Abstract
Early catheter-directed thrombolysis (CDT) for deep vein thrombosis (DVT) can reduce postthrombotic morbidity. Pharmacomechanical thrombolysis (PMT) is a new therapy that can be selected for the treatment of iliofemoral deep vein thrombosis (IFDVT). We performed a meta-analysis of clinical trials comparing PMT versus CDT for treatment of acute IFDVT. Literature on this topic published between January 1, 1990, and June 1, 2018, was identified using PubMed, Embase, Cochrane Library, and Web of Science. Six trials were included in the meta-analysis. Compared to CDT, PMT significantly reduced the Villalta score (P = .007; I 2 = 0%), thrombus score (P = .01; I 2 = 0%), the duration in the hospital (P = .03; I 2 = 64%), and thrombolysis time (P < .00001, I 2 = 0%). There was no significant difference in valvular incompetence events (P = .21; I 2 = 0%), minor bleeding events (P = .59; I 2 = 0%), stent events (P = .09; I 2 = 24%), and clot reduction grade I events (P = .16; I 2 = 43%) between PMT and CDT. Subgroup analysis was performed by dividing the clot reduction grade I events group into PMT plus CDT versus CDT group and significant differences were found (P = .03, I 2 = 0%) as well as for PMT alone versus CDT group (P = .88, I 2 = 37%). This meta-analysis shows that PMT reduces the severity of postthrombotic syndrome (PTS), thrombus score, duration in hospital, and thrombolysis time compared to CDT. More specifically, PMT plus CDT reduces clot reduction grade I events. No significant difference in valvular incompetence events, stent events, and minor bleeding events were found when PMT was compared to CDT.
Keywords: pharmacomechanical thrombectomy, catheter-directed thrombolysis, iliofemoral deep vein thrombosis
Introduction
Iliofemoral deep venous thrombosis (IFDVT) is strongly related to severe postthrombotic morbidity, embodied by a reduction in the quality of life.1 It can impact the daily routine and lead to consequential complications, such as varicosity, limitation in activity, postthrombotic syndrome (PTS), and even pulmonary embolism (PE).2 Treatment options for IFDVT have developed with the increasing utilization of catheter-directed thrombolysis (CDT) and pharmacomechanical thrombolysis (PMT).3,4 Catheter-directed thrombolysis is a therapy that the guidewire passed through the thrombus lesion under the assistance of a supporting catheter, followed by a multiple-sidehole infusion catheter. The catheter was placed within the thrombosed vessel and secured in place. The urokinase solution was infused continuously at a dose of 600 to 1200 U/kg/h over 48 to 72 hours. Catheter-directed thrombolysis has been performed for several years in patients with IFDVT, with positive outcomes. However, the dose of lytic agent needed for therapy is high, and thrombolysis time is long.5 From emerging technology, PMT is a method of the AngioJet device (Possis Medical, Inc, Minneapolis, Minnesota) for rheolytic thrombectomy. The Angiojet device was inserted to the thrombus lesion and the operation continued with a solution of urokinase. The design of AngioJet device is such that it allows for thrombus fragmentation and rapid evacuation through the effluent lumen. This sequence may be repeated in the event that significant residual thrombus remains on subsequent venograms. Pharmacomechanical thrombolysis has been developed with catheter infusion approaches for thrombolysis treatment with considerable success and enthusiasm. Although some studies have demonstrated that PMT is safe and effective with decreased treatment time, hospital stay, and dosage of lytic agent compared to CDT, PMT and CDT have similar venous outcomes in patients with acute IFDVT.6–8 We performed a meta-analysis of 9 studies comparing PMT with CDT for the treatment of IFDVT in an attempt to solve the discrepancy between study outcomes and provide evidence to clinicians.
Methods
Literature Search
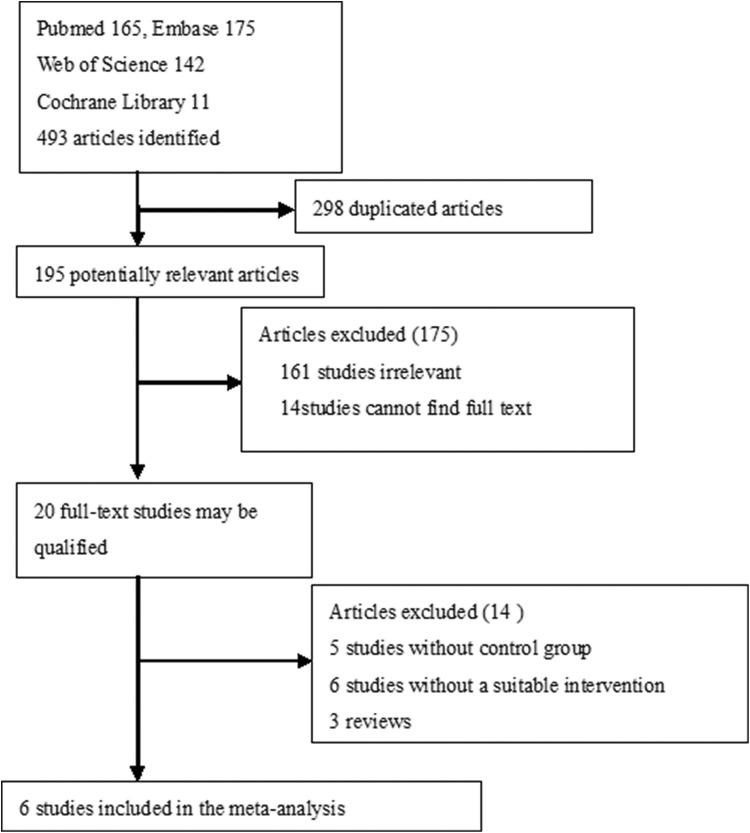
Literature, published between January 1, 1990, and June 1, 2018, was searched using PubMed, Embase, Cochrane Library, and Web of Science. The search terms included the following: CDT or PMT or pharmacomechanical thrombectomy or mechanical thrombectomy or iliofemoral/lower extremity or deep vein thrombosis, and/or comparative studies or randomized controlled trials or cohort studies or retrospective or prospective studies. Inclusion criteria were studies comparing PMT plus CDT or PMT (experimental group) with CDT (control group) and presence of intact clinical data. No language restrictions were enforced. Ten studies were found (Figure 1).
Figure 1.
Flow diagram of literature review.
Two investigators independently extracted data utilizing a data abstraction tool: number of patients in experimental group and control group, study quality, time of follow-up, primary and secondary outcomes. Primary outcomes were the Villalta score (a measure that could be used to diagnose and classify the severity of PTS). Postthrombotic syndrome was classified as mild if the Villalta score is 5 to 9, moderate if the Villalta score is 10 to 14, and severe if the Villalta score is ≥15); clot reduction grade I events, thrombus score, valvular incompetence events, and secondary outcomes included thrombolysis time, stent events, duration in hospital, and bleeding events.
Data Extraction and Quality Assessment
Details of the publication, inclusion and exclusion criteria, demographics of the study participants, interventions, and outcomes (primary and secondary outcomes) were collected and reviewed. Risk of bias in the studies (eg, including masking of participants, intention-to-treat analysis, incomplete or unclear data, and time to follow-up) was also assessed. Study quality was assessed by the Newcastle-Ottawa Scale (NOS).9 Disagreements between reviewers were resolved by consensus.
Statistical Analysis
Statistical analysis was performed using Review Manager (Cochrane Collaboration software, version 5.3). Random effects models were used for some primary outcomes (Villalta score and thrombus score [0-open vein free of thrombus; 1-partially occluded vein with a flow Doppler signal; 2-completely occluded vein with no flow signal]), and some secondary outcomes (thrombolysis time and duration in hospital) and utilized fixed-effects models for outcomes related to clot reduction grade I events (the clot reduction grade I events represented the patients with acute IFDVT after thrombolytic therapy used venography showing achievement of clot lysis of <50%. The clot reduction grade III events implied the patients with acute IFDVT after thrombolytic therapy used venography showing achievement of clot lysis of 90%-100%), stent events, valvular incompetence, and bleeding events. Statistical heterogeneity was assessed by I 2. The level of heterogeneity was defined as low (I 2 = 0%-49%), moderate (I 2 = 50%-74%), and high (I 2 ≥ 75%) heterogeneity. Primary and secondary outcomes were analyzed using odds ratios or standardized mean difference, with a 2-sided significance level of 5%.
Results
Study Characteristics and Quality
The initial search strategy identified 20 full-text articles, and 14 citations were screened. Six trials5–8,10,11 satisfied the appropriate criteria for inclusion in the meta-analysis (Figure 1). Six comparative studies included experimental groups that received PMT therapy for acute IFDVT and control groups that received CDT therapy for acute IFDVT. The qualities of trials were assessed by NOS score. Table 1 shows the baseline characteristics for each study.
Table 1.
Baseline Characteristics of Included Clinical Trials.
| Study | Group | Sample | Follow-Up | Mean Age, Years | Female Sex | Region | Outcomes | Study Quality Score |
|---|---|---|---|---|---|---|---|---|
| Kuo et al7 | CDT | 31 | 24 months | 64.5 | 14 | Taiwan, China | Villalta score, thrombus score, | Retrospective |
| PMT | 30 | 67 | 12 | Clot reduction grade I, valvular incompetence, bleeding, stent events, duration of hospital stay | NOS:8 | |||
| Vogel et al10 | CDT | 20 | 44 months | NA | NA | United States | Valvular incompetence events | Retrospective |
| PMT | 49 | NA | NA | NOS:8 | ||||
| Huang et al11 | CDT | 18 | 12 months | 64 | 6 | Taiwan, China | Villalta score, thrombus score | Retrospective |
| PMT | 16 | 62 | 7 | Clot reduction grade I, valvular incompetence, bleeding, stent events | NOS:8 | |||
| Lin et al8 | CDT | 46 | 38 months | NA | NA | United States | Clot reduction grade I, bleeding events | Retrospective |
| PMT | 52 | NA | NA | Duration of hospital stay | NOS:7 | |||
| Trabal et al6 | CDT | 21 | 28 months | 52 | 7 | United States | Thrombus score, thrombolysis time, clot reduction grade I events | Retrospective NOS:9 |
| PMT ± CDT | 22 | 44 | 15 | Bleeding, stent events | ||||
| Kim et al5 | CDT | 26 | 32 months | 43 | 17 | United States | Thrombus score, clot reduction grade I events | Retrospective |
| PMT + CDT | 19 | 53 | 12 | Bleeding, stent events | NOS:7 |
Abbreviations: CDT, catheter-directed thrombolysis; NA, not available; NOS, Newcastle-Ottawa Scale; PMT, pharmacomechanical thrombolysis.
Primary Outcomes
Primary and secondary outcomes are shown in Table 2. Three studies6,7,11 included the results of thrombus score, 5 studies5–8,11 included the results of clot reduction grade I events, 2 studies7,10 included the results of valvular incompetence events, and 2 studies7,11 included the results of Villalta score. Meta-analysis indicated that PMT reduced the Villalta score (P = .007; I 2 = 0%) and thrombus score (P = .01; I 2 = 0%) compared to CDT and did not result in a significant difference in clot reduction grade I events (P = .16; I 2 = 43%) and valvular incompetence events (P = .21; I 2 = 0%) compared to CDT. There were 2 types of PMT therapy including PMT plus CDT therapy and PMT alone therapy. When the clot reduction grade I events group was divided into PMT plus CDT versus CDT group, and PMT alone versus CDT group, subgroup analysis demonstrated that PMT plus CDT reduced clot reduction grade I events (P = .03, I 2 = 0%) compared to CDT. Pharmacomechanical thrombolysis alone did not result in a significant difference in clot reduction grade I events (P = .88, I 2 = 37%). This meta-analysis showed that PMT plus CDT is more effective in the treatment of IFDVT than CDT alone. Results of the meta-analysis of the primary outcomes are shown in Figures 2 and 3.
Table 2.
Primary and Secondary Outcomes in Clinical Trials.
| Study | Group | Villalta Score | Thrombus Score | Valvular Incompetence Events | Clot Reduction Grade I Events | Duration of Hospital Stay, Days | Thrombolysis Time, Hours | Minor Bleeding Events | Stent Events |
|---|---|---|---|---|---|---|---|---|---|
| Kuo et al7 | CDT | 3.13 ± 3.0 | 1.1 ± 1.8 | 12 | 6 | 10.9 ± 1.5 | NA | NA | 2 |
| PMT | 1.87 ± 2.7 | 0.83 ± 1.4 | 9 | 3 | 9.9 ± 5.0 | NA | NA | 7 | |
| Vogel et al10 | CDT | NA | NA | 13 | NA | NA | NA | NA | NA |
| PMT | NA | NA | 25 | NA | NA | NA | NA | NA | |
| Huang et al11 | CDT | 5.06 ± 4.07 | 2.22 ± 3.49 | NA | 2 | NA | NA | 1 | 6 |
| PMT | 2.06 ± 2.95 | 0.56 ± 0.93 | NA | 0 | NA | NA | 0 | 10 | |
| Lin et al8 | CDT | NA | NA | NA | 6 | 8.4 ± 2.3 | NA | 2 | NA |
| PMT | NA | NA | NA | 11 | 4.6 ± 1.3 | NA | 3 | NA | |
| Trabal et al6 | CDT | NA | 5.74 ± 4.4 | NA | 8 | NA | 55.4 ± 21.3 | 5 | 12 |
| PMT ± CDT | NA | 3.0 ± 3.7 | NA | 2 | NA | 23.4 ± 21.5 | 4 | 14 | |
| Kim et al5 | CDT | NA | NA | NA | 2 | NA | 56.5 ± 27.4 | 1 | 6 |
| PMT + CDT | NA | NA | NA | 0 | NA | 30.3 ± 17.8 | 0 | 3 |
Abbreviations: CDT, catheter-directed thrombolysis, NA, not available; PMT, pharmacomechanical thrombolysis.
Figure 2.
Meta-analysis of primary outcomes of clinical trials (Villata score P = .007, I 2 = 0%, thrombus score P = .01, I 2 = 0%, valvular incompetence events P = .21, I 2 = 0%).
Figure 3.
Meta-analysis of primary outcomes of clot reduction grade I events on primary outcomes with subgroup analysis. (total P = .16, I 2 = 43%), PMT alone versus CDT group (P = .88, I 2 = 37%), and PMT + CDT versus CDT group (P = .03, I 2 = 0%). CDT indicates catheter-directed thrombolysis; M-H, Mantel-Haenszel (a stratification analysis method); PMT, pharmacomechanical thrombolysis.
Secondary Outcomes
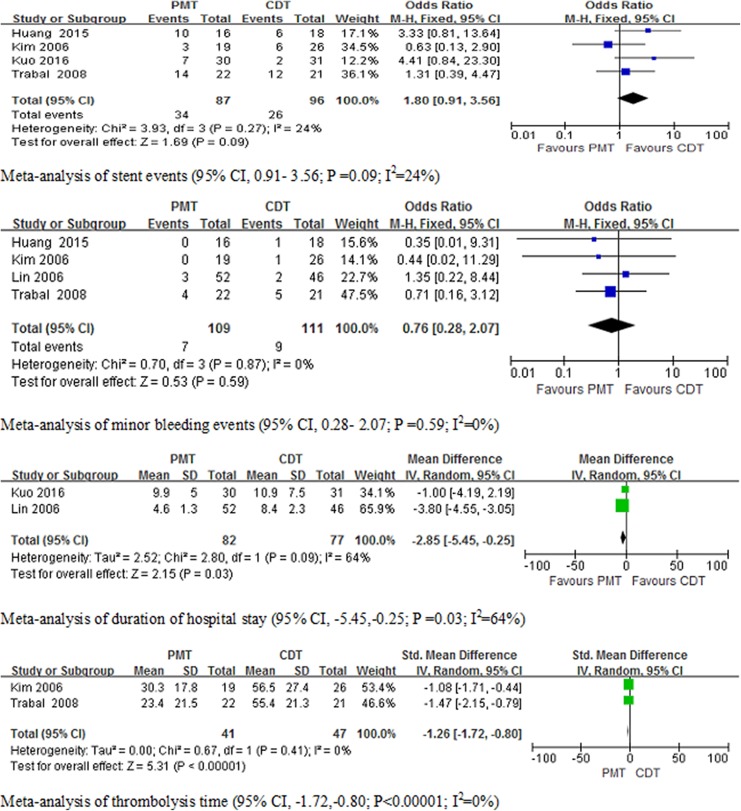
Two articles5,6 had data on thrombolysis time (in hours), 4 articles5,6,8,11 included data on minor bleeding events, 4 studies5–7,11 included the results of stent events, and 2 articles7,8 had data on duration in hospital. There was a statistically significant decrease in duration in the hospital (P = .03; I 2 = 64%) and thrombolysis time (P < .00001, I 2 = 0%) for PMT compared to CDT. There was no significant difference in stent events (P = .09; I 2 = 24%) and minor bleeding events (P = .59; I 2 = 0%) between the treatments. The results of the meta-analysis of secondary outcomes are shown in Figure 4.
Figure 4.
Meta-analysis of secondary outcomes of clinical trials (stent events P = .09, I 2 = 24%; minor bleeding events P = .59, I 2 = 0%, duration of hospital stay P = .03, I 2 = 64%, and thrombolysis time P < .00001, I 2 = 0%).
Discussion
Postthrombotic syndrome is the result of venous outflow obstruction, venous reflux, and calf muscle pump dysfunction after severe DVT.12 Postthrombotic syndrome is also associated with severe clinical symptoms, such as chronic lower limbs pain, intractable edema, varicose veins, skin alterations, and venous ulcer.13 Iliofemoral deep venous thrombosis is a strong risk factor for developing PTS, resulting in a reduction in the quality of life. Treatment with thrombolysis is aimed to lower PTS morbidity.14,15 The rationale to use thrombolytic treatment is based on the efficacy to remove an early thrombus and improve vein patency, prevent valvular incompetence, and potentially reduce the incidence of PTS.16,17 The most recent American College of Chest Physicians guidelines still suggest anticoagulation therapy alone over CDT in acute proximal DVT; however, CDT could be selected as therapy for selected patients with IFDVT who have symptoms for <14 days, good functional status, life expectancy of >1 year, and low risk of bleeding.18,19
Some randomized controlled trials (RCTs)s demonstrated that CDT can improve patency of the iliofemoral vein or decrease severity of PTS compared to anticoagulation therapy alone.20,21 The Acute Venous Thrombosis: Thrombus Removal With Adjunctive Catheter-Directed Thrombolysis Trial (ATTRACT) study22 showed that pharmacomechanical CDT (PCDT)-reduced symptoms of IFDVT and the severity of PTS; however, it may not prevent the incidence of PTS. The efficacy of CDT therapy is verified,23 and PMT therapy also may be a safe and effective alternative treatment for IFDVT.
Our meta-analysis, based on 6 comparative studies,5–8,10,11 compared PMT to CDT for the treatment of acute IFDVT. Only 2 studies7,11 included the results of the Villalta score and 3 studies6,7,11 of the thrombus score. Two studies7,10 included the result of valvular incompetence events: the 24-month follow-up in the study by Kuo et al7 and the 44-month follow-up in the study by Vogel et al.10 The severity of PTS, using the Villalta score, was highly correlated with postoperative thrombus score and severity of venous obstruction and was moderately correlated with severity of valvular incompetence. The postoperative thrombus score also strongly correlated with severity of venous obstruction. Our meta-analysis indicated that PMT reduced the Villalta score (P = .007; I 2 = 0%) and thrombus score (P = .01; I 2 = 0%) compared to CDT. However, there was no significant difference between the PMT and CDT group for valvular incompetence events (P = .21; I 2 = 0%). In the study by Kuo et al,7 all patients with PTS had venous obstruction, but only half of them had valvular incompetence. This shows 2 connotations: (1) valvular incompetence results from venous outflow obstruction. (2) Valvular incompetence is not the main cause of PTS. Our results showed that PMT reduced the severity of PTS compared to CDT.
Five studies included5–8,11 the results of clot reduction grade I events. Although our meta-analysis showed that clot reduction grade I events (P = .16; I 2 = 43%) were not significantly different between the PMT and CDT group, other therapy methods may have affected this result of clot reduction grade I events. The clot reduction grade I events group were divided into PMT plus CDT versus CDT and PMT alone versus CDT. Subgroup analysis indicated that PMT plus CDT reduced clot reduction grade I events (P = .03, I 2 = 0%) compared to CDT. Pharmacomechanical thrombolysis alone did not result in a significant difference for clot reduction grade I events (P = .88, I 2 = 37%) compared to CDT. Definite thrombus removal at an early stage and the amount of residual thrombus are important prognostic factors for developing PTS. It is most likely that a thorough, early intervention to remove a thrombus, either by CDT or PMT, is beneficial to prevent PTS. After PMT procedure, CDT can be used to remove a residual thrombus to prevent PTS.
Four studies5,6,8,11 included the result of minor bleeding events. After a systematic review of relevant studies, the majority of studies reported no major bleeding complications and PE events. In our meta-analysis, minor bleeding was not significantly different (P = .59; I 2 = 0%) between PMT and CDT. Data analysis also demonstrated that the heterogeneity of the results was low. With PMT or CDT therapy, most minor bleeding complications occurred in the puncture site. Four studies5–7,11 included the result of stent events. Meta-analysis showed that there was no significant difference in stent events (P = .09; I 2 = 24%) between the PMT and the CDT group. In 4 studies, the type of patients who needed angioplasty and stenting was not mentioned. Moreover, in the PMT plus CDT group, stenting should be performed after PMT or CDT procedures, but this was not mentioned in the studies. Thrombolytic agent was continuously infused in the CDT group, and lower doses of thrombolytic agents were used in the PMT group, especially in the PMT plus CDT group. Two studies5,6 included the result of thrombolysis time (in hours). In our meta-analysis, thrombolysis time was significantly shorter in the PMT group (P < .00001, I 2 = 0%) compared to CDT. The duration of hospital stay was significantly shorter in the PMT group (P = .03; I 2 = 64%) compared to CDT. This may have been caused by the reduction in thrombolysis time in the PMT group. The shorter duration of hospital stay may decrease the economic burden of patients without health insurance.
Our meta-analysis had limitations. There were no RCTs in this meta-analysis, and the quality of studies was not high. Therefore, the data from the non-RCTs with lower quality may affect the results of the meta-analysis. Although the heterogeneity of most primary and secondary outcomes was not high, we did not carefully explore the sources of heterogeneity. Also, study quality, sample size of the studies, and follow-up time may be important factors influencing the results of the meta-analysis, and we did not eliminate other sources of heterogeneity, such as age, gender, race, dose of thrombolytics and different drugs for thrombolysis. High-quality RCTs are required to reduce heterogeneity and provide more reliable data.
Conclusion
This meta-analysis demonstrates that PMT results in a low severity of PTS compared to CDT therapy alone. Moreover, the average duration of hospital stay and thrombolysis time was shorter in the PMT group compared to the CDT group. However, the PMT group was not significantly different in valvular incompetence events, stent events, and minor bleeding events compared to the CDT group.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by Project of Scientific Research of TaiZhou Science and Technology Agency (grant number 1701KY24).
ORCID iD: Tao Tang  https://orcid.org/0000-0002-9476-2684
https://orcid.org/0000-0002-9476-2684
References
- 1. Kahn SR, Shbaklo H, Lamping DL, et al. Determinants of health-related quality of life during the 2 years following deep vein thrombosis. J Thromb Haemost. 2008;6(7):1105–1112. [DOI] [PubMed] [Google Scholar]
- 2. Comerota AJ, Grewal N, Martinez JT, et al. Postthrombotic morbidity correlates with residual thrombus following catheter-directed thrombolysis for iliofemoral deep vein thrombosis. J Vasc Surg. 2012;55(3):768–773. [DOI] [PubMed] [Google Scholar]
- 3. Bashir R, Zack CJ, Zhao H, Comerota AJ, Bove AA. Comparative outcomes of catheter-directed thrombolysis plus anticoagulation vs anticoagulation alone to treat lower-extremity proximal deep vein thrombosis. JAMA Intern Med. 2014;174(9):1494–1501. [DOI] [PubMed] [Google Scholar]
- 4. Yuksel A, Tuydes O. Midterm outcomes of pharmacomechanical thrombectomy in the treatment of lower extremity deep vein thrombosis with a rotational thrombectomy device. Vasc Endovascular Surg. 2017;51(5):301–306. [DOI] [PubMed] [Google Scholar]
- 5. Kim HS, Patra A, Paxton BE, Khan J, Streiff MB. Adjunctive percutaneous mechanical thrombectomy for lower-extremity deep vein thrombosis: clinical and economic outcomes. J Vasc Interv Radiol. 2006;17(7):1099–1104. [DOI] [PubMed] [Google Scholar]
- 6. Martinez Trabal JL, Comerota AJ, LaPorte FB, Kazanjian S, DiSalle R, Sepanski DM. The quantitative benefit of isolated, segmental, pharmacomechanical thrombolysis (ISPMT) for iliofemoral venous thrombosis. J Vasc Surg. 2008;48(6):1532–1537. [DOI] [PubMed] [Google Scholar]
- 7. Kuo TT, Huang CY, Hsu CP, Lee CY. Catheter-directed thrombolysis and pharmacomechanical thrombectomy improve midterm outcome in acute iliofemoral deep vein thrombosis. J Chin Med Assoc. 2017;80(2):72–79. [DOI] [PubMed] [Google Scholar]
- 8. Lin PH, Zhou W, Dardik A, et al. Catheter-direct thrombolysis versus pharmacomechanical thrombectomy for treatment of symptomatic lower extremity deep venous thrombosis. Am J Surg. 2006;192(6):782–788. [DOI] [PubMed] [Google Scholar]
- 9. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605. [DOI] [PubMed] [Google Scholar]
- 10. Vogel D, Walsh ME, Chen JT, Comerota AJ. Comparison of vein valve function following pharmacomechanical thrombolysis versus simple catheter-directed thrombolysis for iliofemoral deep vein thrombosis. J Vasc Surg. 2012;56(5):1351–1354. [DOI] [PubMed] [Google Scholar]
- 11. Huang CY, Hsu HL, Kuo TT, Lee CY, Hsu CP. Percutaneous pharmacomechanical thrombectomy offers lower risk of post-thrombotic syndrome than catheter-directed thrombolysis in patients with acute deep vein thrombosis of the lower limb. Ann Vasc Surg. 2015;29(5):995–1002. [DOI] [PubMed] [Google Scholar]
- 12. Huang H, Gu JP, Shi HF, et al. Assessment of the probability of post-thrombotic syndrome in patients with lower extremity deep venous thrombosis. Sci Rep. 2018;8(1):12663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Catarinella FS, Nieman FH, de Wolf MA, Toonder IM, de Graaf R, Wittens CH. Quality-of-life in interventionally treated patients with post-thrombotic syndrome. Phlebology. 2015;30(suppl 1):89–94. [DOI] [PubMed] [Google Scholar]
- 14. Denny N, Musale S, Edlin H, Serracino-Inglott F, Thachil J. Iliofemoral deep vein thrombosis and the problem of post-thrombotic syndrome. Acute Med. 2018;17(2):99–103. [PubMed] [Google Scholar]
- 15. Guanella R, Kahn SR. Post-thrombotic syndrome: current prevention and management strategies. Expert Rev Cardiovasc Ther. 2012;10(12):1555–1566. [DOI] [PubMed] [Google Scholar]
- 16. Hofmann LV, Kuo WT. Catheter-directed thrombolysis for acute DVT. Lancet. 2012;379(9810):3–4. [DOI] [PubMed] [Google Scholar]
- 17. Comerota AJ. Randomized trial evidence supporting a strategy of thrombus removal for acute DVT. Semin Vasc Surg. 2010;23(3):192–198. [DOI] [PubMed] [Google Scholar]
- 18. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149(2):315–352. [DOI] [PubMed] [Google Scholar]
- 19. Kearon C, Akl EA, Comerota AJ, et al. Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College Of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(suppl 2):e419S–e496S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Enden T, Haig Y, Klow NE, et al. Long-term outcome after additional catheter-directed thrombolysis versus standard treatment for acute iliofemoral deep vein thrombosis (the CaVenT study): a randomised controlled trial. Lancet. 2012;379(9810):31–38. [DOI] [PubMed] [Google Scholar]
- 21. Lee CY, Lai ST, Shih CC, Wu TC. Short-term results of catheter-directed intrathrombus thrombolysis versus anticoagulation in acute proximal deep vein thrombosis. J Chin Med Assoc. 2013;76(5):265–270. [DOI] [PubMed] [Google Scholar]
- 22. Vedantham S, Goldhaber SZ, Julian JA, et al. Pharmacomechanical catheter-directed thrombolysis for deep-vein thrombosis. N Engl J Med. 2017;377(23):2240–2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lu Y, Chen L, Chen J, Tang T. Catheter-directed thrombolysis versus standard anticoagulation for acute lower extremity deep vein thrombosis: a meta-analysis of clinical trials. Clin Appl Thromb Hemost. 2018;24(7):1134–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]