Summary
A method for the simultaneous (one-step) photochemical conjugation and 89Zr-radiolabeling of antibodies is introduced. A photoactivatable chelate based on the functionalization of desferrioxamine B with an arylazide moiety (DFO-ArN3, [1]) was synthesized. The radiolabeled complex, 89Zr-1+, was produced and characterized. Density functional theory calculations were used to investigate the mechanism of arylazide photoactivation. 89Zr-radiolabeling experiments were also used to determine the efficiency of photochemical conjugation. A standard two-step approach gave a measured conjugation efficiency of 3.5% ± 0.4%. In contrast, the one-step process gave a higher photoradiolabeling efficiency of ∼76%. Stability measurements, cellular saturation binding assays, positron emission tomographic imaging, and biodistribution studies in mice bearing SK-OV-3 tumors confirmed the biochemical viability and tumor specificity of photoradiolabeled [89Zr]ZrDFO-azepin-trastuzumab. Experimental data support the conclusion that the combination of photochemistry and radiochemistry is a viable strategy for producing radiolabeled proteins for imaging and therapy.
Subject Areas: Density Functional Theory (DFT), Medical Imaging, Radiochemicals
Graphical Abstract

Highlights
-
•
Photochemistry is combined with radiochemistry for radiosynthesis in a flash
-
•
Simultaneous photoradiochemistry is achieved with high radiolabeling efficiency
-
•
Photoradiochemistry produces viable 89Zr-radiolabeled antibodies
-
•
Density functional theory calculations elucidate the photoactivation mechanism
Density Functional Theory (DFT); Medical Imaging; Radiochemicals
Introduction
The use of photochemically activated compounds for labeling proteins and other biologically active molecules was introduced by Westheimer and co-workers in 1962 (Singh et al., 1962). Since then, photoaffinity labeling (PAL) has matured, and a wide array of reagents are available for studying the structure and function of biological systems (Bayley and Knowles, 1977, Chowdhry and Westheimer, 1979, Kotzyba-Hilbert et al., 1995). Photochemical activation offers a number of advantages over thermochemical processes. For instance, photoreactive groups can be selected whereby, (1) the reagent is stable under ambient conditions, (2) photoactivation occurs specifically at a wavelength that is not absorbed by the biological vector, and (3) the conjugation step involves a chemoselective reaction with the target molecule. Furthermore, because photochemical activation proceeds via an excited electronic state that generates extremely reactive intermediates like carbenes, nitrenes, and radicals, the rates of photochemical conjugation reactions can be several orders of magnitude faster than those of standard methods (Dennler et al., 2015, Gritsan and Platz, 2006, Klán and Wirz, 2009, Platz, 1995). High reactivity of the photo-induced intermediates presents both advantages and disadvantages. One of the benefits is that photoactive reagents can yield high labeling efficiencies in short reaction times. However, to achieve efficient conjugation, PAL methods often rely on a mechanism in which the photoactive reagent and the target protein form a non-covalent pre-association complex. Pre-association facilitates pseudo-first-order intramolecular bond formation and minimizes the probability of quenching by background media (i.e., by the solvent, oxygen, salts, etc.). However, the problem with this approach is that it restricts most PAL tools to systems that self-assemble.
From the radiochemistry perspective, photochemical reactions are an attractive platform for developing radiotracers. For molecules that undergo radioactive decay, chemical kinetics is one of the main factors in determining if a reaction is suitable for use in radiotracer synthesis (Holland, 2018). As photochemical reactions often proceed with rate constants that tend toward the rate of diffusion, combining photochemistry with radiochemistry (photoradiochemistry) is a logical step.
Several groups have reported the use of photochemical reactions in the synthesis of radiolabeled compounds (Hashizume et al., 1995, Kumar et al., 2015, Kym et al., 1995, Lange et al., 2002, Nishikawa et al., 2003, Pandurangi et al., 1997a, Pandurangi et al., 1997c, Pandurangi et al., 1997b, Pandurangi et al., 1998, Rajagopalan et al., 2002, Stalteri and Mather, 1996, Sykes et al., 1995, Sykes et al., 1997, Wester et al., 1996). However, it is surprising that to date, photochemistry has had minimal impact on radiopharmaceutical science. The main bottlenecks to a more widespread use of photoradiochemistry for labeling proteins, peptides, and small molecules are (1) avoiding the need to form a pre-associated complex, (2) controlling chemoselectivity in the presence of competing nucleophiles, and (3) ensuring that the rate of productive bimolecular conjugation exceeds that of background quenching reactions. If a photochemical process can be tuned to favor bimolecular coupling, then photoradiochemistry may become a more general tool in radiotracer synthesis.
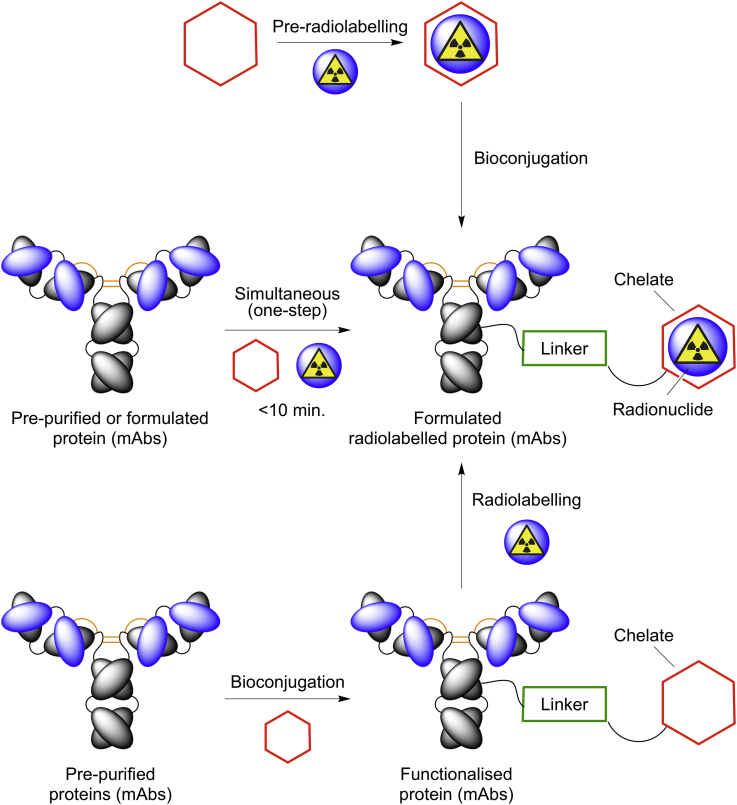
A specific area for potential applications of photoradiochemistry is the synthesis of radiolabeled monoclonal antibodies (mAbs) or immunoglobulin fragments for use in positron emission tomography (immuno-PET) and radioimmunotherapy (Boros and Holland, 2018). Zirconium-89 (t1/2 = 78.41 h) has emerged as the radionuclide of choice for immuno-PET. In almost all available methods for radiolabeling mAbs, the production of 89Zr-mAbs relies on a two-step procedure (Scheme 1). Initially, the antibody is purified from a source and then functionalized with a suitable metal-ion-binding chelate. For 89Zr, the chelate of choice is usually a derivative of desferrioxamine B (DFO), but a number of alternative chelates have been reported (Boros and Holland, 2018). After conjugation, the functionalized mAb is re-purified, characterized, and stored before radiolabeling. Although this two-step approach is highly successful, there are several drawbacks. First, the conjugation chemistry is time consuming and may involve multiple chemical transformations that risk compromising the biological integrity of the mAb. Second, for applications in the clinic, the conjugation chemistry should ideally be performed in accordance with current Good Manufacturing Practice. Third, the conjugated mAb is a new molecular entity, which may be subject to stringent testing, including expensive toxicological test. Finally, storage of the radiolabeling precursor raises concerns over the long-term chemical and biochemical stability.
Scheme 1.
Mechanistic Routes toward Radiolabeled Antibodies and Other Proteins
We postulated that photoradiochemistry could be used to streamline the production of radiolabeled mAbs (and other radiolabeled proteins or peptides) by simplifying the procedure to a one-pot reaction and eliminating the need to isolate or store the functionalized intermediate. Two mechanistically distinct processes can be envisaged for a one-pot procedure: a two-step pre-radiolabeling approach and a one-step pathway in which the radiolabeling and photochemical conjugation reactions occur simultaneously. Here, we present detailed experimental and theoretical data evaluating the photoradiochemical synthesis of 89Zr-labeled trastuzumab.
Results and Discussion
Our initial proof-of-concept studies found that the macrocyclic chelate, (2-(4,7-bis(carboxymethyl)-1,4,7-triazonan-1-yl)pentanedioic acid (NODAGA), when functionalized with a photoreactive arylazide (ArN3) facilitated a two-step photoradiolabeling of trastuzumab with 68Ga3+ ions (Eichenberger et al., 2019, Patra et al., 2019). Although technically feasible, the combination of short-lived 68Ga (t1/2 = 67.71 min) with mAbs (∼150 kDa) is not ideal because of the mismatch between the timescales of radioactive decay and radiotracer pharmacokinetics in vivo. In addition, 68Ga3+ radiochemistry usually requires acidic media (to avoid hydrolysis of the metal ion), which is suboptimal for photochemical conjugation using the ArN3 group (Borden et al., 2000, Gritsan and Platz, 2010, Gritsan and Platz, 2006, Gritsan and Pritchina, 1992). Here, we focused our efforts on developing a photoradiochemical process for producing 89Zr-labeled mAbs. Zirconium-89 radiochemistry normally starts from the zirconium tetraoxalate anion, [89Zr][Zr(C2O4)4]4–(aq.), and complexation by DFO derivatives can be accomplished over a wide pH range (from approximately pH 5–10, with optimal yields attained by using pH 6–8.5) (Holland and Vasdev, 2014, Vosjan et al., 2010). Thus 89Zr-radiochemistry is not only ideal for immuno-PET but also facilitates simultaneous photoradiolabeling.
Chemical and Radiochemical Synthesis
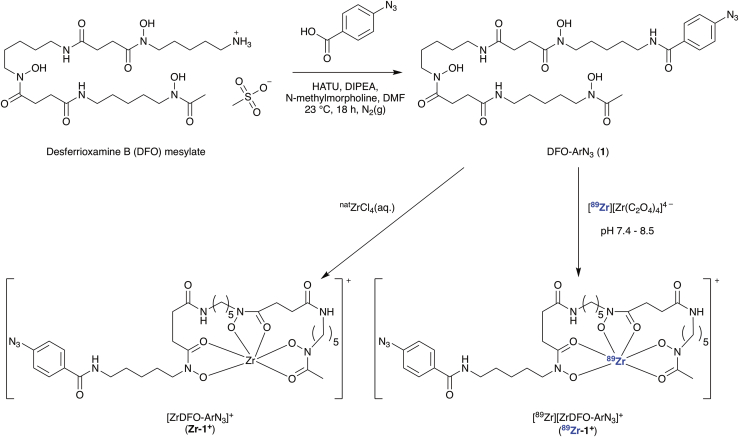
The photoactive chelate DFO-ArN3 (1) was synthesized by using standard chemical transformations (Scheme 2). The compound was isolated in high purity by semi-preparative high-performance liquid chromatography and characterized by ultrahigh-performance liquid chromatography (UHPLC), high-resolution electrospray ionization mass spectrometry (HR-ESI-MS), one- and two-dimensional 1H and 13C nuclear magnetic resonance spectroscopies, and electronic absorption spectroscopy (Transparent Methods Figures S2–S8).
Scheme 2.
Chemical Synthesis of DFO-ArN3 (1) and Complexation of Zr4+ Ions to Give the Non-radioactive (Zr-1+) and Radiolabeled 89Zr-1+) Coordination Complexes
The non-radioactive complex, [ZrDFO-ArN3]+ (Zr-1+), was prepared by the reaction of compound 1 with ZrCl4(aq.), and characterized by HR-ESI-MS and UHPLC (Figure 1 and Transparent Methods Figures S9 and S10). The radiolabeled complex [89Zr][ZrDFO-ArN3]+ (89Zr-1+) was prepared as a single radioactive species, the identity of which was confirmed by comparison of retention times with the authenticated non-radioactive sample and by standard co-injection methods (Figure 1, blue radiotrace).
Figure 1.

Radioactive UHPLC Characterization Data
UHPLC chromatograms showing quantitative radiolabeling to give [89Zr][ZrDFO-ArN3]+ (89Zr-1+, blue trace, RCP >99%, RCC >99%) and electronic absorption chromatograms measured at 220 nm (green), 254 nm (red), and 280 nm (black) showing co-elution with an authenticated sample of non-radioactive Zr-1+.
Photochemical Kinetics
After confirming that 89Zr-1+ could be prepared via standard radiolabeling methods, the photochemical activity of compound 1 was tested under irradiation with UV light. Three separate light sources were tested including two variable-intensity light-emitting diodes (LEDs) with peak emission wavelengths at ∼365 nm and ∼395 nm and a high-powered Rayonet reactor (Transparent Methods Figure S1). The photochemical degradation kinetics of compound 1, induced by using variable light intensities, was monitored by UHPLC (Figure 2 and Transparent Methods Table S2). Experiments confirmed that compound 1 was photoactive. All peaks in the UHPLC associated with photodegraded products were found to be more hydrophilic than the parent compound, which is consistent with the established mechanism of ArN3 activation in aqueous media (Klán and Wirz, 2009). After light absorption, ArN3 releases N2(g) and forms a short-lived arylnitrene species in the singlet (1A2) ground state (Gritsan and Platz, 2006). This arylnitrene undergoes rapid intramolecular rearrangement to give benzazirine or ketenimine intermediates (Platz, 1995). When powerful nucleophiles are present, the ketenimine species reacts to form more polar azepine adducts. In the absence of nucleophiles, hydrolysis reactions can form the 3H-azepin-2-ol or the 1,3-dihydro-2H-azepin-2-one tautomers (Bou-Hamdan et al., 2011). Photo-irradiated samples of compound 1 showed that at least six new species formed (Figure 2A). This observation is consistent with the mechanism of activation. The DFO ligand contains several nucleophilic groups (hydroxamates) that can induce intramolecular reactions with the ketenimine intermediate forming various cycles.
Figure 2.
Kinetic Data on the Photochemically Induced Degradation of Compound 1 during Irradiation with UV Light (365 nm)
(A) Normalized UHPLC chromatograms recorded between 0 and 25 min (50% LED power). * Indicates starting material (compound 1).
(B) Kinetic plot showing the change in concentration of compound 1 versus irradiation time (min.) using different LED intensities. Note, data are fitted with a first-order decay (R2 >0.999 for each dataset), and the observed first-order rate constants, kobs/min−1, are shown in the inset. Error bars correspond to one standard deviation.
(C) Plot of the normalized observed rate constant versus the normalized LED intensity confirming that photodegradation is first order (gradient ∼1.0) with respect to light intensity.
By integrating the peak in the UHPLC associated with compound 1, it was possible to measure the photochemical degradation kinetics (Figure 2B). At room temperature, and using the LED (365 nm) at different intensities (25%, 50%, and 100%), the photochemical degradation of compound 1 fitted a first-order kinetic scheme (non-linear regression coefficient, R2 >0.999 at each light intensity). Changes in the observed rate constant, kobs/min−1, were found to be linearly dependent on the light intensity (Figure 2C). These data are consistent with the anticipated photochemical response of molecules containing ArN3 groups (Gritsan and Platz, 2006, Gritsan and Pritchina, 1992, Klán and Wirz, 2009).
Quantum Yield of Photochemical Activation
How efficient is photochemical activation of the ArN3 in compound 1? This question can be answered by determining the photochemical quantum yield (Φ), which is defined as the ratio of the number of reactions (nr) to the number of photons absorbed (nabs, Equation 1) (Klán and Wirz, 2009)
| (Equation 1) |
An expression for the number of reactions at time t is given by the change in concentration (c) of the starting material multiplied by the volume (V, Equation 2).
| (Equation 2) |
The molar photon flux is defined as the number of photons in moles (i.e., in units of Einstein) incident on a sample per unit time and can be expressed as the light power () divided by the product of Avogadro's constant (NA) and the energy of each photon () as given by Equation 3.
| (Equation 3) |
The fraction of photons absorbed can be expressed as 1 minus the transmittance (T), which can be substituted for the measured absorbance using the Beer-Lambert law (Equation 4).
| (Equation 4) |
The differential photon absorption () at a given time and wavelength (λ) can be calculated by Equation 5.
| (Equation 5) |
Finally, it follows that the quantum yield is given by Equation 6.
| (Equation 6) |
Using experimental values from the kinetics of photochemical degradation of compound 1, a photochemical quantum yield of 4.35% ± 0.43% was calculated. This means that around one in every ∼23 photons that is absorbed (at ∼365 nm) leads to photochemical activation via loss of N2 as opposed to relaxation through other radiative or non-radiative (chemical quenching) pathways. These data indicated that photochemical activation of compound 1, and other molecules functionalized with the ArN3 group, is a highly efficient process.
Density Functional Theory Calculations
Next, to understand the thermochemical stability and photochemical reactivity of compound 1 in more detail, density functional theory (DFT) calculations were performed. The acyclic nature of compound 1 means that the compound has a large degree of conformational freedom. Therefore, to simplify calculation of the reaction coordinate and the electronic excitation profile, the non-substituted arylazide (benzylazide) was used as a model compound. All calculations used the uB3LYP/6-311++G(d,p) level of theory and included a polarizable continuum model (PCM, water). The calculated reaction coordinate is shown in Figure 3. Relative changes in the reaction free energy, ΔG; enthalpy, ΔH; and entropy ΔS of each species, including all transition states between the arylazide starting material and the most stable geometric isomer formed (cis-N-methyl-3H-azepin-2-amine) after addition of methylamine to the ketenimine intermediate, are shown. In addition, relative energies of the possible electronic states of the arylnitrene were calculated.
Figure 3.
DFT-Calculated (uB3LYP/6-311++G(d,p)/PCM) Reaction Coordinate
The relative calculated differences in free energy (ΔG/kJ mol−1), enthalpy (ΔH/kJ mol−1), and entropy (ΔS/J K−1 mol−1, at 298.15 K) of the various intermediates and transition states that connect arylazide (PhN3) with the N-methyl-cis-azepin-2-amine product are shown. Photochemically induced reactivity of arylazides proceeds via the ground-state open-shell singlet nitrene (1A2 state) corresponding to the (px)1(py)1 electronic configuration where the py orbital on the N atom lies in the plane of the C6H5 ring. Note, owing to spin contamination, the energy of the 1A2 state was estimated using the sum method by Ziegler et al. (Ziegler and Rank, 1977).
The first feature to note is that formation of transition state 1 (TS1), which corresponds to loss of N2 from arylazide, has a calculated free energy barrier of 143 kJ mol−1 in solution phase. This high barrier accounts for the thermal stability of the ArN3 group and contributes to the prolonged shelf-life of chelates (like compound 1) that are derivatized with ArN3. Irradiation with UV light can circumvent this thermodynamic barrier (see the time-dependent DFT [TD-DFT)] section below) and leads to the formation of arylnitrene in the lowest energy open-shell singlet state (1A2). The 1A2 ground state has a formal electronic configuration of (px)1(py)1 where the two unpaired electrons are primarily located in the p-orbitals of the nitrogen atom with some delocalization to the aromatic ring. Formation of the more stable triplet state (3A2) is spin forbidden, and the two closed-shell singlet states (both 1A1 with either (px)2 or (py)2 electronic configurations centered on the N atom) are calculated to be higher in energy, with a low probability of populating these states at ambient temperature. The TS2 corresponding to the intramolecular rearrangement of arylnitrene has a calculated barrier of only ΔΔGTS2 = 54 kJ mol−1, which includes destabilization from the loss of aromaticity. After ring insertion occurs, the benzazirine species (ΔG = 22 kJ mol−1) isomerizes to give the more stable ketenimine intermediate (ΔG = −3 kJ mol−1) via a relatively low-energy TS3 (ΔG = 37 kJ mol−1). Nucleophilic attack by methylamine (CH3NH2) at the 2-position of the ketenimine intermediate proceeds via TS4 (ΔG = 51 kJ mol−1) and initially produces the NH-azepin adduct. This NH-azepin then isomerizes to yield the cis-azepin-2-amine product. Formation of the azepin-2-amine from benzylazide and methylamine is calculated to be thermodynamically spontaneous with an overall reaction free energy of ΔG = −166 kJ mol−1. As expected, the main driving force for the initial photoactivation is entropic (N2(g) release, ΔS = +137 J−1 K−1 mol−1). However, nucleophilic addition to the ketenimine, and similarly, conjugation of a photoactive chelate to a biologically active molecule, is driven primarily by changes in enthalpy (ΔH = −173 kJ mol−1).
TD-DFT calculations were used to probe the electronic nature of the photoactivation of arylazide. An overlay of the experimentally measured electronic absorption spectrum of compound 1 (in MeOH) and the TD-DFT-calculated spectrum is presented in Figure 4 (see also Transparent Methods Figure S8 and Table S1). Perhaps surprisingly, compound 1, and indeed arylazide compounds like 4-azidobenzoic acid show almost no absorbance at wavelengths above ∼325 nm. Measurements of the molar absorption coefficients at 365 and 395 nm gave values of 16.9 ± 1.9 M−1 cm−1 and 5.2 ± 1.3 M−1 cm−1, respectively. Nevertheless, TD-DFT calculations of the 16 lowest energy excited states (including both singlets and triplets) of arylazide confirmed that excitation bands are present in the wavelength range used to promote the photochemistry. Electronic transitions to six major singlet excited states (assigned as bands A to F) contribute to the measured absorption profile of compound 1 in the region 200–600 nm. Note that at shorter wavelengths, the fit between the calculated and the experimental spectrum is less accurate because of the use of the model arylazide and the continuum solvation model, which do not account for the full electronic complexity or solvation dynamics of compound 1. However, experimental features associated with absorption at wavelengths >250 nm are well reproduced.
Figure 4.
Experimental Electronic Absorption Spectroscopy and Time-Dependent DFT
Overlay of the experimentally measured electronic absorption spectrum of compound 1 and the TD-DFT (uB3LYP/6-311++G(d,p)/PCM)-calculated spectrum of the model compound arylazide (PhN3). Note that the calculated spectrum was produced by using Lorentzian broadening, 20-nm full-width at half maximum. Calculated energies and oscillator strengths (f/a.u.) of the bands corresponding to transitions to the first six excited singlet states with non-zero expectation values are shown by vertical red lines (band details inset). For reference, band energies to the excited triplet states are shown by vertical green lines. The simulated spectrum and all calculated energies are x-shifted by +12 nm for clarity. Molecular orbital contributions involved in the excitation to the main singlet bands are presented in Table S1.
TD-DFT calculations allow deconvolution of the change in the electronic density associated with the formation of a particular excited state in terms of electronic mixing between the ground-state molecular orbitals (MOs) (Figure 5, and Transparent Methods Table S1). MO analysis from TD-DFT calculations performed on benzylazide found that absorption band A (corresponding to excitation to the lowest energy excited singlet state) involves 96.4% mixing of the electron density from the highest occupied MO to the lowest unoccupied molecular orbital (LUMO). Inspection of the MO isosurfaces showed that this transition involves primarily transfer of electron density from a populated ππ* orbital of the benzyl ring to the unoccupied, in-plane antibonding pπ* orbital centered on the azide group. Populating this excited state weakens the azide bond and stimulates N2 dissociation. Interestingly, our experimental observations indicate that any wavelength that populates the first excited singlet state can induce arylazide activation. However, test experiments using a shorter-wavelength LED (∼275 nm), which populates the second or third excited singlet states (bands B and C, respectively), found negligible photochemical activation of the ArN3 group (data not shown). This observation is consistent with the TD-DFT calculations, which indicate that bands B and C involve electronic transitions to higher-energy virtual orbitals (LUMO+1 and above). Kasha's rule suggests that relaxation of second and third excited singlet states of ArN3 should populate the LUMO, but inspection of the MOs shows that this process is symmetry forbidden (Figure 5).
Figure 5.
Molecular Orbital Diagram of ArN3
DFT-calculated (B3LYP/6-311++G(d,p)/PCM) molecular orbital diagram. Electron density isosurfaces of the three highest occupied molecular orbitals (HOMOs) and three lowest unoccupied molecular orbitals (LUMOs) for the model compound arylazide (PhN3) are shown. Note that the isosurfaces were generated by using a contour value of 0.035 and correspond to 96.5% of the total electron density.
Two-Step Photochemical Conjugation and 89Zr-Radiolabeling of Trastuzumab
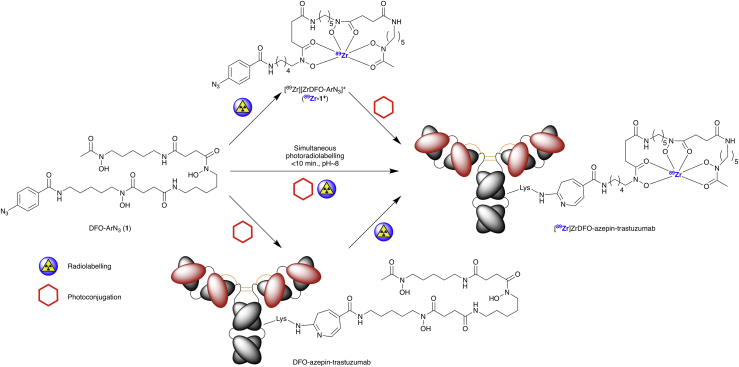
Before investigating a simultaneous one-pot photoradiochemical process, experiments were performed using the traditional two-step approach. This route involved the photochemical conjugation between compound 1 and trastuzumab, followed by 89Zr-radiolabeling (Scheme 3, bottom pathway). The photochemical conjugation was performed at room temperature for 25 min using a Rayonet reactor. The DFO-azepin-trastuzumab conjugate was purified by using a combination of size exclusion chromatography (SEC) methods including spin column centrifugation and preparative PD-10 gel filtration. DFO-azepin-trastuzumab was then radiolabeled with 89Zr using standard conditions (Holland et al., 2010b, Holland et al., 2010a, Holland et al., 2012, Rylova et al., 2016, Verel et al., 2003a, Verel et al., 2003b, Vosjan et al., 2010). Aliquots of the crude radiolabeling mixture were retained, and then the product was purified and formulated in sterile PBS by using standard SEC methods. Analytical measurements on the crude and purified samples of [89Zr]ZrDFO-azepin-trastuzumab were performed using radioactive instant thin-layer chromatography (radio-iTLC), analytical PD-10-SEC, and radioactive SEC-UHPLC (Figure 6).
Scheme 3.
Photoradiochemical Synthesis of [89Zr]ZrDFO-Azepin-Trastuzumab via Three Separate Routes
(Top) Pre-radiolabelling and subsequent photochemical conjugation of [89Zr]ZrDFO-ArN3 (89Zr-1+) with the antibody. (Middle) Simultaneous one-step photoradiochemical labeling. (Bottom) Two-step photochemical conjugation of the antibody and subsequent 89Zr radiolabeling of the isolated DFO-azepin-trastuzumab intermediate.
Figure 6.
Characterization Data for the Radiochemical Synthesis of [89Zr]ZrDFO-Azepin-Trastuzumab
(A) Radio-iTLC chromatograms showing [89Zr]ZrEDTA control; the crude reaction mixture at 15, 40, and 60 min; and the purified product.
(B and C) (B) Analytical PD-10-SEC elution profiles, and (C) SEC-UHPLC chromatograms of the crude and purified product.
Experiments confirmed that the DFO-azepin-trastuzumab was radiolabeled efficiently with 89Zr giving a crude radiochemical conversion (RCC) of >98% after incubating the mixture at room temperature for 15 min. On scaling up the radiolabeling reaction for use in cellular and animal experiments, the final radiochemical yield (RCY) of the purified sample was >99%, and the radiochemical purity (RCP) was measured at >99.5% by analytical PD-10-SEC and >98% by SEC-UHPLC. For the preparations used in the animal studies, the final activity concentration was 29.7 MBq mL−1, with a decay-corrected molar activity (Am) of 13.7 MBq nmol−1 of protein for the stock sample used in the normal doses (vide infra).
Further 89Zr-radiolabeling experiments were performed to measure the radiolabeling kinetics and overall RCC yields of DFO-azepin-trastuzumab samples that were prepared using different initial chelate-to-mAb ratios in the photochemical conjugation step (Figure 7 and Transparent Methods Figure S11). For each sample, the radiolabeling kinetics was monitored by radio-iTLC (Figure 7A) and the RCC (%) versus time was plotted (Figure 7B). These experiments showed a linear relation between the initial chelate-to-mAb ratio and the overall RCC at equilibrium (time points >60 min, Figure S11). Using these data, combined with the experimentally determined molar activity of the stock solution of [89Zr][Zr(C2O4)4]4– (measured by titration with DFO) (Rylova et al., 2016) the photochemical conjugation efficiency between DFO-ArN3 (1) and trastuzumab was estimated to be 3.5% ± 0.4% (n = 3). Hence, for the samples used in the biological studies, an initial chelate-to-mAb ratio of 26.4:1 yielded ∼0.85 accessible chelates per mAb in the final product.
Figure 7.
[89Zr]ZrDFO-azepin-trastuzumab Radiolabeling Kinetics and Stability Data
(A) Radio-iTLC chromatograms showing the kinetics of formation of [89Zr]ZrDFO-azepin-trastuzumab versus time using a pre-functionalized DFO-azepin-trastuzumab sample prepared with an initial chelate-to-mAb ratio of 26.4:1. Data for reactions starting with different initial chelate-to-mAb ratios are presented in Figure S11.
(B) Plot of the percentage radiochemical conversion (RCC) versus time using samples of DFO-azepin-trastuzumab pre-conjugated at different initial chelate-to-mAb ratios.
(C) Radioactive SEC-UHPLC confirming that [89Zr]ZrDFO-azepin-trastuzumab remains stable with respect to change in radiochemical purity during incubation in human serum at 37°C for 92 h.
The radiochemical stability of [89Zr]ZrDFO-azepin-trastuzumab with respect to change in the RCP during incubation in human serum at 37°C for up to 92 h was determined by SEC-UHPLC (Figure 7C). Experiments confirmed that the 89Zr activity remained bound to the mAb (<2% decrease in RCP after 92 h) with essentially no transchelation serum proteins (transferrin, albumin, etc.).
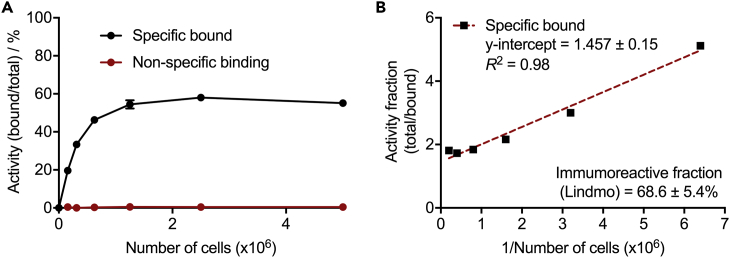
Cellular Saturation Binding Assays (Immunoreactivity)
The biochemical viability of [89Zr]ZrDFO-azepin-trastuzumab was measured by using standard cellular saturation binding assays in accordance with the methods introduced by Lindmo et al.(Junghans, 1999, Konishi et al., 2004, Lindmo et al., 1984). The human ovarian cancer cell line SK-OV-3 shows high expression of the human epidermal growth factor receptor 2 (HER2/neu), which is the target protein of trastuzumab. Saturation binding experiments confirmed that the protein remained biochemically active with an estimated immunoreactivity fraction between 63% and 74% (n = 2, Figure 8). Note that these cellular assays were incubated for only 1 h and the slightly low value of the immunoreactivity is primarily associated with slow binding kinetics. Nevertheless, the immunoreactive fraction is comparable to the reported value of 87% ± 7% obtained with [89Zr]ZrDFO-Nsucc-trastuzumab that was conjugated using one of the standard thermochemical routes (N-succinyl activated ester) (Holland et al., 2010a).
Figure 8.
Measurement of the Immunoreactive Fraction of [89Zr]ZrDFO-Azepin-Trastuzumab as Determined by Cellular Binding to SK-OV-3 (HER2/neu positive) Cells
(A) Saturation binding plot. Error bars correspond to one standard deviation.
(B) Lindmo plot.
Small-Animal PET Imaging and Biodistribution Studies
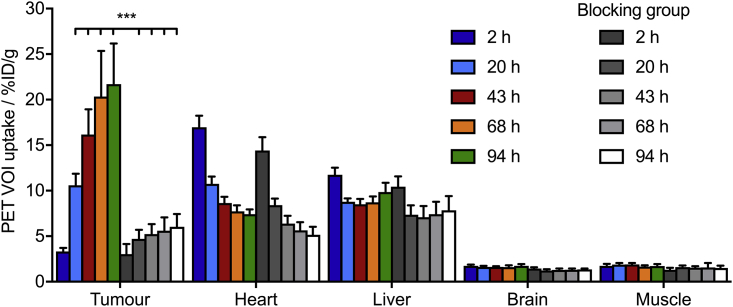
After confirming that [89Zr]ZrDFO-azepin-trastuzumab was chemically stable and biochemically active in vitro, the pharmacokinetics and target specificity were evaluated in athymic nude mice bearing subcutaneous SK-OV-3 tumors. PET images were recorded at multiple time points between 2 and 94 h post-administration. Two groups of mice were used. The normal group (n = 5) received a high molar activity formulation (13.7 MBq nmol−1), and the blocking group received the same amount of radioactivity but a reduced molar activity of 0.14 MBq nmol−1. Representative PET images showing coronal and axial planes taken through the center of the tumors are presented in Figure 9 (see also Transparent Methods Figures S12 and S13). PET imaging data were also quantified by drawing volumes-of-interest over various tissues and plotting the data as time-activity bar charts (Figure 10, based on quantification of the images in units of percentage injected dose per gram (%ID g−1), and Figure S14 for equivalent data using image quantification in units of the mean standardized uptake value). The pharmacokinetic profile and tumor uptake of [89Zr]ZrDFO-azepin-trastuzumab was consistent with previously reported experiments using 89Zr-DFO-radiolabeled trastuzumab produced via traditional coupling chemistries (Holland et al., 2010a).
Figure 9.
Temporal [89Zr]ZrDFO-Azepin-Trastuzumab PET Images Recorded in Mice Bearing SK-OV-3 Tumors on the Right Flank T, tumor; H, heart; L, liver; Sp, spleen
Figure 10.
Time-Activity Bar Chart Showing the Activity Associated with Different Tissues (volumes of Interest, VOI) versus Time
Data presented are based on quantification of the PET images in units of %ID cm−3. Equivalent data using quantification of the PET images in terms of the mean standardized uptake value are shown in Figure S14. Error bars correspond to one standard deviation.
The effective half-life (t1/2(eff)/h) of [89Zr]ZrDFO-azepin-trastuzumab was measured in mice by using a calibrated dosimeter (Transparent Methods Figure S15). A value of t1/2(eff) = 45.7 ± 7.7 h was measured (n = 11; R2 ∼ 0.99) with an estimated biological half-life, t1/2(biol.) = 109.5 ± 18.4 h. No difference was observed between the normal and blocking groups. These data are consistent with previous reports on 89Zr-radiolabeled trastuzumab (Dijkers et al., 2009, Holland et al., 2010a).
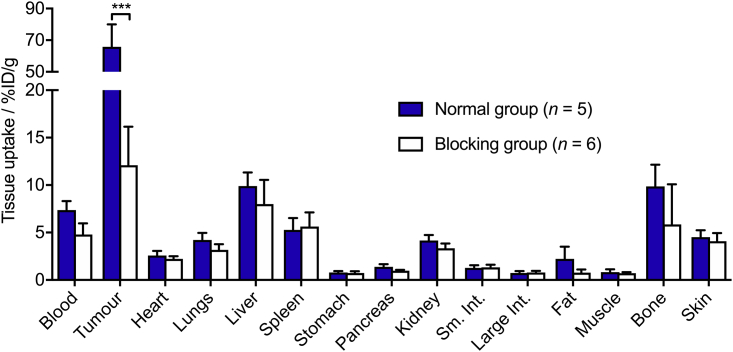
After the final imaging time point, animals were euthanized and a biodistribution analysis was performed to obtain accurate quantification of the accumulation of 89Zr in different tissues (Figure 11, Table 1 and Transparent Methods Figures S16–S19). Comparison of the biodistribution data between the normal and blocking groups showed a specific accumulation of radioactivity in the tumor (65.8% ± 14.2 %ID g−1 in the normal group versus 12.1% ± 4.1%ID g−1 in the blocking group, p value = 0.0006). With the exception of the activity retained in the blood pool (7.4% ± 1.0 %ID g−1 for the normal versus 4.8% ± 1.2 %ID g−1 for the blocking group [p value = 0.003]), no statistically significant differences were observed between radioactivity accumulation in the background tissues of the two groups of mice. Furthermore, a comparison of the tumor-to-tissue contrast ratios recorded from the biodistribution studies performed on the photoradiochemical product [89Zr]ZrDFO-azepin-trastuzumab and on [89Zr]ZrDFO-Nsucc-trastuzumab produced by a conventional conjugation route showed no statistically significant differences between the two radiotracers (Transparent Methods Figure S20).(Holland et al., 2010a) Collectively, the data from experiments performed in vitro, in vivo, and ex vivo indicate that the photoradiochemical route to 89Zr-radiolabeled trastuzumab yields a radiolabeled compound that is of equivalent quality to other 89Zr-mAbs produced via established methods.
Figure 11.
Bar Chart Showing Ex Vivo Biodistribution Data (%ID/g) for the Uptake of [89Zr]ZrDFO-Azepin-Trastuzumab in Mice Bearing Subcutaneous SK-OV-3 Tumors
Data were recorded after the final imaging time point at 94 h post-injection. ***Student's t test p value < 0.001. An equivalent plot using units of standardized uptake value is presented in Figure S17. Data showing tumor-to-tissue contrast ratios are presented in Figure S18. Error bars correspond to one standard deviation.
Table 1.
Ex Vivo Biodistribution Data for [89Zr]ZrDFO-Azepin-Trastuzumab in Mice Bearing SK-OV-3 Tumors
| Tissue | Normal Group (n = 5) |
Blocking Group (n = 6) |
||
|---|---|---|---|---|
| [89Zr]ZrDFO-Azepin-Trastuzumab (94 h)/%ID g−1 ± SDa | Tumor-to-Tissue Contrast Ratio ± SDb | [89Zr]ZrDFO-Azepin-Trastuzumab (94 h)/%ID g−1 ± SDa | Tumour-to-Tissue Contrast Ratio ± SDb | |
| Blood | 7.36 ± 0.96 | 8.94 ± 2.25 | 4.79 ± 1.18 | 2.53 ± 1.05 |
| Tumor | 65.79 ± 14.19 | 1.00 | 12.10 ± 4.06 | 1.00 |
| Heart | 2.58 ± 0.49 | 25.51 ± 7.32 | 2.22 ± 0.28 | 5.45 ± 1.95 |
| Lungs | 4.22 ± 0.75 | 15.57 ± 4.35 | 3.17 ± 0.60 | 3.82 ± 1.47 |
| Liver | 9.90 ± 1.44 | 6.64 ± 1.73 | 7.99 ± 2.56 | 1.51 ± 0.70 |
| Spleen | 5.28 ± 1.24 | 12.45 ± 3.97 | 5.64 ± 1.48 | 2.14 ± 0.91 |
| Stomach | 0.79 ± 0.15 | 83.03 ± 23.85 | 0.74 ± 0.19 | 16.36 ± 6.95 |
| Pancreas | 1.39 ± 0.28 | 47.27 ± 13.91 | 0.98 ± 0.10 | 12.39 ± 4.37 |
| Kidney | 4.16 ± 0.57 | 15.82 ± 4.03 | 3.35 ± 0.50 | 3.61 ± 1.33 |
| Small intestine | 1.28 ± 0.28 | 51.30 ± 15.70 | 1.33 ± 0.28 | 9.13 ± 3.62 |
| Large intestine | 0.74 ± 0.20 | 88.79 ± 30.95 | 0.79 ± 0.17 | 15.29 ± 6.10 |
| Fat | 2.22 ± 1.29 | 29.61 ± 18.31 | 0.78 ± 0.32 | 15.49 ± 8.24 |
| Muscle | 0.82 ± 0.30 | 79.84 ± 33.39 | 0.71 ± 0.12 | 17.05 ± 6.39 |
| Bone | 9.86 ± 2.29 | 6.67 ± 2.12 | 5.85 ± 4.24 | 2.07 ± 1.65 |
| Skin | 4.51 ± 0.73 | 14.58 ± 3.94 | 4.09 ± 0.86 | 2.96 ± 1.17 |
Uptake data are expressed as the mean %ID g−1 ± one standard deviation (SD).
Errors for the tumor-to-tissue ratios are calculated as the geometric mean of the standard deviations.
Simultaneous Photoradiolabeling of Trastuzumab
Previously, we demonstrated that the photoradiochemical approach was successful when radiolabeling either pre-purified mAbs or fully formulated samples (Herceptin) (Patra et al., 2019). In addition to fast reaction and processing times, a one-pot procedure has the unique advantage of avoiding the need to isolate and characterize the conjugated intermediate antibodies. To illustrate the feasibility of the simultaneous photoradiochemical process, experiments were performed to produce [89Zr]ZrDFO-azepin-trastuzumab in a single step.
Reactions were established in which [89Zr][Zr(C2O4)4]4–, compound 1, and trastuzumab (at an initial chelate-to-mAb ratio of ∼29:1) were mixed in water and the pH adjusted to ∼8–9. Control reactions were performed in the absence of either the chelate (1) or the mAb. Mixtures were stirred gently at room temperature and irradiated using the LED source (365 or 395 nm) for 10 min. Note that in this case stirring appeared to be more important than in our previous experiments with 68Ga (Patra et al., 2019). After irradiation, the reactions were quenched by the addition of diethylenetriamine pentaacetic acid (DTPA). Aliquots of the crude samples were retained, and a fraction was purified by SEC methods. Crude and purified samples were then analyzed by using radio-iTLC, analytical PD-10-SEC, and SEC-UHPLC methods (Figure 12 and Transparent Methods Table S3).
Figure 12.
Characterization Data for the Simultaneous One-Pot Photoradiochemical Synthesis of [89Zr]ZrDFO-Azepin-Trastuzumab
(A and B) (A) Radio-iTLC chromatograms showing control reactions in the absence of compound 1 (no chelate, green) or mAb (yellow), 89Zr-1+ before irradiation (purple), and the crude products after irradiation with 365 nm (black) and 395 nm (red). (B) Analytical PD-10-SEC elution profiles showing the [89Zr][ZrDTPA]– control (green, equivalent to the no chelate control confirming no non-specific binding of 89Zr4+ ions to the mAb), a control reaction without mAb (yellow), crude reaction mixtures after irradiation and DTPA quenching at 365 nm (black) and 395 nm (red), and the purified product (blue).
(C) SEC-UHPLC chromatograms of the crude and purified product.
Analysis of the crude reaction mixtures also indicated that ∼72%–73% (n = 2, by analytical PD-10-SEC) and ∼67%–88% (n = 2, by SEC-UHPLC) of the 89Zr-radioactivity was associated with trastuzumab. Control reactions confirmed that the 89Zr radioactivity bound to trastuzumab specifically (Figures 12A and 12B, green and yellow traces). After purification, simultaneous photoradiolabeling gave [89Zr]ZrDFO-azepin-trastuzumab with a decay-corrected RCY of ∼76%, a RCP ∼97% (measured by SEC-UHPLC), and a molar activity of 0.41 MBq nmol−1 of protein (n = 2). Interestingly, both the 365- and 395-nm LED sources gave equivalent radiochemical conversions. Reactions were complete in <10 min, and the entire process, from non-labeled trastuzumab to formulated [89Zr]ZrDFO-azepin-trastuzumab, was accomplished in <15 min. With a higher intensity light source, it is conceivable that the photoradiochemical synthesis could be accomplished in a few seconds, which would mean that radiotracer production times are limited only by the time required for purification and quality control.
Comparison of the final RCYs measured between the two-step process and the simultaneous (one-step) process indicate that the photochemical conjugation efficiency increased from 3.5% to ∼76%. This result shows that the chemical efficiency of simultaneous photoradiolabeling is comparable to many of the most efficient thermally mediated methods, which typically display ∼60%–80% conjugation efficiency (Holland et al., 2010b, Holland et al., 2010a, Holland et al., 2012, Rylova et al., 2016, Verel et al., 2003a, Verel et al., 2003b, Vosjan et al., 2010). Under the conditions employed it is likely that the kinetics of metal ion complexation is equal to, or faster than, that of the photochemical conjugation step. Complexation of 89Zr4+ ions by the DFO-ArN3 chelate reduces the probability of intramolecular reactions between the nucleophilic hydroxamate groups and the photo-generated intermediates. Hence, pre-complexation increases the photochemical conjugation efficiency when compared with the free chelate (1). Notably, the photoradiochemical method is also suitable for use in fully automated radiochemical synthesis modules. Automated photoradiochemical synthesis has the potential to change the way in which radiolabeled mAbs, immunoglobulin fragments, and other proteins are produced in the clinic.
Conclusions
Experiments demonstrated that the photoradiolabeling methods are viable for the synthesis of radiolabeled antibodies for immuno-PET. In contrast to existing (thermochemical) technologies, photoradiochemistry allows for the rapid synthesis of [89Zr]ZrDFO-azepin-trastuzumab in high radiochemical yield and purity using a simultaneous, one-pot approach. Accessing radiolabeled proteins directly from the non-functionalized source eliminates the need to isolate and characterize a radiolabeling precursor (here DFO-azepin-trastuzumab). Our work illustrates the potential of applying photochemistry in radiopharmaceutical science. We continue to investigate the use of photoradiochemistry with different photoactivatable groups, chelates, and radionuclides for producing diagnostic and therapeutic radiopharmaceuticals based on proteins, peptides, small molecules, and nanoparticles.
Limitations of the Study
Although photoradiochemistry offers an exciting alternative for the synthesis of radiolabeled proteins, and we have shown that photoradiochemistry can facilitate processes that are not achievable using standard thermochemical methods, some limitations exist. Namely, the success of the photoradiochemical reaction is dependent on the experimental geometry. The light-activation process was found to be highly efficient, but experimentally, the shape and focus point of the light beam, the photon flux, and the potential absorption or scattering from the reaction vessel or chemical components of the reaction mixture mean that care must be taken to achieve reproducible results. Similar to standard thermochemical methods, we have also discovered that the photochemical conjugation efficiency depends on the nature of the photoactive group, the chelate or complex, and also other factors including the protein, solvent composition, mixing efficiency, and concentration-dependent radiochemical kinetics (Holland, 2018). Much work is required before photoradiochemical methods can be standardized.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
J.P.H. thanks the Swiss National Science Foundation (SNSF Professorship PP00P2_163683), the Swiss Cancer League (Krebsliga Schweiz; KLS-4257-08-2017), and the University of Zurich (UZH) for financial support. This project has received funding from the European Union's Horizon 2020 research and innovation program and from the European Research Council under the Grant Agreement No 676904, ERC-StG-2015, NanoSCAN. We thank all members of the Radiochemistry and Imaging Science group at UZH for helpful discussions. We thank Dr. Benjamin Probst for spectroradiometric measurements. J.P.H. thanks Dr. Douglas J. Fox from Gaussian Inc. for helpful discussions regarding DFT calculations.
Author Contributions
Conceptualization, J.P.H.; Methodology, J.P.H., M.P., S.K., and L.S.E.; Investigation, J.P.H., M.P., S.K., and L.S.E.; Formal Analysis, J.P.H., M.P., and S.K.; Resources, J.P.H.; Writing – Original Draft, J.P.H.; Writing – Review and Editing, J.P.H., M.P., and S.K.; Visualization, J.P.H.; Supervision, J.P.H.; Project Administration, J.P.H.; Funding Acquisition, J.P.H.
Declaration of Interests
J.P.H. and M.P. are listed as inventors on a patent application related to the materials and methods used in this work. Otherwise, the authors declare no competing interests.
Published: March 29, 2019
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2019.03.004.
Supplemental Information
References
- Bayley H., Knowles J.R. Photoaffinity labeling. Methods Enzymol. 1977;46:69–114. doi: 10.1016/s0076-6879(77)46012-9. [DOI] [PubMed] [Google Scholar]
- Borden W.T., Gritsan N.P., Hadad C.M., Karney W.L., Kemnitz C.R., Platz M.S. The interplay of theory and experiment in the study of phenylnitrene. Acc. Chem. Res. 2000;33:765–771. doi: 10.1021/ar990030a. [DOI] [PubMed] [Google Scholar]
- Boros E., Holland J.P. Chemical aspects of metal ion chelation in the synthesis and application antibody-based radiotracers. J. Label. Compd. Radiopharm. 2018;61:652–671. doi: 10.1002/jlcr.3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bou-Hamdan F.R., Lévesque F., O’Brien A.G., Seeberger P.H. Continuous flow photolysis of aryl azides: preparation of 3H-azepinones. Beilstein J. Org. Chem. 2011;7:1124–1129. doi: 10.3762/bjoc.7.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhry V., Westheimer F.H. Photoaffinity labeling of biological systems. Annu. Rev. Biochem. 1979;48:293–325. doi: 10.1146/annurev.bi.48.070179.001453. [DOI] [PubMed] [Google Scholar]
- Dennler P., Fischer E., Schibli R. Antibody conjugates: from heterogeneous populations to defined reagents. Antibodies. 2015;4:197–224. [Google Scholar]
- Dijkers E.C.F., Kosterink J.G.W., Rademaker A.P., Perk L.R., van Dongen G.A.M.S., Bart J., de Jong J.R., de Vries E.G.E., Lub-de Hooge M.N. Development and characterization of clinical-grade 89Zr-trastuzumab for HER2/neu immunopet imaging. J. Nucl. Med. 2009;50:974–981. doi: 10.2967/jnumed.108.060392. [DOI] [PubMed] [Google Scholar]
- Eichenberger L.S., Patra M., Holland J.P. Photoactive chelates for radiolabelling proteins. Chem. Commun. (Camb). 2019;55:2257–2260. doi: 10.1039/c8cc09660k. [DOI] [PubMed] [Google Scholar]
- Gritsan N., Platz M. Photochemistry of Azides: the azide/nitrene interface. In: Bräse S., Banert K., editors. Organic Azides: Syntheses and Applications. John Wiley & Sons; 2010. pp. 311–372. [Google Scholar]
- Gritsan N.P., Platz M.S. Kinetics, spectroscopy, and computational chemistry of arylnitrenes. Chem. Rev. 2006;106:3844–3867. doi: 10.1021/cr040055+. [DOI] [PubMed] [Google Scholar]
- Gritsan N.P., Pritchina E.A. Mechanism of the photolysis of aromatic azides. Russ. Chem. Rev. 1992;61:500–516. [Google Scholar]
- Hashizume K., Hashimoto N., Miyake Y. Synthesis of positron labeled photoactive compounds:18F labeled aryl azides for positron labeling of biochemical molecules. J. Org. Chem. 1995;60:6680–6681. [Google Scholar]
- Holland J.P. Chemical kinetics of radiolabelling reactions. Chem. Eur. J. 2018;24:16472–16483. doi: 10.1002/chem.201803261. [DOI] [PubMed] [Google Scholar]
- Holland J.P., Vasdev N. Charting the mechanism and reactivity of zirconium oxalate with hydroxamate ligands using density functional theory: implications in new chelate design. Dalton Trans. 2014;43:9872–9884. doi: 10.1039/c4dt00733f. [DOI] [PubMed] [Google Scholar]
- Holland J.P., Divilov V., Bander N.H., Smith-Jones P.M., Larson S.M., Lewis J.S. 89Zr-DFO-J591 for immunoPET imaging of prostate-specific membrane antigen (PSMA) expression in vivo. J. Nucl. Med. 2010;51:1293–1300. doi: 10.2967/jnumed.110.076174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland J.P., Caldas-Lopes E., Divilov V., Longo V.A., Taldone T., Zatorska D., Chiosis G., Lewis J.S. Measuring the pharmacodynamic effects of a novel Hsp90 inhibitor on HER2/neu expression in mice using 89Zr-DFO-trastuzumab. PLoS One. 2010;5:e8859. doi: 10.1371/journal.pone.0008859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland J.P., Evans M.J., Rice S.L., Wongvipat J., Sawyers C.L., Lewis J.S. Annotating MYC status with 89Zr-transferrin imaging. Nat. Med. 2012;18:1586–1591. doi: 10.1038/nm.2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junghans R.P. Cruel antibody fictions! Cellular antigen enumeration by “saturation” binding. Immunol. Today. 1999;20:401–406. doi: 10.1016/s0167-5699(97)01178-x. [DOI] [PubMed] [Google Scholar]
- Klán, P., and Wirz, J. (2009). Photochemistry of Organic compounds: from concepts to practice. Wiley. ISBN: 978-1-405-16173-2.
- Konishi S., Hamacher K., Vallabhajosula S., Kothari P., Bastidas D., Bander N., Goldsmith S. Determination of immunoreactive fraction of radiolabeled monoclonal antibodies: what is an appropriate method? Cancer Biother. Radiopharm. 2004;19:706–715. doi: 10.1089/cbr.2004.19.706. [DOI] [PubMed] [Google Scholar]
- Kotzyba-Hilbert F., Kapfer I., Goeldner M. Recent trends in photoaffinity labeling. Angew. Chem. Int. Ed. 1995;34:1296–1312. [Google Scholar]
- Kumar V., Yarravarapu N., Lapinsky D.J., Perley D., Felts B., Tomlinson M.J., Vaughan R.A., Henry L.K., Lever J.R., Newman A.H. Novel azido-iodo photoaffinity ligands for the human serotonin transporter based on the selective serotonin reuptake inhibitor (S)-citalopram. J. Med. Chem. 2015;58:5609–5619. doi: 10.1021/acs.jmedchem.5b00682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kym P.R., Carlson K.E., Katzenellenbogen J.A. Evaluation of a highly efficient aryl azide photoaffinity labeling reagent for the progesterone receptor. Bioconjug. Chem. 1995;6:115–122. doi: 10.1021/bc00031a014. [DOI] [PubMed] [Google Scholar]
- Lange C.W., VanBrocklin H.F., Taylor S.E. Photoconjugation of 3-azido-5-nitrobenzyl-[18F]fluoride to an oligonucleotide aptamer. J. Label. Compd. Radiopharm. 2002;45:257–268. [Google Scholar]
- Lindmo T., Boven E., Cuttitta F., Fedorko J., Bunn P.A. Determination of the immunoreactive function of radiolabeled monoclonal antibodies by linear extrapolation to binding at infinite antigen excess. J. Immunol. Methods. 1984;72:77–89. doi: 10.1016/0022-1759(84)90435-6. [DOI] [PubMed] [Google Scholar]
- Nishikawa M., Nakano T., Okabe T., Hamaguchi N., Yamasaki Y., Takakura Y., Yamashita F., Hashida M. Residualizing indium-111-radiolabel for plasmid DNA and its application to tissue distribution study. Bioconjug. Chem. 2003;14:955–961. doi: 10.1021/bc034032y. [DOI] [PubMed] [Google Scholar]
- Pandurangi R.S., Karra S.R., Katti K.V., Kuntz R.R., Volkert W.A. Chemistry of bifunctional photoprobes. 1. perfluoroaryl azido functionalized phosphorus hydrazides as novel photoreactive heterobifunctional chelating agents: high efficiency nitrene insertion on model solvents and proteins. J. Org. Chem. 1997;62:2798–2807. doi: 10.1021/jo961867b. [DOI] [PubMed] [Google Scholar]
- Pandurangi R.S., Lusiak P., Kuntz R.R., Sun Y., Weber K.T. Chemistry of bifunctional photoprobes II. chemical and photochemical modification of angiotensin converting enzyme inhibitors: implications in the development of cardiac radionuclide imaging agents. Bioorg. Chem. 1997;87:77–87. [Google Scholar]
- Pandurangi R.S., Karra S.R., Kuntzl R.R., Volkert W.A. Symposium-in-print recent trends in the evaluation of photochemical insertion characteristics. Photochem. Photobiol. 1997;65:208–221. [Google Scholar]
- Pandurangi R.S., Lusiak P., Kuntz R.R., Volkert W.A., Rogowski J., Platz M.S. Chemistry of bifunctional photoprobes. 3. Correlation between the efficiency of CH insertion by photolabile chelating agents and lifetimes of singlet nitrenes by flash photolysis: first example of photochemical attachment of 99mTc-complex with human serum. J. Org. Chem. 1998;63:9019–9030. [Google Scholar]
- Patra M., Eichenberger L.S., Fischer G., Holland J.P. Photochemical conjugation and one-pot radiolabelling of antibodies for immuno-PET. Angew. Chem. Int. Ed. 2019;58:1928–1933. doi: 10.1002/anie.201813287. [DOI] [PubMed] [Google Scholar]
- Platz M.S. Comparison of phenylcarbene and phenylnitrene. Acc. Chem. Res. 1995;28:487–492. [Google Scholar]
- Rajagopalan R., Kuntz R.R., Sharma U., Volkert W.A., Pandurangi R.S. Chemistry of bifunctional photoprobes. 6. Synthesis and characterization of high specific activity metalated photochemical probes: development of novel rhenium photoconjugates of human serum albumin and Fab fragments. J. Org. Chem. 2002;67:6748–6757. doi: 10.1021/jo010782u. [DOI] [PubMed] [Google Scholar]
- Rylova S.N., Del Pozzo L., Klingeberg C., Tonnesmann R., Illert A.L., Meyer P.T., Maecke H.R., Holland J.P. Immuno-PET imaging of CD30-positive lymphoma using 89Zr-desferrioxamine-labeled CD30-specific AC-10 antibody. J. Nucl. Med. 2016;57:96–102. doi: 10.2967/jnumed.115.162735. [DOI] [PubMed] [Google Scholar]
- Singh A., Thornton E.R., Westheimer F.H. The photolysis of diazo-acetylchymotrypsin. J. Biol. Chem. 1962;237:PC3006–PC3008. [PubMed] [Google Scholar]
- Stalteri M.A., Mather S.J. Technetium-99m labelling of the anti-tumour antibody PR1A3 by photoactivation. Eur. J. Nucl. Med. 1996;23:178–187. doi: 10.1007/BF01731842. [DOI] [PubMed] [Google Scholar]
- Sykes T.R., Woo T.K., Baum R.P., Qi P., Noujaim A. Direct labeling of monoclonal antibodies with technetium-99m by photoactivation. J. Nucl. Med. 1995;36:1913–1922. [PubMed] [Google Scholar]
- Sykes T.R., Somayaji V.A., Bier S., Woo T.K., Kwok C.S., Snieckus V., Noujaim A. Radiolabeling of monoclonal antibody B43.13 with rhenium-188 for immunoradiotherapy. Appl. Radiat. Isot. 1997;48:899–906. doi: 10.1016/s0969-8043(97)00025-0. [DOI] [PubMed] [Google Scholar]
- Verel I., Visser G.W.M., Boellaard R., Stigter-van Walsum M., Snow G.B., van Dongen G.A.M.S. 89Zr immuno-PET: comprehensive procedures for the production of 89Zr-labeled monoclonal antibodies. J. Nucl. Med. 2003;44:1271–1281. [PubMed] [Google Scholar]
- Verel I., Visser G.W.M., Boerman O.C., van Eerd J.E.M., Finn R., Boellaard R., Vosjan M.J.W.D., Stigter-van Walsum M., Snow G.B., van Dongen G.E.M. Long-lived positron emitters zirconium-89 and iodine-124 for scouting of therapeutic radioimmunoconjugates with PET. Cancer Biother. Radiopharm. 2003;18:655–661. doi: 10.1089/108497803322287745. [DOI] [PubMed] [Google Scholar]
- Vosjan M.J.W.D., Perk L.R., Visser G.W.M., Budde M., Jurek P., Kiefer G.E., van Dongen G.A.M.S. Conjugation and radiolabeling of monoclonal antibodies with zirconium-89 for PET imaging using the bifunctional chelate p-isothiocyanatobenzyl-desferrioxamine. Nat. Protoc. 2010;5:739–743. doi: 10.1038/nprot.2010.13. [DOI] [PubMed] [Google Scholar]
- Wester H.J., Hamacher K., Stöcklin G. A comparative study of N.C.A. fluorine-18 labeling of proteins via acylation and photochemical conjugation. Nucl. Med. Biol. 1996;23:365–372. doi: 10.1016/0969-8051(96)00017-0. [DOI] [PubMed] [Google Scholar]
- Ziegler T., Rank A. On the calculation of multiplet energies by the Hartree-Fock-slater method. Theor. Chim. Acta. 1977;43:261–271. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.