Abstract
In sepsis, systemic coagulation activation frequently causes disseminated intravascular coagulation (DIC), and the uncontrolled activation of the complement system can induce multiple organ dysfunction and poor prognosis. This study aimed to examine the association of DIC with levels of collectin kidney 1 (CL-K1), a novel collectin of the complement system, and mannose-binding lectin (MBL), a classical-type collectin in patients with sepsis. We collected blood samples prospectively from adult patients with sepsis admitted to the intensive care unit (ICU) from day 1 (admission) to day 5. The CL-K1 and MBL levels were measured by enzyme-linked immunosorbent assay, and DIC was diagnosed by using a scoring algorithm. The correlation of CL-K1 and MBL levels with other coagulation markers was analyzed. There were 37 patients with DIC (DIC group) and 15 without DIC (non-DIC group). Compared to the non-DIC group, the DIC group had more severe conditions and higher mortality. During the 5 days after ICU admission, plasma CL-K1 levels were similar between the groups, but plasma MBL levels were significantly lower in the DIC group. Plasma CL-K1 levels were weakly correlated with prothrombin time, activated partial thromboplastin time, and antithrombin levels; plasma MBL levels were weakly correlated with fibrin/fibrinogen degradation product levels and DIC score. In conclusion, during the first 5 days of ICU admission, plasma CL-K1 levels were similar between the DIC and non-DIC groups. However, plasma MBL levels were lower in the DIC group compared to the non-DIC group, and the significance of this difference grew gradually over time.
Keywords: collectin, complement system proteins, disseminated intravascular coagulation, mannose-binding lectin, sepsis
Introduction
In sepsis and septic shock, activation of systemic coagulation often leads to disseminated intravascular coagulation (DIC).1–3 In the intensive care unit (ICU), almost half of the patients with sepsis have complications from DIC, leading to significantly higher mortality compared to sepsis patients without DIC.3,4 Furthermore, simultaneous activation of the systemic complement system1 is often seen. The complement system is an important component of the innate immune system, but its uncontrolled activation induces systemic inflammation and multiple organ dysfunction.1,5,6
Collectin kidney 1 (CL-K1) is a novel pattern recognition molecule of the complement system and is a member of the collectin family, which includes mannose-binding lectin (MBL) and lung surfactant proteins (surfactant protein A and surfactant protein D) as classical collectins.7–9 The CL-K1 binds to lipopolysaccharides and lipoteichoic acid in microorganisms and activates the lectin-complement pathway.7 While MBL is produced in the liver, CL-K1 is widely synthesized in various organs and tissues other than the kidney, such as the liver, lung, brain, and vascular endothelial cells.7,10 However, the normal plasma concentrations of CL-K1 are almost one-tenth that of MLB.11 Recently, the association between elevated CL-K1 levels and DIC has been reported.12
The main functions of MBL, a lectin first identified 4 decades ago,10,13 include binding with mannose and other carbohydrates on the surfaces of pathogens, and activating both the phagocytic clearance of pathogens and the lectin-complement pathway.5,14,15 A recent meta-analysis reported that MBL levels in patients with sepsis were significantly lower than those in healthy participants.15 Huh et al reported that although MBL levels in patients with severe sepsis were higher than those in patients with septic shock, MBL levels in nonsurvivors with septic shock were lower than those in survivors with septic shock.16 However, Zhao et al reported that MBL levels in patients with sepsis with DIC upon arrival at the emergency department were higher than those in patients without DIC.17 Because of these inconsistent results, the association of MBL levels with sepsis and sepsis-induced DIC is not yet well understood.
The present study investigated and compared the association of sepsis-induced DIC with levels of CL-K1 and MBL. Furthermore, the relationships among CL-K1, MBL, and other coagulation markers were also investigated.
Materials and Methods
Study Population
Approval for this study was obtained from the institutional review board of the ethics committee at the Hokkaido University Hospital and the Asahikawa Medical University. Written informed consent was obtained from all patients or acceptable representatives in accordance with the Declaration of Helsinki.
Patients admitted to the ICU for the treatment of sepsis or septic shock were included in this study. Those patients who die within the first 24 hours after admission were excluded. We collected blood samples prospectively from adult patients with sepsis admitted to the ICU at the Hokkaido University Hospital from day 1 to day 5 after admission. The blood samples were collected in tubes containing EDTA-2Na. The samples were immediately centrifuged at 4°C, and the plasma was stored at −80°C until further measurements. From the medical records of enrolled patients, we retrospectively collected data based on patient characteristics and clinical findings.
Definitions
The DIC score was calculated using the scoring algorithm from the Japanese Association for Acute Medicine DIC scoring system.18 Based on the presence or absence of DIC during the observation period, patients were divided into DIC and non-DIC groups, respectively.
Measurements of CL-K1 and MBL Levels
Plasma concentrations of CL-K1 and MBL were measured using a previously established sandwich enzyme-linked immunosorbent assay (ELISA) method, with minor modifications.11,12 Our sandwich ELISA procedure employed both rabbit polyclonal and murine monoclonal antibodies. All the above antibodies were tested to determine the pair that was the best match for capture antibody and biotinylated detection of antibody. The normal ranges for plasma CL-K1 and MBL levels were set at 0.34 ± 0.13 and 1.72 ± 1.51 μg/mL, respectively. This range was determined by testing for these markers in blood samples taken from 220 healthy Japanese volunteers.11
Statistical Analysis
All variables are expressed as median and interquartile range (ie, first to third quartiles) or as number (percentage). Intergroup comparisons were made using the Mann-Whitney U test or χ2 test. For repeated comparisons, a Bonferroni correction was applied. Correlations between the 2 measurements were investigated using Spearman correlation analysis. SPSS 22.0J (SPSS Inc, Chicago, Illinois) was used for all statistical analyses. The level of significance was set at P < .05.
Results
The present study included 37 patients with DIC (DIC group) and 15 patients without DIC (non-DIC group). Table 1 summarizes the characteristics of patients from the 2 groups. The patients in the DIC group were in a more severe condition than those in the non-DIC group. Furthermore, the mortality rate in the DIC group was higher than that in the non-DIC group.
Table 1.
Characteristics of Patients on Admission to Intensive Care Unit.
| DIC, n = 37 | Non-DIC, n = 15 | P Value | |
|---|---|---|---|
| Mean age (range) | 63 (52-70) | 58 (46-66) | 0.196 |
| Men (%) | 23 (62) | 10 (67) | 1.000 |
| Severity scores on day 1 | |||
| APACHE II score | 27 (21-34) | 23 (19-27) | 0.217 |
| SOFA score | 10 (6-13) | 6 (4-9) | 0.003 |
| DIC score | 5 (3-6) | 2 (2-3) | <0.001 |
| SIRS score | 4 (3-4) | 3 (3-4) | 0.730 |
| Infection sites | |||
| Lung | 13 (35%) | 11 (73%) | 0.142 |
| Abdomen | 14 (38%) | 2 (13%) | |
| Urinary tract | 3 (8%) | 0 (0%) | |
| Soft tissue | 3 (8%) | 1 (7%) | |
| Other | 4 (11%) | 1 (7%) | |
| Laboratory test | |||
| White blood cell, ×109/L | 11.0 (5.0-14.8) | 13.1 (4.9-19.6) | 0.538 |
| Hemoglobin, g/dL | 9.5 (7.9-10.7) | 11.2 (8.9-13.2) | 0.026 |
| Platelet, ×109/L | 93 (34-184) | 203 (153-293) | 0.001 |
| PT, seconds | 14.2 (12.9-15.7) | 13.2 (12.5-14.6) | 0.106 |
| APTT, seconds | 42.7 (36.5-67.1) | 34.6 (27.9-48.4) | 0.077 |
| Fibrinogen, mg/dL | 390 (224-488) | 514 (355-603) | 0.095 |
| Antithrombin, % | 62 (47-70) | 74 (63-83) | 0.031 |
| FDP, mg/L | 18.3 (11.3-27.7) | 9.1 (5.3-13.6) | 0.007 |
| d-Dimer, mg/L | 12.1 (5.9-20.1) | 3.7 (2.5-7.2) | 0.003 |
| CRP, mg/L | 18.0 (11.5-25.1) | 22.4 (13.9-29.6) | 0.364 |
| Mortality | |||
| ICU mortality | 9 (24%) | 0 (0%) | 0.046 |
| In-hospital mortality | 15 (41%) | 0 (0%) | 0.002 |
Abbreviations: APACHE, Acute Physiology and Chronic Health Evaluation; APTT, activated partial thromboplastin time; CRP, C-reactive protein; DIC, disseminated intravascular coagulation; FDP, fibrin/fibrinogen degradation products; ICU, intensive care unit; PT, prothrombin time; SIRS, systemic inflammatory response syndrome; SOFA, Sequential Organ Failure Assessment.
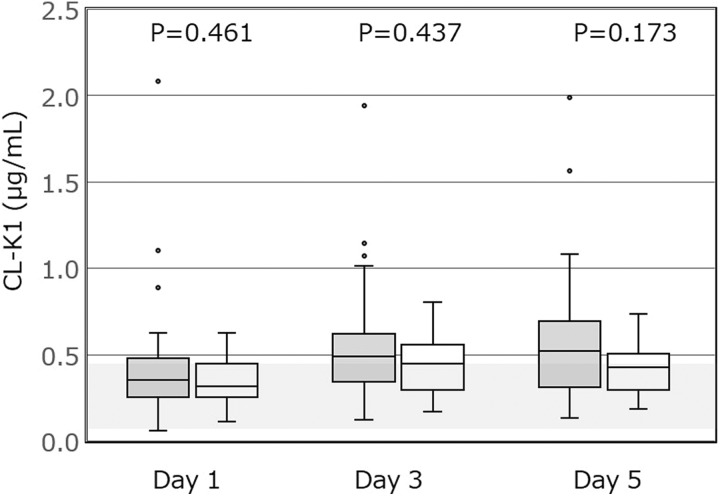
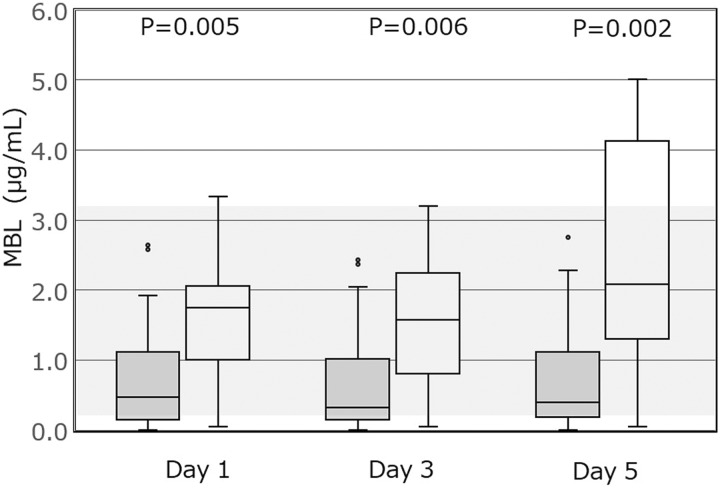
Figures 1 and 2 show the plasma concentrations of CL-K1 and MBL, respectively, in patients from the 2 groups, measured on the first 5 days of admission to the ICU. The plasma levels of CL-K1 showed no significant difference between the DIC and non-DIC groups. However, the plasma levels of MBL were significantly lower in the DIC group compared to the non-DIC group during the 5 days after admission to the ICU.
Figure 1.
The plasma levels of CL-K1 in the DIC and non-DIC groups. Data are presented as box and whisker plots. The plasma levels of CL-K1 are not significantly different between the 2 groups. (Normal range 0.34 ± 0.13 μg/mL,11 as presented by a gray band in the figure). P < .017 is considered statistically significant after Bonferroni correction. CL-K1 indicates collectin kidney 1; DIC, disseminated intravascular coagulation. Gray denotes DIC group; white denotes non-DIC group.
Figure 2.
The plasma levels of MBL in the DIC and non-DIC groups. Data are presented as box and whisker plots. The plasma levels of MBL are statistically different between the 2 groups on days 1, 3, and 5 after ICU admission. (Normal range 1.72 ± 1.51 μg/mL,11 as presented by a gray band in the figure). P < .017 is considered statistically significant after Bonferroni correction. MBL indicates mannose-binding lectin; DIC, disseminated intravascular coagulation; gray, DIC group; ICU, intensive care unit. White denotes non-DIC group.
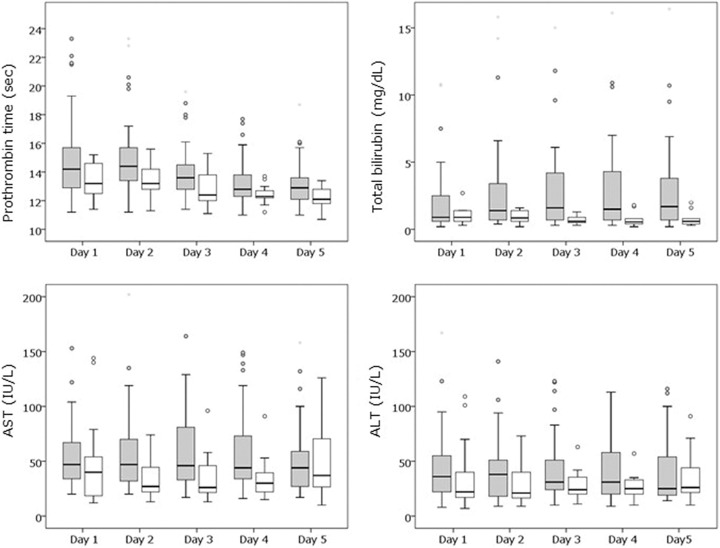
The changes in hepatic function-related markers are presented in Figure 3. During the observation period, hepatic function in the DIC group was worse than that in the non-DIC group. The total bilirubin levels were statistically different between the 2 groups on days 2, 3, and 4 after ICU admission. There was no statistically significant difference between the 2 groups for the other variables.
Figure 3.
Differences in hepatic function–related markers between the DIC and non-DIC groups. Data are presented as box and whisker plots. The total bilirubin levels were statistically different between the 2 groups on days 2, 3, and 4 after ICU admission. There was no statistically significant difference between the 2 groups for the other variables. P < .01 is statistically significant after Bonferroni correction. ALT indicates alanine aminotransferase; AST, aspartate aminotransferase; DIC, disseminated intravascular coagulation; ICU, intensive care unit. Gray denotes DIC group; white denotes non-DIC group.
The correlations between all measurements during the observation period are presented in Table 2. Plasma levels of CL-K1 showed weak correlations with prothrombin time, activated partial thromboplastin time (APTT), and antithrombin levels. On the other hand, plasma levels of MBL were weakly correlated with levels of fibrin/fibrinogen degradation products (FDP) and the DIC score.
Table 2.
Relationships Between CL-K1, MBL, and Laboratory Variables During the Observation Periods.
| CL-K1 | MBL | |||
|---|---|---|---|---|
| Spearman ρ | P Value | Spearman ρ | P Value | |
| CL-K1, ng/mL | NA | NA | −.132 | .101 |
| MBL, ng/mL | −.132 | .101 | NA | NA |
| White blood cells, ×109/L | −.136 | .089 | .092 | .255 |
| Hemoglobin, g/dL | −.055 | .494 | .033 | .678 |
| Platelet counts, ×109/La | −.281 | <.001 | .186 | .020 |
| PT, secondsa | −.356 | <.001 | −.147 | .068 |
| APTT, secondsa | −.257 | .001 | −.020 | .808 |
| Fibrinogen, mg/dL | .102 | .209 | .039 | .630 |
| Antithrombin, %a | .447 | <.001 | .012 | .889 |
| FDP, mg/L | −.040 | .628 | −.225 | .005 |
| d-Dimer, mg/L | −.008 | .922 | −.199 | .014 |
| Total bilirubin, mg/dL | .098 | .226 | .083 | .305 |
| Creatinine, mg/dL | −.006 | .937 | −.046 | .571 |
| CRP, mg/L | .080 | .323 | −.097 | .233 |
| SIRS score | −.097 | .226 | .152 | .058 |
| DIC score | .174 | .030 | −.222 | .005 |
Abbreviations: APTT, activated partial thromboplastin time; CL-K1, collectin kidney 1; CRP, C-reactive protein; DIC, disseminated intravascular coagulation; FDP, fibrin/fibrinogen degradation products; MBL, mannose-binding lectin; NA, not applicable; PT, prothrombin time; SIRS, systemic inflammatory response syndrome.
a Significant correlation with CL-K1. P < .0017 is considered statistically significant after Bonferroni correction.
Discussion
In the present study, during the first 5 days after admission to the ICU, the plasma MBL levels in sepsis patients with DIC were lower than those in patients without DIC. However, the levels of CL-K1 were not different between the 2 groups during the same observational period. Furthermore, the levels of CL-K1 and MBL showed different relationships with the other measurements in this study.
Contrary to an earlier study12 that have reported higher CL-K1 levels in patients with DIC, we did not observe any significant difference in the levels of CL-K1 between patients with sepsis with and without DIC. However, the previous study differed from ours in terms of (1) patient characteristics, (2) definition of DIC, and (3) the ethnicity of the patients.12 First, while the present study evaluated only patients with sepsis, the previous study included patients with various conditions such as infections, respiratory diseases, and neoplasms.12 Second, in the previous study,12 DIC was diagnosed by APTT waveform analysis, which is not the best method for DIC diagnosis19 and is not widely recommended.20–23 Therefore, in the present study, we used the Japanese Association for Acute Medicine DIC scoring system, which has been recommended by various guidelines for the diagnosis and treatment of DIC.20–23 Third, while the present study included only Asian patients, the previous study included mostly Caucasians.12 These differences between the present and previous study could account for the difference in results.
A negative correlation between plasma CL-K1 levels and APTT was observed in the present study. This correlation might have been detected in a previous study, where DIC was diagnosed using APTT waveform analysis.12 The relationship between plasma CL-K1 levels and APTT might be explained as follows. The activation of complement system induces platelet activation,24–26 endothelial cell activation,27–29 and inhibition of the anticoagulation system,30 which in turn may result in coagulation activation and shortening of APTT. However, it is unclear why a relationship was only observed between CL-K1 levels and APTT and not between the MBL levels and APTT.
Activation of the complement system is closely related to activation of coagulation.5,6 In sepsis, both the complement and coagulation systems are activated.1 However, the association between levels of MBL and coagulation is unclear. Huh et al reported a discrepancy regarding the association between MBL levels and sepsis severity.16 In their report, although MBL levels in patients with severe sepsis were higher than those in patients with septic shock, MBL levels in nonsurvivors with septic shock were lower than those in survivors.16 Of course, septic shock is severer than severe sepsis and nonsurvivors have a more critical condition than survivors. Zhao et al had reported that MBL levels in patients with sepsis with DIC were higher than those in patients with sepsis without DIC on arrival at emergency department.17 However, their findings were based on only the initial measurements of MBL on arrival at the emergency department17 and admission to ICU.16 On the other hand, in the present study, we repeatedly measured MBL levels in patients with sepsis on days 1, 3, and 5 and found differences that became more significant with time (Figure 2).
Hakozaki et al previously reported a dysfunction of MBL production in patients with fulminant hepatic failure because MBL is produced in the liver.31 In the present study, the hepatic function in sepsis patients with DIC was worse than that in septic patients without DIC, which was indicated by specific hepatic function-related markers. Therefore, the hepatic dysfunction in sepsis patients with DIC might affect MBL production, thereby resulting in low MBL levels. Furthermore, plasma MBL levels negatively correlated with FDP, which are metabolized by the liver (Table 2), and could be a possible indication of hepatic dysfunction.
The present study has several limitations. First, although the association of CL-K1 and MBL levels with sepsis-induced DIC was elucidated, the underlying pathophysiology of the association is unclear. Second, associations between MBL levels and polymorphisms in the protein-coding region of MBL2 were recently investigated.16,32,33 However, we did not investigate the association between MBL levels and these polymorphisms. Third, the CL-K1 and MBL levels in the patients before the onset of sepsis were not known.
Conclusions
The plasma levels of CL-K1, a novel collectin, in sepsis patients with and without DIC were not significantly different during the first 5 days of admission to the ICU. However, the plasma levels of MBL were lower in sepsis patients with DIC compared to those without DIC during the same observation period, and the differences became more significant with time. However, the plasma levels of neither CL-K1 nor MBL deviated markedly from the normal range.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med. 2013;369(9):840–851. [DOI] [PubMed] [Google Scholar]
- 2. Hunt BJ. Bleeding and coagulopathies in critical care. N Engl J Med. 2014;370(9):847–859. [DOI] [PubMed] [Google Scholar]
- 3. Hayakawa M, Saito S, Uchino S, et al. Characteristics, treatments, and outcomes of severe sepsis of 3195 ICU-treated adult patients throughout Japan during 2011–2013. J Intensive Care. 2016;4:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ogura H, Gando S, Saitoh D, et al. Epidemiology of severe sepsis in Japanese intensive care units: a prospective multicenter study. J Infect Chemother. 2014;20(3):157–162. [DOI] [PubMed] [Google Scholar]
- 5. Keragala CB, Draxler DF, McQuilten ZK, Medcalf RL. Haemostasis and innate immunity – a complementary relationship: a review of the intricate relationship between coagulation and complement pathways. Br J Haematol. 2018;180(6):782–798. [DOI] [PubMed] [Google Scholar]
- 6. Lupu F, Keshari RS, Lambris JD, Coggeshall KM. Crosstalk between the coagulation and complement systems in sepsis. Thromb Res. 2014;133(suppl 1):S28–S31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Keshi H, Sakamoto T, Kawai T, et al. Identification and characterization of a novel human collectin CL-K1. Microbiol Immunol. 2006;50(12):1001–1013. [DOI] [PubMed] [Google Scholar]
- 8. Ohtani K, Suzuki Y, Wakamiya N. Biological functions of the novel collectins CL-L1, CL-K1, and CL-P1. J Biomed Biotechnol. 2012;2012:493945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hansen SW, Ohtani K, Roy N, Wakamiya N. The collectins CL-L1, CL-K1 and CL-P1, and their roles in complement and innate immunity. Immunobiology. 2016;221(10):1058–1067. [DOI] [PubMed] [Google Scholar]
- 10. Kozutsumi Y, Kawasaki T, Yamashina I. Isolation and characterization of a mannan-binding protein from rabbit serum. Biochem Biophys Res Commun. 1980;95(2):658–664. [DOI] [PubMed] [Google Scholar]
- 11. Yoshizaki T, Ohtani K, Motomura W, et al. Comparison of human blood concentrations of collectin kidney 1 and mannan-binding lectin. J Biochem. 2012;151(1):57–64. [DOI] [PubMed] [Google Scholar]
- 12. Takahashi K, Ohtani K, Larvie M, et al. Elevated plasma CL-K1 level is associated with a risk of developing disseminated intravascular coagulation (DIC). J Thromb Thrombolysis. 2014;38(3):331–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kawasaki N, Kawasaki T, Yamashina I. Isolation and characterization of a mannan-binding protein from human serum. J Biochem. 1983;94(3):937–947. [DOI] [PubMed] [Google Scholar]
- 14. Auriti C, Prencipe G, Inglese R, et al. Role of mannose-binding lectin in nosocomial sepsis in critically ill neonates. Hum Immunol. 2010;71(11):1084–1088. [DOI] [PubMed] [Google Scholar]
- 15. Gao DN, Zhang Y, Ren YB, et al. Relationship of serum mannose-binding lectin levels with the development of sepsis: a meta-analysis. Inflammation. 2015;38(1):338–347. [DOI] [PubMed] [Google Scholar]
- 16. Huh JW, Song K, Yum JS, Hong SB, Lim CM, Koh Y. Association of mannose-binding lectin-2 genotype and serum levels with prognosis of sepsis. Crit Care. 2009;13(6):R176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhao X, Chen YX, Li CS. Predictive value of the complement system for sepsis-induced disseminated intravascular coagulation in septic patients in emergency department. J Crit Care. 2015;30(2):290–295. [DOI] [PubMed] [Google Scholar]
- 18. Gando S, Iba T, Eguchi Y, et al. A multicenter, prospective validation of disseminated intravascular coagulation diagnostic criteria for critically ill patients: comparing current criteria. Crit Care Med. 2006;34(3):625–631. [DOI] [PubMed] [Google Scholar]
- 19. Di Nisio M, Thachil J, Squizzato A. Management of disseminated intravascular coagulation: a survey of the international society on thrombosis and haemostasis. Thromb Res. 2015;136(2):239–242. [DOI] [PubMed] [Google Scholar]
- 20. Wada H, Thachil J, Di Nisio M, et al. Guidance for diagnosis and treatment of DIC from harmonization of the recommendations from three guidelines. J Thromb Haemost. 2013;11:716–767. [DOI] [PubMed] [Google Scholar]
- 21. Wada H, Asakura H, Okamoto K, et al. Expert consensus for the treatment of disseminated intravascular coagulation in Japan. Thromb Res. 2010;125(1):6–11. [DOI] [PubMed] [Google Scholar]
- 22. Levi M, Toh CH, Thachil J, Watson HG. Guidelines for the diagnosis and management of disseminated intravascular coagulation. British Committee for Standards in Haematology. Br J Haematol. 2009;145(1):24–33. [DOI] [PubMed] [Google Scholar]
- 23. Di Nisio M, Baudo F, Cosmi B, et al. Diagnosis and treatment of disseminated intravascular coagulation: guidelines of the Italian Society for Haemostasis and Thrombosis (SISET). Thromb Res. 2012;129(5):e177–e184. [DOI] [PubMed] [Google Scholar]
- 24. Sims PJ, Faioni EM, Wiedmer T, Shattil SJ. Complement proteins C5b-9 cause release of membrane vesicles from the platelet surface that are enriched in the membrane receptor for coagulation factor Va and express prothrombinase activity. J Biol Chem. 1988;263(34):18205–18212. [PubMed] [Google Scholar]
- 25. Peerschke EI, Reid KB, Ghebrehiwet B. Platelet activation by C1q results in the induction of alpha IIb/beta 3 integrins (GPIIb-IIIa) and the expression of P-selectin and procoagulant activity. J Exp Med. 1993;178(2):579–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Polley MJ, Nachman RL. Human platelet activation by C3a and C3a des-arg. J Exp Med. 1983;158(2):603–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ikeda K, Nagasawa K, Horiuchi T, Tsuru T, Nishizaka H, Niho Y. C5a induces tissue factor activity on endothelial cells. Thromb Haemost. 1997;77(2):394–398. [PubMed] [Google Scholar]
- 28. Tedesco F, Pausa M, Nardon E, Introna M, Mantovani A, Dobrina A. The cytolytically inactive terminal complement complex activates endothelial cells to express adhesion molecules and tissue factor procoagulant activity. J Exp Med. 1997;185(9):1619–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Platt JL, Dalmasso AP, Lindman BJ, Ihrcke NS, Bach FH. The role of C5a and antibody in the release of heparan sulfate from endothelial cells. Eur J Immunol. 1991;21(11):2887–2890. [DOI] [PubMed] [Google Scholar]
- 30. Nishioka J, Suzuki K. Inhibition of cofactor activity of protein S by a complex of protein S and C4b-binding protein. Evidence for inactive ternary complex formation between protein S, C4b-binding protein, and activated protein C. J Biol Chem. 1990;265(16):9072–9076. [PubMed] [Google Scholar]
- 31. Hakozaki Y, Yoshiba M, Sekiyama K, et al. Mannose-binding lectin and the prognosis of fulminant hepatic failure caused by HBV infection. Liver. 2002;22(1):29–34. [DOI] [PubMed] [Google Scholar]
- 32. Sprong T, Mollnes TE, Neeleman C, et al. Mannose-binding lectin is a critical factor in systemic complement activation during meningococcal septic shock. Clin Infect Dis. 2009;49(9):1380–1386. [DOI] [PubMed] [Google Scholar]
- 33. Luo J, Xu F, Lu GJ, Lin HC, Feng ZC. Low mannose-binding lectin (MBL) levels and MBL genetic polymorphisms associated with the risk of neonatal sepsis: an updated meta-analysis. Early Hum Dev. 2014;90(10):557–564. [DOI] [PubMed] [Google Scholar]