Abstract
Inflammatory diseases of the gastrointestinal tract are often associated with microbial dysbiosis. Thus, dietary interactions with intestinal microbiota, to maintain homeostasis, play a crucial role in regulation of clinical disorders such as colitis. In the current study, we investigated if resveratrol, a polyphenol found in a variety of foods and beverages, would reverse microbial dysbiosis induced during colitis. Administration of resveratrol attenuated colonic inflammation and clinical symptoms in the murine model of TNBS-induced colitis. Resveratrol treatment in mice with colitis led to an increase in CD4+FOXP3+ and CD4+IL-10+ T cells, and a decrease in CD4+IFN-γ+ and CD4+IL-17+ T cells. 16S rRNA gene sequencing to investigate alterations in the gut microbiota revealed that TNBS caused significant dybiosis, which was reversed following resveratrol treatment. Analysis of cecal flush revealed that TNBS administration led to an increase in species such as Bacteroides acidifaciens, but decrease in species such as Ruminococcus gnavus and Akkermansia mucinphilia, as well as a decrease in SCFA i-butyric acid. However, resveratrol treatment restored the gut bacteria back to homeostatic levels, and increased production of i-butyric acid. Fecal transfer experiments confirmed the protective role of resveratrol-induced microbiota against colitis inasmuch as such recipient mice were more resistant to TNBS-colitis and exhibited polarization towards CD4+FOXP3+ T cells and decreases in CD4+IFN-γ+ and CD4+IL-17+ T cells. Collectively, these data demonstrate that resveratrol-mediated attenuation of colitis results from reversal of microbial dysbiosis induced during colitis and such microbiota protect the host from colonic inflammation by inducing Tregs while suppressing inflammatory Th1/Th17 cells.
Keywords: short chain fatty acid; butyrate; fecal transfer; inflammation; 2,4,6-trinitrobenzenesulfonic acid; T helper cells
Summary Sentence:
Gut microbiome composition and SCFA production are altered by resveratrol to prevent development of colitis and induce Tregs which was validated by fecal transfer experiments.
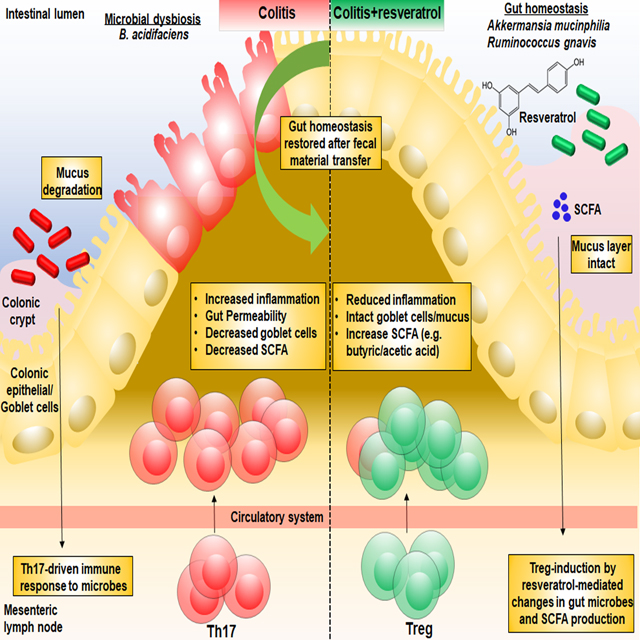
Graphical Abstract
Introduction
Colitis, including ulcerative colitis and Crohn’s disease, is an inflammatory bowel disease (IBD) with unknown etiology [1]. IBD has a high incidence which affects millions of people globally and the prevalence increases annually [2, 3]. There are many factors thought to lead to the development of colitis, such as age, environment, gender, genetics, and diet [4]. Recent advances in next-generation sequencing technology have shown that IBD may also result from alterations in the composition and function of gut microbiota, referred to as dysbiosis. The gut microbiota also interact closely with dietary components to maintain normal immune system homeostasis in the gut. Whether dietary supplements that are effective against colonic inflammation mediate their effects through modulation of gut microbiota is an area of investigation that is novel and highly significant.
Resveratrol (3,4,5-trihydroxy-trans-stilbene) is a natural polyphenol produced by several plants in response to injury or when the plant is under attack by pathogens such as bacteria or fungi [5]. Resveratrol has been extensively studied for its therapeutic benefits against a wide array of diseases including cancer, cardiovascular, neurological and inflammatory diseases [1, 5–11]. Resveratrol mediates these anti-inflammatory effects through multiple pathways [9]. For example, resveratrol has been shown to attenuate colitis by upregulating of silent mating type information regulation-1 (SIRT1) in immune cells which is associated with the T regulatory cells (Treg) induction and activation of hypoxia-inducible Factor 1α (HIF-1α)/MTor signaling pathway [1, 12]. Resveratrol has also been shown to induce unique microRNA that trigger anti-inflammatory pathways as well as induce myeloid-derived suppressor cells (MDSCs) [1, 7, 8, 13]. While resveratrol has been shown to alter the gut microbiome in various disease models [14–17], these studies have captured only an association between resveratrol-induced modulations in gut microbiota and the disease outcome. Thus, conclusive evidence, such as through fecal transfer, is lacking to connect resveratrol-induced modulation in gut microbiota and its beneficial effects against disease pathogenesis.
Here, we demonstrate that during colitis, microbial dysbiosis takes place in the host, which leads to the activation and differentiation of inflammatory effector T cells and inhibition of Tregs. However, upon treatment with resveratrol, these changes are reversed, leading to the development of an anti-inflammatory Treg response. More importantly, we conclusively prove that this mechanism is driven by resveratrol-mediated alterations in the gut microbiome by performing fecal transplant experiments, thereby not only reinforcing the notion that resveratrol is a potential therapeutic against colitis, but also providing a key mechanism through which resveratrol mediates its effects.
Materials and Methods
Animals.
Female BALB/c mice (aged 6–8 weeks) were purchased from the Jackson Laboratories (Bar Harbor, ME). All mice were housed at the AAALAC-accredited animal facility at the University of South Carolina, School of Medicine (Columbia, SC). All procedures were performed according to NIH guidelines under protocols approved by the Institutional Animal Care and Use Committee.
Effects of resveratrol on colitis in mice.
To test the efficacy of treatment with resveratrol in an in vivo TNBS-induced colitis mouse model, we used TNBS, purchased from Sigma-Aldrich (St. Louis, MO). After lightly anesthetizing the mice with controlled isoflurane vaporizer chamber (5% isoflurane with 75% CO2/25% O2), TNBS was administered intrarectally one time into female BALB/c mice at a dose of 1 mg dissolved 0.1 ml of 50% ethanol using a 38 mm catheter, as previously reported [18]. For treatment groups resveratrol, purchased from Sigma-Aldrich (St. Louis, MO), was administered orally using a 30 mm oral gavage needle at 100 mg/kg, a dose established in our previous studies [1], in a total volume of 100μl in appropriate vehicle of 1% carboxymethyl cellulose (CMC). Resveratrol was given 24 hours prior to TNBS injection and given daily this way until completion of the experiment (5 days). Two control groups were used for this study. One control group only received appropriate vehicle (CMC), while the other control group received 100 mg/kg resveratrol dissolved in CMC vehicle. Neither of these control groups received injection of TNBS. The evaluation of colitis clinical signs was done by measuring the weight of mice in all groups daily and performing colonoscopy every other day after TNBS-colitis induction. Colonoscopy scores were determined using a scoring system previously published [19]. In addition, blood was collected prior to experimental endpoint and serum samples were separated and stored at −20°C for colitis-associated biomarker detection. All experimental mice studied were also given intrarectal injections of 50% ethanol to ensure changes in the gut were due to either TNBS or treatment and not attributed to alterations by ethanol. Resveratrol efficacy was also tested in the dextran sodium sulfate (DSS) model of colitis. DSS (3%) was used to induce disease as previously reported[7], and treatment groups (DSS+Resveratrol) were given oral administration of 100 mg/kg of the compound daily throughout the 14-day experiment.
Histology analysis:
Animals were euthanized 5 days after injection of TNBS using the drop jar method containing 5% isoflurane (260 mL in 1 L drop jar) for overdose inhalation, and the proximal portion of colons were excised and cleaned by saline flushing. The length of colon was measured before fixing the excised tissue with 4% paraformaldehyde. Colon pieces were embedded in paraffin, cut into 5μm sections, deparaffinized in xylene, serially diluted in decreasing concentrations of ethanol, and stained with hematoxylin-eosin (H&E) for histopathological examination and Periodic Acid Schiff (PAS) staining to assess mucosal mucin production and presence of goblet cells. Histological scoring of colon sections was determined using previously published criteria [20].
Serum evaluation by enzyme-linked immunoabsorbant assay (ELISA):
Acute phase serum amyloid A (SAA), Lipocalin-2 (Lcn2), myeloid peroxidase (MPO), and interleukin-10 (IL-10) levels in the serum were measured by using enzyme-linked immunosorbent assay (ELISA) kits. SAA ELISA kit was purchased from Abcam (Cambridge, United Kingdom), Lcn-2 ELISA kit was purchased from Thermo-Scientific (Waltham, Massachusetts, USA), MPO ELISA kit was acquired from LifeSpan BioSciences (Seattle, WA) and the IL-10 Luminex ELISA kit was purchased from Biolegend (San Diego, CA). All kits were used in accordance with the respective manufacturer’s protocol.
Flow cytometry staining and analysis:
Cells from mesenteric lymph nodes were isolated and the red blood cells were lysed using lysis buffer (Sigma, St Louis, MO). Cell suspensions were filtered using sterile 70 micron filters (Sigma, St Louis, MO). Four-color flow cytometric analysis was performed following blocking with Fc receptor. All cells were washed with FACS staining buffer (PBS with 1% fetal bovine serum), then stained with FITC-labeled anti-CD3, PE-labeled anti-CD8 and PE-CY7-labeled anti-CD4 at manufacturer suggested concentrations (Biolegend, San Diego, CA). For intracellular staining, cells previously stained for membrane proteins were fixed and permeabilized using a Fix/Perm kit (Biolegend, San Diego, CA). Cells were stained with PE-Cy7-labeled CD4, PE-labeled Foxp3, FITC-labeled IL10, PE-labeled IFN-γ, and FITC-labeled IL-17 (Biolegend, San Diego, CA). Flow cytometry data was analyzed using a CXP FC500 flow cytometer (Beckman Coulter, Brea, CA) and the gating strategy for shown represented plots is shown in Supplemental Figure 1.
Genomic DNA extraction and 16S rRNA gene sequencing:
Colonic flushes were used for pyrosequencing analysis to characterize the gut microbiome composition. The extraction of genomic DNA from colonic flushes was carried out using the QIAamp DNA Stool Mini Kit (Qiagen, Hilden, Germany) according to the manufacture’s instruction. The DNA concentration were determined using a NanoDrop ND-1000 spectrophotometer and stored at −20°C until further processing. Amplification of the 16S rRNA V3-V4 hypervariable gene region was carried out using the 16S V3 314F forward (5′TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGCCTACGGGNGGCWGCAG3′) and V4 805R reverse primers (5′GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGGACTACHVGGGTATCTAATCC3′) with added Illumina adapter overhang nucleotide sequences. The PCR conditions used were as follows: 3 minutes (min) at 95°C, follow by 25 cycles of 30 seconds (s) at 95°C, 30 s at 55°C and 30 s at 72°C, and a final extension at 72°C for 5 min. Each reaction mixture (25 μl) contained 50 ng of genomic DNA, 0.5 μl of amplicon PCR forward primer (0.2 μM), 0.5 μl of amplicon PCR reverse primer (0.2 μM) and 12.5 μl of 2× KAPA Hifi Hot Start Ready Mix. Each reaction was cleaned up with Agencourt AMPure XP beads (Beckman Coulter, Indianapolis, IN). Attachment of dual indices and Illumina sequencing adapters was performed using 5 μl of amplicon PCR DNA product, 5 μl of Illumina Nextera XT Index Primer 1 (N7xx), 5 μl of Nextera XT Index Primer 2 (S5xx), 25 μl of 2× KAPA HiFi Hot Start Ready Mix, and 10 μl of PCR-grade water in this case. Amplification was carried out under the following conditions: 3 min at 95°C, followed by 8 cycles of 30 s at 95°C, 30 s at 55°C, and 30 s at 72°C, and a final extension at 75°C for 5 min. Constructed 16S rRNA gene libraries were purified with Agencourt AMPure XP beads and quantified with Quant-iT PicoGreen dsDNA Assay kit (Thermo Fisher Scientific, Waltham, MA). Library quality control and average size distribution were determined with the Agilent Technologies 2100 Bioanalyzer (Agilent, Santa Clara, CA). Libraries were normalized and pooled to 40 nM based on quantified values. Pooled samples were denatured and diluted to a final concentration of 6 pM with a 30% PhiX (Illumina, San Diego, CA) control. Amplicons were subject to pyrosequencing using the MiSeq Reagent Kit V3 in the Illumina MiSeq System.
Microbial 16S rRNA gene analysis of sequencing data:
The online 16S analysis software from National Institute of Health (NIH, Baltimore, MD), known as Nephele (https://nephele.niaid.nih.gov/), was used to analyze sequencing data collection from the Illumina MiSeq platform. FASTQ sequences were uploaded to Nephele and the 16S metagenomics application was performed. Groups of related DNA sequences were assigned to operational taxonomic units (OTUs), and Nephele-generated output files were analyzed to determine gut microbial composition. Linear Discrimination Analysis Effect Size (LEfSe) was performed on Nephele-generated OTU output data in order to determine microbial biomarkers among experimental groups as previously described [21].
Quantitative Real-Time PCR:
For validation of bacteria identified by 16S rRNA gene analysis, qRT-PCR was used. DNA was extracted from cecal samples using the QIAamp DNA Stool Mini Kit (Qiagen, Hilden, Germany). Samples were analyzed by PCR using primers designed to amplify bacterial 16S rRNA genes. For quantification of Ruminococcus gnavus (5′AGAGGGATGTCAAGACCAGGTA, 3′TACTAGGTGTCGGGTGGAAAAG), Akkermansia muciniphila (5′GTATCTAATCCCTTTCGCTCCC, 3′GACTAGAGTAATGGAGGGGGAA), and Bacteroides acidifaciens (5′GTATGGGATGGGGATGCGTT, 3′CTGCCTCCCGTAGAGTTTGG) the StepOnePlus Real-Time PCR system was used. Fold changes from PCR analysis were obtained by using the delta-delta CT method with comparison to the control group (Vehicle).
SCFAs identification and quantification:
Colonic flushes were collected immediately after euthanasia by excising colon tissues and flushing them with PBS. Samples were collected into in 2 ml Eppendorf tubes under anaerobic conditions. Samples collected were immediately frozen at −80 °C for future analysis. Samples were analyzed for SCFA concentrations using 2-Ethylbutyric acid as internal standard as previously described [22]. Briefly, the cecal samples (100 mg) were suspended and homogenized in water. After centrifugation for 10 min at 12,000 rpm, the supernatant was acidified by addition 25% metaphosphoric acid. The internal standard was added into the supernatant. SCFAs were identified and quantified using a HP 5890 gas chromatograph configured with flame-ionization detectors (GC-FID) and SCFAs were identified using control standard compounds purchased from Sigma-Aldrich.
Fecal transfer experiment:
Fecal material from TNBS+Vehicle or TNBS+Resveratrol mice was collected 48 hours after the last day of oral gavage treatment in the experimental model (day 5) from colonic flushes under anaerobic conditions (using anerobic glove box chamber) prior to inoculation into recipient mice in 200 μl of PBS. Fecal material was collected 48 hours after the last treatment to ensure that resveratrol had been absorbed in the tissues and eliminated from the feces prior to collection, as studies have shown this natural compound is rapidly absorbed and eliminated after consumption [23]. Before the fecal transfer, recipient mice (6 weeks old) were treated with streptomycin and penicillin to deplete endogenous gut microbiota. Penicillin (1g/L) and streptomycin (1g/L) were dissolved in sterile water and 100 μl were fed into mice by oral gavage once a day for four consecutive weeks, as previously described [24]. Depletion of microbiota was validated by PCR analysis using 16S rRNA gene Eubacteria primer (5′ATTACCGCGGCTGCTGGC, 3′ACTCCTACGGGAGGCAGCAGT). Colitis induction was performed as previously described. Briefly, on the last day of antibiotic treatment, prior to disease induction, recipient mice were given feces (5g/L stocks from disease and treated groups) by oral gavage (100 μl) for 5 days. Body weights were measured daily and colonoscopy was performed every other day. At the end of experiment, the mice were sacrificed and colon tissues were taken for histopathology analysis by staining with H&E and PAS as described already. Mesenteric lymph nodes were taken and T cell phenotyping was performed using flow cytometry as described above.
Statistical Analysis:
GraphPad Prism software (San Diego, CA) was used for all statistical analysis. For the in vivo mouse experiments, groups of 5–10 mice were used per experimental group. For in vitro assays, all experiments were performed in triplicate. For statistical differences, one-way ANOVA was used for each experiment, and Tukey’s post-hoc test was performed to analyze differences between groups, unless otherwise indicated. A p value of at least ≤ 0.05 was used to determine statistical significance.
Results
Resveratrol attenuates TNBS-induced colitis
In the current study, we tested the ability of resveratrol to attenuate a well-characterized TNBS-mediated murine model of colitis [25]. We used 4 groups of mice: Vehicle alone, resveratrol alone, TNBS+Vehicle, and TNBS+Resveratrol. TNBS administration caused colitis with significant decrease in body weight (~20%) when compared to Vehicle- or resveratrol-treated only groups, as depicted in Fig. 1A. However, in the TNBS+Resveratrol group, the weight loss was significantly reversed (~8%). Additionally, the TNBS+Vehicle group showed ~60% survival, while TNBS+Resveratrol group showed 100% survival (Fig. 1B). Colitis-induction caused an overall decrease in the colon length in TNBS+Vehicle groups compared to those treated with either Vehicle or Resveratrol alone, as shown in Fig. 1C-D. However, TNBS+Resveratrol groups showed a significant increase in colon length when compared with the disease group (TNBS+Vehicle). Colitis is also characterized by large productions of inflammatory biomarkers such as SAA, Lcn2, and increased MPO activity, which are often used in the diagnosis of the severity of colitis [13, 26]. The level of these biomarkers were significantly elevated in the TNBS+Vehicle group (Fig. 1E-G), but TNBS+Resveratrol showed significant decreases in the levels of all those inflammatory biomarkers, collectively showing that resveratrol was able to ameliorate the colonic inflammatory response induced by TNBS. Similar results were obtained in DSS-induced colitis model with the current resveratrol treatment regimen. Oral administration of resveratrol prevented DSS colitis-induced weight loss (Supplemental Figure 2A) and colon shortening (Supplemental Figure 2B-C).
Figure 1:
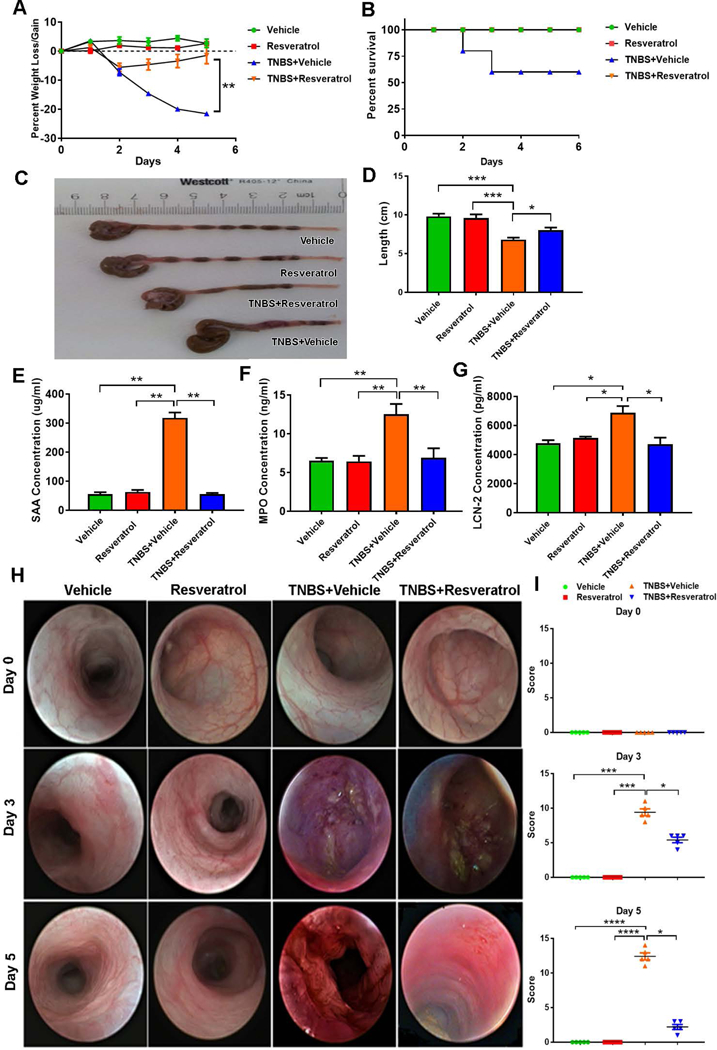
Treatment with resveratrol reduces clinical symptoms associated with TNBS-induced colitis murine model. Balb/c mice were administered intrarectally with 1mg of TNBS to induce colitis. Four groups of mice were used: Vehicle, Resveratrol, TNBS+Vehicle and TNBS+Resveratrol. The percent weight loss (A) was determined over the course of the study. (B) Survival curve of mice up to day 6 with colitis and those treated with RES. Colon lengths (C-D) were measured upon sacrifice (Day 5). Serum levels of SAA (E), MPO (F) and Lcn2 (G) were evaluated by ELISA. Endoscopy (H) was performed on mice on days 0, 3, and 5. Colonoscopy scores are provided (I). Significance (p-value: *<0.05, **<0.01, ***<0.005, ****<0.001) was determined by using one-way ANOVA and post-hoc Tukey’s test. In all data presented in the figure, 5 mice were used in each group. Data presented is representative of at least 3 independent experiments.
Colonoscopic examination at 3 different time points (days 0, 3, and 5) during the experiment gave a clear picture of the development of colitis-associated lesions and tissue sloughing after TNBS injection (TNBS+Vehicle), but TNBS+Resveratrol mice showed marked decrease in tissue disruption (Fig. 1H-I). Histological examination of formalin-fixed colon tissues stained with H&E was also performed (Fig. 2A), which showed a significant amount of cellular infiltration and loss of mucosal architecture in the TNBS+Vehicle group compared to naive mice treated with either just Vehicle or resveratrol alone. In contrast, TNBS+Resveratrol mice showed marked reduction in cellular infiltration, resembling the control groups. We also performed PAS staining on fixed colon tissue to determine normal arrangement and distribution of mucin and goblet cells within the colon mucosa. Mice challenged with TNBS showed high reduction in the number of goblet cells and mucin thickness, which was greatly returned to normal levels and size of mucin thickness in colons excised from TNBS+Resveratrol mice, similarly to naïve mice treated with Vehicle or resveratrol only (Fig. 2B). Histological scores of colons from TNBS mice treated with resveratrol (TNBS+Resveratrol) showed a significant decrease in disease parameters compared to TNBS+Vehicle mice, which had much higher scores than the controls groups (Fig. 2C). These data suggested that resveratrol prevents the colonic tissue damage induced by TNBS, which includes loss of the naturally-occurring protective mucous layer.
Figure 2:
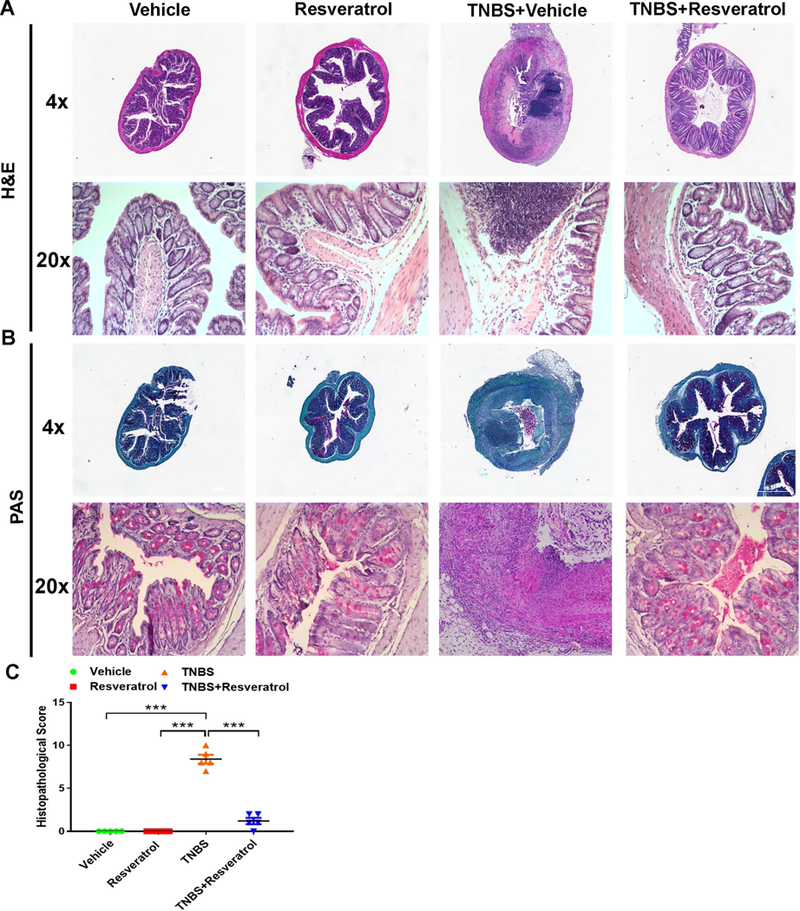
Treatment with resveratrol prevents cellular infiltration and mucin degradation and maintains colon gut structural architecture in TNBS model. The study was designed as described in Fig 1 legend. . Colons (n=5) were excised from experimental mice at the endpoint of experiment, fixed in 10% formaldehyde, and embedded in paraffin blocks. Cross-section slides containing colons from experimental groups were stained using H&E (A) or PAS (B) for histological evaluation. Images of stained tissue were taken using both 4x and 20x objectives, and histological scores were provided (C). Scale bars (white) depicted are at 100 μM. Data is representative of at least 3 independent experiments.
Resveratrol treatment reduces inflammatory T cell subsets and increases anti-inflammatory Tregs
In order to examine the T cell subsets during disease and treatment states, we isolated cells from the mesenteric lymph node of all groups and phenotyped these cells using flow cytometry. First, we looked at expression of the general T cell marker (CD3) which showed a significant increase in the percentage in TNBS+RES mice, while TNBS+Resveratrol treatment led to a marked decrease (Fig. 3A). We next looked at both T helper (CD4+) and cytotoxic (CD8+) T cell subset populations and showed significant increases in both CD4+ and CD8+ T cells in TNBS+Vehicle mice compared with those that were treated with Vehicle or resveratrol alone (Fig. 3B), but this was effectively reduced in TNBS+Resveratrol groups. We then performed intracellular/intranuclear staining to identify the effect of resveratrol on specific CD4+ T cells subsets which include inflammatory IFNγ- and IL17-producing CD4+ T cells, in addition to anti-inflammatory CD4+FOXP3+ and CD4+IL10+ populations. The data showed a significant increase in percentages of both anti-inflammatory CD4+FOXP3+ and CD4+IL10+ cells population in the TNBS+Resveratrol group when compared TNBS+Vehicle, and this increase in CD4+FOXP3+ populations were also observed in naïve mice treated with resveratrol (Fig. 3C-D). In contrast, intracellular staining for CD4+IFNγ+ and CD4+IL17+ showed significant increases in both percentage and absolute cell number in TNBS+Vehicle mice, while those in the TNBS+Resveratrol had a reversal in this effect (Fig. 3E-F). Absolute cell numbers of T cells and T cell subsets in the MLN confirmed these findings (Fig 3G). Collectively, these data showed that resveratrol treatment reduces the inflammatory T cell response during TNBS-induced colitis, while promoting the production of anti-inflammatory T cell subsets, mainly Tregs and IL-10-producing CD4+ cells. This increase in Tregs was also observed in naïve mice that were treated only with resveratrol alone.
Figure 3:
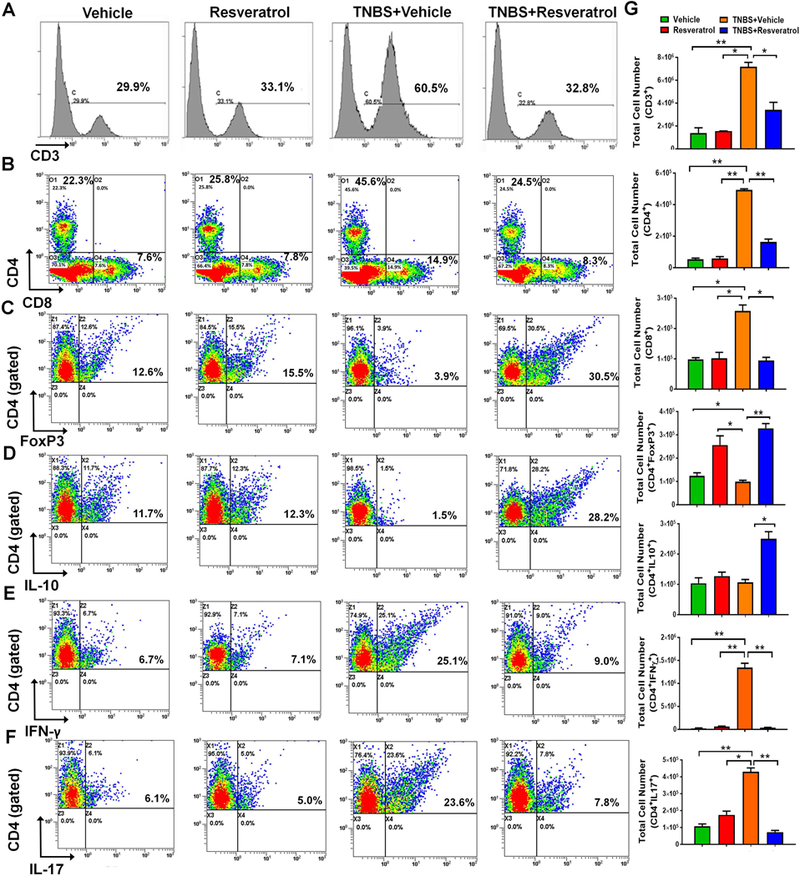
Resveratrol alters T cell subsets during TNBS colitis. The study was designed as described in Fig 1 legend. Flow cytometry histograms/dot plots are shown for the following T cell subsets: CD3+ (A), CD4+ or CD8+ cells (B), CD4+FOXP3+ (C), CD4+IL10+ (D) and CD4+IFNγ+ (E), and CD4+IL-17+ (F) expressing cells. For Figures C-F, cells were gated on the CD4+ population. The gating strategy for the CD4+ populations is detailed in Supplemental Figure 2. Quantitative bar graphs depicting absolute cell numbers of the T cell subsets is provided (G) Each experimental group had at least 5 mice included, and significance (p-value: *<0.05, **<0.01, ***<0.005, ****<0.001) was determined for absolute cell numbers by using one-way ANOVA followed by Tukey’s post-hoc multiple comparisons test. Data is representative of at least 3 independent experiments.
Alterations in gut microbiota and SCFA composition in colitis-induced mice treated with resveratrol
Next, we analyzed the gut microbiota from the all experimental groups to determine whether or not resveratrol altered the gut microbial composition during colitis. From colonic flushes, we isolated genomic DNA and performed 16S rRNA gene sequencing, analyzing the sequenced reads with the NIH-based Nephele online analysis tool. Nephele analysis output showed that the alpha diversity, represented as chao1, in naïve mice treated with resveratrol had the most diverse gut microbial compositions when to the other groups (Fig 4A). In terms of beta diversity, depicted as a PCA plot, samples clustered within their respective groups, with TNBS+Vehicle samples showing more dissimilarity compared to Vehicle-treated, or those groups treated with resveratrol (Resveratrol or TNBS+Resveratrol) (Fig. 4B). 16s rRNA gene sequencing analysis from Nephele allowed sample reads to be classified into OTUs from phylum to the species level, and output data up to the genus level is summarized in Supplemental Figs. 3-5. In order to determine the most divergent and potential microbial biomarkers within experimental groups, LeFSe analysis was performed with comparisons of TNBS+Vehicle vs TNBS+Resveratrol (Fig. 4C-D). The results showed that TNBS+Vehicle mice had increased abundance of Bacteroides acidifaciens compared to the other groups (Fig. 4E), while naïve or TNBS-induced colitis mice treated with resveratrol had enrichment of bacteria belonging to the genus Ruminococcus (Fig. 4C). At the species level, Ruminococcus gnavus and Akkermansia muciniphila showed a significant increase in TNBS+Resveratrol groups when compared to TNBS+Vehicle groups (Fig. 4F-G). It is interesting to note that mice treated with resveratrol alone also showed increases in Ruminococcus gnavus (Fig. 4F). In order to validate our sequencing results at the species level, we performed PCR using bacterial species-specific primers. As shown in Fig. 4H-J, Bacteroides acidifaciens was increased in the TNBS+Vehicle group compared to naïve mice treated with Vehicle or resveratrol only, but this species was reduced in the TNBS+Resveratrol group (Fig. 4H). In addition, Ruminococcus gnavus and Akkermansia muciphila species showed significant increases in abundance in groups treated with resveratrol (Resveratrol or TNBS+Resveratrol) when compared to those treated with only vehicle (Vehicle or TNBS+Vehicle) (Fig. 4I-J).
Figure 4:
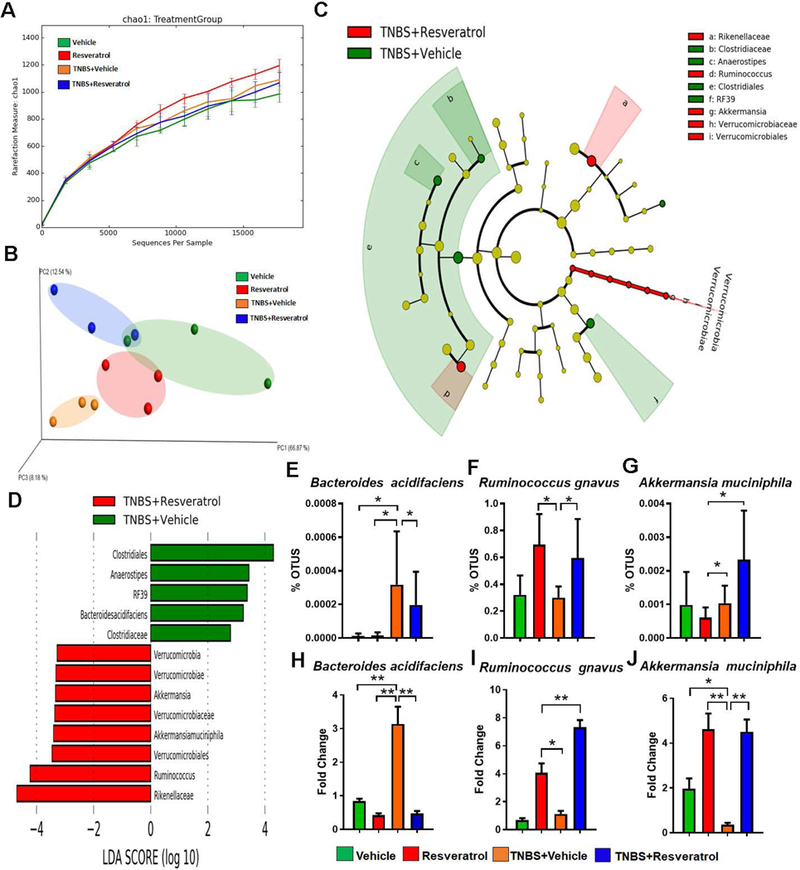
16S rRNA gene sequencing analysis. The study was designed as described in Fig 1 legend. Gut microbiome samples were collected from experimental groups by performing cecal flushes. Genomic DNA was isolated and V3-V4 regions of 16S rRNA gene subunit were sequenced. Three randomly selected mice from each group (n=3) were used for these experiments. All sequencing samples were analyzed using Nephele software 16S metagenomics provided at Nephele website (nephele.niaid.nih.gov). Alpha diversity (A), and Beta diversity (B) are depicted. LeFSe analysis of the Nephele OTU output files generated the cladogram (C) and LDA score bar graph (D) depicting microbial biomarkers among TNBS+Vehicle vs. TNBS+RES groups. OTU percent abundances are shown for the species Bacteroides acidifaciens (E) Ruminococcus gnavus (F) and Akkermansia muciphila (G). Validation of these significantly-altered bacterial species were performed using PCR and the fold changes are calculated using the delta-delta CT method with comparison to Vehicle controls (H-J). For 16S rRNA gene sequencing, 3 representative cecal flushes from each experimental group were processed and sequenced. For PCR validation, 10 mice were used in each group and fold changes were calculated using the delta-delta CT method compared to Vehicle control. Significance (p-value: *<0.05, **<0.01, ***<0.005, ****<0.001) was determined by using one-way ANOVA followed by Tukey’s post-hoc multiple comparisons test. Experiments are representative of 3 independent experiments.
Lastly, we measured the SCFA production in response to these changes in the gut microbiome composition (Fig. 5). The data showed that acetic acid and i-butyric acid concentrations were significantly reduced in the TNBS+Vehicle groups when compared to Vehicle, while naïve mice treated with resveratrol and TNBS+RES groups showed significant increases in these SCFAs (Fig. 5A, 5C). However, propionic acid, n-butyric acid, i-valeric acid, n-valeric acid, and n-copric acid showed no significant changes among the various groups (Fig. 5B, 5D-F). Together, these data suggested that treatment with resveratrol, particularly in colitis induced conditions, significantly altered both the gut microbiome composition and SCFA production.
Figure 5:
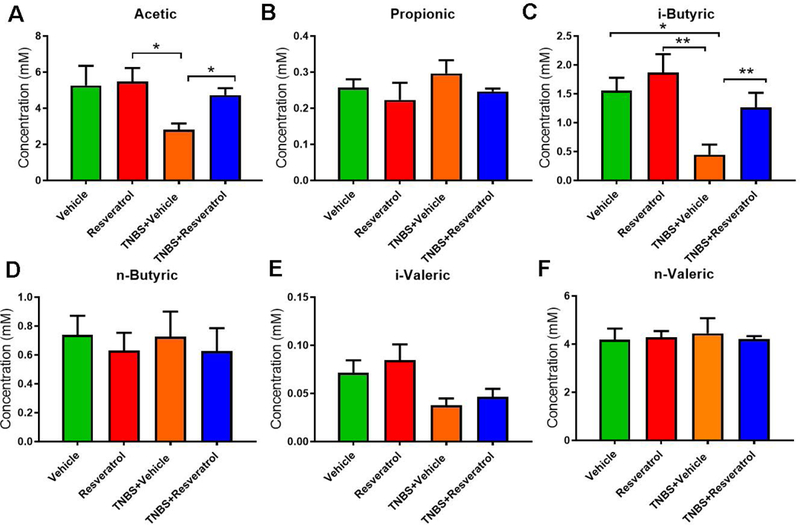
Resveratrol treatment alters SCFA production in TNBS colitis. The study was designed as described in Fig 1 legend. SCFA were isolated from cecal contents of experimental groups through acidification using metaphosphoric acid. GC-FID analysis was performed to determine the concentrations of acetic (A), propionic (B), i-butyric (C), n-buytric (D), i-valeric (E), and n-valeric (F) acids. SCFAs were identified using standard compounds purchased from Sigma-Aldrich. Representative data from two independent experiments with 5 mice in each group is depicted. Significance (p-value: *<0.05, **<0.01, ***<0.005, ****<0.001) was determined using one-way ANOVA followed by Tukey’s post-hoc multiple comparisons test.
Fecal transfer from resveratrol-treated groups attenuates TNBS-induced colitis and alters the immune response
In order to determine whether or not resveratrol-induced alterations in the gut microbiome contributes to the altered immune response in colitis, we performed fecal transfer experiments following treatment of mice with antibiotics to deplete the existing gut microbiota. While there were no significant differences observed in body weight of mice in the wild-type and antibiotic-treated mice (Fig. 6A), PCR for the universal Eubacteria 16S rRNA gene confirmed that the microbiome was depleted in antibiotic-treated mice prior to inoculation with fecal material (Fig. 6B-C). TNBS-exposed mice were inoculated with either feces from TNBS-treated mice ((FT) TNBS+Vehicle), or inoculated with feces from TNBS+Resveratrol-treated mice ((FT) TNBS+Resveratrol). (FT) TNBS+Vehicle mice showed a gradual decrease in body weight until the termination of the experiment (Fig. 6D). However, (FT) TNBS+Resveratrol mice showed resistance to loss of body weight. TNBS-induced colitis mice transferred with feces from TNBS+Vehicle also had shorter colons when compared those receiving fecal transfers from TNBS+Resveratrol-treated mice (Fig. 6E-F). Looking at colitis-associated inflammatory biomarkers such as SAA, Lcn2, and MPO, we found that the (FT) TNBS+Resveratrol group had significantly lower levels of these inflammatory biomarkers compared to the (FT) TNBS+Vehicle group (Fig. 6G-I). In addition, colonoscopy examination showed increased ulceration and sloughing in portions of the colon in (FT) TNBS+Vehicle mice, while (FT) TNBS+Resveratrol groups showed reduced presence of colon tissue destruction (Fig. 6J, top panel). Colonoscopy and histological examination of formalin-fixed colon tissues stained with H&E also showed that (FT) TNBS+Resveratrol mice showed no signs of cellular infiltration and tissue destruction, while (FT) TNBS+Vehicle mice had these colitis-associated observations (Fig. 6J-K).
Figure 6:

Transfer of resveratrol-treated fecal contents leads to amelioration of colitis. Female Balb/c mice were treated for 4 weeks with streptomycin and ampicillin (1g/L) prior to being injected intrarectally with 1mg of TNBS to induce colitis. Antibiotic-treated mice were weighed (A) and PCR performed on colonic flush samples to determine abundance of bacteria in the gut compared to naïve mice (B-C). These mice received fecal transfer (FT) from either colitis disease groups, (FT) TNBS+Vehicle, or from TNBS+Resveratrol-treatment groups, (FT) TNBS+RES. The percent weight loss (D) was determined over the course of the study. Colon lengths were measured upon sacrifice (E-F). Serum biomarkers for SAA (G), MPO (H), LCN2 (I) were detected using ELISA kits. Endoscopic images (J, top panel) and H&E stains of colons (J, bottom panel) are depicted (n=5 per group). Colonoscopy scores (K, top) and histological scores (K, bottom) are provided. Cells were isolated from mesenteric lymph nodes of experimental groups and absolute cell numbers from fecal transfer experiments were determined for CD4+FOXP3+ (L), CD4+IL10+ (M), CD4+IL17+ (N) and CD4+IFNγ+ (O). PCR validation from colonic flushes was performed after fecal transfer to confirm alterations in Akkermansia muciphila (P) Ruminococcus gnavus (Q) and Bacteroides acidifaciens (R), using delta-delta CT method with comparison to Vehicle controls. Each group had 10 recipient mice in this experiment and significance (p-value: *<0.05, **<0.01, ***<0.005, ****<0.001) was determined using one-way ANOVA followed by Tukey’s post-hoc multiple comparisons test.
Lastly, we performed T cell CD4+ phenotyping of the mesenteric lymph nodes in these fecal transfer experiments. The data showed that there was significant increases in CD4+FOXP3+ Tregs (Fig. 6L), though not in CD4+IL10+ (Fig. 6M), populations in the (FT) TNBS+Resveratrol group compared to (FT) TNBS+Vehicle, and the inflammatory Th1 (CD4+IFNγ+) and Th17 (CD4+IL17+) numbers were significantly reduced in these mice as well (Fig. 6N-O). In order to confirm that relevant species were in fact altered during the FT experiments, PCR validation was performed on colonic flushes from these experimental FT groups. PCR validation showed that mice (FT) TNBS+Resveratrol mice did have increased levels of Akkermansia muciniphila (Fig. 6P) and Ruminococcus gnavus (Fig. 6Q) when compared to (FT) TNBS+Vehicle mice. In addition, there was significantly lower levels of Bacteroides acidifaciens in (FT) TNBS+Resveratrol groups compared to (FT) TNBS+Vehicle (Fig. 6R), confirming that prominent species from the sequencing data were transferred successfully. Together, the fecal transfer experiments demonstrated that microbiota from (FT) TNBS+Resveratrol groups provide significant protection from colitis through enhancement of Tregs and suppression of Th17 and Th1 cells.
Discussion
Resversatrol is a potent anti-inflammatory agent. Studies from our lab and elsewhere have shown the ability resveratrol to reduce the symptoms associated with colitis, in different murine models [12, 13, 27–29], as well as in human patient populations [30]. Resveratrol is known to act through multiple pathways. In our previous reports, we were able to reveal some of the mechanisms that made this natural compound such a successful treatment. For example, in the genetic IL-10−/− model of colitis, we showed that resveratrol treatment was able to induce immunosuppressive MDSCs that led to a reduction in clinical parameters in addition to the reduction in CXCR3 expressing T cells [13]. The ability of resveratrol to induce these anti-inflammatory MDSCs has been shown in our lab in other disease models [8–10], and been confirmed by others as well [31, 32]. Other studies have shown that the beneficial effects of resveratrol against colitis can be attributed to other mechanisms, such as targeting sphingosine kinase 1 (SphK1) and apoptosis, restoring nitric oxide levels, reducing neutrophil infiltration, inhibiting nuclear factor-kappaB activation, acting as an anti-oxidant, as well as inhibiting adhesion molecules [33–35]. Resveratrol is also well-known to be a ligand for the aryl hydrocarbon receptor (AhR), and our lab and others have shown this natural compound’s ability to shift T cell differentiation from Th17 to Tregs, which is dependent on this receptor-ligand interaction [5, 36]. Classically, Th1 and Th2 cells were thought to characterize Crohn’s disease and ulcerative colitis respectively, however, Th17 cells are now known to play an important role in gut immunity and inflammation, particularly in regards to IBDs such as colitis [37]. Genome-wide association (GWAS) studies in IBD patients found that IL-17 regulating genes are greatly altered in the disease state, thus suggesting the importance of this factor in IBD such as colitis [37]. In fact, both animal models of colitis and human IBD patients are characterized by increased presence and development of Th17 cells at sites of inflammation [38–40]. Th17 plasticity towards inflammatory (IFN-γ-producing Th1) or anti-inflammatory (Treg) phenotypes make it a very unique cell population involved in intestinal homeostasis [40]. Interestingly, recent research has shown that Th17 cells are greatly influenced by the microenvironment such as the microbiome and microbial-derived byproducts [37].
More recent studies have shown that resveratrol may protect against many clinical disorders by modulating the gut microbiota [41–43]. However, such studies did not perform fecal transfer experiments to demonstrate that the microbiota altered by resveratrol treatment could lead to suppression of colitis-associated inflammation. In the present report, we therefore performed fecal transfer experiments, which conclusively demonstrated that resveratrol-mediated modulations in the gut microbiota is indeed responsible for attenuating colonic inflammation. It is becoming apparent that the gut microbiome contributes significantly to the development and progression of various diseases, particularly in the case of colitis [44–48]. With the gut microbiome playing such an important role in this disease, recent research is even focused on fecal transfer experiments as a therapeutic option [49, 50]. In addition, potential treatments against colitis are being examined more thoroughly to determine what, if any, effects these possible therapeutics have on the gut microbiome [51, 52].
In the current study, we were able to show that bacteria, such as those belonging to the Genus Ruminococcus, are increased during resveratrol treatment, which is consistent with animal studies and human fecal transplant experiments in which bacteria such as Ruminococcus and others were found to be anti-inflammatory, restoring and maintaining normal gastrointestinal tract function and integrity [53]. In fact, Ruminococcus gnavus and Akkermansia muciniphilia are mucolytic bacteria that are found to be reduced in both ulcerative colitis and Crohn’s Disease patients when compared to normal patient controls [54]. Therefore, restoration of these bacteria, which we noted in our data after naïve mice or TNBS-induced colitis mice were treated with resveratrol, could help in restoring or maintaining gut homeostasis, particularly after the microbiome is altered during colitis due to microbial dysbiosis. On the other hand, the current study shows that resveratrol can effectively reduce Bacteroides acidifaciens, which was found to be significantly increased in TNBS-induced colitis, a finding also seen in a murine DSS model [55]. B. acidifaciens have several features which could lead to the progression and development of colitis. This species is known to degrade mucin [56], the protective layer in the colon producing the host epithelial surface from luminal-bound bacteria. B. acidifaciens is also known to increase SCFA production of acetic and succinic acids [56], both of which can contribute to colitis-associated inflammation. Acetic acid, given in high concentrations, can induce colitis in murine models [57]. Succinic acid, which is produced by members of Bacteroidaceae, like B. acidifaciens, was found to be increased in the colons of colitis-induced mice and when administered by enemas can produce ulcers in the colon [58]. B. acidifaciens resembles closely another member of the same genus B. fragilis, and these bacteria have been shown in the literature to trigger a strong inflammatory cascade response, including activation of IL-17-dependent pathways [59]. By decreasing the presence of these bacteria during colitis-induction, resveratrol might be able to suppress the Th17 response which would normally lead to resident tissue destruction and microbial dysbiosis.
From our present study, we were also able to show that not only does resveratrol alter gut microbial composition during colitis disease induction, but these changes in the gut microbiome lead to alterations in the production of SCFAs. In particular, we found that i-butyric acid was significantly upregulated in resveratrol-treated mice during colitis induction and slightly in naïve mice treated with resveratrol compared to those treated only with Vehicle. From the literature, we know that butyrate/butyric acid has potent anti-inflammatory properties [60–62]. There are studies that also show that butyrate plays an important role in regulating the development of colitis, or acting as an agent to mitigate its deleterious effects [63, 64]. For example, oral administration of sodium butyrate into DSS-induced colitis mice led to reduction of inflammation [65]. Butyrate deficiency was shown to increase susceptibility to the development of colitis [66]. Therefore, the fact that resveratrol was able to increase production of this SCFA, particularly during colitis-induced conditions, provides a better understanding of the mechanisms that promote its efficiency against colitis, as well as other inflammatory disorders. Interestingly, we saw an increase in acetic acid in resveratrol treatment only after colitis induction, and this SCFA is often used to induce colitis [67]. It is possible that the increase in butyric acid was able to either negate the effects of increased acetic acid in our model. The uniqueness of our findings lie in the fact that we were able to show through 16S rRNA gene sequencing and fecal transfer experiments that the effectiveness of resveratrol against colitis could be explained by the ability of this natural product to alter and reverse microbial dysbiosis and SCFA production to promote an anti-inflammatory effect (induction of Treg/IL-10) and suppress the inflammatory (Th1/Th17) T cell response, something that has not been reported in the literature thus far. It is particularly interesting to note that the poor bioavailability of resveratrol during oral consumption, which is attributed to the weak aqueous solubility of the compound, has always been an issue in terms to suggesting this natural product as a treatment of various disease [68]. In fact, this observation has led to a wealth of research focusing on how to increase the bioavailability of this potent anti-inflammatory natural product so that it can be absorbed and circulated to various affected organs, such as by way of encapsulation in nanoparticles or some other vehicle [69, 70]. However, our findings suggest that resveratrol alters the microbiome directly and this leads to the anti-inflammatory effects during colitis, even before it becomes bioavailable to various organs after oral consumption.
In summary, the current study demonstrates the efficacy of resveratrol to attenuate colitis may result from its ability to alter gut microbiota that promotes anti-inflammatory T cell induction while suppressing pro-inflammatory T cells as summarized in Supplementary Fig. 6.
Supplementary Material
Treatment with resveratrol reduces clinical symptoms associated with DSS-induced colitis murine model. C57BL/6 mice were given 7 days of 3% DSS ad libitum followed by regular drinking water for 7 more days. Four groups of mice (n=5 per group) were used: Vehicle, Resveratrol, DSS+Vehicle and DSS+Resveratrol. The percent weight loss (A) was determined over the course of the study. Colon lengths (B-C) were measured upon sacrifice (Day 10). Significance of the bar graphs (p-value: *<0.05, **<0.01, ***<0.005, ****<0.001) were determined by using one-way ANOVA followed by Tukey’s post-hoc multiple comparisons test.
Gating strategy for flow cytometry. MLN lymph nodes from control (Vehicle) mice were stained and examined by flow cytometry using the following gating strategy: (A) Unstained negative controls were used to eliminate any non-specific false positive signal. (B) Single color controls were stained with either CD3 (FITC), CD4 (PE), or CD8 (PE-Cy7) to determine appropriate gating for histogram (top) and color dot plots (bottom). (C) Intracellular staining of CD4+ cells was gated as shown and represented in Figure 3.
16S rRNA sequencing analysis at the phylum to order level. Gut microbiome samples (n=3 per group) were collected from experimental groups (Vehicle, Resveratrol, TNBS+Resveratrol, TNBS+Vehicle) by performing cecal flushes. Genomic DNA was isolated and V3-V4 regions of 16S rRNA subunit were sequenced. Three randomly selected mice from each group were used for these experiments. All sequencing samples were analyzed using Nephele software 16S metagenomics provided at Nephele website (nephele.niaid.nih.gov). Stacked bar charts depicting OTU relative expression with corresponding color-coded legend for the following levels: phylum (A), class (B), and order (C).
16S rRNA sequencing analysis at the family level. Gut microbiome samples were collected from experimental groups (Vehicle, Resveratrol, TNBS+Resveratrol, TNBS+Vehicle) by performing cecal flushes. Genomic DNA was isolated and V3-V4 regions of 16S rRNA subunit were sequenced. Three randomly selected mice from each group were used for these experiments. All sequencing samples were analyzed using Nephele software 16S metagenomics provided at Nephele website (nephele.niaid.nih.gov). (A) Stacked bar charts depicting OTU relative expression with corresponding color-coded legend. (B) Bar graphs representing percent OTU abundance. Significance of the bar graphs (p-value: *<0.05, **<0.01, ***<0.005, ****<0.001) were determined by using one-way ANOVA followed by Tukey’s post-hoc multiple comparisons test.
16S rRNA sequencing analysis at the genus level. Gut microbiome samples were collected from experimental groups (Vehicle, Resveratrol, TNBS+Resveratrol, TNBS+Vehicle) by performing cecal flushes. Genomic DNA was isolated and V3-V4 regions of 16S rRNA subunit were sequenced. Three randomly selected mice from each group were used for these experiments. All sequencing samples were analyzed using Nephele software 16S metagenomics provided at Nephele website (nephele.niaid.nih.gov). (A) Stacked bar charts depicting OTU relative expression with corresponding color-coded legend. (B) Bar graphs representing percent OTU abundance. Significance of the bar graphs (p-value: *<0.05, **<0.01, ***<0.005, ****<0.001) were determined by using one-way ANOVA followed by Tukey’s post-hoc multiple comparisons test.
Graphical Abstract. TNBS-induced colitis results in microbial dysbiosis, with increased abundance of Bacteroides acidifaciens. However, treatment with resveratrol prevents this colitis-associated gut microbial shift, leading to increased abundance of bacteria such as Akkermansia muciphila and Ruminococcus gnavus, and production of SCFA butyrate. Increased presence of Akkermansia muciphila, Ruminococcus gnavus, and butyrate shifts the CD4+ T helper response from Th17 to anti-inflammatory Tregs. Fecal transfer of resveratrol-treated mice confirms these alterations in the immune response were the result of changes in the gut microbial profile mediated by resveratrol.
Acknowledgments:
This study used the Nephele platform from the National Institute of Allergy and Infectious Diseases (NIAID) Office of Cyber Infrastructure and Computational Biology (OCICB) in Bethesda, MD. Special thanks to Mike Walla, Director of Mass Spectrometry Services at the University of South Carolina, for his assistance in SCFA quantification presented in these studies. The studies were supported in part by NIH grants P01AT003961, R01AT006888, R01AI123947, R01AI129788, R01MH094755, and P20GM103641.
The funding agency had no role in the experimental design, data collection and analysis, decision to publish, or preparation of the manuscript.
Abbreviations:
- CMC
carboxymethyl cellulose
- FT
fecal transfer
- GC-FID
gas chromatograph configured with flame-ionization detectors
- H&E
hematoxylin and eosin
- IBD
inflammatory bowel disease
- IFNγ
interferon gamma
- IL
interleukin
- Lcn2
lipocalin-2
- LDA
Linear Discrimination Analysis
- LEfSe
Linear Discrimination Analysis Effect Size
- MDSC
Myeloid-derived suppressor cells
- MPO
myeloid peroxidase
- PAS
periodic acid schiff
- PBS
phosphate buffer saline
- PCR
polymerase chain reaction
- SAA
acute phase serum amyloid A
- SCFA
short chain fatty acid
- Th17
T helper 17 cells
- TNBS
2,4,6-Trinitrobenzenesulfonic acid
- Tregs
regulatory T cells
Footnotes
Conflicts of Interest Statement: The authors declare no competing financial interests.
References
- 1.Singh UP, Singh NP, Singh B, Hofseth LJ, Price RL, Nagarkatti M, Nagarkatti PS (2010) Resveratrol (trans-3,5,4’-trihydroxystilbene) induces silent mating type information regulation-1 and down-regulates nuclear transcription factor-kappaB activation to abrogate dextran sulfate sodium-induced colitis. The Journal of pharmacology and experimental therapeutics 332, 829–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carbonnel F and Boutron MC (2017) Incidence, phenotype and mortality of inflammatory bowel disease twenty years after. Journal of Crohn’s & colitis. [DOI] [PubMed] [Google Scholar]
- 3.Bequet E, Sarter H, Fumery M, Vasseur F, Armengol-Debeir L, Pariente B, Ley D, Spyckerelle C, Coevoet H, Laberenne JE, Peyrin-Biroulet L, Savoye G, Turck D, Gower-Rousseau C, Group E (2017) Incidence and Phenotype at Diagnosis of Very-early-onset Compared with Later-onset Paediatric Inflammatory Bowel Disease: A Population-based Study [1988–2011]. Journal of Crohn’s & colitis 11, 519–526. [DOI] [PubMed] [Google Scholar]
- 4.Yadav P, Ellinghaus D, Remy G, Freitag-Wolf S, Cesaro A, Degenhardt F, Boucher G, Delacre M, International IBDGC, Peyrin-Biroulet L, Pichavant M, Rioux JD, Gosset P, Franke A, Schumm LP, Krawczak M, Chamaillard M, Dempfle A, Andersen V (2017) Genetic Factors Interact With Tobacco Smoke to Modify Risk for Inflammatory Bowel Disease in Humans and Mice. Gastroenterology 153, 550–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singh NP, Hegde VL, Hofseth LJ, Nagarkatti M, Nagarkatti P (2007) Resveratrol (trans-3,5,4’-trihydroxystilbene) ameliorates experimental allergic encephalomyelitis, primarily via induction of apoptosis in T cells involving activation of aryl hydrocarbon receptor and estrogen receptor. Molecular pharmacology 72, 1508–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de la Lastra CA and Villegas I (2005) Resveratrol as an anti-inflammatory and anti-aging agent: mechanisms and clinical implications. Molecular nutrition & food research 49, 405–30. [DOI] [PubMed] [Google Scholar]
- 7.Cui X, Jin Y, Hofseth AB, Pena E, Habiger J, Chumanevich A, Poudyal D, Nagarkatti M, Nagarkatti PS, Singh UP, Hofseth LJ (2010) Resveratrol suppresses colitis and colon cancer associated with colitis. Cancer prevention research 3, 549–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Altamemi I, Murphy EA, Catroppo JF, Zumbrun EE, Zhang J, McClellan JL, Singh UP, Nagarkatti PS, Nagarkatti M (2014) Role of microRNAs in resveratrol-mediated mitigation of colitis-associated tumorigenesis in Apc(Min/+) mice. The Journal of pharmacology and experimental therapeutics 350, 99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rieder SA, Nagarkatti P, Nagarkatti M (2012) Multiple anti-inflammatory pathways triggered by resveratrol lead to amelioration of staphylococcal enterotoxin B-induced lung injury. British journal of pharmacology 167, 1244–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guan H, Singh NP, Singh UP, Nagarkatti PS, Nagarkatti M (2012) Resveratrol prevents endothelial cells injury in high-dose interleukin-2 therapy against melanoma. PloS one 7, e35650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu SL, Yu L, Meng KW, Ma ZH, Pan CE (2005) Resveratrol prolongs allograft survival after liver transplantation in rats. World journal of gastroenterology 11, 4745–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yao J, Wei C, Wang JY, Zhang R, Li YX, Wang LS (2015) Effect of resveratrol on Treg/Th17 signaling and ulcerative colitis treatment in mice. World journal of gastroenterology 21, 6572–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singh UP, Singh NP, Singh B, Hofseth LJ, Taub DD, Price RL, Nagarkatti M, Nagarkatti PS (2012) Role of resveratrol-induced CD11b(+) Gr-1(+) myeloid derived suppressor cells (MDSCs) in the reduction of CXCR3(+) T cells and amelioration of chronic colitis in IL-10(−/−) mice. Brain, behavior, and immunity 26, 72–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tung YC, Lin YH, Chen HJ, Chou SC, Cheng AC, Kalyanam N, Ho CT, Pan MH (2016) Piceatannol Exerts Anti-Obesity Effects in C57BL/6 Mice through Modulating Adipogenic Proteins and Gut Microbiota . Molecules 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jung MJ, Lee J, Shin NR, Kim MS, Hyun DW, Yun JH, Kim PS, Whon TW, Bae JW (2016) Chronic Repression of mTOR Complex 2 Induces Changes in the Gut Microbiota of Diet-induced Obese Mice. Scientific reports 6, 30887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Etxeberria U, Arias N, Boque N, Macarulla MT, Portillo MP, Martinez JA, Milagro FI (2015) Reshaping faecal gut microbiota composition by the intake of trans-resveratrol and quercetin in high-fat sucrose diet-fed rats. The Journal of nutritional biochemistry 26, 651–60. [DOI] [PubMed] [Google Scholar]
- 17.Diaz-Gerevini GT, Repossi G, Dain A, Tarres MC, Das UN, Eynard AR (2016) Beneficial action of resveratrol: How and why? Nutrition 32, 174–8. [DOI] [PubMed] [Google Scholar]
- 18.Elson CO, Beagley KW, Sharmanov AT, Fujihashi K, Kiyono H, Tennyson GS, Cong Y, Black CA, Ridwan BW, McGhee JR (1996) Hapten-induced model of murine inflammatory bowel disease: mucosa immune responses and protection by tolerance. J Immunol 157, 2174–85. [PubMed] [Google Scholar]
- 19.Kodani T, Rodriguez-Palacios A, Corridoni D, Lopetuso L, Di Martino L, Marks B, Pizarro J, Pizarro T, Chak A, Cominelli F (2013) Flexible colonoscopy in mice to evaluate the severity of colitis and colorectal tumors using a validated endoscopic scoring system. J Vis Exp, e50843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Akgun E, Caliskan C, Celik HA, Ozutemiz AO, Tuncyurek M, Aydin HH (2005) Effects of N-acetylcysteine treatment on oxidative stress in acetic acid-induced experimental colitis in rats. J Int Med Res 33, 196–206. [DOI] [PubMed] [Google Scholar]
- 21.Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, Huttenhower C (2011) Metagenomic biomarker discovery and explanation. Genome biology 12, R60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chitrala KN, Guan H, Singh NP, Busbee B, Gandy A, Mehrpouya-Bahrami P, Ganewatta MS, Tang C, Chatterjee S, Nagarkatti P, Nagarkatti M (2017) CD44 deletion leading to attenuation of experimental autoimmune encephalomyelitis results from alterations in gut microbiome in mice. European journal of immunology 47, 1188–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Busbee PB, Rouse M, Nagarkatti M, Nagarkatti PS (2013) Use of natural AhR ligands as potential therapeutic modalities against inflammatory disorders. Nutrition reviews 71, 353–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khosravi A, Yanez A, Price JG, Chow A, Merad M, Goodridge HS, Mazmanian SK (2014) Gut microbiota promote hematopoiesis to control bacterial infection. Cell host & microbe 15, 374–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim HS and Berstad A (1992) Experimental colitis in animal models. Scandinavian journal of gastroenterology 27, 529–37. [DOI] [PubMed] [Google Scholar]
- 26.Martinez-Moya P, Ortega-Gonzalez M, Gonzalez R, Anzola A, Ocon B, Hernandez-Chirlaque C, Lopez-Posadas R, Suarez MD, Zarzuelo A, Martinez-Augustin O, Sanchez de Medina F (2012) Exogenous alkaline phosphatase treatment complements endogenous enzyme protection in colonic inflammation and reduces bacterial translocation in rats. Pharmacological research 66, 144–53. [DOI] [PubMed] [Google Scholar]
- 27.Wagnerova A, Babickova J, Liptak R, Vlkova B, Celec P, Gardlik R (2017) Sex Differences in the Effect of Resveratrol on DSS-Induced Colitis in Mice. Gastroenterology research and practice 2017, 8051870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Youn J, Lee JS, Na HK, Kundu JK, Surh YJ (2009) Resveratrol and piceatannol inhibit iNOS expression and NF-kappaB activation in dextran sulfate sodium-induced mouse colitis. Nutrition and cancer 61, 847–54. [DOI] [PubMed] [Google Scholar]
- 29.Martin AR, Villegas I, Sanchez-Hidalgo M, de la Lastra CA (2006) The effects of resveratrol, a phytoalexin derived from red wines, on chronic inflammation induced in an experimentally induced colitis model. British journal of pharmacology 147, 873–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Samsami-Kor M, Daryani NE, Asl PR, Hekmatdoost A (2015) Anti-Inflammatory Effects of Resveratrol in Patients with Ulcerative Colitis: A Randomized, Double-Blind, Placebo-controlled Pilot Study. Archives of medical research 46, 280–5. [DOI] [PubMed] [Google Scholar]
- 31.Chen L, Yang S, Liao W, Xiong Y (2015) Modification of Antitumor Immunity and Tumor Microenvironment by Resveratrol in Mouse Renal Tumor Model. Cell biochemistry and biophysics 72, 617–25. [DOI] [PubMed] [Google Scholar]
- 32.Hong EH, Heo EY, Song JH, Kwon BE, Lee JY, Park Y, Kim J, Chang SY, Chin YW, Jeon SM, Ko HJ (2017) Trans-scirpusin A showed antitumor effects via autophagy activation and apoptosis induction of colorectal cancer cells. Oncotarget 8, 41401–41411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abdin AA (2013) Targeting sphingosine kinase 1 (SphK1) and apoptosis by colon-specific delivery formula of resveratrol in treatment of experimental ulcerative colitis in rats. European journal of pharmacology 718, 145–53. [DOI] [PubMed] [Google Scholar]
- 34.Abdallah DM and Ismael NR (2011) Resveratrol abrogates adhesion molecules and protects against TNBS-induced ulcerative colitis in rats. Canadian journal of physiology and pharmacology 89, 811–8. [DOI] [PubMed] [Google Scholar]
- 35.Yao J, Wang JY, Liu L, Zeng WS, Li YX, Xun AY, Zhao L, Jia CH, Feng JL, Wei XX, Wang LS (2011) Polydatin ameliorates DSS-induced colitis in mice through inhibition of nuclear factor-kappaB activation. Planta medica 77, 421–7. [DOI] [PubMed] [Google Scholar]
- 36.Wang B, Sun J, Li X, Zhou Q, Bai J, Shi Y, Le G (2013) Resveratrol prevents suppression of regulatory T-cell production, oxidative stress, and inflammation of mice prone or resistant to high-fat diet-induced obesity. Nutrition research 33, 971–81. [DOI] [PubMed] [Google Scholar]
- 37.Ueno A, Jeffery L, Kobayashi T, Hibi T, Ghosh S, Jijon H (2018) Th17 plasticity and its relevance to inflammatory bowel disease. Journal of autoimmunity 87, 38–49. [DOI] [PubMed] [Google Scholar]
- 38.Lee SJ, McLachlan JB, Kurtz JR, Fan D, Winter SE, Baumler AJ, Jenkins MK, McSorley SJ (2012) Temporal expression of bacterial proteins instructs host CD4 T cell expansion and Th17 development. PLoS pathogens 8, e1002499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jiang W, Su J, Zhang X, Cheng X, Zhou J, Shi R, Zhang H (2014) Elevated levels of Th17 cells and Th17-related cytokines are associated with disease activity in patients with inflammatory bowel disease. Inflammation research : official journal of the European Histamine Research Society ... [et al. ] 63, 943–50. [DOI] [PubMed] [Google Scholar]
- 40.Galvez J (2014) Role of Th17 Cells in the Pathogenesis of Human IBD. ISRN inflammation 2014, 928461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bird JK, Raederstorff D, Weber P, Steinert RE (2017) Cardiovascular and Antiobesity Effects of Resveratrol Mediated through the Gut Microbiota. Advances in nutrition 8, 839–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sung MM, Kim TT, Denou E, Soltys CM, Hamza SM, Byrne NJ, Masson G, Park H, Wishart DS, Madsen KL, Schertzer JD, Dyck JR (2017) Improved Glucose Homeostasis in Obese Mice Treated With Resveratrol Is Associated With Alterations in the Gut Microbiome. Diabetes 66, 418–425. [DOI] [PubMed] [Google Scholar]
- 43.Chen ML, Yi L, Zhang Y, Zhou X, Ran L, Yang J, Zhu JD, Zhang QY, Mi MT (2016) Resveratrol Attenuates Trimethylamine-N-Oxide (TMAO)-Induced Atherosclerosis by Regulating TMAO Synthesis and Bile Acid Metabolism via Remodeling of the Gut Microbiota. mBio 7, e02210–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rapozo DC, Bernardazzi C, de Souza HS (2017) Diet and microbiota in inflammatory bowel disease: The gut in disharmony. World journal of gastroenterology 23, 2124–2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Autenrieth DM and Baumgart DC (2017) [Microbiome and Gut Inflammation]. Deutsche medizinische Wochenschrift 142, 261–266. [DOI] [PubMed] [Google Scholar]
- 46.Kanauchi O, Mitsuyama K, Araki Y, Andoh A (2003) Modification of intestinal flora in the treatment of inflammatory bowel disease. Current pharmaceutical design 9, 333–46. [DOI] [PubMed] [Google Scholar]
- 47.Conte MP, Schippa S, Zamboni I, Penta M, Chiarini F, Seganti L, Osborn J, Falconieri P, Borrelli O, Cucchiara S (2006) Gut-associated bacterial microbiota in paediatric patients with inflammatory bowel disease. Gut 55, 1760–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nishikawa J, Kudo T, Sakata S, Benno Y, Sugiyama T (2009) Diversity of mucosa-associated microbiota in active and inactive ulcerative colitis. Scandinavian journal of gastroenterology 44, 180–6. [DOI] [PubMed] [Google Scholar]
- 49.Paramsothy S, Paramsothy R, Rubin DT, Kamm MA, Kaakoush NO, Mitchell HM, Castano-Rodriguez N (2017) Faecal Microbiota Transplantation for Inflammatory Bowel Disease: A Systematic Review and Meta-analysis. Journal of Crohn’s & colitis. [DOI] [PubMed] [Google Scholar]
- 50.Meighani A, Hart BR, Bourgi K, Miller N, John A, Ramesh M (2017) Outcomes of Fecal Microbiota Transplantation for Clostridium difficile Infection in Patients with Inflammatory Bowel Disease. Digestive diseases and sciences. [DOI] [PubMed] [Google Scholar]
- 51.Yang Y, Chen G, Yang Q, Ye J, Cai X, Tsering P, Cheng X, Hu C, Zhang S, Cao P (2017) Gut microbiota drives the attenuation of dextran sulphate sodium-induced colitis by Huangqin decoction. Oncotarget 8, 48863–48874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jang SH, Park J, Kim SH, Choi KM, Ko ES, Cha JD, Lee YR, Jang H, Jang YS (2017) Oral administration of red ginseng powder fermented with probiotic alleviates the severity of dextran-sulfate sodium-induced colitis in a mouse model. Chinese journal of natural medicines 15, 192–201. [DOI] [PubMed] [Google Scholar]
- 53.Satokari R, Fuentes S, Mattila E, Jalanka J, de Vos WM, Arkkila P (2014) Fecal transplantation treatment of antibiotic-induced, noninfectious colitis and long-term microbiota follow-up. Case reports in medicine 2014, 913867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Png CW, Linden SK, Gilshenan KS, Zoetendal EG, McSweeney CS, Sly LI, McGuckin MA, Florin TH (2010) Mucolytic bacteria with increased prevalence in IBD mucosa augment in vitro utilization of mucin by other bacteria. The American journal of gastroenterology 105, 2420–8. [DOI] [PubMed] [Google Scholar]
- 55.Kang CS, Ban M, Choi EJ, Moon HG, Jeon JS, Kim DK, Park SK, Jeon SG, Roh TY, Myung SJ, Gho YS, Kim JG, Kim YK (2013) Extracellular vesicles derived from gut microbiota, especially Akkermansia muciniphila, protect the progression of dextran sulfate sodium-induced colitis. PloS one 8, e76520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Miyamoto Y and Itoh K (2000) Bacteroides acidifaciens sp. nov., isolated from the caecum of mice. International journal of systematic and evolutionary microbiology 50 Pt 1, 145–8. [DOI] [PubMed] [Google Scholar]
- 57.Karakoyun B, Ertas B, Yuksel M, Akakin D, Cevik O, Sener G (2017) Ameliorative effects of riboflavin on acetic acid-induced colonic injury in rats. Clinical and experimental pharmacology & physiology. [DOI] [PubMed] [Google Scholar]
- 58.Ariake K, Ohkusa T, Sakurazawa T, Kumagai J, Eishi Y, Hoshi S, Yajima T (2000) Roles of mucosal bacteria and succinic acid in colitis caused by dextran sulfate sodium in mice. Journal of medical and dental sciences 47, 233–41. [PubMed] [Google Scholar]
- 59.Chung L, Thiele Orberg E, Geis AL, Chan JL, Fu K, DeStefano Shields CE, Dejea CM, Fathi P, Chen J, Finard BB, Tam AJ, McAllister F, Fan H, Wu X, Ganguly S, Lebid A, Metz P, Van Meerbeke SW, Huso DL, Wick EC, Pardoll DM, Wan F, Wu S, Sears CL, Housseau F (2018) Bacteroides fragilis Toxin Coordinates a Pro-carcinogenic Inflammatory Cascade via Targeting of Colonic Epithelial Cells. Cell host & microbe 23, 203–214 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.van der Beek CM, Dejong CHC, Troost FJ, Masclee AAM, Lenaerts K (2017) Role of short-chain fatty acids in colonic inflammation, carcinogenesis, and mucosal protection and healing. Nutrition reviews 75, 286–305. [DOI] [PubMed] [Google Scholar]
- 61.Dai H, Liu X, Yan J, Aabdin ZU, Bilal MS, Shen X (2017) Sodium Butyrate Ameliorates High-Concentrate Diet-Induced Inflammation in the Rumen Epithelium of Dairy Goats. Journal of agricultural and food chemistry 65, 596–604. [DOI] [PubMed] [Google Scholar]
- 62.Wang F, Liu J, Weng T, Shen K, Chen Z, Yu Y, Huang Q, Wang G, Liu Z, Jin S (2017) The Inflammation Induced by Lipopolysaccharide can be Mitigated by Short-chain Fatty Acid, Butyrate, through Upregulation of IL-10 in Septic Shock. Scandinavian journal of immunology 85, 258–263. [DOI] [PubMed] [Google Scholar]
- 63.Cobo ER, Kissoon-Singh V, Moreau F, Holani R, Chadee K (2017) MUC2 Mucin and Butyrate Contribute to the Synthesis of the Antimicrobial Peptide Cathelicidin in Response to Entamoeba histolytica- and Dextran Sodium Sulfate-Induced Colitis. Infection and immunity 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang T, Ding C, Zhao M, Dai X, Yang J, Li Y, Gu L, Wei Y, Gong J, Zhu W, Li N, Li J (2016) Sodium Butyrate Reduces Colitogenic Immunoglobulin A-Coated Bacteria and Modifies the Composition of Microbiota in IL-10 Deficient Mice. Nutrients 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Simeoli R, Mattace Raso G, Pirozzi C, Lama A, Santoro A, Russo R, Montero-Melendez T, Berni Canani R, Calignano A, Perretti M, Meli R (2017) An orally administered butyrate-releasing derivative reduces neutrophil recruitment and inflammation in dextran sulphate sodium-induced murine colitis. British journal of pharmacology 174, 1484–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Meisel M, Mayassi T, Fehlner-Peach H, Koval JC, O’Brien SL, Hinterleitner R, Lesko K, Kim S, Bouziat R, Chen L, Weber CR, Mazmanian SK, Jabri B, Antonopoulos DA (2017) Interleukin-15 promotes intestinal dysbiosis with butyrate deficiency associated with increased susceptibility to colitis. The ISME journal 11, 15–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sadraei H, Asghari G, Khanabadi M, Minaiyan M (2017) Anti-inflammatory effect of apigenin and hydroalcoholic extract of Dracocephalum kotschyi on acetic acid-induced colitis in rats. Research in pharmaceutical sciences 12, 322–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Peng RM, Lin GR, Ting Y, Hu JY (2018) Oral delivery system enhanced the bioavailability of stilbenes: Resveratrol and pterostilbene. BioFactors 44, 5–15. [DOI] [PubMed] [Google Scholar]
- 69.Zu Y, Overby H, Ren G, Fan Z, Zhao L, Wang S (2018) Resveratrol liposomes and lipid nanocarriers: Comparison of characteristics and inducing browning of white adipocytes. Colloids and surfaces. B, Biointerfaces 164, 414–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Borges SC, Ferreira PEB, da Silva LM, de Paula Werner MF, Irache JM, Cavalcanti OA, Buttow NC (2018) Evaluation of the treatment with resveratrol-loaded nanoparticles in intestinal injury model caused by ischemia and reperfusion. Toxicology 396–397, 13–22. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Treatment with resveratrol reduces clinical symptoms associated with DSS-induced colitis murine model. C57BL/6 mice were given 7 days of 3% DSS ad libitum followed by regular drinking water for 7 more days. Four groups of mice (n=5 per group) were used: Vehicle, Resveratrol, DSS+Vehicle and DSS+Resveratrol. The percent weight loss (A) was determined over the course of the study. Colon lengths (B-C) were measured upon sacrifice (Day 10). Significance of the bar graphs (p-value: *<0.05, **<0.01, ***<0.005, ****<0.001) were determined by using one-way ANOVA followed by Tukey’s post-hoc multiple comparisons test.
Gating strategy for flow cytometry. MLN lymph nodes from control (Vehicle) mice were stained and examined by flow cytometry using the following gating strategy: (A) Unstained negative controls were used to eliminate any non-specific false positive signal. (B) Single color controls were stained with either CD3 (FITC), CD4 (PE), or CD8 (PE-Cy7) to determine appropriate gating for histogram (top) and color dot plots (bottom). (C) Intracellular staining of CD4+ cells was gated as shown and represented in Figure 3.
16S rRNA sequencing analysis at the phylum to order level. Gut microbiome samples (n=3 per group) were collected from experimental groups (Vehicle, Resveratrol, TNBS+Resveratrol, TNBS+Vehicle) by performing cecal flushes. Genomic DNA was isolated and V3-V4 regions of 16S rRNA subunit were sequenced. Three randomly selected mice from each group were used for these experiments. All sequencing samples were analyzed using Nephele software 16S metagenomics provided at Nephele website (nephele.niaid.nih.gov). Stacked bar charts depicting OTU relative expression with corresponding color-coded legend for the following levels: phylum (A), class (B), and order (C).
16S rRNA sequencing analysis at the family level. Gut microbiome samples were collected from experimental groups (Vehicle, Resveratrol, TNBS+Resveratrol, TNBS+Vehicle) by performing cecal flushes. Genomic DNA was isolated and V3-V4 regions of 16S rRNA subunit were sequenced. Three randomly selected mice from each group were used for these experiments. All sequencing samples were analyzed using Nephele software 16S metagenomics provided at Nephele website (nephele.niaid.nih.gov). (A) Stacked bar charts depicting OTU relative expression with corresponding color-coded legend. (B) Bar graphs representing percent OTU abundance. Significance of the bar graphs (p-value: *<0.05, **<0.01, ***<0.005, ****<0.001) were determined by using one-way ANOVA followed by Tukey’s post-hoc multiple comparisons test.
16S rRNA sequencing analysis at the genus level. Gut microbiome samples were collected from experimental groups (Vehicle, Resveratrol, TNBS+Resveratrol, TNBS+Vehicle) by performing cecal flushes. Genomic DNA was isolated and V3-V4 regions of 16S rRNA subunit were sequenced. Three randomly selected mice from each group were used for these experiments. All sequencing samples were analyzed using Nephele software 16S metagenomics provided at Nephele website (nephele.niaid.nih.gov). (A) Stacked bar charts depicting OTU relative expression with corresponding color-coded legend. (B) Bar graphs representing percent OTU abundance. Significance of the bar graphs (p-value: *<0.05, **<0.01, ***<0.005, ****<0.001) were determined by using one-way ANOVA followed by Tukey’s post-hoc multiple comparisons test.
Graphical Abstract. TNBS-induced colitis results in microbial dysbiosis, with increased abundance of Bacteroides acidifaciens. However, treatment with resveratrol prevents this colitis-associated gut microbial shift, leading to increased abundance of bacteria such as Akkermansia muciphila and Ruminococcus gnavus, and production of SCFA butyrate. Increased presence of Akkermansia muciphila, Ruminococcus gnavus, and butyrate shifts the CD4+ T helper response from Th17 to anti-inflammatory Tregs. Fecal transfer of resveratrol-treated mice confirms these alterations in the immune response were the result of changes in the gut microbial profile mediated by resveratrol.


