Abstract
Proprioceptive and motor impairments commonly occur after stroke. Relationships between corticospinal tract (CST) fractional anisotropy (FA) and motor recovery have been identified. However, the relationship between sensory tract microstructure and proprioceptive recovery remains unexplored. Using probabilistic tractography, we examined the relationship between diffusion metrics in three tracts known to contain proprioceptive information (a) dorsal‐column medial‐lemniscal (DCML), (b) postcentral gyrus to supramarginal gyrus (POCG‐SMG), (c) postcentral gyrus to Heschl's gyrus (POCG‐HG) and proprioception at 1 (n = 26) and 6 months (n = 19) poststroke. Proprioception was assessed using two robotic tasks. Motor performance was also assessed robotically and compared to CST diffusion metrics. At 1‐month poststroke, a nonsignificant relationship (r = −0.43, p = 0.05) was observed between DCML‐FA and proprioceptive impairment. A moderate relationship was identified between POCG‐SMG FA and POCG‐HG FA and proprioceptive impairment (r = −0.47, p = 0.001 and r = −0.51, p = 0.008, respectively). No relationships were significant at 6 months poststroke. Similar to previous studies, lower CST‐FA correlated with motor impairment at 1 month poststroke (r = −0.58, p = 0.002). While CST‐FA is considered a predictor of motor impairment, our findings suggest that the relationship between FA and tracts containing proprioceptive information is not as straightforward and highlights the importance of sensory association areas in proprioception.
Keywords: diffusion tractography, stroke, recovery, proprioception, motor skills
1. INTRODUCTION
Proprioception, the sense of limb position and motion (Sherrington, 1907), is impaired in up to 69% of individuals after stroke (Connell, Lincoln, & Radford, 2008; Welmer, Holmqvist, & Sommerfeld, 2008). Proprioceptive impairments lead to problems performing activities of daily living, longer hospital stays, and generally worse poststroke outcomes (Carey, 1995; Sommerfeld & von Arbin, 2004; Zeman & Yiannikas, 1989). Despite the fact that proprioceptive impairments occur in over half of all cases of stroke, they receive less attention than motor impairments, both clinically and in the literature. Compared to the motor system, the anatomy underlying proprioception is less well understood. Our group has recently examined cortical and subcortical lesion locations associated with poor proprioception in the subacute (Findlater et al., 2016; Kenzie et al., 2016) and chronic phases poststroke (Findlater et al., 2018) and have highlighted the importance of the primary somatosensory cortex, the supramarginal gyrus (SMG) and Heschl's gyrus (HG; also referred to as the transverse temporal gyrus) for proprioception. The relevance of the white matter tracts thought to underlie proprioception has yet to be investigated after stroke, despite numerous (>20) investigations into the relationship of the corticospinal tracts (CST) and motor impairment (see Kim & Winstein, 2017 for a review).
Fractional anisotropy (FA) of the CST is considered a biomarker for predicting motor recovery after stroke (Boyd et al., 2017). Biomarkers, such as CST‐FA, are anticipated to one‐day guide treatment content and timing, as well as inform inclusion criteria for clinical trials (Boyd et al., 2017; Cassidy, Tran, Quinlan, & Cramer, 2018; Ward, 2017). The recommendation to include CST‐FA as a biomarker has largely resulted from two different types of studies. First, cross‐sectional studies have identified that low CST‐FA is correlated with poor motor scores on clinical assessments (Lindenberg et al., 2010; Schaechter et al., 2009; Schulz et al., 2012). Second, longitudinal observational studies have suggested that higher CST‐FA is predictive of better motor recovery (Puig et al., 2010; Yu et al., 2009). Given the reported importance of proprioception in recovery, experts have recommended examination of sensory tracts (Boyd et al., 2017). Here we explore three tracts that are involved in proprioceptive processing, (a) the dorsal‐column medial‐lemniscal (DCML) pathway, (b) the postcentral gyrus to supramarginal gyrus association tract (POCG‐SMG), and (c) the postcentral gyrus to Heschl's gyrus association tract (POCG‐HG). The DCML carries proprioceptive and cutaneous information from the periphery, ascending the dorsal columns of the spinal cord through the medial lemniscus to the thalamus, and finally, the primary somatosensory cortex via the posterior limb of the internal capsule (PLIC). While one group has examined the DCML in patients with multiple sclerosis (Fling, Dutta, Schlueter, Cameron, & Horak, 2014) and members of our team have studied the DCML in children with cerebral palsy (Kuczynski et al., 2017), the importance of DCML microstructure after adult stroke remains unexplored. Furthermore, little is known about connections between the POCG and SMG or HG. The SMG is a somatosensory association area (Iwamura, 2003; Pandya & Seltzer, 1982) that is important for awareness of hand position (Ben‐Shabat, Matyas, Pell, Brodtmann, & Carey, 2015; Brozzoli, Gentile, Petkova, & Ehrsson, 2011). HG is an association area which appears to be involved in proprioception (Findlater et al., 2016, 2018) and is also involved in sensorimotor integration of listening and repeating (Bajada et al., 2017) as well as spatial localization of sound (Altmann, Bledowski, Wibral, & Kaiser, 2007; Baumgart, Gaschler‐Markefski, Woldorff, Heinze, & Scheich, 1999; Zatorre & Penhune, 2001). HG also has extensive connections with the parietal lobe (Jung, Cloutman, Binney, & Lambon Ralph, 2017).
In exploring relationships between tract microstructure and poststroke impairments, most studies rely on observer‐based ordinal scales to quantify impairments. The common scales used to measure proprioception suffer from challenges regarding precision, accuracy, and reliability (Carey, Oke, & Matyas, 1996; Connell & Tyson, 2012; Garraway, Akhtar, Gore, Prescott, & Smith, 1976; Lincoln et al., 1991). These flaws create significant challenges when comparing clinical findings with measures from imaging studies. Problems with the clinical measures of proprioception led our team to develop robotic proprioceptive assessment tools that have better precision, accuracy, and reliability than the common observer‐based ordinal scales (Dukelow et al., 2010; Semrau, Herter, Scott, & Dukelow, 2013). Our robotic assessment tools can quantify deficits in upper extremity position sense and kinesthesia (sense of limb movement). Our group has shown that not only can sensory and motor impairments be independent of one another (Dukelow, Herter, Bagg, & Scott, 2012), but they can follow entirely different recovery timelines (Semrau, Herter, Scott, & Dukelow, 2015). This work led us to question the role of the DCML in proprioceptive function and recovery.
In the present study, we evaluated the diffusion metrics of the DCML, POCG‐SMG, POCG‐HG, as well as the CST and made comparisons to robotically administered assessments of position sense, kinesthesia, and reaching. We were specifically interested in the relationship between metrics of white matter microstructure (fractional anisotropy [FA], mean diffusivity [MD], radial diffusivity [RD], and axial diffusivity [AD]) and proprioceptive and motor function over the first 6 months of recovery. We hypothesized that altered diffusion metrics of the DCML, POCG‐SMG, and POCG‐HG would correlate with poor performance in our robotic proprioceptive tasks. Furthermore, based on previous studies, we hypothesized that altered diffusion metrics of the CST would correlate with poor performance on the robotic reaching task. Finally, we predicted that altered DCML, POCG‐SMG, and POCG‐HG metrics would be associated with poor recovery of proprioception.
2. MATERIALS AND METHODS
2.1. Participants
Over the course of two and one‐half years, 26 individuals with stroke and nine healthy control subjects were recruited to participate in this study. All participants with stroke were recruited at the Foothills Medical Centre in Calgary, Alberta, Canada. All participants provided informed consent and the University of Calgary Conjoint Health Research Ethics Board approved this study. The inclusion criteria for participants with stroke were as follows: first clinical presentation of stroke, stroke onset less than 4 weeks prior to recruitment, stroke lesion overlapped subcortical structures, 18 years of age or older, no prior history of neurological conditions, ability to understand and follow instructions for the robotic and clinical assessments, and eligible and willing to undergo an MRI. Participants with stroke were excluded if clinical testing revealed visuospatial neglect (score < 129) as determined by the conventional sub‐tests of the Behavioral Inattention Test (Wilson, Cockburn, & Halligan, 1988) or apraxia as determined by a clinical apraxia assessment (van Heugten, Dekker, Deelman, Stehmann‐Saris, & Kinebanian, 1999).
2.2. Study design
Imaging, robotic, and clinical assessments for the study's first timepoint were conducted at 3–4 weeks poststroke. This timeframe was chosen to keep our sample as homogenous as possible in terms of edema resolution (Baird et al., 1997; Schwamm et al., 1998), and to allow enough time for the lesion to be detectable with diffusion parameters such as FA (Puig et al., 2010; Puig et al., 2013; Takenobu et al., 2014). Nineteen of these participants returned for follow up imaging, robotic, and clinical evaluations at 6 months poststroke. Nine healthy control participants also completed the imaging, robotic, and clinical assessment protocol.
2.3. Image acquisition
Participants were imaged using a 3 T MRI scanner (Discovery MR750, GE Healthcare, Waukesha, WI) equipped with a 12 channel head coil. A 3D inversion recovery‐prepared fast spoiled gradient echo sequence was acquired to obtain T1‐weighted anatomical images with the following parameters: repetition time (TR) = 6.656 ms, echo time (TE) = 2.9 ms, inversion time (TI) = 650 ms, flip angle = 10°, matrix size = 256 × 256 × 192, sagittal acquisition, voxel size = 1 mm3, GRAPPA (ARC) acceleration factor of two in the phase encode direction. Diffusion‐weighted data were acquired with the following parameters: TR = 8,000 ms, TE = 61 ms, matrix size = 110 × 110, 80 axial slices, voxel size = 2.2 mm3, and SENSE (ASSET) acceleration factor of two in the phase encode direction. Diffusion weighting was isotropically distributed along 90 directions using a b value of 1,000 s mm−2. Nine volumes with no diffusion weighting were acquired at the beginning of the acquisition. Additionally, a nine‐volume diffusion sequence was acquired with the reverse phase encoding polarity (six diffusion‐weighted volumes with a b value of 1,000 s mm−2 and three volumes without diffusion weighting). The total time for the diffusion‐weighted protocol was 13 min, 40 s.
2.4. Image analysis
Image preprocessing and probabilistic tractography were completed using tools from the FMRIB Software Library (FSL; http://www.fmrib.ox.ac.uk/fsl). The TOPUP tool was used to calculate the susceptibility induced off‐resonance field (Andersson, Skare, & Ashburner, 2003; Smith et al., 2004). This information was used in the subsequent preprocessing step (eddy) that completed a combined correction for susceptibility, eddy currents, and head motion.
FA, MD, and AD (λ1) were calculated by DTIfit. Radial diffusivity (RD) was calculated by averaging λ2 and λ3.
The FLIRT tool (affine [12 degrees of freedom] model, correlation ratio cost function) was used to register between T1 space, diffusion space, and the MNI152 2 mm template. As our linear registration results were excellent, we opted not to perform nonlinear registration, which could introduce errors related to lesion pathology. Importantly, data were visually inspected at each step for every participant. Registration was checked by overlaying the participant's transformed image over the MNI template to verify the alignment of five anatomical locations (central sulcus, left and right caudate, left and right putamen).
2.5. Probabilistic tractography
FSL's Bayesian Estimation of Diffusion Parameters Obtained Using Sampling Techniques (BEDPOSTX) tool was used to model diffusion distributions at each voxel. Two fiber directions were modeled per voxel. The BEDPOSTX output was used by the PROBTRACKX tool to generate probabilistic streamlines (curvature threshold = 0.2; number of steps per sample = 2,000; step length 0.5 mm) for our tracts of interest (Behrens et al., 2003; Behrens, Berg, Jbabdi, Rushworth, & Woolrich, 2007).
For each study participant, the DCML, POCG‐SMG, POCG‐HG, and CST were reconstructed in both hemispheres. To define the DCML, the POCG was used as the seed mask and the medial lemniscus was used as a waypoint and termination mask (Gardner & Johnson, 2013; Johnson, Babis, Soultanis, & Soucacos, 2008). To define the POCG‐SMG, the POCG was used as the seed mask and the SMG was used as a waypoint and termination mask. To define the POCG‐HG, the POCG was used as the seed mask and HG was used as a waypoint and termination mask. For the CST, the precentral gyrus was used as the seed mask and the CST in the mid‐pons was used as a waypoint and termination mask (Kim et al., 2015; Kuczynski et al., 2017). The Harvard–Oxford Cortical Structural Atlas provided with FSL was used to define the postcentral, supramarginal, Heschl's, and precentral gyri. These masks were converted to each subject's diffusion space for tractography. The medial lemniscus and CST masks were manually defined in the pons using the individual's color‐coded FA map at anatomically guided locations based on known anatomy as has been done previously for DCML (Kuczynski et al., 2017) and CST tractography (Lindenberg, Zhu, Ruber, & Schlaug, 2012; Puig et al., 2013). Contralateral hemisphere exclusion masks were used for tracking the DCML and CST. The probabilistic DCML, POCG‐SMG, POCG‐HG, and CST tracts were thresholded at 1% to create binary tract‐derived regions of interest (ROIs). Average FA, MD, RD, and AD were then calculated for the DCML, POCG‐SMG, POCG‐HG, and CST ROIs.
2.6. Lesion masks
A trained assessor (SF) manually delineated lesions on the T1‐weighted image of each stroke participant using MRIcron (Rorden, Karnath, & Bonilha, 2007) (http://www.mccauslandcenter.sc.edu/mricro/mricron/). The previously defined affine matrices were applied using FLIRT to transform lesion masks to diffusion and standard space. Individual lesion masks were combined to create lesion overlap maps for the sample.
2.7. Tract/lesion overlap
Lesioned voxels that overlapped the DCML, POCG‐SMG, POCG‐HG, or CST were identified for each subject at both timepoints. The number of overlapping voxels was calculated by multiplying the individual's DCML, POCG‐SMG, POCG‐HG, or CST mask with their lesion mask for each timepoint. The result (total voxels of overlap between the tract and lesion) was then divided by the total number of voxels in the subject's tract of interest to calculate the proportion of overlapping voxels.
2.8. Robotic proprioceptive and motor assessment
A KINARM exoskeleton robot was used to assess two aspects of proprioception (position matching and kinesthetic matching) and motor performance (visually guided reaching). Participants were seated on the wheelchair base of the exoskeleton with their arms supported by forearm troughs. A trained assessor ensured participants were centered in the exoskeleton and fit the arm troughs to each subject's height and limb geometry. Once the participant was in position, the assessor moved the exoskeleton to a virtual reality screen. For the proprioceptive tasks, vision of the arms was occluded by a bib. The robot passively moved the stroke‐affected arm for the subjects with stroke. We defined the arm moved by the robot as the “passive” arm and the arm that the subject moved as the “active” arm. Healthy control participants completed the tasks twice, once with each are acting as the active arm.
The first proprioceptive task, position matching (Figure 1a), assessed the individual's ability to sense the static position of their arm. This task has been described previously (Dukelow et al., 2010; Dukelow et al., 2012; Findlater et al., 2016). With vision of the arms occluded, the robot moved the passive (stroke affected) arm to one of nine spatial locations. The subject was instructed to mirror‐match the movement. In total, the passive arm was moved to each spatial location six times for a total of 54 trials. A task score was calculated that accounts for the parameters that quantify performance of the position matching task including (a) Variability—the trial‐to‐trial variability of the matching hand's position to each target; (b) Contraction/expansion—ratio of the area subtended by the subject's matched positions as the outer eight targets divided by the area of the square made by outer eight targets with the robotically‐moved arm; (c) spatial shift—the mean error between the matching and passive hands was calculated to indicate whether the subject perceived a systematic shift in the x, y, or xy directions of the workspace. Details of these parameters have been previously published (Dukelow et al., 2010, 2012). The overall task score calculation is detailed below.
Figure 1.
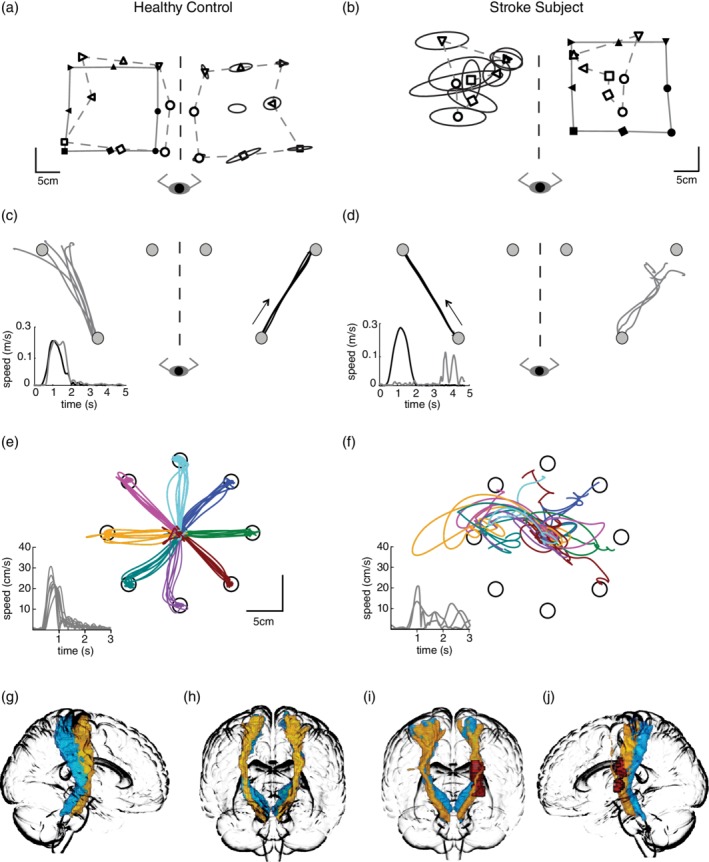
Exemplars—Robotic task performance and probabilistic DCML and CST. For the proprioceptive tasks, the robot moved the respective participant's right (the stroke participant's affected) arm. Subjects matched with the opposite arm. For the position matching task (a and b), a solid line joins the spatial locations that the robot moved the passive arm to (filled symbols). A dashed line connects the workspace locations where the participant actively matched (unfilled symbols). The data from the matching arm have been mirror transformed onto the passive movement to visualize discrepancies between arms. Trial to trial variability is symbolized by ellipses around each location—each ellipse represents 1 SD. For the kinesthetic matching task (c and d), black lines represent the path of the passively moved arm, the gray lines represent the active mirror‐matched movements by the participant. The arrow indicates the direction that the passive arm was moved. Temporal data is also provided. Only one of the six movement directions are presented in this figure. For the visually guided reaching task (e and f), data for the left arm of the control participant and the right arm of the participant with stroke are shown. Temporal data is also provided. Of note, in comparison to the healthy control, the stroke subject demonstrated high variability (denoted by ellipses size at spatial locations) and a contracted workspace on the position matching task (b), poor spatial accuracy, and slow response latency on the kinesthetic matching task (d), and poor spatial accuracy with several missed targets and slow reaction time on the visually guided reaching task (f). The DCML (blue) and CST (yellow) are displayed in the sagittal view (g and j) and coronal view (h and i) for the exemplar control and stroke subjects. Red represents the lesion location for the stroke subject in i and j
The kinesthetic‐matching task (Figure 1c) was used to assess the individual's sense of limb motion. This task has been described previously (Semrau et al., 2013). With vision occluded, the robot moved the passive arm to one of three locations. The participant placed a white circle that represented the tip of the index finger of their active into a red circle. This ensured that limbs were mirrored across the midline. The visual targets would then extinguish and the trial would start. The robot then initiated movement of the passive arm and the participant mirror‐matched the direction, speed, and length of the robotic movement with their opposite (active) arm. In total, six movement directions were completed between the three spatial locations for a total of 36 trials. If the matching movement was not completed within 10 s, that trial was considered a nonmovement. An overall task score was calculated which summarizes the parameters used to quantify performance on the kinesthetic matching task including (a) peak speed ratio—ratio of peak hand speed of the active arm versus the passive arm; (b) response latency—the difference between movement onset for the active and passive arms; (c) initial direction error—the angular difference between the active and passive arms at the peak speed of the movement; and (d) path length ratio—ratio of the distance traveled by the active arm versus the distance traveled by the passive arm. Details of these four parameters have been published previously (Kenzie, Semrau, Hill, Scott, & Dukelow, 2017; Semrau et al., 2013). The overall task score calculation is detailed below.
The visually guided reaching task (Figure 1e) was completed as an indicator of motor performance. It assessed the ability to make accurate and timely reaching movements and has been described previously (Coderre et al., 2010). For this task, participants had full vision of their arms. They positioned the tip of their index finger within a central target for 1,250–1,750 ms until 1 of 8 peripheral targets was illuminated. Participants were told to reach the peripheral targets as quickly and accurately as possible. Participants had 3,000 ms to reach the target. The peripheral targets were presented randomly within a block that was repeated eight times for a total of 64 trials. In this study, we considered 11 parameters of movement during task performance: (a) No initial stabilization—count of trials where the subject failed to stabilize at the starting target; (b) no end movement—number of trials for which movement offset is not detected; (c) posture speed—the mean hand speed, at rest, for 500 ms before the peripheral target illuminates; (d) reaction time—the time between target illumination to movement onset; (e) initial movement direction error—calculates the angular deviation between the (i) a straight line between the central to peripheral target and (ii) the participant's hand path in the initial phase of movement; (f) initial distance ratio—ratio of (i) the distance the hand traveled during the participant's initial phase of movement to (ii) the distance the hand traveled between movement onset and offset; (g) speed maxima count—the number of hand speed maxima between movement onset and offset; (h) Min–Max speed—Mean difference between pairs of adjacent local hand speed minima and maxima, for all such pairs between the time of Max Speed, and movement offset; (i) total movement time—the total time elapsed from movement onset to offset; (j) path length ratio—ratio of (i) the distance traveled by the hand between movement onset and movement offset and (ii) the straight line distance between those two hand positions; (k) Max speed—maximum hand speed between movement onset and offset (BKIN Technologies, 2018). Details of these parameters have been previously published (Coderre et al., 2010; Semrau et al., 2015). An overall task score was calculated (detailed below) that considers all parameters of the visually guided reaching task.
Performance on each parameter of the three robotic tasks was normalized to a z‐score based on previously collected healthy control data that accounted for age, sex, and handedness. This data set included 494 individuals for the position matching task (mean age = 50), 164 individuals for the kinesthetic matching task (mean age = 52), and 178 individuals for the visually guided reaching task (mean age = 49). Control data sets for each task parameter were transformed to a normal distribution using a Box–Cox transformation (Box & Cox, 1964). Outliers beyond ±3.29 standard deviations (SDs) were removed from the sample (on average ~1% of control subjects were removed per parameter; BKIN Technologies, 2018; Simmatis, Krett, Scott, & Jin, 2017). Z‐scores represent the distance from the mean performance of healthy control subjects in SDs. The overall task scores were calculated from the root‐mean‐square of all parameter z‐scores for each task and renormalized based on the performance of healthy controls (BKIN Technologies, 2018; Kenzie et al., 2017; Simmatis et al., 2017). Task scores greater than 1.96 were considered abnormal/failure.
2.9. Clinical assessment
In addition to the robotic assessment, participants with stroke also completed clinical assessments at 1 and 6 months poststroke. These assessments were performed by a research physiotherapist blinded to tractography results. The Modified Edinburgh Handedness Inventory was a self‐report scale used to determine handedness (Oldfield, 1971). The Functional Independence Measure (FIM) provided an overall measure of an individual's level of independence in activities of daily living (Keith, Granger, Hamilton, & Sherwin, 1987). Eighteen activity categories (i.e., dressing) were scored on an ordinal scale where seven indicated complete independence and the total possible score was 126. The Thumb Localizer Test was used to evaluate the position sense of the arms (Hirayama, Fukutake, & Kawamura, 1999). For this test, the therapist moved and held the participant's contralesional limb away from midline. With vision occluded, the participant aimed to touch their thumb with their unaffected hand. Perfect thumb localization received a score of 0; inability to locate thumb received a score of 3. The Chedoke McMaster Stroke Assessment (CMSA) Impairment Inventory of the Arm and Hand was used to measure motor impairment (Gowland et al., 1993). This ordinal scale evaluated the ability to perform predetermined movements on a scale of 0 (flaccid arm) to 7 (normal movement). The Modified Ashworth measured spasticity in the arm (Bohannon & Smith, 1987). On this five‐point scale, 0 indicated normal muscle tone and 4 indicated contracture.
2.10. Statistical analysis
One‐way analysis of variance (ANOVA) was used to determine whether FA, MD, RD, or AD were different between control participants and participants with stroke at either timepoint 1 or timepoint 2 for the DCML, POCG‐SMG, POCG‐HG, and CST. Repeated measure ANOVAs were used to determine whether FA, MD, RD, or AD were different between timepoints 1 and 2 for participants with stroke. Levene's tests were conducted to verify equality of variances (p > 0.5). In cases where the variances were unequal, post hoc tests were conducted using the Games–Howell procedure (p < 0.05). In cases where variances were equal, post hoc t tests were employed when differences between group means were detected. Benjamini–Hochberg corrections were used to control for multiple comparisons for post hoc testing (Benjamini & Hochberg, 1995). Paired t tests were conducted to compare individual diffusion metrics at timepoint 1 and timepoint 2 in the 19 subjects who completed both timepoints. Pearson's correlations were used to examine the relationship between diffusion metrics and performance on the proprioceptive and motor robotic tasks. The significance level was set at p < 0.05. The Benjamini–Hochberg procedure was used to control for multiple comparisons. Statistical analyses were conducted using MATLAB 2014b (MathWorks, Natick, MA) and SPSS 24 (IBM, Armonk, NY).
3. RESULTS
We examined 26 individuals at 1 month poststroke (timepoint 1) and repeated our measurements on 19 of those individuals at 6 months poststroke (timepoint 2). We included participants with either ischemic (n = 19 at timepoint 1; n = 14 at timepoint 2) or hemorrhagic stroke. Further demographics and clinical assessment are presented in Table 1. Participants with stroke had varied reasons for not completing the 6‐month follow up: one did not consent to a second MRI, one had a second stroke, one had a surgery that resulted in MRI contraindications, and the remaining four subjects were lost to follow up. Nine healthy control participants also completed our neuroimaging protocol. An independent t test revealed that the control participants and participants with stroke did not differ in age (p = 0.41). The lesion volume of the timepoint 2 cohort was larger than that of the timepoint 1 cohort (Table 1) because six of the seven participants who did not return for timepoint 2 had very small strokes (mean = 3.3 ml, SD = 2.2).
Table 1.
Demographics
| Control subjects | Subjects with stroke (1 month) | Subjects with stroke (6 months) | |
|---|---|---|---|
| Number | 9 | 26 | 19 |
| Age mean(range) | 60 (42–70) | 62.8 (27–84) | 62.2 (27–84) |
| Sex (F/M) | 4/5 | 10/16 | 9/10 |
| Handedness (R/A/L) | 9/0/0 | 25/1/0 | 18/1/0 |
| Ischemic/hemorrhagic | – | 19/7 | 14/5 |
| Lesioned hemisphere (R/L) | – | 20/6 | 15/4 |
| Vascular territory (MCA/PCA) | – | 21/5 | 17/3 |
| Lesion volume ml mean(SD) | – | 8.8 (8.9)/5.4 (0.1–24.2) | 9.8 (9.2)/5.4 (0.1–24.2) |
| Stroke to imaging (days) | – | 28 (9.7)/26.5 (15–38) | 187 (21.4)/184 (149–255) |
| Stroke to clinical/robotic session (days) | – | 24 (10)/22.5 (9–42) | 187 (21.3)/184 (149–255) |
| Functional Independence measure | – | 101 (18.9)/104 (65–126)a | 119.4 (7.7)/122 (97–126) |
| Thumb localizer test 0/1/2/3 | – | 17/1/5/3 | 14/2/3/0 |
| Chedoke McMaster 7/6/5/4/3/2/1 | – | 4/5/5/3/4/5/0 | 11/2/4/1/1/0 |
| Modified Ashworth scale 0/1/1+/2/3/4 | – | 21/4/0/1/0/0 | 13/4/1/1/0/0 |
Mean(SD)/Median(range) unless otherwise indicated.
Thumb Localizer Test, Chedoke McMaster Stroke Assessment, and Modified Ashworth Scale are ordered such that the left‐most number is the best score.
Missing data from one subject.
3.1. Proprioceptive and motor performance
The behavior of an exemplar control participant on the position matching task (1a), the kinesthetic matching task (1c), and the visually guided reaching task (1e) is presented in Figure 1. In Figure 1b, the exemplar participant with stroke demonstrated high variability about the individual targets and spatial contraction while performing the position matching task. In Figure 1d, the exemplar participant with stroke demonstrated difficulty mirror matching the path of the robotic movement and demonstrated a significantly prolonged response latency while performing the kinesthetic matching task. In Figure 1f, the participant with stroke showed significant spatial and temporal reaching abnormalities. Figure 1g and h presents the DCML and CST in an exemplar control participant, whereas Figure 1i and j demonstrate an exemplar stroke participant with the red representing the lesion.
With respect to behavior on the robotic tasks, 15 participants with stroke failed the position matching task at timepoint 1 and six failed at timepoint 2. Furthermore, 13 participants with stroke failed the kinesthetic matching task at timepoint 1 and five failed at timepoint 2. Finally, 21 participants with stroke failed the visually guided reaching task at timepoint 1 and 12 failed at timepoint 2.
3.2. Diffusion metrics for the DCML, POCG‐SMG, POCG‐HG, and CST
We measured FA in the DCML of control participants and found it was not different between hemispheres [left hemisphere mean(SD) 0.47(0.02), right hemisphere 0.47(0.03); Figure 2a]. Ipsilesional DCML‐FA in stroke participants at timepoints 1 and 2 was significantly lower than the DCML‐FA in either hemisphere in controls. Ipsilesional DCML‐FA was also significantly lower than the contralesional DCML‐FA at both timepoints.
Figure 2.

FA, MD, RD, and AD for the DCML, POCG‐SMG, POCG‐HG. The DCML is presented in the top row, the POCG‐SMG in the middle row, and the POCG‐HG in the bottom row. For each tract, the FA, MD, RD, and AD are presented for the left hemisphere of controls (CL), the right hemisphere of controls (CR), the ipsilesional (Ips), and contralesional (Con) hemisphere of participants with stroke at 1 (1 mo) and 6 (6 mo) months poststroke. Asterisks indicate whether paired (**) or unpaired (*) testing was conducted. Only significant relationships that survived the Benjamini–Hochberg correction for multiple comparisons are noted, p values are provided. Boxplots provide the 25th and 75th percentiles and median. Outliers are indicated by + [Color figure can be viewed at http://wileyonlinelibrary.com]
In addition to FA in the DCML, we measured MD, RD, and AD. Neither ipsilesional nor contralesional DCML‐MD (Figure 2b) was significantly different than controls at timepoints 1 or 2. However, at timepoint 2, ipsilesional DCML‐MD was significantly higher than the contralesional DCML‐MD. Ipsilesional DCML‐RD (Figure 2c) was significantly higher than either hemisphere of controls at both timepoints. There were no significant differences between controls and either the ipsilesional or contralesional hemisphere for DCML‐AD. However, at timepoint 1, ipsilesional DCML‐AD (Figure 2d) was significantly lower than contralesional DCML‐AD.
We also measured the FA, MD, RD, and AD in the POCG‐SMG. FA was not different between hemispheres of control participants [left hemisphere; mean (SD) 0.40(0.04), right hemisphere 0.30(0.03); Figure 2e]. We did not observe any significant differences between groups for FA (Figure 2e), MD (Figure 2f), RD (Figure 2g), or AD (Figure 2h). Furthermore, we did not observe any differences between the ipsilesional and contralesional hemispheres of the participants with stroke at either timepoint for FA, MD, RD, and AD.
We also measured the FA, MD, RD, and AD in the POCG‐HG. FA was not different between hemispheres of control participants [left hemisphere mean (SD) 0.28(0.03), right hemisphere 0.34(0.03); Figure 2i)]. Ipsilesional POCG‐HG FA in stroke participants was significantly lower than POCG‐HG FA in either hemisphere of controls at both timepoints. Ipsilesional POCG‐HG MD in stroke participants was significantly higher than POCG‐HG MD in either hemisphere of controls at both timepoints (Figure 2j). Ipsilesional POCG‐HG RD in stroke participants was significantly higher than POCG‐HG RD in either hemisphere of controls at both timepoints (Figure 2k). There were no significant differences between groups or between hemispheres for AD (Figure 2l).
We also measured CST‐FA [(left hemisphere mean (SD) 0.50(0.02), right hemisphere 0.51 (0.03); Figure 3a] in control subjects and found it was not different between control hemispheres. Ipsilesional CST‐FA in stroke participants at timepoints 1 and 2 was significantly lower than CST‐FA in either hemisphere in controls (Figure 3a). Ipsilesional CST‐FA was also significantly lower than contralesional CST‐FA at both timepoints. CST‐MD (Figure 3b) was not significantly different between the left and right hemispheres of control participants. Ipsilesional CST‐MD was significantly higher than CST‐MD in control participants at timepoints 1 and 2. Contralesional CST‐MD at timepoint 1 was higher than control participants. Ipsilesional CST‐MD was significantly higher than contralesional CST‐MD at timepoint 2. CST‐RD (Figure 3c) was not different between hemispheres in control participants. Ipsilesional and contralesional CST‐RD was higher than controls at both timepoints. Ipsilesional CST‐RD was higher than contralesional CST‐RD at both timepoints. Finally, there were no significant differences between controls and either the ipsilesional or contralesional hemisphere for CST‐AD (Figure 3d).
Figure 3.

FA, MD, RD, and AD for the CST. The FA, MD, RD, and AD for the left hemisphere of controls (CL), the right hemisphere of controls (CR), the ipsilesional (Ips), and contralesional (Con) hemisphere of participants with stroke at 1 (1 mo) and 6 (6 mo) months poststroke is presented. Asterisks indicate whether paired (**) or unpaired (*) testing was conducted. Only significant relationships that survived the Benjamini–Hochberg correction for multiple comparisons are noted, p values are provided. Boxplots provide the 25th and 75th percentiles and median. Outliers are indicated by + [Color figure can be viewed at http://wileyonlinelibrary.com]
3.3. Relationship between proprioceptive or motor performance and FA
We investigated the relationship between the robotic task scores and ipsilesional FA of the DCML, POCG‐SMG, POCG‐HG, and CST using Pearson's correlations which were corrected for multiple comparisons. Figure 4a (solid line) demonstrates a nonsignificant relationship between DCML‐FA and stroke participant performance on the position match task score at timepoint 1 (r = −0.29, p = 0.14). A nonsignificant relationship between DCML‐FA and stroke participant performance on the kinesthetic matching task score was also observed at timepoint 1 (r = −0.30, p = 0.13). The relationship between DCML‐FA and stroke participant performance on both the position (r = −0.19, p = 0.43) and kinesthetic matching tasks (r = −0.17, p = 0.48) was nonsignificant at timepoint 2.
Figure 4.
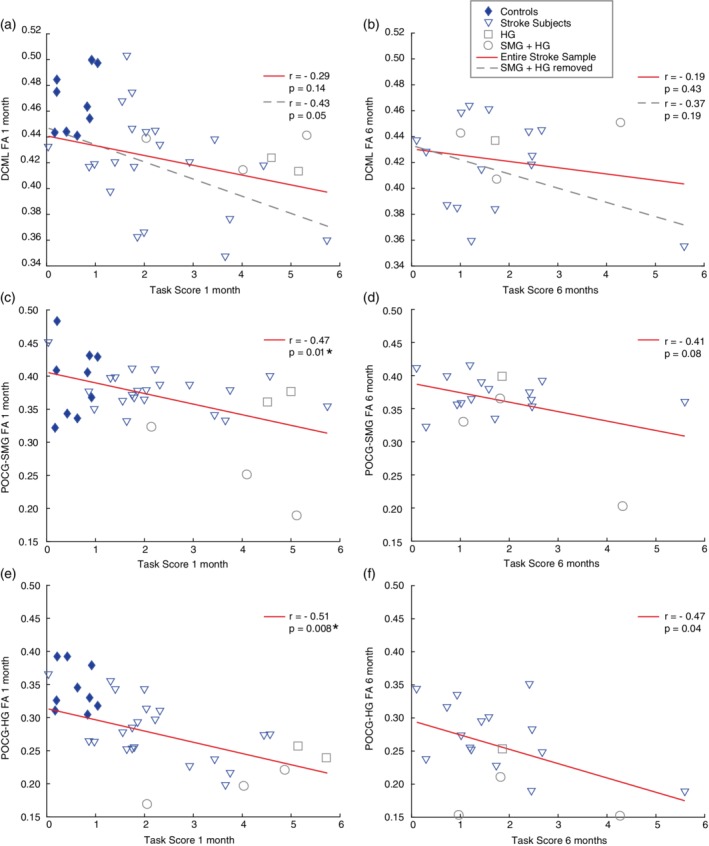
Relationship between FA and position matching performance. Scatterplots and Pearson's correlation results for FA of each tract and position matching task scores at both timepoints are presented (DCML is presented in the top row, POCG‐SMG in the middle row, and POCG‐HG in the bottom row). Control subjects are represented by filled diamond symbols, and stroke subjects are represented by open symbols. Open square symbols represent subjects with stroke lesions overlapping Heschl's gyrus. Open circle symbols represent subjects with stroke lesions overlapping the supramarginal gyrus and Heschl's gyrus. For the DCML, two correlations were conducted—first with the entire stroke sample (n = 26 at 1 month, n = 19 at 6 months poststroke) and secondly without the stroke subjects whose lesion overlapped SMG and/or HG (n = 5 at 1 month poststroke, n = 4 at 6 months poststroke). The solid line corresponds with the correlation that included the entire stroke sample while the dashed line corresponds to the stroke sample without individuals who had lesions overlapping SMG and/or HG. Asterisks indicate significant results that survived the Benjamini–Hochberg correction for multiple comparisons [Color figure can be viewed at http://wileyonlinelibrary.com]
A moderate significant relationship was observed between POCG‐SMG FA and stroke participant performance on the position match task scores at timepoint 1 (r = −0.47, p = 0.01) (Figure 4c). POCG‐SMG FA and stroke participant performance on the kinesthetic matching task was not significantly correlated at timepoint 1 (r = −0.39, p = 0.05). POCG‐SMG FA was not significantly correlated with stroke participant performance on the position matching task (r = −0.41, p = 0.08; Figure 4d) or the kinesthetic matching task (r = −0.38, p = 0.11) at timepoint 2.
A moderate significant relationship was observed between POCG‐HG FA and stroke participant performance on the position matching task scores at timepoint 1 (r = −0.51, p = 0.008; Figure 4e). In contrast, FA of the POCG‐HG was not significantly correlated with stroke participant performance on the kinesthetic matching task at timepoint 1 (r = 0.31, p = 0.12). POCG‐HG FA was not significantly correlated with stroke participant performance on the position matching task after correcting for multiple comparisons (r = −0.47, p = 0.04) at timepoint 2 (Figure 4f). POCG‐HG FA was not significantly correlated with stroke participant performance on the kinesthetic matching task (r = −0.20, p = 0.41) at timepoint 2.
A moderate (r = −0.58, p = 0.002; Figure 5a), a significant relationship was observed between CST‐FA and performance on the visually guided reaching task at timepoint 1, although the relationship was nonsignificant at timepoint 2 (Figure 5b). Furthermore, there was no significant relationship between CST‐FA and either position matching task scores or kinesthetic matching task scores at either timepoint.
Figure 5.

Relationship between FA and motor performance. Scatterplots and Pearson's correlation results for CST‐FA and scores on the visually guided reaching task at 1 month poststroke (a) and 6 months poststroke (b). Control subjects are represented by filled diamond symbols, and stroke subjects are represented by open symbols. Open square symbols represent subjects with stroke lesions overlapping Heschl's gyrus. Open circle symbols represent subjects with stroke lesions overlapping the supramarginal gyrus and Heschl's gyrus. At each timepoint, two correlations were conducted—first with the entire stroke sample (n = 26 at 1 month, n = 19 at 6 months poststroke) and secondly without the subjects whose lesion overlapped SMG and/or HG (n = 5 at 1 month poststroke, n = 4 at 6 months poststroke). The solid line corresponds with the correlation that included the entire stroke sample while the dashed line corresponds to the stroke sample without individuals who had lesions overlapping SMG and/or HG. Asterisks indicate significant results that survived the Benjamini–Hochberg correction for multiple comparisons [Color figure can be viewed at http://wileyonlinelibrary.com]
We also examined the relationships between MD, RD, and AD for the DCML, POCG‐HG, POCG‐SMG, and CST across the three robotic task scores at timepoints 1 and 2. We saw no significant relationships between any of these diffusion metrics and any of the robotic task scores. Changes from timepoint 1 to timepoint 2 for all four diffusion metrics are presented in Supporting Information Figure SS1 (DCML, POCG‐SMG, and POCG‐HG), and Supporting Information Figure SS2 (CST).
Our previous work has documented that damage to the SMG and HG is related to poor performance on the position matching and kinesthetic matching tasks (Findlater et al., 2016; Kenzie et al., 2016). Thus, we queried whether participants with SMG and HG lesions in the present sample would also demonstrate poor performance on the robotic proprioception tasks. Furthermore, we questioned whether damage to these regions affected the relationship between DCML‐FA and proprioception. We identified individuals with lesions that overlapped the SMG or HG using the Harvard–Oxford cortical atlas included with FSL. All subjects (n = 5) with damage to the SMG and/or HG had failing task scores on both of the proprioceptive tasks at timepoint 1 and yet had a DCML‐FA (0.44 ± 0.01) which was not significantly different than the DCML‐FA for the contralesional hemisphere in the same group (p = 0.1, paired t test, mean DCML‐FA in the contralesional hemisphere was 0.46 ± 0.02). We then removed individuals who had SMG/HG damage (n = 5 at timepoint 1 and n = 4 at timepoint 2) and re‐examined the relationship between the robotic tasks and ipsilesional DCML‐FA. When subjects with SMG/HG lesions were removed, the correlation observed between DCML‐FA and performance on the position matching task at timepoint 1 was r = −0.43, but this was not significant (p = 0.05, Figure 4a). We saw a similar relationship between DCML‐FA and performance on the kinesthetic matching task (r = −0.40, p = 0.07). No significant relationships between DCML‐FA and performance on the robotic proprioception tasks were seen at timepoint 2 when the subjects with SMG and HG lesions were removed. Furthermore, no significant relationships were observed between DCML MD, RD, AD, and position matching or kinesthetic matching performance when the subjects with SMG and HG lesions were removed from the analysis.
Given the potential confound of ipsilesional motor impairments on performance of the robotic position and kinesthetic matching tasks, we performed a supplementary analysis and excluded two subjects from the analysis above that had abnormal ipsilesional CMSA arm scores (both received scores of 6) at timepoint 1 and one subject that had a CMSA score of 6 at timepoint 2. Our findings were largely unchanged (see Supporting Information Results).
3.4. Proportion of tract overlapped by lesion
Finally, we investigated whether the amount of tract overlapped by a lesion was related to (a) FA values or (b) robotic proprioceptive or motor task scores. At timepoint 2, we found that the proportion of POCG‐SMG lesion/overlap was significantly correlated with POCG‐SMG FA (r = −0.85, p = 5.06 × 10−6) after correcting for multiple comparisons. We also found that the proportion of POCG‐HG lesion/overlap was significantly correlated with POCG‐HG FA (r = −0.63, p = 0.004) at timepoint 2 after correcting for multiple comparisons. However, no other significant relationships were identified. The specifics of these results are detailed in the Supplemental Results. Lesion overlap maps are presented in Figure 6 for the whole sample (Figure 6a) as well as for the five subjects with lesions overlapping the HG and/or SMG (Figure 6b) at timepoint 1.
Figure 6.

Overlap maps, SMG, and HG regions. (a) The overlap of the lesions for all 26 participants with stroke. The highest region of overlap was in the internal capsule and thalamus of the right hemisphere (n = 9). (b) The lesion overlap map of the five subjects who had damage to HG (region traced in yellow) and/or SMG (region traced in magenta). The DCML (of a representative control subject) is traced in orange. (c) A render image presenting SMG and HG regions
4. DISCUSSION
Our study examined the relationship between proprioception and diffusion metrics of three sensory tracts at 1 and 6 months poststroke. We observed significant relationships between lower FA in the association tracts (POCG‐HG and POCG‐SMG) and proprioceptive performance. Interestingly, we failed to see a significant relationship between DCML‐FA and proprioceptive task performance. Closer inspection revealed that participants with lesions involving the supramarginal and Heschl's gyri demonstrated abnormal proprioceptive task performance despite having relatively high DCML‐FA. The relationship between motor performance on the visually guided reaching task and CST‐FA in our present study, however, demonstrated a moderate significant correlation. Whereas numerous previous studies have found that CST‐FA is predictive of motor function (cross‐sectional) or motor recovery (improvement over time), our findings imply that it is possible to have poor proprioception without low DCML‐FA. This reinforces the importance of intact cortical sensory association areas and tracts that do not have direct connections to the DCML.
While we were unable to find other studies that investigated the relationship between sensory tract FA and proprioception after adult stroke, a number of studies have investigated lower CST‐FA and motor function (at an acute/subacute timepoint) or motor recovery (improvement from within 1‐month poststroke to approximately 6 months poststroke) at various timepoints poststroke. Our finding that lower CST‐FA was associated with poorer motor performance at 1 month poststroke has also been observed by other groups (Liang et al., 2007; Yu et al., 2009). A number of studies have reported a relationship between CST‐FA and motor performance at 6 months (Groisser, Copen, Singhal, Hirai, & Schaechter, 2014; Koyama et al., 2013; Kwon et al., 2012; Lindenberg et al., 2012). However, the present study and a number of others have failed to observe a significant relationship between CST‐FA and motor function at 6 months poststroke (Grassel et al., 2010; Puig et al., 2011; Yu et al., 2009). These discrepant findings may be due to between‐study variability across a number of methodological factors. First, the timing of diffusion imaging collection differs between studies from days (Cho, Kim, Kim, et al., 2007; Jang et al., 2010; Kusano et al., 2009) to weeks (Kim et al., 2015; Koyama et al., 2013). Individual diffusion metrics have been shown to evolve over time (Liang et al., 2007; Puig et al., 2011; Yu et al., 2009). It has been suggested that higher mean diffusivity after stroke may be related to loss of structural integrity in white matter over time (Lindenberg et al., 2012; Yu et al., 2009). FA appears to be associated with axonal degeneration which is a process that takes days to weeks to occur in animal studies (Shereen et al., 2011). In the present study, we chose to collect diffusion imaging at 3–4 weeks poststroke as others have reported that CST‐FA was stable by this point (Puig et al., 2011). Second, many studies used ordinal scales such as the Modified Rankin Scale [scores range from 0 to 5 (Grassel et al., 2010; Koyama et al., 2013; Kusano et al., 2009)], the National Institutes of Health Stroke Scale [arm portion, scores range from 0 to 6 (Liang et al., 2007; Song et al., 2014)] or the Medical Research Council strength scale [scores range from 0 to 5 (Cho, Kim, Kim, et al., 2007; Koyama, Tsuji, Miyake, Ohmura, & Domen, 2012)]. Scales such as these are prone to floor and ceiling effects which have the potential to bias relationships. Third, some studies dichotomized motor impairment scores (Puig et al., 2011) or tract integrity (Jang et al., 2010; Kim et al., 2015) which may have influenced their results by increasing the risk of type I error. Fourth, differing acquisition parameters, preprocessing methods, and tractography algorithms can affect the ability to estimate diffusion parameters such as FA (Jeurissen, Descoteaux, Mori, & Leemans, 2017). In terms of the latter, various methods have been used to explore the relationship between FA metrics of the CST and motor impairment [FA of the ipsilesional region (Liang et al., 2007; Lindenberg et al., 2012; Puig et al., 2010), FA ratio of ipsilesional to contralesional region (Koyama et al., 2013; Puig et al., 2010), and FA asymmetry (Lindenberg et al., 2010; Stinear et al., 2007)]. Finally, some studies restricted inclusion criteria, and only included subjects with severe motor deficits (Cho, Kim, Kim, et al., 2007; Jang et al., 2010; Kwon et al., 2012; Liu et al., 2012; Song et al., 2014). Our selection criteria led to a more heterogenous group, representative of a typical inpatient rehabilitation sample at our center. Finally, we observed participants who had normal reaching performance and low CST‐FA and others with abnormal reaching performance and normal (within the control range) CST‐FA, suggesting that CST‐FA may not be a reliable biomarker for all individuals.
Our findings also indicated that tract/lesion overlap was not related either to performance on the proprioceptive or motor tasks. This is consistent with Cassidy et al. (2018) who investigated FA of the posterior limb of the internal capsule and motor recovery in a chronic stroke sample. Additionally, our findings are in agreement with Lindenberg et al. (2010) who reported that diffusivity changes were present in chronic stroke subjects with motor deficits even if the lesion did not directly overlap the tract. They, and others, suggest that such findings are consistent with remote changes in the motor network, possibly due to diaschisis (Peters et al., 2018; Seitz Rüdiger et al., 1999; Thomas et al., 2005).
Lesions of the SMG and HG are known to lead to impairments in proprioceptive function (Findlater et al., 2016, 2018; Kenzie et al., 2016). The SMG, as part of the parietal sensory association area, is an important region for integration of somatosensory information (Iwamura, 2003; Lynch, 1980; Pandya & Seltzer, 1982). Recent functional MRI studies have demonstrated the SMG's role in awareness of hand position in healthy individuals (Ben‐Shabat et al., 2015; Brozzoli et al., 2011) in addition to individuals with stroke (Ben‐Shabat et al., 2015). Therefore, it is not entirely surprising that damage to the tract communicating with the POCG‐SMG was associated with poor proprioceptive performance in the present study. HG is involved in the frontoparietal network (Shinn, Baker, Cohen, & Ongur, 2013) and is known to contribute to the spatial localization of sound (Altmann et al., 2007; Zatorre & Penhune, 2001) but based on the results of Findlater et al. (2018), it may also contribute to somatosensory processing. The present study adds to our previous results as lower FA in the POCG‐HG was also associated with poor proprioceptive performance. As such, the DCML appears to be only part of the story with respect to proprioceptive function. The cortical network responsible for proprioceptive processing is critical for function and is not well elucidated by looking at the DCML in isolation.
The present study is the only one we know of that has examined the relationship between DCML diffusion metrics and proprioceptive function in adults poststroke, something called for in a recent review of stroke biomarkers (Boyd et al., 2017). While we were able to make novel conclusions based on our results, we recognize that this study has some limitations. First, we question whether our findings at 6 months would have been more informative with larger sample size. Our sample was smaller than eight of the studies we are aware of that have demonstrated an association between CST‐FA and motor function (Cho, Kim, Kim, et al., 2007; Cho, Kim, Choi, et al., 2007; Jang et al., 2010; Kim et al., 2015; Kwon et al., 2012; Liu et al., 2012; Puig et al., 2013; Puig et al., 2011), and slightly larger than seven others (Grassel et al., 2010; Koyama et al., 2012; Kusano et al., 2009; Kuzu et al., 2012; Liang et al., 2007; Lindenberg et al., 2012; Ma, Liu, Li, Zhou, & Zhou, 2014). We also included participants with either ischemic or hemorrhagic stroke. In doing so, we were able to recruit more subjects and our sample is representative of the mix of individuals with stroke on a typical rehabilitation unit.
Our findings indicate that DCML damage as measured by diffusion tractography does not necessarily predict proprioceptive performance after stroke, but FA of the POCG‐SMG and POCG‐HG may be more useful. Improved understanding of proprioceptive processing is important because approximately one‐third of participants with stroke continued to have proprioceptive deficits at 6 months poststroke (Findlater et al., 2018; Semrau et al., 2015). As we enter the era of personalized medicine, these results will hopefully inform clinicians and researchers who aim to provide targeted treatment to rehabilitate proprioceptive deficits. Given the importance of cortical processing, these results may inform treatments such as noninvasive brain stimulation.
Supporting information
Appendix S1: Supporting information
Figure S1 Timepoint comparison of diffusion metrics for the DCML, POCG‐SMG, and POCG‐HG
One‐month and 6‐month values for FA, MD, RD, and AD for the 19 participants who attended both timepoints. Each line represents an individual subject in each plot. Diffusion metrics for the DCML are presented in the left column, POCG‐SMG in the middle column, and POCG‐HG in the right column. The two timepoints for each diffusion metric were compared using paired t tests and the Benjamini–Hochberg procedure was used to correct for multiple comparisons. p values are presented for significant results that survived the Benjamini–Hochberg multiple comparison correction. Comparisons for FA, MD, RD, and AD are presented for each tract.
Figure S2 Timepoint comparison of diffusion metrics for the CST
One‐month and six‐month values for FA, MD, RD, and AD for the 19 participants who attended both timepoints. The two timepoints for each diffusion metric were compared using paired t tests and the Benjamini–Hochberg procedure was used to correct for multiple comparisons. p values are presented for significant results that survived the Benjamini–Hochberg multiple comparison correction. Comparisons for FA (A), MD (B), RD (C), and AD (D) are presented.
ACKNOWLEDGMENTS
The authors wish to thank Mr. Mark Piitz and Ms. Janice Yajure for their assistance with participant recruitment and assessment. The present work was supported by a Canadian Institutes of Health Research Grant (MOP 106662). SEF was supported by an Alberta Innovates Health Solutions Clinical Fellowship.
Findlater SE, Mazerolle EL, Pike GB, Dukelow SP. Proprioception and motor performance after stroke: An examination of diffusion properties in sensory and motor pathways. Hum Brain Mapp. 2019;40:2995–3009. 10.1002/hbm.24574
Deceased: Sonja E. Findlater
Funding information Alberta Innovates Health Solutions Clinician Fellowship; Canadian Institutes of Health Research, Grant/Award Number: MOP 106662
REFERENCES
- Altmann, C. F. , Bledowski, C. , Wibral, M. , & Kaiser, J. (2007). Processing of location and pattern changes of natural sounds in the human auditory cortex. NeuroImage, 35(3), 1192–1200. 10.1016/j.neuroimage.2007.01.007 [DOI] [PubMed] [Google Scholar]
- Andersson, J. L. R. , Skare, S. , & Ashburner, J. (2003). How to correct susceptibility distortions in spin‐echo echo‐planar images: Application to diffusion tensor imaging. NeuroImage, 20(2), 870–888. 10.1016/s1053-8119(03)00336-7 [DOI] [PubMed] [Google Scholar]
- Baird, A. E. , Benfield, A. , Schlaug, G. , Siewert, B. , Lovblad, K. O. , Edelman, R. R. , & Warach, S. (1997). Enlargement of human cerebral ischemic lesion volumes measured by diffusion‐weighted magnetic resonance imaging. Annals of Neurology, 41(5), 581–589. 10.1002/ana.410410506 [DOI] [PubMed] [Google Scholar]
- Bajada, C. J. , Haroon, H. A. , Azadbakht, H. , Parker, G. J. M. , Lambon Ralph, M. A. , & Cloutman, L. L. (2017). The tract terminations in the temporal lobe: Their location and associated functions. Cortex, 97, 277–290. 10.1016/j.cortex.2016.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgart, F. , Gaschler‐Markefski, B. , Woldorff, M. G. , Heinze, H. J. , & Scheich, H. (1999). A movement‐sensitive area in auditory cortex. Nature, 400(6746), 724–726. 10.1038/23385 [DOI] [PubMed] [Google Scholar]
- Behrens, T. E. , Berg, H. J. , Jbabdi, S. , Rushworth, M. F. , & Woolrich, M. W. (2007). Probabilistic diffusion tractography with multiple fibre orientations: What can we gain? NeuroImage, 34(1), 144–155. 10.1016/j.neuroimage.2006.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens, T. E. , Woolrich, M. W. , Jenkinson, M. , Johansen‐Berg, H. , Nunes, R. G. , Clare, S. , … Smith, S. M. (2003). Characterization and propagation of uncertainty in diffusion‐weighted MR imaging. Magnetic Resonance in Medicine, 50(5), 1077–1088. 10.1002/mrm.10609 [DOI] [PubMed] [Google Scholar]
- Benjamini, Y. , & Hochberg, Y. (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society: Series B: Methodological, 57(1), 289–300. [Google Scholar]
- Ben‐Shabat, E. , Matyas, T. A. , Pell, G. S. , Brodtmann, A. , & Carey, L. M. (2015). The right supramarginal gyrus is important for proprioception in healthy and stroke‐affected participants: A functional MRI study. Frontiers in Neurology, 6, 248 10.3389/fneur.2015.00248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- BKIN Technologies . (2018). Dexterit‐E 3.7 User Guide. Kingston, ON: BKIN Technologies. [Google Scholar]
- Bohannon, R. W. , & Smith, M. B. (1987). Interrater reliability of a modified Ashworth scale of muscle spasticity. Physical Therapy, 67(2), 206–207. [DOI] [PubMed] [Google Scholar]
- Box, G. E. P. , & Cox, D. R. (1964). An analysis of transformations. Journal of the Royal Statistical Society: Series B: Methodological, 26(2), 211–252. [Google Scholar]
- Boyd, L. A. , Hayward, K. S. , Ward, N. S. , Stinear, C. M. , Rosso, C. , Fisher, R. J. , … Cramer, S. C. (2017). Biomarkers of stroke recovery: Consensus‐based core recommendations from the stroke recovery and rehabilitation roundtable. International Journal of Stroke, 12(5), 480–493. 10.1177/1747493017714176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brozzoli, C. , Gentile, G. , Petkova, V. I. , & Ehrsson, H. H. (2011). FMRI adaptation reveals a cortical mechanism for the coding of space near the hand. Journal of Neuroscience, 31(24), 9023–9031. 10.1523/JNEUROSCI.1172-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey, L. M. (1995). Somatosensory loss after stroke. Critical Reviews in Physical and Rehabilitation Medicine, 7(1), 51–91. 10.1615/CritRevPhysRehabilMed.v7.i1.40 [DOI] [Google Scholar]
- Carey, L. M. , Oke, L. E. , & Matyas, T. A. (1996). Impaired limb position sense after stroke: A quantitative test for clinical use. Archives of Physical Medicine and Rehabilitation, 77, 1271–1278. [DOI] [PubMed] [Google Scholar]
- Cassidy, J. M. , Tran, G. , Quinlan, E. B. , & Cramer, S. C. (2018). Neuroimaging identifies patients Most likely to respond to a restorative stroke therapy. Stroke, 49(2), 433–438. 10.1161/strokeaha.117.018844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho, S. H. , Kim, D. G. , Kim, D. S. , Kim, Y. H. , Lee, C. H. , & Jang, S. H. (2007). Motor outcome according to the integrity of the corticospinal tract determined by diffusion tensor tractography in the early stage of corona radiata infarct. Neuroscience Letters, 426(2), 123–127. 10.1016/j.neulet.2007.08.049 [DOI] [PubMed] [Google Scholar]
- Cho, S. H. , Kim, S. H. , Choi, B. Y. , Cho, S. H. , Kang, J. H. , Lee, C. H. , … Jang, S. H. (2007). Motor outcome according to diffusion tensor tractography findings in the early stage of intracerebral hemorrhage. Neuroscience Letters, 421(2), 142–146. 10.1016/j.neulet.2007.04.052 [DOI] [PubMed] [Google Scholar]
- Coderre, A. M. , Zeid, A. A. , Dukelow, S. P. , Demmer, M. J. , Moore, K. D. , Demers, M. J. , … Scott, S. H. (2010). Assessment of upper‐limb sensorimotor function of subacute stroke patients using visually guided reaching. Neurorehabilitation and Neural Repair, 24(6), 528–541. 10.1177/1545968309356091 [DOI] [PubMed] [Google Scholar]
- Connell, L. A. , Lincoln, N. B. , & Radford, K. A. (2008). Somatosensory impairment after stroke: Frequency of different deficits and their recovery. Clinical Rehabilitation, 22(8), 758–767. 10.1177/0269215508090674 [DOI] [PubMed] [Google Scholar]
- Connell, L. A. , & Tyson, S. F. (2012). Measures of sensation in neurological conditions: A systematic review. Clinical Rehabilitation, 26(1), 68–80. 10.1177/0269215511412982 [DOI] [PubMed] [Google Scholar]
- Dukelow, S. P. , Herter, T. M. , Bagg, S. D. , & Scott, S. H. (2012). The independence of deficits in position sense and visually guided reaching following stroke. Journal of Neuroengineering and Rehabilitation, 9, 72 10.1186/1743-0003-9-72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dukelow, S. P. , Herter, T. M. , Moore, K. D. , Demers, M. J. , Glasgow, J. I. , Bagg, S. D. , … Scott, S. H. (2010). Quantitative assessment of limb position sense following stroke. Neurorehabilitation and Neural Repair, 24(2), 178–187. 10.1177/1545968309345267 [DOI] [PubMed] [Google Scholar]
- Findlater, S. E. , Desai, J. A. , Semrau, J. A. , Kenzie, J. M. , Rorden, C. , Herter, T. M. , … Dukelow, S. P. (2016). Central perception of position sense involves a distributed neural network: Evidence from lesion‐behavior analyses. Cortex, 79(79), 42–56. 10.1016/j.cortex.2016.03.008 [DOI] [PubMed] [Google Scholar]
- Findlater, S. E. , Hawe, R. L. , Semrau, J. A. , Kenzie, J. M. , Yu, A. Y. , Scott, S. H. , & Dukelow, S. P. (2018). Lesion locations associated with persistent proprioceptive impairment in the upper limbs after stroke. NeuroImage: Clinical, 20, 955–971. 10.1016/j.nicl.2018.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fling, B. W. , Dutta, G. G. , Schlueter, H. , Cameron, M. H. , & Horak, F. B. (2014). Associations between proprioceptive neural pathway structural connectivity and balance in people with multiple sclerosis. Frontiers in Human Neuroscience, 8, 814 10.3389/fnhum.2014.00814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner, E. , & Johnson, K. (2013). The somaotosensory system: Receptors and central pathways In Kandell E., Schwartz J., Jessell T., Siegelbaum S., & Hudspeth A. (Eds.), Principles of neural science (pp. 473–497). New York: McGraw‐Hill. [Google Scholar]
- Garraway, W. M. , Akhtar, A. J. , Gore, S. M. , Prescott, R. J. , & Smith, R. G. (1976). Observer variation in the clinical assessment of stroke. Age and Ageing, 5(4), 233–240. [DOI] [PubMed] [Google Scholar]
- Gowland, C. , Stratford, P. , Ward, M. , Moreland, J. , Torresin, W. , Van Hullenaar, S. , … Plews, N. (1993). Measuring physical impairment and disability with the Chedoke‐McMaster stroke assessment. Stroke, 24(1), 58–63. 10.1161/01.str.24.1.58 [DOI] [PubMed] [Google Scholar]
- Grassel, D. , Ringer, T. M. , Fitzek, C. , Fitzek, S. , Kohl, M. , Kaiser, W. A. , … Axer, H. (2010). Wallerian degeneration of pyramidal tract after paramedian pons infarct. Cerebrovascular Diseases, 30(4), 380–388. 10.1159/000319573 [DOI] [PubMed] [Google Scholar]
- Groisser, B. N. , Copen, W. A. , Singhal, A. B. , Hirai, K. K. , & Schaechter, J. D. (2014). Corticospinal tract diffusion abnormalities early after stroke predict motor outcome. Neurorehabilitation and Neural Repair, 28(8), 751–760. 10.1177/1545968314521896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirayama, K. , Fukutake, T. , & Kawamura, M. (1999). "Thumb localizing test" for detecting a lesion in the posterior column‐medial lemniscal system. Journal of the Neurological Sciences, 167(1), 45–49. [DOI] [PubMed] [Google Scholar]
- Iwamura, Y. (2003). Somatosensory association cortices. International Congress Series, 1250, 3–14. 10.1016/S0531-5131(03)00971-3 [DOI] [Google Scholar]
- Jang, S. H. , Ahn, S. H. , Sakong, J. , Byun, W. M. , Choi, B. Y. , Chang, C. H. , … Son, S. M. (2010). Comparison of TMS and DTT for predicting motor outcome in intracerebral hemorrhage. Journal of the Neurological Sciences, 290(1–2), 107–111. 10.1016/j.jns.2009.10.019 [DOI] [PubMed] [Google Scholar]
- Jeurissen, B. , Descoteaux, M. , Mori, S. , & Leemans, A. (2017). Diffusion MRI fiber tractography of the brain. NMR in Biomedicine. 10.1002/nbm.3785 [DOI] [PubMed] [Google Scholar]
- Johnson, E. O. , Babis, G. C. , Soultanis, K. C. , & Soucacos, P. N. (2008). Functional neuroanatomy of proprioception. Journal of Surgical Orthopaedic Advances, 17(3), 159–164. [PubMed] [Google Scholar]
- Jung, J. , Cloutman, L. L. , Binney, R. J. , & Lambon Ralph, M. A. (2017). The structural connectivity of higher order association cortices reflects human functional brain networks. Cortex, 97, 221–239. 10.1016/j.cortex.2016.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith, R. A. , Granger, C. V. , Hamilton, B. B. , & Sherwin, F. S. (1987). The functional independence measure: A new tool for rehabilitation. Advances in Clinical Rehabilitation, 1, 6–18. [PubMed] [Google Scholar]
- Kenzie, J. M. , Semrau, J. A. , Findlater, S. E. , Yu, A. Y. , Desai, J. A. , Herter, T. M. , … Dukelow, S. P. (2016). Localization of impaired kinesthetic processing post‐stroke. Frontiers in Human Neuroscience, 10, 505 10.3389/fnhum.2016.00505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenzie, J. M. , Semrau, J. A. , Hill, M. D. , Scott, S. H. , & Dukelow, S. P. (2017). A composite robotic‐based measure of upper limb proprioception. Journal of Neuroengineering and Rehabilitation, 14(1), 114 10.1186/s12984-017-0329-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, B. , & Winstein, C. (2017). Can neurological biomarkers of brain impairment be used to predict poststroke motor recovery? A systematic review. Neurorehabilitation and Neural Repair, 31(1), 3–24. 10.1177/1545968316662708 [DOI] [PubMed] [Google Scholar]
- Kim, K. H. , Kim, Y. H. , Kim, M. S. , Park, C. H. , Lee, A. , & Chang, W. H. (2015). Prediction of motor recovery using diffusion tensor tractography in supratentorial stroke patients with severe motor involvement. Annals of Rehabilitation Medicine, 39(4), 570–576. 10.5535/arm.2015.39.4.570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama, T. , Tsuji, M. , Miyake, H. , Ohmura, T. , & Domen, K. (2012). Motor outcome for patients with acute intracerebral hemorrhage predicted using diffusion tensor imaging: An application of ordinal logistic modeling. Journal of Stroke and Cerebrovascular Diseases, 21(8), 704–711. 10.1016/j.jstrokecerebrovasdis.2011.03.004 [DOI] [PubMed] [Google Scholar]
- Koyama, T. , Tsuji, M. , Nishimura, H. , Miyake, H. , Ohmura, T. , & Domen, K. (2013). Diffusion tensor imaging for intracerebral hemorrhage outcome prediction: Comparison using data from the corona radiata/internal capsule and the cerebral peduncle. Journal of Stroke and Cerebrovascular Diseases, 22(1), 72–79. 10.1016/j.jstrokecerebrovasdis.2011.06.014 [DOI] [PubMed] [Google Scholar]
- Kuczynski, A. M. , Carlson, H. L. , Lebel, C. , Hodge, J. A. , Dukelow, S. P. , Semrau, J. A. , & Kirton, A. (2017). Sensory tractography and robot‐quantified proprioception in hemiparetic children with perinatal stroke. Human Brain Mapping, 38(5), 2424–2440. 10.1002/hbm.23530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusano, Y. , Seguchi, T. , Horiuchi, T. , Kakizawa, Y. , Kobayashi, T. , Tanaka, Y. , … Hongo, K. (2009). Prediction of functional outcome in acute cerebral hemorrhage using diffusion tensor imaging at 3T: A prospective study. AJNR. American Journal of Neuroradiology, 30(8), 1561–1565. 10.3174/ajnr.A1639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzu, Y. , Inoue, T. , Kanbara, Y. , Nishimoto, H. , Fujiwara, S. , Ogasawara, K. , & Ogawa, A. (2012). Prediction of motor function outcome after intracerebral hemorrhage using fractional anisotropy calculated from diffusion tensor imaging. Cerebrovascular Diseases, 33(6), 566–573. 10.1159/000338904 [DOI] [PubMed] [Google Scholar]
- Kwon, Y. H. , Jeoung, Y. J. , Lee, J. , Son, S. M. , Kim, S. , Kim, C. , & Jang, S. H. (2012). Predictability of motor outcome according to the time of diffusion tensor imaging in patients with cerebral infarct. Neuroradiology, 54(7), 691–697. 10.1007/s00234-011-0972-x [DOI] [PubMed] [Google Scholar]
- Liang, Z. , Zeng, J. , Liu, S. , Ling, X. , Xu, A. , Yu, J. , & Ling, L. (2007). A prospective study of secondary degeneration following subcortical infarction using diffusion tensor imaging. Journal of Neurology, Neurosurgery and Psychiatry, 78(6), 581–586. 10.1136/jnnp.2006.099077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lincoln, N. B. , Crow, J. L. , Jackson, J. M. , Waters, G. R. , Adams, S. A. , & Hodgson, P. (1991). The unreliability of sensory assessments. Clinical Rehabilitation, 5(4), 273–282. 10.1177/026921559100500403 [DOI] [Google Scholar]
- Lindenberg, R. , Renga, V. , Zhu, L. L. , Betzler, F. , Alsop, D. , & Schlaug, G. (2010). Structural integrity of corticospinal motor fibers predicts motor impairment in chronic stroke. Neurology, 74(4), 280–287. 10.1212/WNL.0b013e3181ccc6d9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenberg, R. , Zhu, L. L. , Ruber, T. , & Schlaug, G. (2012). Predicting functional motor potential in chronic stroke patients using diffusion tensor imaging. Human Brain Mapping, 33(5), 1040–1051. 10.1002/hbm.21266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, X. , Tian, W. , Qiu, X. , Li, J. , Thomson, S. , Li, L. , & Wang, H. Z. (2012). Correlation analysis of quantitative diffusion parameters in ipsilateral cerebral peduncle during Wallerian degeneration with motor function outcome after cerebral ischemic stroke. Journal of Neuroimaging, 22(3), 255–260. 10.1111/j.1552-6569.2011.00617.x [DOI] [PubMed] [Google Scholar]
- Lynch, J. C. (1980). The functional organization of posterior parietal association cortex. Behavioral and Brain Sciences, 3(4), 485–499. 10.1017/S0140525X00006324 [DOI] [Google Scholar]
- Ma, C. , Liu, A. , Li, Z. , Zhou, X. , & Zhou, S. (2014). Longitudinal study of diffusion tensor imaging properties of affected cortical spinal tracts in acute and chronic hemorrhagic stroke. Journal of Clinical Neuroscience, 21(8), 1388–1392. 10.1016/j.jocn.2013.11.032 [DOI] [PubMed] [Google Scholar]
- Oldfield, R. C. (1971). The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia, 9(1), 97–113. 10.1016/0028-3932(71)90067-4 [DOI] [PubMed] [Google Scholar]
- Pandya, D. N. , & Seltzer, B. (1982). Association areas of the cerebral cortex. Trends in Neurosciences, 5, 386–390. [Google Scholar]
- Peters, D. M. , Fridriksson, J. , Stewart, J. C. , Richardson, J. D. , Rorden, C. , Bonilha, L. , … Fritz, S. L. (2018). Cortical disconnection of the ipsilesional primary motor cortex is associated with gait speed and upper extremity motor impairment in chronic left hemispheric stroke. Human Brain Mapping, 39(1), 120–132. 10.1002/hbm.23829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puig, J. , Blasco, G. , Daunis, I. E. J. , Thomalla, G. , Castellanos, M. , Figueras, J. , … Pedraza, S. (2013). Decreased corticospinal tract fractional anisotropy predicts long‐term motor outcome after stroke. Stroke, 44(7), 2016–2018. 10.1161/strokeaha.111.000382 [DOI] [PubMed] [Google Scholar]
- Puig, J. , Pedraza, S. , Blasco, G. , Daunis, I. E. J. , Prados, F. , Remollo, S. , … Serena, J. (2011). Acute damage to the posterior limb of the internal capsule on diffusion tensor tractography as an early imaging predictor of motor outcome after stroke. American Journal of Neuroradiology, 32(5), 857–863. 10.3174/ajnr.A2400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puig, J. , Pedraza, S. , Blasco, G. , Daunis, I. E. J. , Prats, A. , Prados, F. , … Serena, J. (2010). Wallerian degeneration in the corticospinal tract evaluated by diffusion tensor imaging correlates with motor deficit 30 days after middle cerebral artery ischemic stroke. American Journal of Neuroradiology, 31(7), 1324–1330. 10.3174/ajnr.A2038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorden, C. , Karnath, H. O. , & Bonilha, L. (2007). Improving lesion‐symptom mapping. Journal of Cognitive Neuroscience, 19(7), 1081–1088. 10.1162/jocn.2007.19.7.1081 [DOI] [PubMed] [Google Scholar]
- Schaechter, J. D. , Fricker, Z. P. , Perdue, K. L. , Helmer, K. G. , Vangel, M. G. , Greve, D. N. , & Makris, N. (2009). Microstructural status of ipsilesional and contralesional corticospinal tract correlates with motor skill in chronic stroke patients. Human Brain Mapping, 30(11), 3461–3474. 10.1002/hbm.20770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz, R. , Park, C. H. , Boudrias, M. H. , Gerloff, C. , Hummel, F. C. , & Ward, N. S. (2012). Assessing the integrity of corticospinal pathways from primary and secondary cortical motor areas after stroke. Stroke, 43(8), 2248–2251. 10.1161/STROKEAHA.112.662619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwamm, L. H. , Koroshetz, W. J. , Sorensen, A. G. , Wang, B. , Copen, W. A. , Budzik, R. , … Gonzalez, R. G. (1998). Time course of lesion development in patients with acute stroke: Serial diffusion‐ and hemodynamic‐weighted magnetic resonance imaging. Stroke, 29(11), 2268–2276. [DOI] [PubMed] [Google Scholar]
- Seitz Rüdiger, J. , Azari Nina, P. , Knorr, U. , Binkofski, F. , Herzog, H. , & Freund, H.‐J. (1999). The role of diaschisis in stroke recovery. Stroke, 30(9), 1844–1850. 10.1161/01.STR.30.9.1844 [DOI] [PubMed] [Google Scholar]
- Semrau, J. A. , Herter, T. M. , Scott, S. H. , & Dukelow, S. P. (2013). Robotic identification of kinesthetic deficits after stroke. Stroke, 44(12), 3414–3421. 10.1161/STROKEAHA.113.002058 [DOI] [PubMed] [Google Scholar]
- Semrau, J. A. , Herter, T. M. , Scott, S. H. , & Dukelow, S. P. (2015). Examining differences in patterns of sensory and motor recovery after stroke with robotics. Stroke, 46(12), 3459–3469. 10.1161/STROKEAHA.115.010750 [DOI] [PubMed] [Google Scholar]
- Shereen, A. , Nemkul, N. , Yang, D. , Adhami, F. , Dunn, R. S. , Hazen, M. L. , … Kuan, C. Y. (2011). Ex vivo diffusion tensor imaging and neuropathological correlation in a murine model of hypoxia‐ischemia‐induced thrombotic stroke. Journal of Cerebral Blood Flow and Metabolism, 31(4), 1155–1169. 10.1038/jcbfm.2010.212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrington, C. (1907). On the proprioceptive system, especially in its reflex aspect. Brain, 29, 467–482. [Google Scholar]
- Shinn, A. K. , Baker, J. T. , Cohen, B. M. , & Ongur, D. (2013). Functional connectivity of left Heschl's gyrus in vulnerability to auditory hallucinations in schizophrenia. Schizophrenia Research, 143(2–3), 260–268. 10.1016/j.schres.2012.11.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmatis, L. , Krett, J. , Scott, S. H. , & Jin, A. Y. (2017). Robotic exoskeleton assessment of transient ischemic attack. PLoS One, 12(12), e0188786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, S. M. , Jenkinson, M. , Woolrich, M. W. , Beckmann, C. F. , Behrens, T. E. , Johansen‐Berg, H. , … Matthews, P. M. (2004). Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage, 23(Suppl 1), S208–S219. 10.1016/j.neuroimage.2004.07.051 [DOI] [PubMed] [Google Scholar]
- Sommerfeld, D. K. , & von Arbin, M. H. (2004). The impact of somatosensory function on activity performance and length of hospital stay in geriatric patients with stroke. Clinical Rehabilitation, 18(2), 149–155. 10.1191/0269215504cr710oa [DOI] [PubMed] [Google Scholar]
- Song, J. , Young, B. M. , Nigogosyan, Z. , Walton, L. M. , Nair, V. A. , Grogan, S. W. , … Prabhakaran, V. (2014). Characterizing relationships of DTI, fMRI, and motor recovery in stroke rehabilitation utilizing brain‐computer interface technology. Frontiers in Neuroengineering, 7, 31 10.3389/fneng.2014.00031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinear, C. M. , Barber, P. A. , Smale, P. R. , Coxon, J. P. , Fleming, M. K. , & Byblow, W. D. (2007). Functional potential in chronic stroke patients depends on corticospinal tract integrity. Brain, 130(Pt 1, 170–180. 10.1093/brain/awl333 [DOI] [PubMed] [Google Scholar]
- Takenobu, Y. , Hayashi, T. , Moriwaki, H. , Nagatsuka, K. , Naritomi, H. , & Fukuyama, H. (2014). Motor recovery and microstructural change in rubro‐spinal tract in subcortical stroke. Neuroimage: Clinical, 4, 201–208. 10.1016/j.nicl.2013.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas, B. , Eyssen, M. , Peeters, R. , Molenaers, G. , Van Hecke, P. , De Cock, P. , & Sunaert, S. (2005). Quantitative diffusion tensor imaging in cerebral palsy due to periventricular white matter injury. Brain, 128(11), 2562–2577. 10.1093/brain/awh600 [DOI] [PubMed] [Google Scholar]
- van Heugten, C. M. , Dekker, J. , Deelman, B. G. , Stehmann‐Saris, F. C. , & Kinebanian, A. (1999). A diagnostic test for apraxia in stroke patients: Internal consistency and diagnostic value. The Clinical Neuropsychologist, 13(2), 182–192. 10.1076/clin.13.2.182.1966 [DOI] [PubMed] [Google Scholar]
- Ward, N. S. (2017). Restoring brain function after stroke ‐ bridging the gap between animals and humans. Nature Reviews. Neurology, 13(4), 244–255. 10.1038/nrneurol.2017.34 [DOI] [PubMed] [Google Scholar]
- Welmer, A. K. , Holmqvist, L. W. , & Sommerfeld, D. K. (2008). Limited fine hand use after stroke and its association with other disabilities. Journal of Rehabilitation Medicine, 40(8), 603–608. 10.2340/16501977-0218 [DOI] [PubMed] [Google Scholar]
- Wilson, B. , Cockburn, J. , & Halligan, P. (1988). Behavioural inattention test. Fareham, UK: Thames Valley Test Company. [Google Scholar]
- Yu, C. , Zhu, C. , Zhang, Y. , Chen, H. , Qin, W. , Wang, M. , & Li, K. (2009). A longitudinal diffusion tensor imaging study on Wallerian degeneration of corticospinal tract after motor pathway stroke. NeuroImage, 47(2), 451–458. 10.1016/j.neuroimage.2009.04.066 [DOI] [PubMed] [Google Scholar]
- Zatorre, R. J. , & Penhune, V. B. (2001). Spatial localization after excision of human auditory cortex. Journal of Neuroscience, 21(16), 6321–6328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeman, B. , & Yiannikas, C. (1989). Functional prognosis in stroke: Use of somatosensory evoked potentials. Journal of Neurology, Neurosurgy & Psychiatry, 52, 242–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting information
Figure S1 Timepoint comparison of diffusion metrics for the DCML, POCG‐SMG, and POCG‐HG
One‐month and 6‐month values for FA, MD, RD, and AD for the 19 participants who attended both timepoints. Each line represents an individual subject in each plot. Diffusion metrics for the DCML are presented in the left column, POCG‐SMG in the middle column, and POCG‐HG in the right column. The two timepoints for each diffusion metric were compared using paired t tests and the Benjamini–Hochberg procedure was used to correct for multiple comparisons. p values are presented for significant results that survived the Benjamini–Hochberg multiple comparison correction. Comparisons for FA, MD, RD, and AD are presented for each tract.
Figure S2 Timepoint comparison of diffusion metrics for the CST
One‐month and six‐month values for FA, MD, RD, and AD for the 19 participants who attended both timepoints. The two timepoints for each diffusion metric were compared using paired t tests and the Benjamini–Hochberg procedure was used to correct for multiple comparisons. p values are presented for significant results that survived the Benjamini–Hochberg multiple comparison correction. Comparisons for FA (A), MD (B), RD (C), and AD (D) are presented.


