioavailability of Pae is relatively low due to insufficient absorption across the gastrointestinal epithelium after oral administration (Takeda et al., 1995). Extensive studies have been conducted to improve the bioavailability of Pae. Two well-known P-glycoprotein (P-gp) inhibitors, verapamil, and quinidine, can significantly elevate the absorption of Pae (Chan et al., 2006; Chen Y. et al., 2012). Furthermore, modification of acetylation improves the absorption and lipophilicity of Pae in vitro (Yang et al., 2013) and the bioavailability of benzoylpaeoniflorin sulfonate was increased and tested in a mouse model (Cheng et al., 2010). Based on this principle, we prepared paeoniflorin-6′-O-benzene sulfonate (CP-25). The oral bioavailability of CP-25 was much better than Pae in rats, and its anti-inflammatory and immunoregulatory effects were also significantly higher than Pae and TGP.
In this review, we summarize the recent progress in the use of Pae in immunoregulatory and anti-inflammatory activities, including the regulation of RA, kidney/liver injury and other immune-related diseases. In addition, we also review the pharmaceutical effects of CP-25 on inflammation and immune-associated diseases to highlight the use of Pae and its derivative CP-25 as potential agents for subsequent research and clinical application (Figure 1).
FIGURE 1.
The developmental process of TGP-Pae-CP-25.
The Pharmacological Effect of Pae in Inflammatory Pathologies
Arthritis
The anti-inflammatory and immunoregulatory effects of Pae on mesenteric lymph node (MLN) lymphocytes and the underlying mechanisms were investigated in an adjuvant arthritis (AA) rat model. Pae greatly reduced arthritis scores and secondary hind paw swelling, pro-inflammatory cytokine production and the proliferation of MLN lymphocytes. Pae induced the expression of β2-adrenergic receptor (ADRB2) and decreased that of β-arrestin1/2 in MLN lymphocytes. In addition, Pae reversed the pro-inflammatory cAMP of MLN lymphocytes in vitro. The effects of Pae on ADRB2 desensitization and β2-cAMP signal transduction in MLN lymphocytes is essential for arthritis pathogenesis (Wu et al., 2007). In another publication using AA rat model, Pae repressed the malondialdehyde and induced superoxide dismutase, catalase, and glutathione peroxidase in blood. Moreover, Pae inhibited nuclear factor-κB (NF-κB) p65 unit, tumor necrosis factor (TNF)-α, interleukin (IL)-1β and IL-6, and reduced the COX-2 (Jia and He, 2016). These reports suggested that Pae ameliorates disease of AA rat.
Zhai et al. (2018) showed that Pae relieved Collagen-Induced Arthritis (CIA) rats by repressing Rho kinase (ROCK), p-NF-κB p65 and inflammatory cytokines, such as TNF- α and IL-6, in the joint synovial tissues. Wu et al. (2013) also showed that the expressions of inflammatory cytokines were repressed in Pae-treated CIA rat. Additionally, Pae could repress TNF- α and IL-1β in serum and ameliorate bone erosion of CIA rat. Inflammatory mediators and G protein-coupled signaling were associated with the pathogenesis of synovitis in CIA rats that was attenuated by Pae. Our group demonstrated that Pae could inhibit hyperpalsy and inflammatory cytokine production of fibroflast-like synoviocytes (FLS) from CIA via inhibiting Gi expression and restoring cAMP level and PKA activity (Zhang et al., 2008).
GRK2 and β-arrestin1/2 also belong to G-protein-coupled receptors (GPCRs) signaling family. GRK2 increases in synovium from CIA rats and GRK2 inhibitor suppresses proliferation and induces the cAMP/PKA activity of FLS. Moreover, Pae shows similar effects in the CIA model via inhibiting GRK2 expression in FLS which mirrors the effects of a GRK2 inhibitor. These results suggest that Pae could ameliorate the inflammatory status in CIA via regulating GRK2 in FLS (Chen J.-Y. et al., 2012). To further explore the effect of Pae on β-arrestin 2 in humans, FLS were isolated and cultured in vitro. It was shown that IL-1β-induced β-arrestin 2 suppresses the cAMP-PKA signaling pathway and promotes FLS proliferation. Addition of Pae inhibits FLS proliferation and up-regulates expression of β-arrestin 2 in human FLS. This implies that Pae could repress IL-1β-induced human FLS proliferation via modulation of β-arrestin 2-cAMP-PKA pathway (Wu et al., 2012).
Besides inhibition of FLS proliferation and suppression of inflammatory cytokines through activating the E-prostanoid (EP4) receptor protein expression and modulating intracellular cAMP level, Pae also inhibited thymocyte and splenocyte proliferation in CIA rats (Chang et al., 2011). PI3K/Akt/mTOR signaling mediated by BAFF/BAFF-R participates in antibody production by B lymphocytes of CIA rats. Pae had therapeutic effects on CIA rats via regulating PI3K/Akt/mTOR signal mediated by B cell-activating factor belonging to the TNF family (BAFF)/BAFF-R. Thus Pae could repress antibodies production from B cells (Li et al., 2012). Taken together, Pae exerts powerful anti-inflammatory effects via mainly modulating PEG2-β-arrestin 2-cAMP-PKA in various immune cells, such as B cells, FLS, thymocytes, and splenocytes. Taken together, these results from AA and CIA rat models laid the foundation for further study of Pae in RA therapy.
Osteoarthritis (OA) is another main type of arthritis. Pae treatment also showed therapeutic effects on OA chondrocytes. Pae repressed IL-1β-induced inflammatory factors, including NO, PGE2, iNOS and COX-2, in chondrocytes from OA patients. Moreover, Pae repressed the IL-1β-induced metalloproteinase-3 (MMP-3), MMP-13 and NF-κB p65 in OA patient-derived chondrocytes. Therefore Pae may ameliorate IL-1β-stimulated infammatory factors in chondrocytes from OA patients by inhibiting the activation of the NF-κB pathway (Zhao L. et al., 2018). What’s more, Pae repressed the production of lactate dehydrogenase (LDH) and IL-1β-stimulated apoptosis of rat chondrocytes via activating Akt signaling pathway (Kogut et al., 2005). To summarize, Pae may exert its therapeutical effect by inhibiting harmful effects of chondrocytes and thus, may be a potential agent in the future treatment of OA.
Liver Diseases
Pae treatment showed protective effect for several liver diseases. Ma et al. (2016) demonstrated that the Pae repressed serum alanine transferase (ALT), aspartate transferase (AST) and total levels of cholesterol (TC), low-density lipoprotein (LDL), and TNF-α, from a non-alcoholic steatohepatitis (NASH) rat model via inhibiting Rho kinase (ROCK) and NF-κB pathway. Zhao et al. (Zhao et al., 2017) evaluated the effect of Pae on α-naphthylisothiocyanate (ANIT)-induced cholestasis rat model. Pae inhibited neutrophils infiltration, edema and necrosis in liver tissue. Additionally, Pae repressed the as total bilirubin (TBIL), direct bilirubin (DBIL), AST, ALT, alkaline phosphatase (ALP), γ-glutamyltranspeptidase (γ-GT), total bile acid (TBA) from serum samples that isolated from ANIT-treated rats. The liver expression of NF-κB and IL-1β were repressed and the hepatocyte transporters such as Na+/taurocholate cotransporting polypeptide (NTCP), bile salt export pump (BSEP), multidrug resistance-associated protein 2 (MRP2) were reduced by Pae treatment. The alleviating effect of Pae on the liver seems to be closely associated with down-regulation of activated NF-κB pathway.
The elevated IL-8 is positively related to inflammatory liver diseases, implying that IL-8 inhibition may be a potential treatment of inflammatory liver diseases. Pae ameliorated IL-8-induced liver damage by exerting anti-inflammatory effects on primary human hepatic sinusoidal endothelial cells (HHSECs) through inhibiting IL-8 via repression of ERK1/2 and Akt pathway (Gong et al., 2015). Pae pretreatment reduced the elevated plasma aminotransferase expression and liver necrosis in Concanavalin A (Con A)-induced hepatitis mice model. Moreover, Pae pretreatment repressed the proinflammatory cytokines and infiltration of CD4+, CD8+ and NKT cells in liver. Pae pretreatment also could inhibit the Toll-like receptor (TLR) 4 and NF-κB pathway in Con A-induced liver. These results suggested that Pae pretreatment protects mice against Con A-induced liver damage via suppression of several inflammatory factors and infiltration of CD4+, CD8+ and NKT cells in liver, and Pae might exert this therapeutical effect through inhibition of TLR4 and NF-κB pathway (Chen et al., 2014).
Hepatic ischemia/reperfusion (I/R) injury could induce several side effects and even death after liver resection and transplantation. Pae treatment significantly inhibited I/R-induced serum ALT and AST, hepatic damages/apoptosis, and neutrophils infiltration in liver. The secretion of pro-inflammatory cytokines and expression of high mobility group box-1 (HMGB1), TLR4, phosphorylated ERK1/2, JNK1/2, p38, and NF-κB was repressed by Pae treatment in the I/R-operated mice, suggesting that that Pae also could protect liver I/R injury via suppressing HMGB1-TLR4 pathway (Xie et al., 2018).
Zhang et al. (2015) evaluated the effects of Pae on Nonalcoholic fatty liver disease (NAFLD) by using a high-fat diet mice model. Pae prevented NAFLD development by down-regulating inflammation (phosphoenolpyruvate carboxykinase and G6Pase), hyperlipidemia (lipid synthesis pathway [3-hydroxy-3-methyl glutaryl coenzyme A reductase (HMG-CoAR) and peroxisome proliferator-activated receptor (PPAR) pathways], insulin resistance and body weight. In addtion, Pae also protected the cardiovascular system of NAFLD mice. Kim et al. investigated the protective effects of Pae on lipopolysaccharide (LPS)-induced inflammation in rat liver. Pae pretreatment repressed LPS-induced glutamate oxaloacetate transaminase, lactate dehydrogenase, glutamate pyruvate transaminase, and malondialdehyde. Additionally, Pae treatment resotred LPS-repressed superoxide dismutase, glutathione peroxidase, and catalase. Pae protected LPS-stimulated damage of liver tissue, suggesting that Pae indeed could ameliorate LPS-induced liver inflammation (Kim and Ha, 2010).
In addition, a potentially important feature of Pae treatment is its ability to protect against hyperplasia of hepatic stellate cells (HSCs) and significant depositions of collagen type I (Col I) and type III (Col III) in experimental schistosomiasis by modulation of TNF-α, IL-6, lipopolysaccharide binding protein (LBP) and CD14 expressions (Liu et al., 2006).
Murine peritoneal macrophages secrete Transforming growth factor beta (TGF-β) 1 after activation with Soluble Egg Antigen (SEA) of Schistosoma japonicum. This causes HSCs proliferation and secretion of Col I and III. Addition of Pae to macrophage-conditioned medium inhibits these pathological features of hepatic fibrosis HSCs (Chu et al., 2007). IL-13 is closely associated with the development of schistosome fibrosis. While IL-13 receptor (R) a2 is an effective target in attenuation of fibrosis. A mouse model for liver fibrosis was established by subcutaneous infection with S. japonicum cercariae. Pae had suppressive effect on the increase of both hepatic hydroxyproline and Col I and III, which are the main components of extracellular matrix (ECM). Moreover, Pae inhibited IL-13 production and elevates IL-13Ra2 in Pae-treated groups. Therefore Pae meliorated liver fibrosis via rebalancing of IL-13 and IL-13Ra2 (Li et al., 2009). In another IL-13 related study, Pae inhibited IL-13-induced collagen synthesis in the in vitro culture of primary hepatic stellate cells (HSCs), implying that Pae could alleviate the hepatic granulomas and fibrosis via modulating IL-13 signaling pathway in HSCs (Li et al., 2010). Moreover, IL-13 secretion was up-regulated from liver alternative activated macrophages. Pae repressed Signal transducer and activator of transcription (STAT) 6, phosphorylations of janus-activated kinase 2 (JAK2), and Arginase-1 in alternative activation of macrophages, then causing repression of IL-13 secretion. Therefore, Pae is a promising prophylactic agent for hepatic granuloma and fibrosis of schistosomiasis japonica (Chu et al., 2011).
Prostaglandin E2 (PGE2) and its four prostanoid receptors (EP1-4) are involved in tumor development and progression (Aoki and Narumiya, 2017). Pae significantly inhibited the proliferation and induced apoptosis in butaprost-stimulated HepG2 and SMMC-7721 cells. Pae induced apoptosis in hepatocellular carcinoma cells by moudulating PGE2-EP2 pathway and inducing the Bax-to-Bcl-2 ratio, suggesting that Pae might be a promising agent in the treatment of liver cancer (Hu et al., 2013).
Kidney Diseases
High glucose activated macrophages mainly through TLR2-dependent pathway which aggravated the severity of renal inflammation and eventually contributed to diabetic nephropathy (DN). Pae might be used as a potential therapeutic agent against progressive DN (Shao et al., 2016). In vivo, Pae reduced the urinary albumin excretion rate and inhibit macrophage infiltration and activation through inhibition of the TLR2/4 pathway. In vitro, Pae reduced the advanced glycation end products (AGEs)-induced TLR2/4 activation and inflammatory responses. These findings indicated that Pae prevents macrophage activation via regulating TLR2/4 signaling activation in DN (Zhang T. et al., 2017). The effects of Pae on the kidneys of mice with streptozotocin-induced type 1 diabetes mellitus was evaluated by using TLR2 knockout mice (TLR2-/-). After 12 weeks of Pae treatment, diabetic mice had significantly reduced albuminuria and attenuated renal histopathology. These changes were associated with substantially alleviated macrophage infiltration and down-regulation of TLR2 signaling pathway. These data supported the idea that the curative effects of Pae on the kidney of diabetic mice are associated with the regulation of the TLR2 pathway. Pae thus shows therapeutic potential for the prevention and treatment of DN (Shao et al., 2017).
Zhang et al. (2013) evaluated the protective activities of Pae on advanced glycation end product-induced oxidative stress and inflammation in mesangial cells. Pae pretreatment induced advanced glycation end product-induced glutathione peroxidase and catalase and repressed the macrophages migration in a co-culture system of mesangial cells and macrophages. In addition, the advanced glycation end products-induced IL-6 and monocyte chemoattractant protein (MCP)-1 was repressed by Pae pretreatment. These results showed that Pae could ameliorate advanced glycation end products-induced oxidative damage and inflammation in mesangial cells.
The potential treatment effects of Pae on acute renal injury induced by acute necrotizing pancreatitis (ANP) were investigated in a rat model. Pae repressed acute renal injury by suppressing inflammatory reaction and apoptosis of renal cell via modulaing p38MAPK and NF-κB pathway (Wang et al., 2016). Another paper (Liu et al., 2016) reported the protecetive effects of Pae on renal function by using a cyclophosphamide (CYP)-induced mice model. Pae ameliorated the damage of kidney tissues, such as apoptosis, caused by CYP. Pae decreased the uric acid and creatinine in urine and inflammatory cytokines in serum through up-regulating AMPK pathway and down-regulating NF-κB pathway. Therefore Pae is a potential agent for kidney toxicity.
Other Inflammatory Related Conditions
Recombinant human interleukin-1b (rhIL-1β) was used to treat primary monocytes to imitate inflammatory condition in vitro. Pae showed low cytotoxicity on rhIL-1β-treated monocytes. Pae significantly suppressed phagocytic function of rhIL-1β-induced monocytes, and decreased the levels of TNF-α and PGE2 production. Administration of Pae significantly inhibited the HLA-DR and CD80 with rhIL-1β-stimulated monocytes. These results indicated that Pae could inhibit activation and normal function of monocytes in human peripheral blood (Wang D. et al., 2012).
Another similar study focused on the effect of Pae on rhIL-1β-stimulated human peripheral blood mononuclear cells (PBMCs). Pae inhibited the proliferation of rhIL-1β-treated PBMCs and production of IL-17 and IL-10. rhIL-1β-induced down-regulation of PBMCs CD4+CD25+Foxp3+ subpopulation numbers was also repressed by Pae. Therefore, Pae exerts its anti-inflammatory effects via regulating IL-17/IL-10 secretion (Dai et al., 2015).
Topical application of dinitrochlorobenzene (DNCB) induced cutaneous inflammation. Thymocyte proliferation in the mice with allergic contact dermatitis (ACD) was significantly inhibited by Pae. IL-4/IL-10 production was induced and IL-2/IL-17 was redued in Pae-treated thymocyte and splenocyte. Therefore anti-inflammatory action of Pae in the murine model of allergic ACD is through its regulation of the imbalanced cytokine production (Wang et al., 2013). In summary, the anti-inflammaotry and immune regulatroy roles of Pae was summarized in Table 1.
Table 1.
The summary of curative effects of Pae.
| Disorder’s and related models | Related cells | Related target of pathway | Reference | |
|---|---|---|---|---|
| Arthritis | AA rats | Mesenteric lymph node (MLN) lymphocytes | B2-AR and β-arrestinl/2-cAMP | Wu et al., 2007 |
| Blood sample and joint tissue | NF-kB pathway | Jia and He, 2016 | ||
| CIA rats | FLS | Gi-cAMP-PKA | Zhang et al., 2008 | |
| GRK2 | Chen Y. et al., 2012 | |||
| Thymocyte and sptenocyte | EP4-CAMP | Chang et al., 2011 | ||
| B lymphocytes | TNF family (BAFF)/BAFF-R-P13K/Akt/mTOR | Li et al., 2012 | ||
| Joint synovium | Rho kinase, NF-KB pathway | Zhai et al., 2018 | ||
| Serum | TNF-a, IL-1β | Wu et al., 2013 | ||
| OA | Human chondrocyte | NF-KB pathway | Zhao M. et al., 2018 | |
| Rat chondrocyte | Akt pathway | Kogut et al., 2005 | ||
| IL-1β-induced inflammation | Human FLS | β-arrestin 2-cAMP-PKA | Wu et al., 2012 | |
| Liver disease | Immunological liver injury | Mice liver | TNF-a, IL-6, LBP and CD14 | Liu et al., 2006 |
| Non-alcoholic steatohepatitis | Rat liver | ROCK/NF-KB pathway | Ma et al., 2016 | |
| Cholestasis | Rat liver | NF-KB pathway and hepatocyte transporters | Zhao et al., 2017 | |
| IL-8 induced inflammatory damage | Primary human hepatic sinusoidal endothelial cells | ERK1/2 and Akt pathway | Gong et al., 2015 | |
| Con A-induced hepatitis | Mice liver | TLR4 and NF-KB pathway | Chen et al., 2014 | |
| Hepatic ischemia/reperfusion injury | Mice liver | HMGB1-TLR4 pathway | Xie et al., 2018 | |
| Nonalcoholic fatty liver disease | Rat liver | PPAR pathway | Zhang et al., 2015 | |
| LPS-induced liver inflammation | Rat liver | Oxidative stress markers | Kim and Ha, 2010 | |
| Fibrosis | Macrophages conditional medium-treated HSCs | TGF-β1 signaling | Chu et al., 2007 | |
| Mice liver | IL-13 and IL-13Ra2 | Li et al., 2009 | ||
| Hepatic stellate cells | IL-13 pathway | Li et al., 2010 | ||
| Hepatic granuloma | Macrophages | JAK-STAT pathway | Chu et al., 2011 | |
| Liver cancer | HepG2 and SMMC-7721 cells | PGE2-EP4 | Hu et al., 2013 | |
| Kidney disease | Diabetic nephropathy (ON) | Macrophage | TLR2/4 signaling | Shao et al., 2016; Zhang T. et al., 2017; Shao et al., 2017 |
| Advanced glycation end product-induced oxidative stress and inflammation in mesangial cells | Mesangial cells and macrophages | None | Zhang et al., 2013 | |
| Acute renal injury | Kidney tissue | MAPK and NF-KB pathway | Wang et al., 2016 | |
| Cyclophosphamide - induced renal damage | Kidney tissue | MAPK and NF-KB pathway | Liu et al., 2016 | |
| Other disorders | rhIL-16-induced inflammation | Circulating Monocyte | HLA-DR and CD80 | Wang D. et al., 2012 |
| PBMC | 1L-17 and 1L-10 | Dai et al., 2015 | ||
| Allergic contact dermatitis | Thymocyte and splenocyte | IL-4/IL-10andlL-2/IL-17 | Wang et al., 2013 | |
Pharmacokinetics (PK)
The pharmacokinetics of Pae microemulsion and Pae saline was compared by our group. Pae microemulsion and Pae were given to AA rats for 10 days. Compared to the Pae group, the area under the plasma concentration-time curve [AUC(0-t)], maximum concentration [C(max)] and mean retention time MRT(0-infinity))(h) of Pae microemulsion were up-regulated, while volume of distribution (Vd) and clearance rate (CL/F) decreased, suggesting that microemulsion significantly improves the absorption of Pae in AA rats (Wang C. et al., 2012).
The Pharmacological Effects of CP-25
Arthritis
By using a T cell and FLS co-culture system, CP-25 repressed the proliferation and production of pro-inflammatory cytokines of FLS via inhibiting BAFF-R in CD4+ T cells, suggesting that CP-25 could interfere in the crosstalk between T cells and FLS in vitro (Jia et al., 2016). The AA model was used to investigate the anti-arthritic activity of CP-25. In general, CP-25 repressed both the clinical and the histopathological scores of arthritis. The levels of pro-inflammatory cytokines, including IL-1β, IL-6 and TNF-α, were decreased and after CP-25 treatment the anti-inflammatory cytokine TGF-β1 could be detected in serum. Furthermore, CP-25 treatment polarized peritoneal macrophages from a M1 to a M2 phenotype, inhibited Th17-IL-17, suppressed the Th17-associated transcription factor RAR-related orphan receptor gamma (ROR-γt), the receptor activator of nuclear factor kappa B ligand (RANKL) and matrix metalloproteinase (MMP) 9 in AA rats (Chang et al., 2016).
Other Chronic Inflammatory Diseases
Bone marrow dendritic cells (DCs) were isolated from BALB/c mice and stimulated by PGE2 and TNF-α, respectively, which induced CD40, CD80, CD83, CD86, and MHC-II and suppressed the antigen uptake by DCs. Additionally, the proliferation of T cells was induced using a co-culture system. The expression of surface markers, DC antigen uptake and DC-mediated proliferation of T cells were inhibited by CP-25 treatment. Moreover, CP-25 decreased PGE2-induced EP4 and NF-κB and induced PGE2-suppressed increase of cAMP in DCs. TNF-α-induced TNFR1, TRADD, TRAF2, and NF-κB were also inhibited by CP-25 in DC, suggesting that CP-25 modulates DCs immune function via regulating PGE2-EP4-cAMP and TNF-α-TNFR1-TRADD-TRAF2-NF-κB pathways (Li et al., 2015). While BAFF or TNF-α could induce B lymphocytes proliferation in vitro additional CP-25 treatment suppressed B lymphocytes proliferation. Moreover, CP-25 also reduced the numbers of B lymphocytes subtypes, including CD19+ B lymphocytes, CD19+CD20+ B lymphocytes, CD19+CD27+ B lymphocytes and CD19+CD20+CD27+ B lymphocytes, and down-regulated BAFF or TNF-α-induced expression of BAFFR, BCMA, and TACI. Interestingly, this study also compared the effects between Rituximab, Etanercept and CP-25 treatments. Results showed that addition of CP-25 moderately restored a hyper-activated B lymphocyte function to a physiological level by regulating the classical and alternative NF-κB signaling pathway mediated by BAFF. On the contrary, the inhibitory effects on BAFF-BAFFR-NF-κB pathway are much more obvious in Rituximab and Etanercept treatment groups. Therefore CP-25 is a promising anti-inflammatory immune agent as a modifying drug that minimize the potential side effects (Zhang F. et al., 2017).
A hallmark in patients with autoimmune diseases is the elevated presence of immunoglobulin D (IgD) (Wu et al., 2016, 2017). IgD binds to IgD receptor (IgDR) on CD4+ T cells from human PBMC, which leads to activation/proliferation of T cells by enhancing phosphorylation of the activating tyrosine residue of Lck (Tyr394). CP-25 treatment could repress the IgD-induced activation/proliferation of CD4+ T cells by repressing of Lck (Tyr 394) phosphorylation. These results demonstrate CP-25 is a novel potential therapeutic agent for human autoimmune diseases via modulating T cells function (Wu et al., 2018).
Primary Sjögren’s syndrome (pSS) is a chronic inflammatory autoimmune disease that is featured by various immune abnormalities in moisture-producing glands. CP-25 alimeorated the clinical manifestations, including salivary flow and histopathological scores, of in NOD/Ltj mice (a mice model of pSS). Compared to control group, lymphocyte viability and the infiltration of Th1/Th2 cells in salivary glands were repressed in CP-25-treated NOD/Ltj mice. Moreover, CP-25 treatment skewed the ratio of Th17/regulatory T (Treg) cells in the spleen on NOD/Ltj mice. A concentration of inflammatory cytokines and anti-La/SSB and IgG antibodies in the serum from NOD/Ltj mice was repressed by CP-25. This study suggested that CP-25 is a potential agent for pSS by regulating T lymphocyte subsets (Fang et al., 2018). Taken together, CP-25 was developed which enhanced lipophilicity and strong anti-inflammatory and immune regulatory properties (Table 2).
Table 2.
The summary of curative effects of CP-25.
| Disorders and related models | Related cells | Related target or pathway | Reference | |
|---|---|---|---|---|
| Arthritis | AA rats | Macrophages | Thl7-IL-17, ROR-yt, RANKL | Kim and Ha, 2010 |
| CIA mice | T lymphocytes and FLS | β2-AR pathway | Liu et al., 2006 | |
| Other disorders | BAFF-induced inflammation | T lymphocytes and FLS | BAFF-R pathway | Chu et al., 2007 |
| PGE2 or TNF-α induced inflammation | DC and T lymphocytes | PGE2-EP4-cAMP and TNF-α-TNFR1-TRADD-TRAF2-NF-KB pathways | Li et al., 2009 | |
| BAFF or TNF-α could induce inflammation | B lymphocytes | BAFF-BAFFR-NF-KB pathway | Li et al., 2010 | |
| IgD-induced T cells activation | T lymphocytes | Lck (Tyr 394) | Chu et al., 2011 | |
| Sjögren’s syndrome | Thl7/regulatory T (Treg) cells | Anti-SSB/La and IgG antibody | Aoki and Narumiya, 2017 | |
Absorption and Excretion
As a bioavailable derivative of Pae, CP-25 improves the absorption of Pae. This has been attributed to both the lipid solubility enhancement and its resistance to P-gp-mediated efflux (Yang et al., 2016). With regard to tissue distribution, CP-25 concentration was higher in most tissues when compared to Pae via an oral route with the highest concentration in the liver at 3 h after oral treatment. Other tissues, including intestine, synovium, muscle, lung, and brain in both male and female rat, also showed high concentration of CP-25. Following a single oral dose of 50 mg/kg in rats, CP-25 was primarily excreted in the feces. There is a gender-related difference in the tissue distribution and excretion (Zhao M. et al., 2018).
Outlook
The mixture of glycosides Pae has been shown to be anti-inflammatory, anti-neoplastic, anti-hyperglycemia, and neuroprotective. To improve oral bioavailability of Pae. Paeoniflorin-6′-O-benzene sulfonate (CP-25) was developed which enhanced lipophilicity and significant increases could be shown in animal experiments. Current basic and preclinical studies imply that these natural compounds with their strong anti-inflammatory properties should be considered in the clinic. As shown in various preclinical studies, Pae and its derivative CP-25 demonstrated positive effects on chronic inflammatory diseases, such as arthritis, and in different models of liver injury and kidney injury. The use in therapy modulated inflammatory mediators such as cytokines (IL-1β, IL-6, TNF-α), chemokines (CCL8), pattern recognition receptors and their relevant transcription factors (STAT, NF-κB). Although the effectiveness of Pae and CP-25 has been demonstrated, aspects such as pharmacokinetics and product safety need to be considered. While CIA or AA rat models have been used widely the joint inflammation model K/BxN which is achieved by serum transfer would be a more reliable model to investigate the pathological mechanism of RA.
Author Contributions
JT drafted the manuscript. YG, WH, YF, DH, PZ, XW, HK, and WW revised the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This study was supported by the National Natural Science Foundation of China (81330081 and 81673444), Natural Science Foundation of Anhui Province for Young Scholars (1708085QH200), and Grants for Scientific Research of BSKY from Anhui Medical University (4501041101).
References
- Absolute T. H. E., Of S., Chinese F., Root P. (1972). Chemical studies on the oriental plant drugs—XXXIII: the absolute structures of paeoniflorin, albiflorin, oxypaeoniflorin and benzoylpaeoniflorin isolated from chinese paeony root. Tetrahedron 28 4309–4317. [Google Scholar]
- Aoki T., Narumiya S. (2017). Prostaglandin E2-EP2 signaling as a node of chronic inflammation in the colon tumor microenvironment. Inflamm. Regen. 37:4. 10.1186/s41232-017-0036-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan K., Liu Z. Q., Jiang Z. H., Zhou H., Wong Y. F., Xu H. X., et al. (2006). The effects of sinomenine on intestinal absorption of paeoniflorin by the everted rat gut sac model. J. Ethnopharmacol. 103 425–432. 10.1016/j.jep.2005.08.020 [DOI] [PubMed] [Google Scholar]
- Chang Y., Jia X., Wei F., Wang C., Sun X., Xu S., et al. (2016). CP-25, a novel compound, protects against autoimmune arthritis by modulating immune mediators of inflammation and bone damage. Sci. Rep. 6:262394. 10.1038/srep26239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y., Zhang L., Wang C., Jia X. Y., Wei W. (2011). Paeoniflorin inhibits function of synoviocytes pretreated by rIL-1α and regulates EP4receptor expression. J. Ethnopharmacol. 137 1275–1282. 10.1016/j.jep.2011.07.057 [DOI] [PubMed] [Google Scholar]
- Chen J.-Y., Wu H.-X., Chen Y., Zhang L.-L., Wang Q.-T., Sun W.-Y., et al. (2012). Paeoniflorin inhibits proliferation of fibroblast-like synoviocytes through suppressing g-protein-coupled receptor kinase 2. Planta Med. 78 665–671. 10.1055/s-0031-1298327 [DOI] [PubMed] [Google Scholar]
- Chen M., Cao L., Luo Y., Feng X., Sun L., Wen M., et al. (2014). Paeoniflorin protects against concanavalin A-induced hepatitis in mice. Int. Immunopharmacol. 24 42–49. 10.1016/j.intimp.2014.11.006 [DOI] [PubMed] [Google Scholar]
- Chen Y., Wang J., Wang L., Chen L., Wu Q. (2012). Absorption and interaction of the main constituents from the traditional chinese drug pair shaoyao-gancao via a caco-2 cell monolayer model. Molecules 17 14908–14917. 10.3390/molecules171214908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Li X. P., Li Z. J., Xu L., Li X. M. (2013). Reduced hepatotoxicity by total glucosides of paeony in combination treatment with leflunomide and methotrexate for patients with active rheumatoid arthritis. Int. Immunopharmacol. 15 474–477. 10.1016/j.intimp.2013.01.021 [DOI] [PubMed] [Google Scholar]
- Cheng Y., Peng C., Wen F., Zhang H. (2010). Pharmacokinetic comparisons of typical constituents in white peony root and sulfur fumigated white peony root after oral administration to mice. J. Ethnopharmacol. 129 167–173. 10.1016/j.jep.2009.12.040 [DOI] [PubMed] [Google Scholar]
- Chu D., Du M., Hu X., Wu Q., Shen J. (2011). Paeoniflorin attenuates schistosomiasis japonica-associated liver fibrosis through inhibiting alternative activation of macrophages. Parasitology 138 1259–1271. 10.1017/S0031182011001065 [DOI] [PubMed] [Google Scholar]
- Chu D., Luo Q., Li C., Gao Y., Yu L., Wei W., et al. (2007). Paeoniflorin inhibits TGF-β1-mediated collagen production by Schistosoma japonicum soluble egg antigen in vitro. Parasitology 134 1611–1621. 10.1017/S0031182007002946 [DOI] [PubMed] [Google Scholar]
- Dai X., Wang L. W., Jia X. Y., Chang Y., Wu H. X., Wang C., et al. (2015). Paeoniflorin regulates the function of human peripheral blood mononuclear cells stimulated by rhIL-1β by up-regulating Treg expression. Immunopharmacol. Immunotoxicol. 37 252–257. 10.3109/08923973.2015.1026603 [DOI] [PubMed] [Google Scholar]
- Fang G., Shixia X., Zhang P., Chen X., Wu Y., Wang C., et al. (2018). CP-25 alleviates experimental sjögren’s syndrome features in NOD/Ltj mice and modulates T lymphocyte subsets. Basic Clin. Pharmacol. Toxicol. 123 423–434. 10.1111/bcpt.13025 [DOI] [PubMed] [Google Scholar]
- Gong W. G., Lin J. L., Niu Q. X., Wang H. M., Zhou Y. C., Chen S. Y., et al. (2015). Paeoniflorin diminishes ConA-induced IL-8 production in primary human hepatic sinusoidal endothelial cells in the involvement of ERK1/2 and Akt phosphorylation. Int. J. Biochem. Cell. Biol. 62 93–100. 10.1111/imr.12374 [DOI] [PubMed] [Google Scholar]
- Hu S., Sun W., Wei W., Wang D., Jin J., Wu J., et al. (2013). Involvement of the prostaglandin E receptor EP2 in paeoniflorin-induced human hepatoma cell apoptosis. Anticancer Drugs 24 140–149. 10.1097/CAD.0b013e32835a4dac [DOI] [PubMed] [Google Scholar]
- Jia X., Wei F., Sun X., Chang Y., Xu S., Yang X., et al. (2016). CP-25 attenuates the inflammatory response of fibroblast-like synoviocytes co-cultured with BAFF-activated CD4+T cells. J. Ethnopharmacol. 189 194–201. 10.1016/j.jep.2016.05.034 [DOI] [PubMed] [Google Scholar]
- Jia Z., He J. (2016). Paeoniflorin ameliorates rheumatoid arthritis in rat models through oxidative stress, inflammation and cyclooxygenase 2. Exp. Ther. Med. 11 655–659. 10.3892/etm.2015.2908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim I. D., Ha B. J. (2010). The effects of paeoniflorin on LPS-induced liver inflammatory reactions. Arch. Pharm. Res. 33 959–966. 10.1007/s12272-010-0620-8 [DOI] [PubMed] [Google Scholar]
- Kogut S. J., El-Maouche D., Abughosh S. M. (2005). Paeoniflorin inhibits IL-1β-induced chondrocyte apoptosis by regulating the Bax/Bcl-2/caspase-3 signaling pathway. Pharmacotherapy 25 1729–1735. 10.3892/mmr.2018.8631 [DOI] [PubMed] [Google Scholar]
- Li C. L., He J., Li Z. G., Zheng L. W., Hua H. (2013). Effects of total glucosides of paeony for delaying onset of Sjogren’s syndrome: an animal study. J. Craniomaxillofac. Surg 41 610–615. 10.1016/j.jcms.2012.11.042 [DOI] [PubMed] [Google Scholar]
- Li J., Chen C. X., Shen Y. H. (2011). Effects of total glucosides from paeony (Paeonia lactiflora Pall) roots on experimental atherosclerosis in rats. J. Ethnopharmacol. 135 469–475. 10.1016/j.jep.2011.03.045 [DOI] [PubMed] [Google Scholar]
- Li P. P., Liu D. D., Liu Y. J., Song S. S., Wang Q. T., Chang Y., et al. (2012). BAFF/BAFF-R involved in antibodies production of rats with collagen-induced arthritis via PI3K-Akt-mTOR signaling and the regulation of paeoniflorin. J. Ethnopharmacol. 141 290–300. 10.1016/j.jep.2012.02.034 [DOI] [PubMed] [Google Scholar]
- Li X., Shen J., Zhong Z., Peng J., Wen H., Li J., et al. (2010). Paeoniflorin ameliorates schistosomiasis liver fibrosis through regulating IL-13 and its signalling molecules in mice. Parasitology 137 1213–1225. 10.1017/S003118201000003X [DOI] [PubMed] [Google Scholar]
- Li X., Shen J., Zhong Z., Wen H., Luo Q., Wei W. (2009). Paeoniflorin: a monomer from traditional Chinese medical herb ameliorates Schistosoma japonicum egg-induced hepatic fibrosis in mice. J. Parasitol. 95 1520–1524. 10.1645/GE-1994.1 [DOI] [PubMed] [Google Scholar]
- Li Y., Sheng K., Chen J., Wu Y., Zhang F., Chang Y., et al. (2015). Regulation of PGE2 signaling pathways and TNF-alpha signaling pathways on the function of bone marrow-derived dendritic cells and the effects of CP-25. Eur. J. Pharmacol. 769 8–21. 10.1016/j.ejphar.2015.09.036 [DOI] [PubMed] [Google Scholar]
- Liu D. F., Wei W., Song L. H. (2006). Protective effect of paeoniflorin on immunological liver injury induced by bacillus Calmette-Guerin plus lipopolysaccharide: modulation of tumour necrosis factor-α and interleukin-6 mRNA. Clin. Exp. Pharmacol. Physiol. 33 332–339. 10.1111/j.1440-1681.2006.04371.x [DOI] [PubMed] [Google Scholar]
- Liu D. Z., Xie K. Q., Ji X. Q., Ye Y., Jiang C. L., Zhu X. Z. (2005). Neuroprotective effect of paeoniflorin on cerebral ischemic rat by activating adenosine A1receptor in a manner different from its classical agonists. Br. J. Pharmacol. 146 604–611. 10.1038/sj.bjp.0706335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q., Lin X., Li H., Yuan J., Peng Y., Dong L., et al. (2016). Paeoniflorin ameliorates renal function in cyclophosphamide-induced mice via AMPK suppressed inflammation and apoptosis. Biomed. Pharmacother. 84 1899–1905. 10.1016/j.biopha.2016.10.097 [DOI] [PubMed] [Google Scholar]
- Ma Z., Chu L., Liu H., Li J., Zhang Y., Liu W., et al. (2016). Paeoniflorin alleviates non-alcoholic steatohepatitis in rats: Involvement with the ROCK/NF-κB pathway. Int. Immunopharmacol. 38 377–384. 10.1016/j.intimp.2016.06.023 [DOI] [PubMed] [Google Scholar]
- Shao Y. X., Xu X., Wang K., Qi X. M., Wu Y. G. (2017). Paeoniflorin attenuates incipient diabetic nephropathy in streptozotocin-induced mice by the suppression of the toll-like receptor-2 signaling pathway. Drug Des. Devel. Ther. 11 3221–3233. 10.2147/DDDT.S149504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Y. X., Xu X. X., Li Y. Y., Qi X. M., Wang K., Wu Y. G., et al. (2016). Paeoniflorin inhibits high glucose-induced macrophage activation through TLR2-dependent signal pathways. J. Ethnopharmacol. 193 377–386. 10.1016/j.jep.2016.08.035 [DOI] [PubMed] [Google Scholar]
- Su J., Zhang P., Zhang J. J., Qi X. M., Wu Y. G., Shen J. J. (2010). Effects of total glucosides of paeony on oxidative stress in the kidney from diabetic rats. Phytomedicine 17 254–260. 10.1016/j.phymed.2009.07.005 [DOI] [PubMed] [Google Scholar]
- Sun W.-Y. (2013). A standardized extract from Paeonia lactiflora and Astragalus membranaceus induces apoptosis and inhibits the proliferation, migration and invasion of human hepatoma cell lines. Int. J. Oncol. 43 1643–1651. 10.3892/ijo.2013.2085 [DOI] [PubMed] [Google Scholar]
- Takeda S., Isono T., Wakui Y., Matsuzaki Y., Sasaki H., Amagaya S., et al. (1995). Absorption and excretion of paeoniflorin in rats. J. Pharm. Pharmacol. 47 1036–1040. 10.1111/j.2042-7158.1995.tb03293.x [DOI] [PubMed] [Google Scholar]
- Wang C., Yuan J., Wu H. X., Chang Y., Wang Q. T., Wu Y. J., et al. (2013). Paeoniflorin inhibits inflammatory responses in mice with allergic contact dermatitis by regulating the balance between inflammatory and anti-inflammatory cytokines. Inflamm. Res. 62 1035–1044. 10.1007/s00011-013-0662-8 [DOI] [PubMed] [Google Scholar]
- Wang C., Yuan J., Yang Z., Nie X., Song L., Wei W. (2012). Pharmacokinetics of paeoniflorin microemulsion after repeated dosing in rats with adjuvant arthritis. Pharmazie 67 997–1001. 10.1691/ph.2012.2026 [DOI] [PubMed] [Google Scholar]
- Wang D., Yuan F., Wang L., Wei W. (2012). Paeoniflorin inhibits function and down-regulates HLA-DR and CD80 expression of human peripheral blood monocytes stimulated by RhIL-1β. Int. Immunopharmacol. 14 172–178. 10.1016/j.intimp.2012.07.005 [DOI] [PubMed] [Google Scholar]
- Wang P., Wang W., Shi Q., Zhao L., Mei F., Li C., et al. (2016). Paeoniflorin ameliorates acute necrotizing pancreatitis and pancreatitis-induced acute renal injury. Mol. Med. Rep. 14 1123–1131. 10.3892/mmr.2016.5351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. N., Zhang Y., Wang Y., Zhu D. X., Xu L. Q., Fang H., et al. (2014). The beneficial effect of total glucosides of paeony on psoriatic arthritis links to circulating tregs and th1 cell function. Phyther. Res. 28 372–381. 10.1002/ptr.5005 [DOI] [PubMed] [Google Scholar]
- Wu D., Chen J., Zhu H., Xiong X. G., Liang Q. H., Zhang Y., et al. (2013). UPLC-PDA determination of paeoniflorin in rat plasma following the oral administration of radix paeoniae alba and its effects on rats with collagen-induced arthritis. Exp. Ther. Med. 7 209–217. 10.3892/etm.2013.1358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H., Wei W., Song L., Zhang L., Chen Y., Hu X. (2007). Paeoniflorin induced immune tolerance of mesenteric lymph node lymphocytes via enhancing beta 2-adrenergic receptor desensitization in rats with adjuvant arthritis. Int. Immunopharmacol. 7 662–673. 10.1016/j.intimp.2007.01.019 [DOI] [PubMed] [Google Scholar]
- Wu H. X., Chen J. Y., Wang Q. T., Sun W. Y., Liu L. H., Zhang L. L., et al. (2012). Expression and function of β-arrestin 2 stimulated by IL-1β in human fibroblast-like synoviocytes and the effect of paeoniflorin. Int. Immunopharmacol. 12 701–706. 10.1016/j.intimp.2012.01.018 [DOI] [PubMed] [Google Scholar]
- Wu Y., Chen W., Chen H., Zhang L., Chang Y., Yan S., et al. (2016). The elevated secreted immunoglobulin D enhanced the activation of peripheral blood mononuclear cells in rheumatoid arthritis. PLoS One 11:e0147788. 10.1371/journal.pone.0147788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y. J., Chen H. S., Chen W. S., Dong J., Dong X. J., Dai X., et al. (2018). CP-25 attenuates the activation of CD4+T cells stimulated with immunoglobulin D in human. Front. Pharmacol. 9:4. 10.3389/fphar.2018.00004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y. J., Chen W. S., Chen H. S., Dai X., Dong J., Wang Y., et al. (2017). The immunoglobulin D Fc receptor expressed on fibroblast-like synoviocytes from patients with rheumatoid arthritis contributes to the cell activation. Acta Pharmacol. Sin. 38 1466–1474. 10.1038/aps.2017.105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie T., Li K., Gong X., Jiang R., Huang W., Chen X., et al. (2018). Paeoniflorin protects against liver ischemia/reperfusion injury in mice via inhibiting HMGB1-TLR4 signaling pathway. Phyther. Res. 32 2247–2255. 10.1002/ptr.6161 [DOI] [PubMed] [Google Scholar]
- Yang H. O., Ko W. K., Kim J. Y., Ro H. S. (2004). Paeoniflorin: An antihyperlipidemic agent from Paeonia lactiflora. Fitoterapia 75 45–49. 10.1016/j.fitote.2003.08.016 [DOI] [PubMed] [Google Scholar]
- Yang X. D., Wang C., Zhou P., Yu J., Asenso J., Ma Y., et al. (2016). Absorption characteristic of paeoniflorin-6’-O-benzene sulfonate (CP-25) in in situ single-pass intestinal perfusion in rats. Xenobiotica 46 775–783. 10.3109/00498254.2015.1121553 [DOI] [PubMed] [Google Scholar]
- Yang X.-W., Guo J., Xu W. (2013). Absorption and transport characteristic of paeoniflorin and its derivatives in model of Caco-2 cell monolayers. Chin. Tradit. Herbal Drugs 44 2097–2104 10.7501/j.issn.0253-2670.2013.15.014 [DOI] [Google Scholar]
- Zhai W., Ma Z., Wang W., Song L., Yi J. (2018). Paeoniflorin inhibits Rho kinase activation in joint synovial tissues of rats with collagen-induced rheumatoid arthritis. Biomed. Pharmacother. 106 255–259. 10.1016/j.biopha.2018.06.130 [DOI] [PubMed] [Google Scholar]
- Zhang F., Shu J.-L., Li Y., Wu Y.-J., Zhang X.-Z., Han L., et al. (2017). CP-25, a novel anti-inflammatory and immunomodulatory drug, inhibits the functions of activated human b cells through Regulating BAFF and TNF-alpha Signaling and comparative efficacy with biological agents. Front. Pharmacol. 8:933. 10.3389/fphar.2017.00933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L. J., Yang B., Yu B. P. (2015). Paeonillorin protects against nonalcoholic fatty liver disease induced by a high-fat diet in mice. Biol. Pharm. Bull. 38 1005–1011. 10.1248/bpb.b14-00892 [DOI] [PubMed] [Google Scholar]
- Zhang L. L., Wei W., Wang N. P., Wang Q. T., Chen J. Y., Chen Y., et al. (2008). Paeoniflorin suppresses inflammatory mediator production and regulates G protein-coupled signaling in fibroblast - Like synoviocytes of collagen induced arthritic rats. Inflamm. Res. 57 388–395. 10.1007/s00011-007-7240-x [DOI] [PubMed] [Google Scholar]
- Zhang M. H., Feng L., Zhu M. M., Gu J. F., Wu C., Jia X. (2013). Antioxidative and anti-inflammatory activities of paeoniflorin and oxypaeoniflora on AGEs-induced mesangial cell damage. Planta Med. 79 1319–1323. 10.1055/s-0033-1350649 [DOI] [PubMed] [Google Scholar]
- Zhang T., Zhu Q., Shao Y., Wang K., Wu Y. (2017). Paeoniflorin prevents TLR2/4-mediated inflammation in type 2 diabetic nephropathy. Biosci. Trends 11 308–318. 10.5582/bst.2017.01104 [DOI] [PubMed] [Google Scholar]
- Zhang W., Zhao L., Su S.-Q., Xu X.-X., Wu Y.-G. (2014). Total glucosides of paeony attenuate renal tubulointerstitial injury in STZ-induced diabetic rats: role of toll-like receptor 2. J. Pharmacol. Sci. 125 59–67. 10.1254/jphs.13173FP [DOI] [PubMed] [Google Scholar]
- Zhao L., Chang Q., Huang T., Huang C. (2018). Paeoniflorin inhibits IL-1β-induced expression of inflammatory mediators in human osteoarthritic chondrocyte. Mol. Med. Rep. 17 3306–3311. 10.3892/mmr.2017.8222 [DOI] [PubMed] [Google Scholar]
- Zhao M., Zhou P., Yu J., James A., Xiao F., Wang C., et al. (2018). The tissue distribution and excretion study of paeoniflorin-6’-O-benzene sulfonate (CP-25) in rats. Inflammopharmacology 25 1–6. 10.1007/s10787-018-0463-3 [DOI] [PubMed] [Google Scholar]
- Zhao Y., He X., Ma X., Wen J., Li P., Wang J., et al. (2017). Paeoniflorin ameliorates cholestasis via regulating hepatic transporters and suppressing inflammation in ANIT-fed rats. Biomed. Pharmacother. 89 61–68. 10.1016/j.biopha.2017.02.025 [DOI] [PubMed] [Google Scholar]