Abstract
Background and Purpose
Thrombectomy in late time windows leads to improved outcomes in patients with ischemic stroke due to large vessel occlusion (LVO). We determined if patients with rapid neurologic improvement (RNI) 24 hours (24h) after thrombectomy were more likely to have a favorable clinical outcome in the DEFUSE 3 study.
Methods
All patients who underwent thrombectomy in DEFUSE 3 were included. RNI was defined as a reduction of ≥8 NIHSS or NIHSS 0–1 24-hours after thrombectomy. Clinical outcomes were assessed by an ordinal analysis modified Rankin Scale (mRS) score and a dichotomous analysis for 90-day independence (mRS 0–2).
Results
91 patients in DEFUSE 3 underwent thrombectomy with follow up data; 31 patients (34%) experienced RNI (RNI+) after thrombectomy and 60 patients (66%) did not (RNI−). Patient demographics and stroke presentation and imaging details were similar between RNI+ and RNI− patients. Reperfusion (TICI IIB-III) after thrombectomy was achieved in 26 (84%) RNI+ and 43 (72%) RNI− (p=0.2). Symptomatic intracranial hemorrhage occurred in no RNI+ and 8% of RNI− patients (p=0.2). RNI was associated with a favorable mRS shift at day 90 (OR 3.8 [CI 1.7–8.6]; p=0.001) and higher rates of mRS 0–2 (61% vs 37%) OR 2.7 [CI 1.1–6.7]; p=0.03. Mortality was 3% in RNI+ vs 18% in RNI− (p=0.05). RNI+ patients had lower median 24h NIHSS (5 [IQR 1–7] vs 13 [IQR 7.5–21]; p<0.001), smaller 24h infarction volume (21 ml [IQR 5–32] vs 65 [IQR 27–145]; p<0.001), and less 24h infarct growth (8 ml [IQR 1–18] vs 37 [IQR 16–105]; p<0.001) compared to RNI− patients. Hospital stay was shorter in RNI+ (3.7 days [IQR 2.9–7.1] vs 7.4 [IQR 5.2–12.1] in RNI−; p<0.001).
Conclusions
RNI following thrombectomy correlates with favorable clinical and radiographic outcomes and reduced hospital length of stay. RNI was a favorable prognostic sign following late window thrombectomy in DEFUSE 3.
Keywords: stroke, thrombectomy, rapid neurologic improvement
Introduction
Endovascular thrombectomy is an effective therapy for acute ischemic stroke caused by occlusions of the internal carotid artery or first or second segment of the middle cerebral artery.1–7 Some patients experience rapid neurologic improvement (RNI) following stroke treatment by thrombectomy or intravenous thrombolysis, which has emerged as an early indicator of a favorable long-term clinical outcome.8–14 However, RNI has only been described for the treatment of patients between zero and six hours after last known well.
The DEFUSE 3 (Endovascular Therapy Following Imaging Evaluation for Ischemic Stroke 3) study demonstrated the effectiveness of thrombectomy in patients who were treated 6 to 16 hours after last seen normal with a target mismatch profile on perfusion imaging.7 The purpose of this study was to determine the frequency of RNI in patients undergoing thrombectomy in late time windows and whether RNI is an early predictor of a favorable clinical outcome 90-days after treatment.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
DEFUSE 3 Trial and patients
DEFUSE 3 was a multicenter, randomized trial that compared thrombectomy with medical management for the treatment of ischemic stroke due to an anterior circulation large vessel occlusion (LVO) in patients who were last known to be normal 6 to 16 hours prior to enrollment. The study protocol15 and primary manuscript7 have been previously published. Patients were selected for enrollment following computed tomography (CT) or magnetic resonance imaging (MRI) studies with perfusion imaging; enrolled patients had a target mismatch between the core infarction and the salvageable penumbra.15 This is a post hoc analysis of all DEFUSE 3 patients who underwent thrombectomy. All included patients or their next of kin provided informed consent for inclusion in the DEFUSE 3, and this study was approval by our institutional review board. The DEFUSE 3 trial registration is found at: https://clinicaltrials.gov/ct2/show/NCT02586415
Clinical Definitions and outcomes
Rapid neurologic improvement (RNI) was defined as a reduction of ≥8 on the National Institutes of Health Stroke Scale (NIHSS) or NIHSS 0–1 24 hours after thrombectomy.12, 13 All imaging outcomes were determined by the DEFUSE 3 core laboratory in a blinded manner.7, 15 Collaterals were rated by CT angiography (CTA) with the binary modified Tan collateral scale;16, 17 “good collaterals” were defined as those that filled >50% of the vascular territory distal of the occluded MCA of ICA, and poor collaterals were those that filled ≤50% of the territory distal to the arterial occlusion. Post thrombectomy angiographic revascularization was quantified using the Thrombolysis in Cerebral Infarction (TICI) score, and successful revascularization was defined as TICI 2b or 3.18 Pre- and post-thrombectomy core and penumbra volumes were quantified by automated software (RAPID, iSchemaView, Menlo Park, CA).15 Reperfusion was assessed on 24-hour MR or CT perfusion studies as described previously.15 Infarction growth was determined by subtracting the baseline core infarction volume from the 24-hour core infarction volume by the DEFUSE 3 core laboratory. Favorable discharge location was defined as patient discharge to home, acute rehabilitation unit, home with home care, or subacute rehabilitation. Unfavorable discharge location was defined as discharge to hospice, skilled nursing facility, acute care facility or death. Hospital length of stay was reported ordinally and considered in a binary manner (≤4 days [favorable] versus >4 days [unfavorable] for the regression analysis). Clinical outcomes were assessed 90 days after treatment using the ordinal modified Rankin Scale (mRS). Favorable clinical outcome was defined as mRS 0–2 at 90-days.
Statistical Analysis
Patient demographics, clinical variables, and neuroimaging data were compared between patients with RNI (RNI+) and those without RNI (RNI−) using the χ2 and Wilcoxon rank-sum tests. The association between RNI and neurologic outcome was assessed using a logistic regression model that was fitted to the primary outcome and ordinal logistic regression models for the secondary outcomes. The model was adjusted for additional covariates that were independently associated with the primary outcome: patient age, presentation NIHSS and serum glucose at enrollment, and time from last known normal to randomization. For the ordinal logistic regression models, the proportional-odds assumption had to be met (p>0.05) before further analysis. To test the hypothesis that RNI+ patients were more likely to achieve a favorable clinical outcome, we derived odds ratios for more favorable 90-day functional outcome stratified by RNI. Alpha was set at the 0.05 level, and all reported results are two-sided. Statistical analysis was done using SAS 9.4.
Results
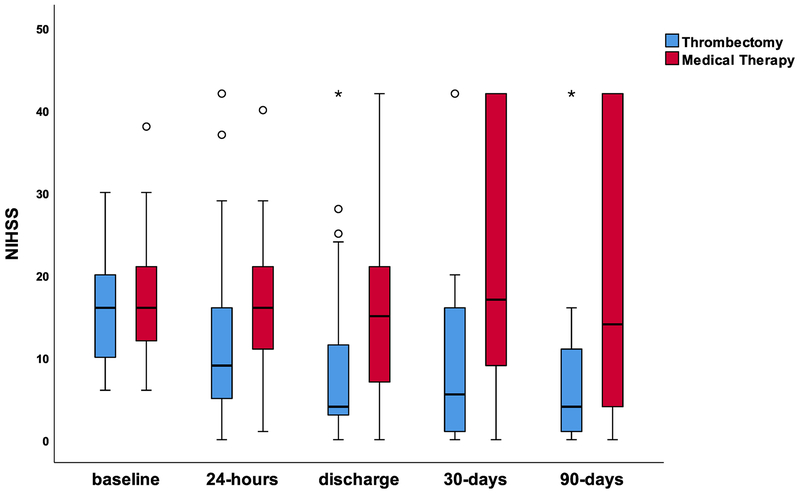
Changes in NIHSS following enrollment were first compared between patients treated by medical therapy versus thrombectomy (Figure 1). There was no difference in median presentation NIHSS (16 [IQR, 11–21] for thrombectomy and 16 [IQR, 12–21] for medical therapy). Patients who underwent thrombectomy had significantly lower median NIHSS scores at 24 hours, discharge, 30 days, and 90 days compared to medical therapy patients (Figure 1).
Figure 1. Changes in NIHSS in the medical therapy and thrombectomy groups in the DEFUSE 3 study.
Median and IQR NIHSS scores at baseline, 24-hours, discharge, 30-days, and 90-days for the medical therapy group (red) and the thrombectomy group (blue). There were no differences between these groups at baseline (p=0.39). Significant differences between these two groups were present at 24-hours (p<0.001), discharge (p<0.001), 30-days (p=0.001), and 90-days (p<0.001). 13 patients died before discharge, which included 6 in the medical therapy group (5 RNI−, 1 RNI+) and seven in thrombectomy group (6 RNI− and 1 excluded due no NIHSS assessment).
Thrombectomy patients were then dichotomized into RNI+ (31 patients, 34%) and RNI− (60 patients, 65%). A single patient who suffered a large reperfusion hemorrhage and died without a 24-hour NIHSS being recorded was excluded from the analysis. There were no differences in age, sex, or medical comorbidities between RNI+ and RNI− patients (Table 1). Median presentation NIHSS was similar between RNI+ (17 [IQR, 13–21]) and RNI− (15.5 [IQR, 9.5–20]; p=0.2), and there were no differences in the frequency of intravenous tPA administration, right hemisphere stroke, frequency of internal carotid artery occlusion, presentation ischemic core volume, perfusion lesion volume, ASPECTS, or time from last seen normal to qualifying imaging between these groups (Table 1). There were no differences in the frequency of good collaterals on the baseline CTA between RNI+ (79%; 19/24) and RNI− (73%; 29/40; p=0.55) groups.
Table 1:
Patient characteristics and stroke presentation details dichotomized by Rapid Neurologic Improvement.
| All Thrombectomy (N=92)* |
RNI+ (N=31) |
RNI− (N=60) |
p-value | |
|---|---|---|---|---|
| Median age (years), (IQR) | 70 (59–79) | 68 (61–76) | 71 (59–81) | 0.44 |
| Female sex, n (%) | 46 (50) | 16 (52) | 29 (48) | 0.77 |
| Ethnicity Hispanic, n (%) | 14 (15) | 3 (10) | 11 (18) | 0.37 |
| Race White, n (%) | 78 (85) | 25 (81) | 53 (88) | 0.35 |
| Hypertension, n (%) | 71 (77) | 23 (74) | 48 (80) | 0.53 |
| Hyperlipidemia, n (%) | 49 (53) | 17 (55) | 32 (53) | 0.89 |
| Atrial fibrillation, n (%) | 34 (37) | 10 (32) | 24 (40) | 0.45 |
| Diabetes mellitus, n (%) | 28 (30) | 8 (26) | 20 (33) | 0.46 |
| Prior stroke, n (%) | 13 (14) | 4 (13) | 9 (15) | 1.00 |
| Median presentation NIHSS (IQR) | 16 (10–20) | 17 (13–21) | 15.5 (9.5–20) | 0.23 |
| Treated with IV tPA, n (%) | 10 (11) | 3 (10) | 7 (12) | 1.00 |
| Occlusion site internal carotid artery, n (%) | 32 (35) | 13 (42) | 18 (30) | 0.26 |
| Good collaterals on qualifying CTA, n/total (%) | 48/64 (75) | 19/24 (79) | 29/40 (73) | 0.55 |
| Median ischemic core (IQR), mL | 9.4 (2.3–25.6) | 7.8 (2.0–16.8) | 9.9 (2.7–34.2) | 0.38 |
| Median perfusion lesion (IQR), mL | 114.7 (79.3–146.3) | 108.6 (82.6–181.5) | 114.7 (72.3–141.1) | 0.42 |
| Median time from stroke onset to qualifying imaging (IQR), hours | 10:29 (8:09–11:40) | 9:53 (8:12–11:30) | 10:39 (7:47–11:47) | 0.43 |
| Median ASPECTS (IQR) | 8 (7–9) | 8 (7–9) | 8 (7–9) | 0.30 |
| Right hemisphere stroke, n (%) | 43 (47) | 11 (35) | 32 (53) | 0.11 |
A single patient died without a 24-hour NIHSS being recorded and was excluded from the analysis.
Thrombectomy treatment details are presented in Table 2. Final TICI scores were more favorable in the RNI+ compared with the RNI− patients (p=0.04). Revascularization (TICI 2b/3) on cerebral angiography was achieved in 84% of RNI+ and 72% of RNI− patients, but these differences were not significant (p=0.2). After thrombectomy, median NIHSS at 24-hours was lower in RNI+ patients (5 [IQR, 1–7] versus 13 [IQR, 7.5–21] in RNI−; p=0.001). RNI+ patients had a smaller median infarct volume (21.1 ml [IQR, 4.9–31.5] versus 64.8 ml [IQR, 27.2–145.2]; p<0.001) and less infarct growth (8.2 ml [IQR, 1.1–17.5 ml] versus 36.9 ml [IQR, 15.9–104.8]; p<0.001). Reperfusion on the 24-hour post-randomization MR or CT perfusion studies occurred in 26 (93%) RNI+ and 33 (70%) RNI− patients (p=0.02), and reperfusion or complete revascularization 24-hours after randomization on MR or CT perfusion and angiography, was present in 26 (93%) of RNI+ and 39 (68%) of RNI− patients (p=0.01). There was no difference in the frequency of symptomatic hemorrhage after thrombectomy in these groups (Table 2).
Table 2:
Stroke treatment and outcome details.
| All Thrombectomy N=91* |
RNI+ N=31 |
RNI− N=60 |
p-value | |
|---|---|---|---|---|
| General anesthesia, n (%) | 26 (29) | 8 (26) | 18 (30) | 0.67 |
| Median time from stroke onset to groin puncture (IQR), hours | 11:27 (9:12–12:50) | 10:58 (9:16–12:33) | 11:42 (9:02–13:06) | 0.31 |
| Median time from baseline imaging to groin puncture (IQR), hours | 0:59 (0:39–1:27) | 0:53 (0:39–1:26) | 1:00 (0:41–1:29) | 0.41 |
| Median time from stroke onset to reperfusion (IQR), hours ψ | 12:16 (9:48–13:42) | 11:34 (9:48–13:15) | 12:34 (9:38–14:50) | 0.37 |
| Mean number thrombectomy passes (sd) | 2.2 (1.5) | 2.1 (1.2) | 2.3 (1.6) | 0.50 |
| Number single pass, n (%) (N=29 [RNI+] & 58 [RNI−]) | 32 (37) | 11 (38) | 21 (36) | 0.88 |
| Final TICI score, n (%) | 0.04 | |||
| 0 | 10 (11) | 1 (3) | 9 (15) | |
| I | 0 (0) | 0 | 0 | |
| IIa | 12 (13) | 4 (13) | 8 (13) | |
| IIb | 52 (57) | 15 (48) | 36 (60) | |
| III | 18 (20) | 11 (35) | 7 (12) | |
| Revascularization (TICI IIB/III), n (%) | 70 (76) | 26 (84) | 43 (72) | 0.20 |
| Reperfusion on 24-hour MR or CT perfusion**, n (%) (N=28 [RNI+] & 47 [RNI−]) | 59 (79) | 26 (93) | 33 (70) | 0.022 |
| Reperfusion on 24-hour MR or CT perfusion or complete revascularization on CTA/MRA, n (%) (N=28 [RNI+] & 57 [RNI−]) | 65 (76) | 26 (93) | 39 (68) | 0.014 |
| Median 24-hour NIHSS (IQR) | 9 (5–16) | 5 (1–7) | 13 (7.5–21) | <0.001 |
| Median infarct volume at 24 hours (IQR) | 35.0 (17.6–81.6) | 21.1 (4.9–31.5) | 64.8 (27.2–145.2) | <0.001 |
| Median infarct growth (IQR), mL | 23.4 (10.0–74.5) | 8.2 (1.1–17.5) | 36.9 (15.9–104.8) | <0.001 |
| SICH, n (%) | 6 (7) | 0 (0) | 5 (8) | 0.16 |
| Median mRS at 90 days (IQR) | 3 (1–4) | 2 (1–3) | 3.5 (2–5) | 0.001 |
| mRS 0–2 at 90 days, n (%) | 41 (45) | 19 (61) | 22 (37) | 0.03 |
| Hospital stay (including initial time at the transfer hospital), days | 6.5 (3.7–9.3) | 3.7 (2.9–7.1) | 7.4 (5.2–12.1) | <0.001 |
| Hospital stay (only enrolling hospital), days | 6.5 (3.7–9.2) | 3.7 (2.8–7.0) | 7.2 (5.2–12.1) | <0.001 |
| Favorable discharge location, n (%) | 61/91 (67) | 25/31 (81) | 37/60 (62) | 0.07 |
| Mortality, n (%) | 13 (14) | 1 (3) | 11 (18) | 0.05 |
TICI IIB/III reperfusion achieved in 69 patients who underwent thrombectomy and in 26 (large NIHSS shift group) and 43 (small NIHSS group) patients.
A single patient died without a 24-hour NIHSS being recorded and was excluded from the analysis.
24 hours refers to imaging performed 24 hours after randomization
RNI+ patients had a more favorable median mRS at 90 days (2 [IQR, 1–3]) compared to RNI− patients (3.5 [IQR, 2–5]; p=0.001) and were more likely to be functionally independent at 90 days (61% versus 37% in RNI−; p=0.03). Hospital length of stay was reduced in RNI+ patients (3.7 days [IQR 2.8–7.0] vs. 7.2 days [5.2–12.1]; p<0.001) (Table 2). There was a non-significant trend toward reduced mortality in RNI+ patients (3% versus 18% in RNI−; p=0.05).
In the primary outcome and secondary outcome regression analysis (Table 3), RNI was associated with better mRS shift at 90 days (OR, 3.8; 95% CI, 1.7–8.6; p=0.001), mRS 0–2 at 90 days (OR, 2.7; 95% CI, 1.1–6.7; p=0.03), and a reduced hospital length of stay at enrolling hospitals (OR 7.2, 95% CI, 2.5–20.7; p<0.001). After adjustment for presentation age, baseline NIHSS, blood glucose level, and time to randomization, RNI had an adjusted OR of 6.3 (95% CI, 2.7–15.2; p<0.001) for a favorable mRS shift at 90 days, 5.9 for mRS 0–2 at 90 days (95% CI, 1.6–21.3; p=0.01), and 14.4 for hospital length of stay ≤4 days (95% CI, 3.6–57.3; p=<0.001). RNI was not associated with mortality in the unadjusted (OR, 0.15; 95% CI, 0.02–1.2; p=0.08) or adjusted analysis (OR, 0.12; 95% CI, 0.01–1.2; p=0.07).
Table 3:
Logistic regression for primary and secondary outcome measures.
| OR (unadjusted) | 95% CI | p value | OR (adjusted) | 95% CI | p value | |
|---|---|---|---|---|---|---|
| Ordinal logistic regression for mRS shift at 90-days | ||||||
| Rapid Neurologic Improvement (RNI) | 3.8 | 1.7–8.6 | 0.001 | 6.3 | 2.7–15.2 | <0.001 |
| Logistic regression for mRS 0–2 at 90-days | ||||||
| Rapid Neurologic Improvement (RNI) | 2.7 | 1.1–6.7 | 0.027 | 5.9 | 1.6–21.3 | 0.01 |
| Logistic regression for hospital length of stay ≤4 days | ||||||
| Rapid Neurologic Improvement (RNI) | 7.2 | 2.5–20.7 | <0.001 | 14.4 | 3.6–57.3 | <0.001 |
| Logistic regression for mortality | ||||||
| Rapid Neurologic Improvement (RNI) | 0.15 | 0.02–1.21 | 0.075 | 0.12 | 0.01–1.23 | 0.07 |
Adjusted for presentation NIHSS, age, blood glucose, and time to randomization.
Discussion
We found that patients who experience RNI 24 hours following thrombectomy in late time windows are more likely to achieve a favorable ordinal mRS score and a favorable clinical outcome (mRS 0–2) at 90 days. Prior studies have identified RNI, which is also termed early neurologic improvement or dramatic neurologic improvement, as a marker of excellent long-term clinical outcomes following intravenous tPA administration12–14, 19 or endovascular stroke treatment8–10 within 0–6 hours of symptom onset. In the DEFUSE 3 study, RNI occurred in 34% of patients who underwent thrombectomy, which is similar to the reported 26–42% frequency of RNI in early time window studies9–11. This study is the first to identify RNI as an important early predictor of excellent long-term outcomes following thrombectomy in extended time windows.
In early time window studies, RNI has been associated with good pial collaterals10, 11, a small core infarct volume10, 11, and shorter time to reperfusion10, 11. By contrast, RNI was not associated with good collaterals, core infarction volume at presentation, or time to reperfusion in the DEFUSE 3 study. We hypothesize that the selection of patients with a target mismatch between core infarction and penumbra in DEFUSE 3 may account for these differences.
It is possible that the modern thrombectomy techniques (stent retrievers and/or aspiration thrombectomy) used in DEFUSE 3 might influence the likelihood of RNI. We identified a significant difference in TICI scores after thrombectomy between RNI+ and RNI− patients, and there was a non-significant trend toward greater revascularization rates in RNI+ patients (84% versus 72%). In addition, perfect revascularization (TICI III) was achieved in 35% of RNI+ patients compared to only 12% in RNI− patients. These findings also likely explain the higher percentage of cerebral reperfusion follow-up MR or CT perfusion studies in RNI+ patients (93% versus 73%). These differences likely account for the larger core infarct volumes and greater degree of core infarct growth at 24-hours post-randomization in RNI− patients.
Our observation that RNI was associated with a shorter hospital stay and favorable discharge location may have additional prognostic importance and implications for discharge planning following thrombectomy. RNI may also be a marker for decreased costs associated with stroke care, although we did not address cost issues in this study.
When we considered the entire DEFUSE 3 cohort, we found thrombectomy to be associated with substantial NIHSS improvement at 24-hours, discharge, 30-days, and 90-days. Interestingly, the largest difference in NIHSS between the endovascular and medical treatment groups was at 30-days (11.5 points on the NIHSS). However, patients treated by thrombectomy had NIHSS that were at least seven points lower at all time points examined, which is consistent with the large treatment effect of thrombectomy.
This study is limited by its small sample size for analysis.
Conclusion
RNI following thrombectomy correlates with favorable clinical and radiographic outcomes and a reduced hospital length of stay. RNI was an early favorable prognostic sign following late window thrombectomy in the DEFUSE 3 cohort.
Acknowledgements
The authors thank the DEFUSE 3 Primary Investigators and the National Institutes of Neurological Disorders and Stroke.
Sources of Funding
Funding was provided by grants from the National Institutes of Neurological Disorders and Stroke (U10NS086487 and U01NS092076).
Disclosures
MGL: Novo Nordisk, Genentech/Roche, Biogen and Moleac. Principal investigator on research grants from the NIH and from Neofect
GWA: iSchemaView, Inc (equity and consulting); Medtronic (consulting).
JJH: Medtronic (consulting); MicroVention (consulting), iSchemaView, Inc (Medical and Scientific Advisory Board)
SOG: Stryker Neurovascular (consulting); Medtronic (consulting)
Footnotes
Clinical Trial Registration
Unique Identifier
References
- 1.Berkhemer OA, Fransen PS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. The New England journal of medicine. 2015;372:11–20 [DOI] [PubMed] [Google Scholar]
- 2.Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. The New England journal of medicine. 2015;372:1019–1030 [DOI] [PubMed] [Google Scholar]
- 3.Jovin TG, Chamorro A, Cobo E, de Miquel MA, Molina CA, Rovira A, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. The New England journal of medicine. 2015;372:2296–2306 [DOI] [PubMed] [Google Scholar]
- 4.Saver JL, Goyal M, Bonafe A, Diener HC, Levy EI, Pereira VM, et al. Stent-retriever thrombectomy after intravenous t-pa vs. T-pa alone in stroke. The New England journal of medicine. 2015;372:2285–2295 [DOI] [PubMed] [Google Scholar]
- 5.Campbell BC, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. The New England journal of medicine. 2015;372:1009–1018 [DOI] [PubMed] [Google Scholar]
- 6.Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. The New England journal of medicine. 2018;378:11–21 [DOI] [PubMed] [Google Scholar]
- 7.Albers GW, Marks MP, Kemp S, Christensen S, Tsai JP, Ortega-Gutierrez S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. The New England journal of medicine. 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Christoforidis G, Mohammad YM, Khadir M, Yang M, Slivka AP. Factors associated with rapid neurological improvement 24 h following intra-arterial thrombolytic treatment for acute ischemic stroke. Journal of neurointerventional surgery. 2013;5:35–39 [DOI] [PubMed] [Google Scholar]
- 9.Prabhakaran S, Chen M, Choi JH, Mangla S, Lavine SD, Pile-Spellman J, et al. Major neurologic improvement following endovascular recanalization therapy for acute ischemic stroke. Cerebrovasc Dis. 2008;25:401–407 [DOI] [PubMed] [Google Scholar]
- 10.Jeong HS, Kwon HJ, Kang CW, Song HJ, Koh HS, Park SM, et al. Predictive factors for early clinical improvement after intra-arterial thrombolytic therapy in acute ischemic stroke. J Stroke Cerebrovasc Dis. 2014;23:e283–289 [DOI] [PubMed] [Google Scholar]
- 11.Cao Y, Wang S, Sun W, Dai Q, Li W, Cai J, et al. Prediction of favorable outcome by percent improvement in patients with acute ischemic stroke treated with endovascular stent thrombectomy. J Clin Neurosci. 2017;38:100–105 [DOI] [PubMed] [Google Scholar]
- 12.Brown DL, Johnston KC, Wagner DP, Haley EC Jr. Predicting major neurological improvement with intravenous recombinant tissue plasminogen activator treatment of stroke. Stroke; a journal of cerebral circulation. 2004;35:147–150 [DOI] [PubMed] [Google Scholar]
- 13.Saposnik G, Di Legge S, Webster F, Hachinski V. Predictors of major neurologic improvement after thrombolysis in acute stroke. Neurology. 2005;65:1169–1174 [DOI] [PubMed] [Google Scholar]
- 14.Nam HS, Lee KY, Han SW, Kim SH, Lee JY, Ahn SH, et al. Prediction of long-term outcome by percent improvement after the first day of thrombolytic treatment in stroke patients. Journal of the neurological sciences. 2009;281:69–73 [DOI] [PubMed] [Google Scholar]
- 15.Albers GW, Lansberg MG, Kemp S, Tsai JP, Lavori P, Christensen S, et al. A multicenter randomized controlled trial of endovascular therapy following imaging evaluation for ischemic stroke (defuse 3). International journal of stroke : official journal of the International Stroke Society. 2017;12:896–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tan IY, Demchuk AM, Hopyan J, Zhang L, Gladstone D, Wong K, et al. Ct angiography clot burden score and collateral score: Correlation with clinical and radiologic outcomes in acute middle cerebral artery infarct. AJNR. American journal of neuroradiology. 2009;30:525–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yeo LL, Paliwal P, Teoh HL, Seet RC, Chan BP, Ting E, et al. Assessment of intracranial collaterals on ct angiography in anterior circulation acute ischemic stroke. AJNR. American journal of neuroradiology. 2015;36:289–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zaidat OO, Yoo AJ, Khatri P, Tomsick TA, von Kummer R, Saver JL, et al. Recommendations on angiographic revascularization grading standards for acute ischemic stroke: A consensus statement. Stroke. 2013;44:2650–2663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alexandrov AV, Demchuk AM, Felberg RA, Christou I, Barber PA, Burgin WS, et al. High rate of complete recanalization and dramatic clinical recovery during tpa infusion when continuously monitored with 2-mhz transcranial doppler monitoring. Stroke. 2000;31:610–614 [DOI] [PubMed] [Google Scholar]