Abstract
Aims
Dietary inorganic nitrate (NO3 −) lowers peripheral blood pressure (BP) in healthy volunteers, but lacks such effect in individuals with, or at risk of, type 2 diabetes mellitus (T2DM). Whilst this is commonly assumed to be a consequence of chronic hyperglycaemia/hyperinsulinaemia, we hypothesized that acute physiological elevations in plasma [glucose]/[insulin] blunt the haemodynamic responses to NO3 −, a pertinent question for carbohydrate‐rich Western diets.
Methods
We conducted an acute, randomized, placebo‐controlled, double‐blind, crossover study on the haemodynamic and metabolic effects of potassium nitrate (8 or 24 mmol KNO3) vs. potassium chloride (KCl; placebo) administered 1 hour prior to an oral glucose tolerance test in 33 healthy volunteers.
Results
Compared to placebo, there were no significant differences in systolic or diastolic BP (P = 0.27 and P = 0.30 on ANOVA, respectively) with KNO3, nor in pulse wave velocity or central systolic BP (P = 0.99 and P = 0.54 on ANOVA, respectively). Whilst there were significant elevations from baseline for plasma [glucose] and [C‐peptide], no differences between interventions were observed. A significant increase in plasma [insulin] was observed with KNO3 vs. KCl (n = 33; P = 0.014 on ANOVA) with the effect driven by the high‐dose cohort (24 mmol, n = 13; P < 0.001 on ANOVA; at T = 0.75 h mean difference 210.4 pmol/L (95% CI 28.5 to 392.3), P = 0.012).
Conclusions
In healthy adults, acute physiological elevations of plasma [glucose] and [insulin] result in a lack of BP‐lowering with dietary nitrate. The increase in plasma [insulin] without a corresponding change in [C‐peptide] or [glucose] suggests that high‐dose NO3 − decreases insulin clearance. A likely mechanism is via NO‐dependent inhibition of insulin‐degrading enzyme.
Keywords: blood pressure, cardiology, cardiovascular, nitric oxide, nutrition, physiology
What is already known about this subject
Inorganic nitrate lowers blood pressure and pulse wave velocity in healthy individuals.
These effects are absent in those with, or at risk of, T2DM.
This is assumed to be a consequence of chronic hyperglycaemia/hyperinsulinaemia.
What this study adds
Acute physiological elevations of plasma [glucose] and [insulin] result in a lack of BP‐lowering with dietary nitrate.
High‐dose inorganic nitrate reduced insulin clearance, probably via NO‐dependent inhibition of insulin‐degrading enzyme.
1. INTRODUCTION
The role of dietary inorganic nitrate (NO3 −) as an alternative source of nitric oxide (NO) via the enterosalivary nitrate–nitrite–NO pathway is recognized as a physiological mediator of blood pressure (BP), endothelial function and platelet aggregation.1, 2, 3 In both healthy individuals and those with chronic cardiovascular conditions, NO3 − supplementation has been shown to increase exercise capacity.4, 5, 6, 7, 8 This beneficial effect is thought to arise from the action of NO on skeletal muscle where it modulates excitation‐contraction coupling, mitochondrial respiration, autoregulation of blood flow, and glucose homeostasis.9 However, individuals with, or at risk of, type 2 diabetes mellitus (T2DM) fail to exhibit a reduction in peripheral BP or pulse wave velocity (PWV) in response to NO3 − supplementation.10, 11, 12 There are a number of mechanisms that might contribute to this lack of effect including dysfunctional NO synthesis, increased NO scavenging and altered redox balance.13 To what extent this is a consequence of acute or chronic hyperglycaemia/hyperinsulinaemia is unknown.
Carbohydrate (CHO) ingestion also has established benefits on exercise performance.14 However, the effects of concurrent NO3 − and CHO intake on cardiovascular haemodynamics and glucose homeostasis (both important determinants of exercise capacity) have not been studied in detail.
Type two diabetes mellitus is a condition associated with excess CHO intake,15 although the aetiology of the condition is more complex.16 It has been observed that in both healthy individuals and those with T2DM, plasma [nitrate] and [nitrite] fall acutely in response to an oral glucose tolerance test (OGTT),17, 18 likely reflecting an increase in NO consumption. However, there is a lack of agreement with regard to basal plasma [nitrate] and [nitrite], with conflicting results reported.17, 19 This lack of agreement regarding basal concentrations may be the result of the use of the Griess test, which measures combined plasma [nitrate/nitrite] and is not sufficiently sensitive to measure physiological plasma [nitrite].
Systemic inhibition of NO synthesis results in a deterioration in glucose tolerance in non‐diabetic individuals in response to an OGTT, accompanied by an elevation in BP.20, 21 However, the effects of NO3 − supplementation on glucose homeostasis are less clear. In healthy individuals, NO3 − supplementation appears to result in lower plasma [glucose] post‐exercise,22, 23 but without changing homeostatic responses to glucose at rest.24, 25, 26 In those with, or at risk of T2DM, studies are heterogeneous in their design and report either an improvement or null effect of nitrate on insulin sensitivity following glucose administration.26, 27, 28, 29
In studies investigating the haemodynamic effects of NO3 − supplementation in individuals with T2DM there is greater consistency, as neither peripheral BP nor exercise tolerance are improved10, 11, 12; although we have demonstrated a lowering of central SBP with 6 months' dietary nitrate [24], with a decrease in left ventricular volumes.30 This lack of effect in those with impaired glucose tolerance may be due to impaired insulin‐mediated vasodilation,31, 32, 33 but whether this is a consequence of acute or chronic hyperglycaemia/hyperinsulinaemia has not been established.
The purpose of this study was to determine whether there is an interaction between NO3 − and glucose on BP and glucose homeostasis in healthy individuals. We hypothesized that acute physiological elevations in plasma [glucose] and [insulin] would blunt the haemodynamic responses to NO3 −. This study was therefore conducted to address two complementary questions: (i) is the BP response to NO3 − supplementation affected by concurrent glucose ingestion? and (ii) is the metabolic response to an OGTT affected by NO3 − supplementation?
2. METHODS
2.1. Participants
Participants were healthy, normotensive volunteers aged 18–45 years. All participants had a body mass index (BMI) 18–35 kg/m2, no current or recent illness and were not taking systemic medication other than the oral contraceptive pill. A negative urine dipstick result for nitrite was required on the morning of each visit.
The study was approved by the South East London Research Ethics Committee (10/H0802/52). Written informed consent was obtained from all participants.
2.2. Study protocol
We conducted an acute, randomized, placebo‐controlled, double‐blind, crossover study of potassium nitrate (KNO3) vs. potassium chloride (KCl; placebo) (both Martindale Pharma) followed by an OGTT performed 1 hour later. The study consisted of two independent cohorts based on the dose of KNO3/KCl ingested: (i) a ‘high‐dose’ cohort received 24 mmol, and (ii) a ‘low‐dose’ cohort received 8 mmol. Each study visit lasted 4 hours and was separated by a minimum of 7 days. The allocation to KNO3 or KCl for each participant was performed using a random, computer‐generated order produced by an independent researcher.
Participants were asked to fast overnight (>12 hours) and to avoid nitrate‐rich foods, strenuous exercise, smoking and the use of mouthwash for 24 hours before the study. To minimize any dietary confounders, participants were asked to consume the same meals for the day prior to each arm of the study.
On the day of the study and following an hour's equilibration period during which baseline measurements were taken (see below), participants were randomized to receive KNO3 vs. KCl at Time −1 h. Both were administered with low‐nitrate water (300 ml; Buxton Water) and an antacid (10–20 mL repeated if necessary; Gaviscon, GSK) to minimize gastrointestinal discomfort from the potassium supplement. A standard OGTT (75 g glucose as Lucozade, GSK) was performed at Time 0 h. A schematic of the events is presented in Figure 1.
Figure 1.
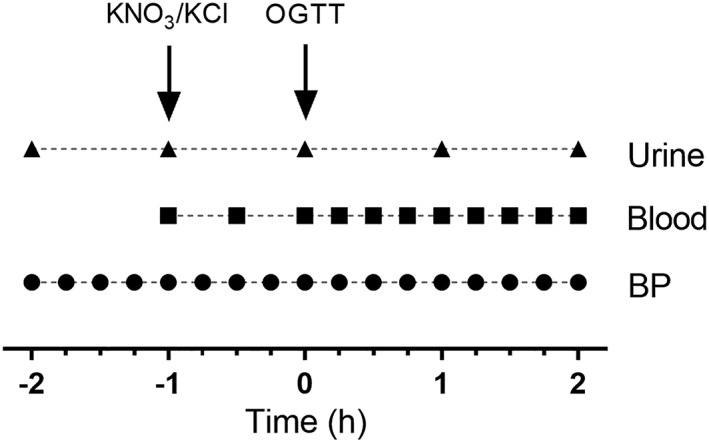
Schematic of events. After acclimatization (−2 h to −1 h), participants received KNO3 or KCl tablets (Time −1 h) followed by an oral glucose tolerance test (OGTT; 75 mg glucose) at Time 0 h. Blood pressure (BP) measurement, blood tests and urine collection occurred as indicated
2.3. Measurements
Blood pressure and heart rate (HR) readings were taken in triplicate every 15 min using an oscillometric BP monitor (Omron 705CP, UK) according to guidelines. The average of the second and third readings was used for analysis to diminish the impact of any alerting response. Central systolic blood pressure (cSBP), pulse wave velocity (PWV) and augmentation index (AIx) were measured (Time −1 h and Time 2 h) using Finometer (Finopress Medical Systems, Netherlands) and Vicorder (SMT Medical, Germany) devices according to manufacturers' instructions.
Blood samples were taken from a cannula in the antecubital vein at time intervals shown in Figure 1. An initial 2 mL of blood was discarded, before 6 mL of blood was collected and transferred into chilled lithium heparin blood collection tubes. Blood samples were immediately centrifuged at a relative centrifugal force of 2,000× g for 5 min at 4°C (Hettich Mikro 220R, Germany). Plasma was stored in duplicate in 1 mL aliquots at −80°C prior to analysis.
Plasma concentrations of glucose, insulin and C‐peptide were measured using standardized clinical assays (Viapath, St Thomas' Hospital). Nitrate and nitrite concentrations in urine and plasma were measured by ozone‐based chemiluminescence as previously described.1, 34 The coefficient of variation was <10% for both nitrite and nitrate quantification. Exhaled NO (eNO) was measured using a NObreath monitor (Bedfont Scientific, UK), according to the manufacturer's instructions.
Insulin sensitivity during each study arm was calculated via the Matsuda index, where a higher value represents greater insulin sensitivity.35
2.4. Data and statistical analyses
All data were analysed with GraphPad Prism software (v7.03), and are expressed as mean ± SEM unless otherwise stated. Repeated‐measures two‐way ANOVA with Sidak's post‐test was used for comparison of the data between the two interventions. Repeated‐measures one‐way ANOVA with Dunn's post‐test was used for comparison with baseline. Correlation was assessed using Pearson's correlation. Where data were non‐parametric, appropriate equivalent statistical tests were used. P < 0.05 was considered statistically significant.
2.5. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY,36 and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18.37, 38
3. RESULTS
Thirty‐three participants completed both parts of the study, of which 13 received high‐dose (24 mmol) and 20 received low‐dose (8 mmol) KNO3/KCl. Mild gastrointestinal discomfort lasting <15 min was reported by 42.4% (14/33) of participants following dosing, with no significant difference between dose or intervention. Demographic data for participants are summarized in Table 1.
Table 1.
Demographic data for participants
| All participants | Subgroups | ||
|---|---|---|---|
| 24 mmol | 8 mmol | ||
| Number of participants (n) | 33 | 13 | 20 |
| Gender (n male) | 15 | 6 | 9 |
| Age (years) | 27.1 ± 6.5 | 27.8 ± 7.2 | 26.5 ± 6.0 |
| Weight (kg) | 70.1 ± 13.9 | 69.4 ± 9.9 | 70.5 ± 16.1 |
| Height (m) | 1.7 ± 0.1 | 1.7 ± 0.1 | 1.7 ± 0.1 |
| BMI (kg/m2) | 23.3 ± 2.9 | 23.7 ± 3.2 | 23.1 ± 2.8 |
| SBP (mmHg) | 113.4 ± 10.1 | 115.0 ± 11.1 | 112.4 ± 9.6 |
| DBP (mmHg) | 71.2 ± 5.8 | 72.0 ± 5.3 | 70.7 ± 6.2 |
| HR (bpm) | 67.9 ± 9.4 | 69.4 ± 10.3 | 67.1 ± 8.0 |
| Fasting glucose (mmol/L) | 4.7 ± 0.6 | 4.7 ± 0.4 | 4.7 ± 0.7 |
| Fasting insulin (pmol/L) | 44.1 ± 22.2 | 39.9 ± 16.2 | 46.9 ± 25.3 |
Data expressed as mean ± SD. BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate.
3.1. Nitrate metabolism
The metabolism of ingested NO3 − was confirmed by a significant time‐dependent increase in plasma and urinary [nitrate] and [nitrite] and eNO following KNO3 compared to KCl (Figure 2). In the high‐dose cohort, plasma [nitrite] was significantly increased for KNO3 vs. KCl at both the time of the OGTT (Time 0 h, 399 ± 104 vs. 81 ± 16 nmol/L; P < 0.01) and at peak plasma [glucose] (Time 1 h, 721 ± 95 vs. 60 ± 13 nmol/L; P < 0.001); see Figure 2.
Figure 2.

Effect of 24 mmol KNO3 versus KCl (n = 13) on: A, plasma [nitrate], B, plasma [nitrite], C, urine [nitrate], D, urine [nitrite] and E, exhaled nitric oxide (NO). Effect of 8 mmol KNO3 versus KCl (n = 20) on F, exhaled NO. Data expressed as mean ± SEM. Significance shown as: †P < 0.05, ††P < 0.01, †††P < 0.001 on ANOVA, and *P < 0.05, **P < 0.01, ***P < 0.001, Sidak's post‐test of KNO3 vs. KCl. OGTT, oral glucose tolerance test
3.2. Haemodynamic response
Haemodynamic parameters pre‐intervention (Time −2 h to −1 h) were similar for KNO3 vs. KCl interventions (Table 2). There were no significant differences in BP or HR for KNO3 vs. KCl throughout the study (Time −2 h to +2 h; SBP P = 0.27; DBP P = 0.30; PP P = 0.74; HR P = 0.12) (Figure 3). Similarly, there were no significant differences in PWV, cSBP or AIx pre‐ and post‐OGTT (Time −1 h vs. +2 h; all P > 0.05; Figure 4).
Table 2.
Baseline haemodynamic parameters. Time −2 h to −1 h
| KNO 3 | KCl | |
|---|---|---|
| SBP (mmHg) | 113.1 ± 10.0 | 113.2 ± 10.9 |
| DBP (mmHg) | 70.8 ± 6.7 | 71.1 ± 6.4 |
| PP (mmHg) | 42.3 ± 7.3 | 41.2 ± 7.6 |
| HR (bpm) | 66.0 ± 7.2 | 65.7 ± 6.1 |
Data expressed as mean ± SD. SBP, systolic blood pressure; DBP, diastolic blood pressure; PP, pulse pressure; HR, heart rate.
Figure 3.

Effect of KNO3 vs. KCl (n = 33) on A, systolic blood pressure (SBP), B, diastolic blood pressure (DBP), C, pulse pressure (PP) and D, heart rate (HR). Data expressed as mean ± SEM. OGTT, oral glucose tolerance test
Figure 4.
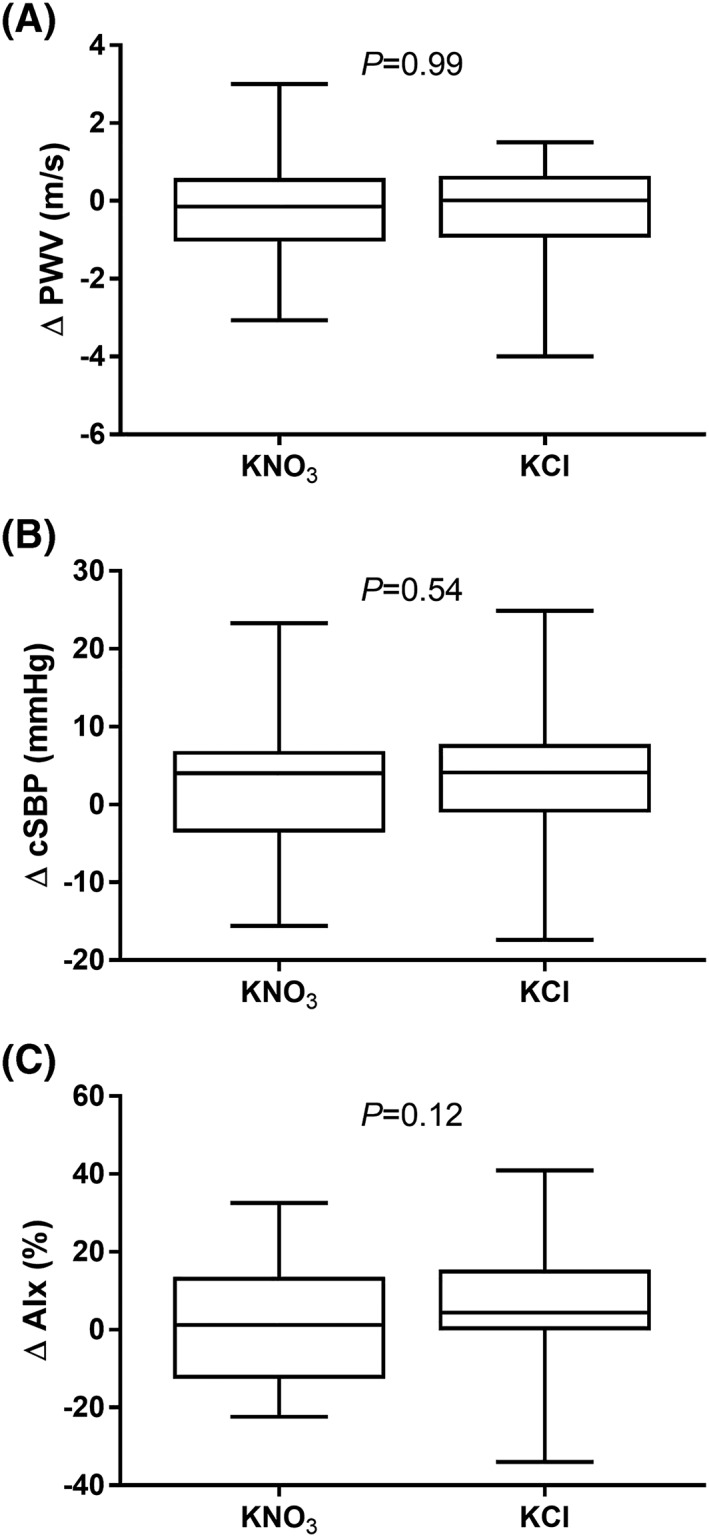
Effect of KNO3 vs. KCl (n = 29) on A, pulse wave velocity (PWV), B, central systolic blood pressure (cSBP) and C, augmentation index (AIx). Plots show range, median and 25th to 75th percentiles
Subgroup analyses of high‐dose (24 mmol) and low‐dose (8 mmol) cohorts also revealed similar haemodynamic parameters at baseline (data not shown). However, in contrast to the main analysis, significant differences in HR were observed between interventions within each cohort. In the high‐dose cohort, HR was reduced with KNO3 vs. KCl (mean 67.29 ± 0.55 vs. 68.36 ± 0.56 mmHg; P = 0.01) (Figure 5).
Figure 5.
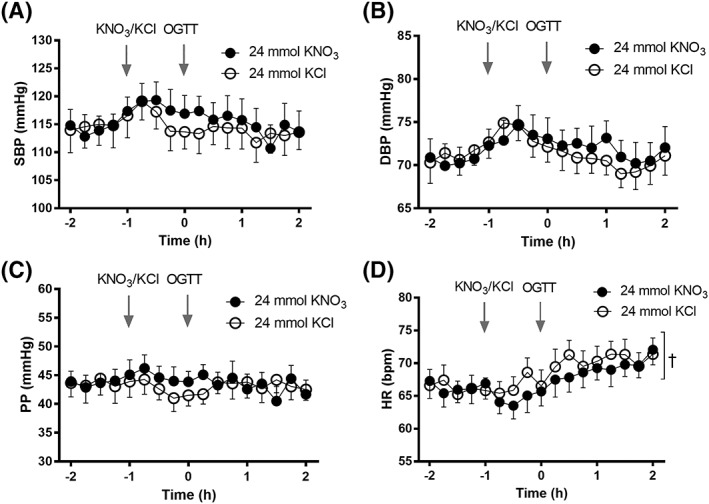
Effect of 24 mmol KNO3 vs. KCl (n = 13) on A, systolic blood pressure (SBP), B, diastolic blood pressure (DBP), C, pulse pressure (PP) and D, heart rate (HR). Data expressed as mean ± SEM. Significance shown as: †P < 0.05 on ANOVA. OGTT, oral glucose tolerance test
For the low‐dose cohort, the opposite effect was observed with a significantly higher HR with KNO3 vs. KCl (mean 65.89 ± 0.62 vs. 64.40 ± 0.40 mmHg; P < 0.01) (Figure 6). There were no significance differences in ΔPWV, ΔcSBP or ΔAIx for the interventions within either cohort (all P > 0.05; data not shown).
Figure 6.

Effect of 8 mmol KNO3 vs. KCl (n = 20) on A, systolic blood pressure (SBP), B, diastolic blood pressure (DBP), C, pulse pressure (PP) and D, heart rate (HR). Data expressed as mean ± SEM. Significance shown as: †††P < 0.001 on ANOVA. OGTT: oral glucose tolerance test
3.3. Glucose homeostasis
There were no significant differences between interventions for plasma [glucose] (P = 0.58) or [C‐peptide] (P = 0.84), but significantly higher plasma [insulin] was observed for KNO3 vs. KCl (P = 0.01) (Figure 7). Insulin sensitivity, as represented by the Matsuda index, was not significantly different for KNO3 vs. KCl (mean 4.26 ± 0.48 vs. 4.37 ± 0.48; P = 0.59).
Figure 7.

Effect of KNO3 vs. KCl (n = 33) on A, plasma [glucose], B, plasma [insulin] and C, plasma [C‐peptide]. Data expressed as mean ± SEM. Significance shown as: †P < 0.05 on ANOVA and *P < 0.05, Sidak's post‐test of KNO3 vs. KCl. ¥ P < 0.01 on ANOVA for KNO3 vs. baseline (−1 h) with Dunn's post‐test. ‡ P < 0.01 on ANOVA for KCl vs. baseline with Dunn's post‐test. OGTT, oral glucose tolerance test
Subgroup analyses of high‐dose (24 mmol) and low‐dose (8 mmol) cohorts revealed that the significant difference in plasma [insulin] was driven by the high‐dose cohort (P < 0.001 on ANOVA; at t = 0.75 h mean difference 210.4 pmol/L (95% CI 28.5–392.3), P = 0.012) (Figure 8). There was no significant difference in the Matsuda index for KNO3 vs. KCl with either 24 mmol (mean 5.42 ± 0.86 vs. 5.60 ± 0.75; P = 0.77) or 8 mmol (mean 3.50 ± 0.51 vs. 3.57 ± 0.57; P = 0.31). No significant correlation was observed between ΔSBP/DBP and Δ[insulin]/[glucose] at Time 1 h and 2 h (data not shown).
Figure 8.

Effect of KNO3 vs. KCl (24 mmol, n = 13; A, C and E; 8 mmol, n = 20; B, D and F) on: A, and B, plasma [glucose], C, and D, plasma [insulin], and E, and F, plasma [C‐peptide]. Data expressed as mean ± SEM. Significance shown as: †††P < 0.001 on ANOVA, and *P < 0.05, Sidak's post‐test of KNO3 vs. KCl. OGTT, oral glucose tolerance test
4. DISCUSSION
This acute, crossover study investigated the effects of concurrent inorganic nitrate and glucose ingestion on blood pressure and glucose homeostasis in healthy individuals. The principal findings of this study were as follows: (i) physiological elevation of plasma [glucose] and [insulin] resulted in a lack of BP‐lowering with inorganic nitrate, despite elevated plasma [nitrite], and (ii) the increase in plasma [insulin] without a corresponding change in [C‐peptide] or [glucose] suggests that high‐dose NO3 − decreases insulin clearance.
A dose–response relationship has previously been demonstrated between NO3 − ingestion (as beetroot juice or nitrate capsules) and peripheral BP reduction.1, 39, 40 Doses as low as 5.1 mmol have been shown to cause significant SBP reductions,39, 40 with higher doses (up to 22 mmol as beetroot juice, and 24 mmol as potassium nitrate, as used here) resulting in SBP/DBP reductions of 10.4/8.0 mmHg, and 9.4/6.0 mmHg, respectively.1, 39 Reductions in arterial stiffness have also occurred with both acute and chronic dosing.41, 42 Whilst several studies in healthy individuals failed to show a peripheral BP decrease with NO3 − supplementation, this is the first study with a neutral effect for ≥12 mmol/d NO3 −. There is a strong correlation between PWV and PP, and so the lack of change in PWV is consistent with the peripheral measurements.43 Based on our previous work in those with, or at risk of, T2DM we would have expected to observe a reduction in cSBP following NO3 − ingestion through a selective dilatory effect on medium‐sized conduit vessels.12, 44 However, nitrate had no effect on cSBP with an acute glucose load.
The lack of effect on both peripheral and central haemodynamics suggests that normal, physiological responses to glucose are sufficient to prevent the BP‐lowering effects of NO3 − supplementation. The observed differences in HR between interventions were small and, as the magnitude of change was opposite to that expected for the two doses, their biological validity is uncertain. The lack of BP‐lowering is consistent with other studies that have demonstrated inhibition of NO‐dependent flow‐mediated dilatation of conduit and small resistance arteries following acute physiological elevations in plasma [glucose] and [insulin].45, 46, 47 Furthermore, in a study of overweight men, Joris et al. reported that co‐ingestion of beetroot juice (approximately 8 mmol NO3 −) counteracted the decrease in FMD associated with the intake of a mixed meal, without differences in PWV or peripheral BP between groups.48 Whilst our study was not designed to disentangle the relative contributions from glucose and insulin, we hypothesize that lack of effect was modulated by elevated plasma glucose given that insulin‐mediated vasodilatation within skeletal muscle is NO‐dependent.31 The elevated exhaled NO demonstrated an increase in systemic NO availability following nitrate supplementation, and that the lack of BP‐lowering was therefore unlikely due to interruption of the nitrate–nitrite–NO pathway.
In agreement with previous studies, NO3 − supplementation did not lower resting plasma [glucose] or improve insulin sensitivity as assessed by the Matsuda index.24, 25, 26 However, in the high‐dose cohort we did observe an increase in plasma [insulin] without a corresponding increase in [C‐peptide], thus suggesting decreased insulin clearance. A change in plasma [insulin] without a corresponding change in [glucose] is consistent with the multifaceted mechanisms responsible for glucose homeostasis.49, 50 Dietary nitrate has been demonstrated to enhance glucose uptake in skeletal muscle independent of insulin via translocation of glucose transporter 4 (GLUT4).51 It is therefore possible that high‐dose dietary nitrate facilitated glucose uptake via insulin‐independent mechanisms, thus reducing insulin clearance at the same site. Our finding is also consistent with a previous study which showed that systemic inhibition of nitric oxide synthase (NOS) with N G‐monomethyl‐l‐arginine (l‐NMMA) in healthy volunteers increased insulin clearance without an effect on peripheral insulin sensitivity.21 The mechanism of increased insulin clearance following NOS inhibition was attributed to activation of the specific protease hepatic insulin‐degrading enzyme (IDE), which is largely responsible for whole‐body insulin clearance.52 IDE is dose‐dependently inhibited by NO in vitro and provides a plausible mechanism for our observation of decreased insulin clearance. Furthermore, as NO mediates glucose uptake by skeletal muscle in vitro through insulin‐independent mechanisms, decreased insulin clearance may also occur peripherally following NO3 − intake.53, 54
This study differs from those previously conducted with regard to the nitrate dose, glucose load and relative timing of ingestion. Our use of high‐dose nitrate, a full OGTT and coordination of peak plasma [glucose] with elevated [nitrite], optimized any interaction and may explain why other studies did not observe changes in plasma [insulin]. Furthermore, we opted to deliver NO3 − via capsules rather than beetroot juice, to avoid additional uncontrolled CHO ingestion (37.5 g sugar per 500 mL; James White Drinks Ltd). It is a limitation of this study that although Lucozade is routinely used to administer OGTTs in clinical practice, we cannot exclude confounders mediated by other ingredients. However, the ingredients of Lucozade are similar to those in many other sports drinks and so the potential impact on exercise may represent a “class effect”. Thus, the lack of an effect of concomitant administration of glucose with nitrate on BP suggests the possibility that glucose might also negate the beneficial effects of nitrate on exercise performance.
In summary, our findings describe decreased insulin clearance as a previously unidentified consequence of NO3 − supplementation and provide further information regarding how diet can acutely modulate blood pressure. Further investigation is required into the potentially antagonistic interaction between glucose and NO3 −.
COMPETING INTERESTS
A.J.W. holds shares in HeartBeet Ltd, which receive a royalty from James White Drinks Ltd who manufacture beetroot juice (source of dietary nitrate). The other authors have stated explicitly that there are no conflicts of interest in connection with this article.
CONTRIBUTORS
C.N.F., S.L., J.H., S.A.O., K.M., A.J.W. made substantial contributions to conception and design, or acquisition of data, or analysis and interpretation of data; and were involved in drafting the manuscript or revising it critically for important intellectual content; and gave final approval of the version to be published; and have participated sufficiently in the work to take public responsibility for appropriate portions of the content; and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
ACKNOWLEDGEMENTS
There was no support from any external organization specifically for the submitted work. We acknowledge internal infrastructure financial support from King's College London British Heart Foundation Centre (pump‐priming funding); National Institute for Health Research (NIHR) Clinical Research Facility at Guy's & St Thomas' NHS Foundation Trust and NIHR Biomedical Research Centre based at Guy's and St Thomas' NHS Foundation Trust and King's College London. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
Floyd CN, Lidder S, Hunt J, Omar SA, McNeill K, Webb AJ. Acute interaction between oral glucose (75 g as Lucozade) and inorganic nitrate: Decreased insulin clearance, but lack of blood pressure‐lowering. Br J Clin Pharmacol. 2019;85:1443–1453. 10.1111/bcp.13913
The authors confirm that the Principle Investigator for this paper is Dr A.J. Webb and that he had direct clinical responsibility for patients.
REFERENCES
- 1. Webb AJ, Patel N, Loukogeorgakis S, et al. Acute blood pressure lowering, vasoprotective, and antiplatelet properties of dietary nitrate via bioconversion to nitrite. Hypertension. 2008;51(3):784‐790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Khatri J, Mills CE, Maskell P, Odongerel C, Webb AJ. It is rocket science—Why dietary nitrate is hard to 'beet'! Part I: twists and turns in the realization of the nitrate–nitrite–NO pathway. Br J Clin Pharmacol. 2017;83(1):129‐139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mills CE, Khatri J, Maskell P, Odongerel C, Webb AJ. It is rocket science—Why dietary nitrate is hard to 'beet'! Part II: further mechanisms and therapeutic potential of the nitrate–nitrite–NO pathway. Br J Clin Pharmacol. 2017;83(1):140‐151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Larsen FJ, Weitzberg E, Lundberg JO, Ekblom B. Effects of dietary nitrate on oxygen cost during exercise. Acta Physiol (Oxf). 2007;191(1):59‐66. [DOI] [PubMed] [Google Scholar]
- 5. Bailey SJ, Winyard P, Vanhatalo A, et al. Dietary nitrate supplementation reduces the O2 cost of low‐intensity exercise and enhances tolerance to high‐intensity exercise in humans. J Appl Physiol (1985). 2009;107(4):1144‐1155. [DOI] [PubMed] [Google Scholar]
- 6. Zamani P, Rawat D, Shiva‐Kumar P, et al. Effect of inorganic nitrate on exercise capacity in heart failure with preserved ejection fraction. Circulation. 2015;131(4):371‐380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Eggebeen J, Kim‐Shapiro DB, Haykowsky M, et al. One week of daily dosing with beetroot juice improves submaximal endurance and blood pressure in older patients with heart failure and preserved ejection fraction. JACC Heart Fail. 2016;4(6):428‐437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Coggan AR, Broadstreet SR, Mahmood K, et al. Dietary nitrate increases VO2peak and performance but does not alter ventilation or efficiency in patients with heart failure with reduced ejection fraction. J Card Fail. 2017;24(2):65‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stamler JS, Meissner G. Physiology of nitric oxide in skeletal muscle. Physiol Rev. 2001;81(1):209‐237. [DOI] [PubMed] [Google Scholar]
- 10. Gilchrist M, Winyard PG, Aizawa K, Anning C, Shore A, Benjamin N. Effect of dietary nitrate on blood pressure, endothelial function, and insulin sensitivity in type 2 diabetes. Free Radic Biol Med. 2013;60:89‐97. [DOI] [PubMed] [Google Scholar]
- 11. Shepherd AI, Gilchrist M, Winyard PG, et al. Effects of dietary nitrate supplementation on the oxygen cost of exercise and walking performance in individuals with type 2 diabetes: A randomized, double‐blind, placebo‐controlled crossover trial. Free Radic Biol Med. 2015;86:200‐208. [DOI] [PubMed] [Google Scholar]
- 12. Mills CE, Govoni V, Faconti L, et al. Reducing arterial stiffness independently of blood pressure: The VaSera trial. J Am Coll Cardiol. 2017;70(13):1683‐1684. [DOI] [PubMed] [Google Scholar]
- 13. Omar SA, Webb AJ, Lundberg JO, Weitzberg E. Therapeutic effects of inorganic nitrate and nitrite in cardiovascular and metabolic diseases. J Intern Med. 2016;279(4):315‐336. [DOI] [PubMed] [Google Scholar]
- 14. Jeukendrup AE. Carbohydrate intake during exercise and performance. Nutrition. 2004;20(7–8):669‐677. [DOI] [PubMed] [Google Scholar]
- 15. Alhazmi A, Stojanovski E, McEvoy M, Garg ML. Macronutrient intakes and development of type 2 diabetes: A systematic review and meta‐analysis of cohort studies. J Am Coll Nutr. 2012;31(4):243‐258. [DOI] [PubMed] [Google Scholar]
- 16. Taylor R. Pathogenesis of type 2 diabetes: Tracing the reverse route from cure to cause. Diabetologia. 2008;51(10):1781‐1789. [DOI] [PubMed] [Google Scholar]
- 17. Derosa G, D'Angelo A, Salvadeo SA, et al. Modification of vascular and inflammation biomarkers after OGTT in overweight healthy and diabetic subjects. Microvasc Res. 2010;79(2):144‐149. [DOI] [PubMed] [Google Scholar]
- 18. Weiss EP, Park JJ, McKenzie JA, et al. Plasma nitrate/nitrite response to an oral glucose load and the effect of endurance training. Metabolism. 2004;53(5):673‐679. [DOI] [PubMed] [Google Scholar]
- 19. Ghasemi A, Zahediasl S, Azizi F. Nitric oxide and clustering of metabolic syndrome components in pediatrics. Eur J Epidemiol. 2010;25(1):45‐53. [DOI] [PubMed] [Google Scholar]
- 20. Gentilcore D, Visvanathan R, Russo A, et al. Role of nitric oxide mechanisms in gastric emptying of, and the blood pressure and glycemic responses to, oral glucose in healthy older subjects. Am J Physiol Gastrointest Liver Physiol. 2005;288(6):G1227‐G1232. [DOI] [PubMed] [Google Scholar]
- 21. Natali A, Ribeiro R, Baldi S, et al. Systemic inhibition of nitric oxide synthesis in non‐diabetic individuals produces a significant deterioration in glucose tolerance by increasing insulin clearance and inhibiting insulin secretion. Diabetologia. 2013;56(5):1183‐1191. [DOI] [PubMed] [Google Scholar]
- 22. Wylie LJ, Mohr M, Krustrup P, et al. Dietary nitrate supplementation improves team sport‐specific intense intermittent exercise performance. Eur J Appl Physiol. 2013;113(7):1673‐1684. [DOI] [PubMed] [Google Scholar]
- 23. Vasconcellos J, Henrique Silvestre D, Dos Santos Baiao D, et al. A single dose of beetroot gel rich in nitrate does not improve performance but lowers blood glucose in physically active individuals. J Nutr Metab. 2017;2017:7853034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Larsen FJ, Schiffer TA, Ekblom B, et al. Dietary nitrate reduces resting metabolic rate: A randomized, crossover study in humans. Am J Clin Nutr. 2014;99(4):843‐850. [DOI] [PubMed] [Google Scholar]
- 25. Shepherd AI, Wilkerson DP, Fulford J, et al. Effect of nitrate supplementation on hepatic blood flow and glucose homeostasis: A double‐blind, placebo‐controlled, randomized control trial. Am J Physiol Gastrointest Liver Physiol. 2016;311(3):G356‐G364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Beals JW, Binns SE, Davis JL, et al. Concurrent beet juice and carbohydrate ingestion: Influence on glucose tolerance in obese and nonobese adults. J Nutr Metab. 2017;2017:6436783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cermak NM, Hansen D, Kouw IW, et al. A single dose of sodium nitrate does not improve oral glucose tolerance in patients with type 2 diabetes mellitus. Nutr Res. 2015;35(8):674‐680. [DOI] [PubMed] [Google Scholar]
- 28. Fuchs D, Nyakayiru J, Draijer R, et al. Impact of flavonoid‐rich black tea and beetroot juice on postprandial peripheral vascular resistance and glucose homeostasis in obese, insulin‐resistant men: A randomized controlled trial. Nutr Metab. 2016;13(1):34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ashor AW, Chowdhury S, Oggioni C, et al. Inorganic nitrate supplementation in young and old obese adults does not affect acute glucose and insulin responses but lowers oxidative stress. J Nutr. 2016;146(11):2224‐2232. [DOI] [PubMed] [Google Scholar]
- 30. Faconti L, Mills CE, Govoni V, et al. Cardiac effects of 6 months' dietary nitrate and spironolactone in patients with hypertension and with/at risk of type 2 diabetes, in the factorial design, double‐blind, randomized controlled VaSera trial. Br J Clin Pharmacol. 2019;85(1):169‐180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Barrett EJ, Eggleston EM, Inyard AC, et al. The vascular actions of insulin control its delivery to muscle and regulate the rate‐limiting step in skeletal muscle insulin action. Diabetologia. 2009;52(5):752‐764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Laakso M, Edelman SV, Brechtel G, Baron AD. Decreased effect of insulin to stimulate skeletal muscle blood flow in obese man: A novel mechanism for insulin resistance. J Clin Invest. 1990;85(6):1844‐1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Laakso M, Edelman SV, Brechtel G, Baron AD. Impaired insulin‐mediated skeletal muscle blood flow in patients with NIDDM. Diabetes. 1992;41(9):1076‐1083. [DOI] [PubMed] [Google Scholar]
- 34. Nair A, Khan S, Omar S, et al. Remote ischaemic preconditioning suppresses endogenous plasma nitrite during ischaemia‐reperfusion: A randomized controlled crossover pilot study. Br J Clin Pharmacol. 2017;83(7):1416‐1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: Comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22(9):1462‐1470. [DOI] [PubMed] [Google Scholar]
- 36. Southan C, Sharman JL, Benson HE, et al. The IUPHAR/BPS guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucleic Acids Res. 2016;44(D1):D1054‐D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Alexander SPH, Benson HE, Faccenda E, et al. The Concise Guide to PHARMACOLOGY 2013/14: Enzymes. Br J Pharmacol. 2013;170(8):1797‐1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Alexander SPH, Benson HE, Faccenda E, et al. The Concise Guide to PHARMACOLOGY 2013/14: Transporters. Br J Pharmacol. 2013;170(8):1706‐1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kapil V, Milsom AB, Okorie M, et al. Inorganic nitrate supplementation lowers blood pressure in humans: Role for nitrite‐derived NO. Hypertension. 2010;56(2):274‐281. [DOI] [PubMed] [Google Scholar]
- 40. Bailey SJ, Fulford J, Vanhatalo A, et al. Dietary nitrate supplementation enhances muscle contractile efficiency during knee‐extensor exercise in humans. J Appl Physiol (1985). 2010;109(1):135‐148. [DOI] [PubMed] [Google Scholar]
- 41. Hughes WE, Ueda K, Treichler DP, Casey DP. Effects of acute dietary nitrate supplementation on aortic blood pressure and aortic augmentation index in young and older adults. Nitric Oxide. 2016;59:21‐27. [DOI] [PubMed] [Google Scholar]
- 42. Kapil V, Khambata RS, Robertson A, Caulfield MJ, Ahluwalia A. Dietary nitrate provides sustained blood pressure lowering in hypertensive patients: A randomized, phase 2, double‐blind, placebo‐controlled study. Hypertension. 2015;65(2):320‐327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kim EJ, Park CG, Park JS, et al. Relationship between blood pressure parameters and pulse wave velocity in normotensive and hypertensive subjects: Invasive study. J Hum Hypertens. 2007;21(2):141‐148. [DOI] [PubMed] [Google Scholar]
- 44. Omar SA, Fok H, Tilgner KD, et al. Paradoxical normoxia‐dependent selective actions of inorganic nitrite in human muscular conduit arteries and related selective actions on central blood pressures. Circulation. 2015;131(4):381‐389. discussion 389 [DOI] [PubMed] [Google Scholar]
- 45. Akbari CM, Saouaf R, Barnhill DF, Newman PA, LoGerfo FW, Veves A. Endothelium‐dependent vasodilatation is impaired in both microcirculation and macrocirculation during acute hyperglycemia. J Vasc Surg. 1998;28(4):687‐694. [DOI] [PubMed] [Google Scholar]
- 46. Title LM, Cummings PM, Giddens K, Nassar BA. Oral glucose loading acutely attenuates endothelium‐dependent vasodilation in healthy adults without diabetes: An effect prevented by vitamins C and E. J Am Coll Cardiol. 2000;36(7):2185‐2191. [DOI] [PubMed] [Google Scholar]
- 47. Watanabe K, Oba K, Suzuki T, et al. Oral glucose loading attenuates endothelial function in normal individual. Eur J Clin Invest. 2011;41(5):465‐473. [DOI] [PubMed] [Google Scholar]
- 48. Joris PJ, Mensink RP. Beetroot juice improves in overweight and slightly obese men postprandial endothelial function after consumption of a mixed meal. Atherosclerosis. 2013;231(1):78‐83. [DOI] [PubMed] [Google Scholar]
- 49. Kahn SE, Prigeon RL, McCulloch DK, et al. The contribution of insulin‐dependent and insulin‐independent glucose uptake to intravenous glucose tolerance in healthy human subjects. Diabetes. 1994;43(4):587‐592. [DOI] [PubMed] [Google Scholar]
- 50. Roder PV, Wu B, Liu Y, et al. Pancreatic regulation of glucose homeostasis. Exp Mol Med. 2016;48(3):e219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Jiang H, Torregrossa AC, Potts A, et al. Dietary nitrite improves insulin signaling through GLUT4 translocation. Free Radic Biol Med. 2014;67:51‐57. [DOI] [PubMed] [Google Scholar]
- 52. Cordes CM, Bennett RG, Siford GL, Hamel FG. Redox regulation of insulin degradation by insulin‐degrading enzyme. PLoS ONE. 2011;6(3):e18138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Etgen GJ Jr, Fryburg DA, Gibbs EM. Nitric oxide stimulates skeletal muscle glucose transport through a calcium/contraction‐ and phosphatidylinositol‐3‐kinase‐independent pathway. Diabetes. 1997;46(11):1915‐1919. [DOI] [PubMed] [Google Scholar]
- 54. Deshmukh AS, Long YC, de Castro Barbosa T, et al. Nitric oxide increases cyclic GMP levels, AMP‐activated protein kinase (AMPK)alpha1‐specific activity and glucose transport in human skeletal muscle. Diabetologia. 2010;53(6):1142‐1150. [DOI] [PMC free article] [PubMed] [Google Scholar]


