Abstract
Graft-versus-host disease (GVHD) is a life-threatening consequence of allogeneic haematopoietic stem cell transplantation, a curative therapy for haematological malignancies. The ATP-gated P2X7 receptor channel is implicated in the development of GVHD. P2X7 activity on human leukocytes can be influenced by gain-of-function (GOF) and loss-of-function (LOF) single nucleotide polymorphisms (SNPs) in the P2RX7 gene. In this study, the P2RX7 gene was sequenced in 25 human donors and the P2X7 activity on subsets of peripheral blood T cells, natural killer (NK) cells and monocytes was measured using an ATP-induced dye uptake assay. GOF and LOF SNPs representing 10 of the 17 known P2RX7 haplotypes were identified, and correlated with P2X7 activity on all leukocyte subsets investigated. Notably, invariant (i) NK T cells displayed the highest P2X7 activity amongst all cell types studied. To determine if donor P2X7 activity influenced the development of GVHD, immunodeficient NOD-SCID-IL2Rγnull (NSG) mice were injected with human peripheral blood mononuclear cells isolated from donors of either GOF (hP2X7GOF mice) or LOF (hP2X7LOF mice) P2RX7 genotype. Both hP2X7GOF and hP2X7LOF mice demonstrated similar human leukocyte engraftment, and showed comparable weight loss, GVHD clinical score and overall survival. Donor P2X7 activity did not affect human leukocyte infiltration or GVHD-mediated tissue damage, or the relative expression of human P2X7 or human interferon-γ (hIFNγ) in tissues. Finally, hP2X7GOF and hP2X7LOF mice demonstrated similar concentrations of serum hIFNγ. This study demonstrates that P2X7 activity correlates with donor P2RX7 genotype on human leukocyte subsets important in GVHD development, but does not affect GVHD development in a humanised mouse model of this disease.
Keywords: P2X7 receptor, P2RX7 gene single nucleotide polymorphism, Purinergic receptor, Xenogeneic graft-versus-host disease, Bone marrow transplantation, Leukocyte, Humanised mice
Introduction
Allogeneic haematopoietic stem cell transplantation (allo-HSCT) is one of the few established curative therapies for life-threatening haematological malignancies, which aims to eliminate host tumour cells using myeloablative therapy and transplanted donor leukocytes [1]. However, donor leukocytes can also trigger inflammatory damage against healthy host tissues, resulting in graft-versus-host disease (GVHD) [2]. The initial source of inflammatory damage that promotes GVHD is often the myeloablative regime used to treat the haematological malignancy [3]. This promotes activation of GVHD effector leukocytes which release pro-inflammatory cytokines, such as interferon-γ (IFNγ), that enhance GVHD development [4]. GVHD remains a problem in about half of allo-HSCT patients due to ineffective therapies to treat or prevent this disease [5], and the absence of validated biomarkers to predict GVHD onset [6]. Recently, donor single nucleotide polymorphisms (SNPs) in genes important in inflammation have been recognised as possible risk factors for GVHD [7, 8]. Donor SNPs may have potential as future GVHD biomarkers, but these SNPs are not currently screened in allo-HSCT donors. Combined, this highlights a need for new GVHD biomarkers and therapeutics.
The purinergic signalling cascade is a key mediator of the inflammatory immune response, and has an emerging role in the development of GVHD through activation of the P2X7 receptor, an ATP-gated cation channel [9]. P2X7 activation by extracellular ATP, which can be released during tissue damage, initiates the release of pro-inflammatory cytokines which mediate cell death and proliferation, and can trigger inflammatory tissue damage [10]. P2X7 blockade can reduce GVHD severity in allogeneic mouse models [11–13], and reduce concentrations of serum human IFNγ in humanised mice with GVHD [14]. This indicates an important role for P2X7 in mouse models of GVHD, but there are limited studies investigating its effects on GVHD development in humans.
In humans, P2X7 is predominantly expressed on leukocytes, with the highest expression on monocytes followed by natural killer (NK) cells, B cells and T cells [15, 16]. P2X7 activation causes formation of a pore that allows the flux of organic cations including fluorescent dyes such as YO-PRO-12+, providing a method to reliably quantify P2X7 activity on human leukocytes with minimal variation [17, 18]. P2X7 activation on these cells can drive their differentiation into many effector subsets, which can have important roles in GVHD. For example, P2X7 activation on naive CD4+ T cells can increase expression of IFNγ, interleukin (IL)-17 and IL-6, which collectively promote T helper (Th) 17 cell responses [19] that are key in GVHD progression [20]. Moreover, P2X7 activation also impacts regulatory T cells (Tregs), which limit GVHD development [21], by potentially regulating Treg numbers [22] and their suppressive functions [23].
One of the biggest influences on human P2X7 activity is SNPs in the human P2RX7 gene. To date, 16 missense P2RX7 SNPs have been identified which can cause either a gain-of-function (GOF) or loss-of-function (LOF) in human P2X7 [24]. Notably, four of these SNPs originally defined five haplotype variants (denoted P2X7–1 to 5) which give rise to altered P2X7 activity [25, 26]. Subsequently, analysis of a further seven SNPs revealed that these five haplotypes can be segregated into 17 haplotypes (H1 to H17) [27]. Notably, some P2RX7 SNPs are associated with various inflammatory diseases such as multiple sclerosis [28] and rheumatoid arthritis [29]. Moreover, the LOF SNP rs3751143 (E496A) in either donors or recipients is associated with poorer clinical outcomes in HSCT [30], although this finding was not supported by a larger study [31]. Therefore, further investigation into the role of P2RX7 SNPs in GVHD is warranted. The current study investigated the effect of human donor P2RX7 genotype on P2X7 activity on leukocyte subsets important in GVHD, and whether altered P2X7 activity affects the development of GVHD in a pre-clinical humanised mouse model of this disease.
Materials and methods
Antibodies
R-Phycoerythrin (PE) conjugated mouse anti-human (h) CD4 (clone RPA-T4) and mouse anti-hCD25 (clone M-A251) monoclonal antibodies (mAb); PE-cyanine 7 (PE-Cy7) conjugated mouse anti-hCD8 (clone RPA-T8), mouse anti-hCD56 (clone B159) and mouse anti-hCD3 (clone SK7) mAb; peridinin chlorophyll protein-cyanine 5.5 (PerCP-Cy5.5) conjugated mouse anti-hCD4 (clone SK3), mouse anti-hCD16 (clone 3G8) and rat anti-mouse (m) CD45 (clone 30-F11) mAb; allophycocyanin (APC) conjugated mouse anti-hCD3 (clone UCHT1), mouse anti-hCD45 (clone HI30) and mouse anti-hCD19 (clone HIB19) mAb; Brilliant Violet 711 (BV711) conjugated mouse anti-hCD3 (clone UCHT1) mAb; Brilliant Violet 421 (BV421) conjugated mouse anti-hCD127 (clone HL-7R-M21) and mouse anti-hCD14 (clone MΦPg) mAb were obtained from BD Biosciences (San Jose, USA). PE-Cy7 conjugated mouse anti-hVα24-Jα18 (clone 6B11) mAb was obtained from BioLegend (San Diego, CA, USA).
Mice
Experiments involving mice were approved by the University of Wollongong Animal Ethics Committee (Wollongong, Australia). Female NOD-SCID-IL2Rγnull (NSG) mice (aged 6–8 weeks) were obtained from Australian BioResources (Moss Vale, Australia) and housed in individually ventilated cages (Techniplast; Buguggiate, Italy) with a 12-h light/12-h dark cycle. Mice were provided with autoclaved food and water, ad libitum.
Isolation of genomic DNA
Experiments involving human blood were approved by the University of Wollongong Human Ethics Committee (Wollongong, Australia). Whole blood was collected from consenting donors, by venepuncture, into VACUETTE® lithium heparin tubes (Greiner Bio-One; Frickenhausen, Germany). Genomic DNA was isolated from peripheral blood using a Wizard® Genomic DNA Isolation Kit (Promega; Madison, USA) as per the manufacturer’s instructions. DNA was stored at − 20 °C. Blood was collected from a total of 25 healthy adult donors (range 20–44 years, mean 28 years; 12 males and 13 females; 24 Caucasian and 1 Asian).
P2RX7 gene sequencing
Exons 1–13 of the human P2RX7 gene were amplified and sequenced by PCR [32] using 12 primer pairs (Invitrogen; Carlsbad, USA) as originally described [33]. PCR was conducted using Mango Taq DNA polymerase (Bioline; London, UK) with an initial denaturation step of 94 °C for 1 min followed by 35 cycles of denaturation at 94 °C for 30 s, annealing at 60 °C for 30 s (for exons 1, 2 and exons 4–13) or 55 °C for 30 s (for exon 3) and extension at 72 °C for 1 min, before a final extension for 5 min at 72 °C using a GeneAmp PCR System 9700 (Applied Bio Systems; Carlsbad, USA). Amplicons were visualised using a 1.5% v/v Tris acetate EDTA (TAE) (20 mM acetic acid, 1 mM EDTA, 40 mM Tris, pH 8) agarose gel stained with GelRed™ (Biotium; Hayward, USA). Amplicons for exons 1–12 for 23 donors were purified using ExoSAP-IT enzyme (Invitrogen) before being sequenced with the above primers and the ABI PRISM BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems) as per the manufacturer’s instructions, using an Applied Biosystems 2130xl Genetic Analyzer. For two donors, amplicons for exons 1–12 were purified using the QIAquick® Purification Kit (Qiagen; Venlo, Netherlands) as per the manufacturer’s instructions, and sequenced with the above primers by the Australian Genome Research Facility (AGRF) (Melbourne, Australia). For all donors, exon 13 was sequenced by AGRF using samples excised from TAE agarose gels using a QIAquick ® Gel Extraction Kit (Qiagen) as per the manufacturer’s instructions. Sequences were analysed using Chromas Pro software (Technelysium; Brisbane, Australia) by comparing to the NCBI wild-type reference sequence for the human P2RX7 gene (NG_011471.2).
Isolation of human peripheral blood mononuclear cells
Donor peripheral blood was diluted in an equal volume of sterile Dulbecco’s phosphate buffered-saline (PBS) (Thermo Fisher Scientific; Waltham, USA), and human peripheral blood mononuclear cells (hPBMCs) isolated by density-gradient centrifugation (560×g for 30 min) using Ficoll-Paque PLUS (GE Healthcare; Uppsala, Sweden). hPBMCs were washed twice in two volumes of PBS (300×g for 10 min), resuspended in PBS and counted using an improved bright line haemacytometer (Boeco Neubauer; Hamburg, Germany).
ATP-induced YO-PRO-12+ uptake assay
P2X7 activity on human leukocyte subsets was quantified using an ATP-induced YO-PRO-12+ uptake assay, adapted from Spildrejorde et al. [32]. hPBMCs were resuspended at 1 × 106 cells/mL in NaCl medium (145 mM NaCl, 5 mM KCl, 5 mM glucose and 10 mM HEPES, pH 7.5). Cells were pre-incubated at 37 °C for 5 min before being incubated with 1 μM YO-PRO-1 iodide (Molecular Probes; Eugene, USA) in the absence or presence of 1 mM ATP (Sigma-Aldrich; St. Louis, USA) for 5 min. Reactions were stopped with 1 mL ice-cold NaCl medium containing 20 mM MgCl2 and centrifugation (300×g for 3 min). Cells were washed in PBS (300×g for 3 min), incubated with fluorochrome-conjugated mAb at RT for 15 min and washed in PBS (300×g for 3 min). In some experiments, hPBMCs were incubated in the absence or presence of 60 μM 2-(phenylthio)-N-[[tetrahydro-4-(4-phenyl-1-piperazinyl)-2H-pyran-4-yl]methyl-3 pyridinecarboxamide (JNJ-47965567) (Tocris Bioscience, Bristol, UK) at 37 °C 15 min prior to the addition of YO-PRO-12+. Data was collected using calibration beads (Spherotech; Lake Forest, USA) and a BD Bioscience LSR Fortessa X-20 flow cytometer (using the band pass filters 450/50 for BV421, 710/50 for BV711, 515/20 for YO-PRO-12+, 586/15 for PE, 780/60 for PE-Cy7, 670/30 for APC and 695/40 for PerCP) and BD Biosciences FACSDiva software v8.0. Geometric mean fluorescence intensity of YO-PRO-12+ uptake was analysed with FlowJo software v8.7.1 (TreeStar Inc.; Ashland, USA). ATP-induced YO-PRO-12+ uptake (P2X7 activity) was determined as the difference in YO-PRO-12+ in the presence and absence of ATP from duplicate samples. P2X7 activity was determined prior to P2RX7 genotyping. Donor numbers used to determine P2X7 activity in different immune cell subsets varied due to availability of mAb at the time of experiments.
Humanised mouse model of GVHD
A humanised mouse model of GVHD was used as described by Geraghty et al. [14]. NSG mice were injected intra-peritoneally (i.p.) with 10 × 106 hPBMCs, isolated from either GOF or LOF P2RX7 genotype donors (matched for age and sex), in 200 μL sterile PBS. These mice were subsequently classified as hP2X7GOF or hP2X7LOF mice, respectively. Mice were checked for the engraftment of human cells at 3 weeks post-injection by immunophenotyping tail blood. Mice were assessed in a blinded fashion for clinical signs of GVHD for 10 weeks post-injection using a monitoring system that scored 5 criteria (weight loss, hunched posture, decreased activity, fur ruffling and skin scaling) out of 3 (total score out of 15) (Table 1). Mice were euthanised if they demonstrated a score of 3 in any category (excluding fur ruffling), exhibited a total score ≥ 10, displayed ≥ 10% weight loss over a 7-day period or survived 10 weeks post-injection, according to the approved animal ethics protocol. At euthanasia, samples of spleens were collected for immunophenotyping. Samples of spleen, liver, skin and small intestine were stored in either RNAlater (Sigma-Aldrich) at − 20 °C for quantitative real-time PCR (qPCR) analysis, or fixed overnight in neutral buffered (10%) formalin (Sigma-Aldrich) and embedded in paraffin for histological analysis. Serum was isolated from mouse blood collected by cardiac puncture, following incubation at RT for 1 h and centrifugation (1700×g for 10 min). Sera were stored at − 80 °C.
Table 1.
Clinical scoring criteria for the assessment of GVHD in humanised NSG mice. NSG mice, injected (i.p.) with 10 × 106 hPBMCs, were monitored for GVHD development by scoring for weight loss, posture, activity, fur loss and skin integrity for up to 10 weeks post-PBMC injection
| Score1 | 0 | 1 | 2 | 3 |
|---|---|---|---|---|
| Weight loss 2, 3 | < 5% | 5 to 9.9% | 10 to 15% | > 15% |
| Hunched posture | Normal | Hunching noted at rest only | Hunching noted at rest and movement | Severe hunching |
| Activity | Normal | Mild to moderately decreased | Stationary unless stimulated | Permanently stationary |
| Fur loss | Normal | Mild to moderate ruffling | Severe ruffling and hair loss (thinning) | Areas of hair loss (balding) |
| Skin integrity | Normal | Scaling of paws and/or tail | Scaling at additional areas | Areas of denuded skin (loss of surface layers) |
1Mice were euthanised if they scored 3 in any category (excluding fur) or at a total score ≥ 10
2Weight loss determined as percentage loss from starting weight (weight on day 0)
3Acute weight loss determined as > 10% weight loss over any 7-day period and assigned a score of 3 for weight
Immunophenotyping
Immunophenotyping was performed on cells isolated from mouse tail blood at 3 weeks post-injection and on splenocytes from euthanised mice. Tail blood was collected via tail snip into 200 μL of citrate solution (Sigma-Aldrich). Blood was diluted with PBS and centrifuged (300×g for 5 min). Mouse spleens were dissociated in PBS, passed through a 70-μm nylon filter (Falcon Biosciences; New York, USA) and centrifuged (300×g for 5 min). Cells were incubated in ammonium chloride potassium lysis buffer (150 mM NH4Cl, 1 mM KHCO3, 0.1 mM Na2CO3, pH 7.3) for 5 min at RT, washed twice in PBS containing foetal bovine serum (Bovogen Biologicals; Keilor East, Australia) (300×g for 5 min) and incubated with fluorochrome conjugated mAb for 15 min at RT. Cells were washed in PBS (300×g for 3 min) and data were collected using a BD LSR Fortessa X-20 flow cytometer (using the band pass filters 450/50 for BV421, 710/50 for BV711, 586/15 for PE, 780/60 for PE-Cy7, 670/30 for APC and 695/40 for PerCP) and FACSDiva software v8.0. Cell populations were analysed using FlowJo software v8.7.1.
Histological analysis
Embedded tissue was sectioned (5 μm) and stained with haematoxylin and eosin (POCD; Artarmon, Australia) as previously described [14]. Histology was assessed using a Leica (Wetzlar, Germany) DM750 inverted light microscope at × 10 objective and images captured using a Leica ICC50 HD camera and processed using Leica application suite software version 4.7.
RNA isolation
RNA was isolated, from tissue stored in RNAlater, using TRIzol reagent (Thermo Fisher Scientific) as per the manufacturer’s instructions. RNA was converted to complementary DNA (cDNA) using a qScript cDNA Synthesis Kit (Quanta Biosciences, Beverly, USA) as per the manufacturer’s instructions and stored at − 80 °C. cDNA was checked by PCR using primers (Invitrogen) to the housekeeping gene GAPDH (forward primer 5′-TGCACCACCAACTGCTTAGC-3′; reverse primer 5′–GGAAGGCCATGCCAGTGA-3′). PCR was performed with an initial cycle of 95 °C followed by 35 cycles of 95 °C for 1 min, 55 °C for 1 min and 72 °C for 1 min and a final extension of 72 °C for 10 min. Amplicons were visualised with a 1.5% TAE agarose gel stained with GelRed™.
Quantitative real-time PCR
Quantitative real-time PCR (qPCR) was performed on cDNA using TaqMan Universal Master Mix II (Thermo Fisher Scientific) according to the manufacturer’s instructions. qPCR was conducted with FAM™-labelled human IFNG (Hs00989291_m1) or human P2X7B (AIOIXC2) primers, with VIC®-labelled human HPRT-1 (Hs99999909_m1) primers used for this housekeeping gene (Thermo Fisher Scientific). qPCR cycles consisted of an initial step of 50 °C for 2 min, a subsequent 50 °C step for 10 min and 50 cycles of 95 °C for 15 s and 60 °C for 1 min. Reactions were conducted in triplicate using a Roche Diagnostics (Indianapolis, USA) LightCycler 480 with LightCycler480 software v1.5.1. Gene expression was quantified using the ΔΔCt method and was calculated relative to the gene expression in cDNA generated from freshly isolated hPBMCs from one randomly selected individual.
ELISA
The concentration of serum hIFNγ was measured using an IFNγ Human Uncoated ELISA Kit (Thermo Fisher Scientific) as per the manufacturer’s instructions. Absorbance (570 nm and 450 nm) was measured using a SpectraMax Plus 384 (Molecular Devices; Sunnyvale, USA).
Statistical analysis
All data is given as mean ± standard error of the mean (SEM). Statistical differences between means were calculated using a Student’s t test for single comparisons or a one-way analysis of variance (ANOVA) with Tukey’s post hoc test for multiple comparisons. Comparisons of mouse weight and clinical score over time were completed using a two-way ANOVA. Mouse survival was analysed using a log-rank (Mantel-Cox) test. All statistical analyses and graphs were generated using GraphPad Prism software version 7.0 (GraphPad Software, La Jolla, USA). For all analysis, differences were considered significant if P < 0.05.
Results
P2RX7 genotype varies between donors
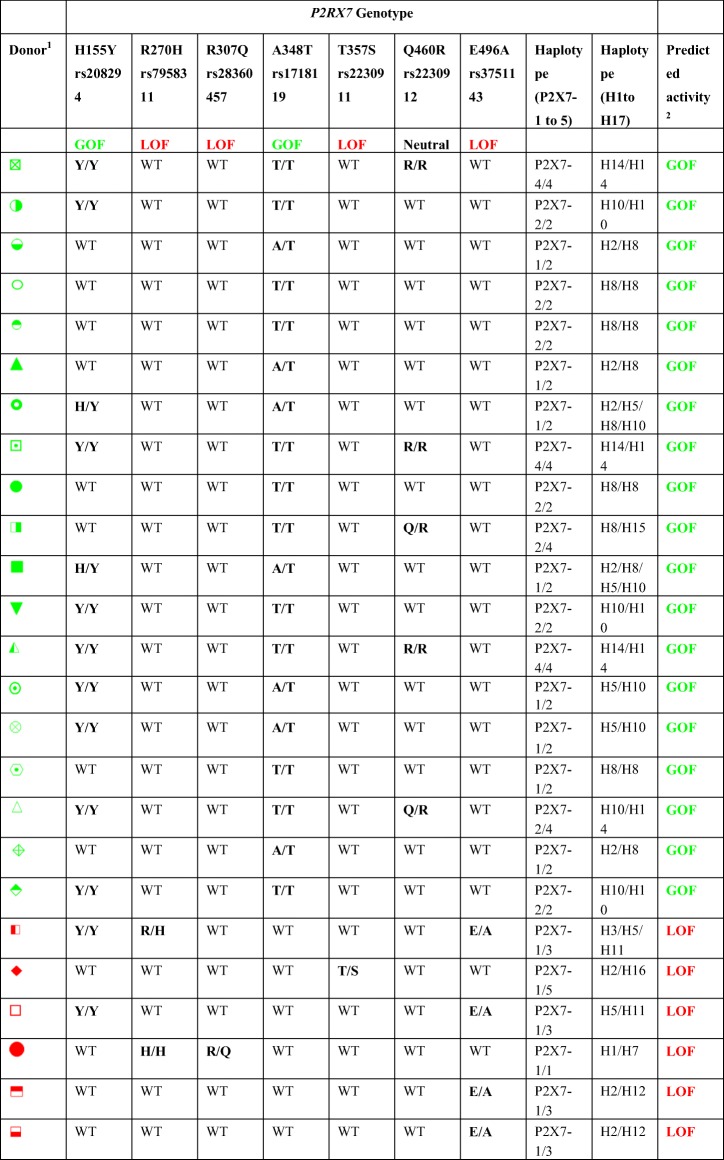
The P2RX7 genotype and haplotype, as originally described by Stokes et al. [25] (P2X7–1 to 5) and Jørgensen et al. [27] (H1 to H17), as well as the predicted P2X7 activity of 25 randomly selected human donors, are listed in Table 2. As expected, P2RX7 haplotype varied between donors. In relation to haplotypes H1 to H17, four donors were homozygous for H8 and another three homozygous for H14. Exact haplotypes could be assigned to 22 of the 25 donors, but exact haplotypes could not be assigned in the remaining three donors. Donors with one wild-type allele were defined as GOF or LOF based on their mutant allele. No donors demonstrated mixed GOF or LOF haplotypes. The presence of different GOF and LOF P2RX7 SNPs in each donor allowed their P2X7 activity to be predicted. Nineteen donors were predicted to have GOF P2X7 activity and six predicted to have LOF P2X7 activity.
Table 2.
P2RX7 genotype and predicted P2X7 activity of healthy human donors. Genomic DNA was isolated from whole blood of 25 human donors. Donor P2RX7 genotype was determined by sequencing the 13 exons in the human P2RX7 gene using exon-specific primers. Donors were classed into P2RX7 haplotypes based on the presence or absence of different P2RX7 SNPs according to Stokes et al. [25] (P2X7–1 to 5) and Jørgensen et al. [27] (H1-H17). Predicted P2X7 activity is based on the P2RX7 SNPs found in each donor
P2RX7 genotype correlates with P2X7 activity on human immune cell subsets
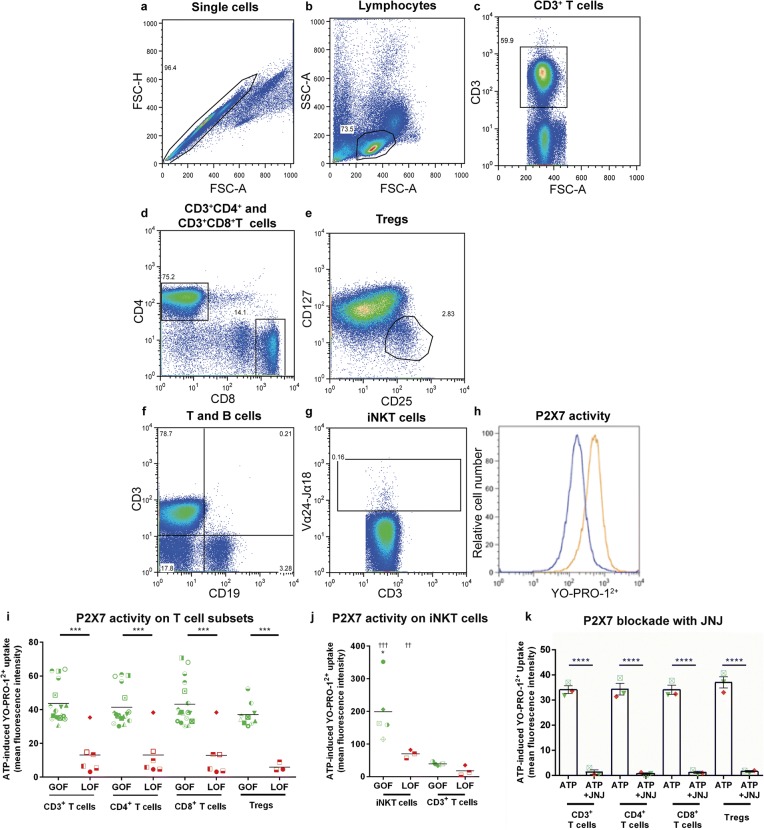
P2X7 activity on human CD3+ T cells [15], CD4+ and CD8+ T cells [34] and classical, non-classical and intermediate monocytes [35] has been previously assessed; however, there are limited studies on P2X7 activity on human NK cell subsets [15], Tregs [23, 36] or invariant (i) NKT cells. Moreover, correlation between various P2RX7 genotypes and P2X7 activity on these leukocyte subsets is lacking. Therefore, P2X7 activity on these cells, from randomly selected donors, was determined by flow cytometric measurements of ATP-induced YO-PRO-12+ uptake [32]. P2X7 activity was measured on CD3+ T cells, CD3+CD4+ and CD3+CD8+ T cells, CD3+CD4+CD25+CD127lo Tregs and CD3+CD19−Vα24−Jα18+ iNKT cells using a defined gating strategy (Fig. 1 a–h) and was found to be similar between CD3+ T cells, CD3+CD4+ and CD3+CD8+ T cells and Tregs in each donor (P > 0.05) (Fig. 1 i). Moreover, donor P2RX7 genotype corresponded with P2X7 activity on these cells as ATP-induced YO-PRO-12+ uptake in GOF donors was significantly greater compared to corresponding LOF donors in each T cell subset (P < 0.0001). This was also true in iNKT cells, where GOF donors demonstrated significantly higher P2X7 activity than LOF donors (P < 0.05). Notably, P2X7 activity on iNKT cells was significantly higher than on CD3+ T cells in each corresponding donor analysed, regardless of P2RX7 genotype (P < 0.005) (Fig. 1 j).
Fig. 1.
Donor P2X7 activity correlates with P2RX7 genotype on human T cell subsets. a-k hPBMCs, from donors of either GOF or LOF P2RX7 genotype, resuspended in NaCl medium were incubated with 1 μM YO-PRO-12+ in the absence or presence of 1 mM ATP for 5 min at 37 °C. YO-PRO-12+ uptake into human T cell subsets was analysed by flow cytometry using the gating strategy shown. a Single cells were gated by forward scatter area (FSC-A) and height (FSC-H). b Viable single lymphocytes were gated using FSC-A and side scatter (SSC-A). Single viable cells were then used to identify c CD3+ T cells, d CD3+CD4+ and CD3+CD8+ T cells and e CD3+CD4+CD25+CD127lo Tregs. Single, viable cells were also used to gate f CD3+CD19− cells to subsequently identify g CD3+Vα24−Jα18+ iNKT cells. h P2X7 activity was determined by comparing YO-PRO-12+ uptake in the absence (blue histograms) and presence (orange histogram) of ATP; histograms for CD3+ T cells are shown as examples. P2X7 activity in i CD3+, CD3+CD4+ and CD3+CD8+ T cells and Tregs was determined in multiple donors and P2X7 activity in j iNKT cells and corresponding CD3+ T cells was determined in some donors. k hPBMCs from three donors were pre-incubated with 60 μM JNJ-47965567 (JNJ) at 37 °C for 15 min to block P2X7 before incubation in the absence or presence of 1 mM ATP. Symbols correlate to symbols in Table 2 and represent i–j mean values of duplicate ATP-induced YO-PRO-12+ uptake measurements into CD3+, CD3+CD4+, CD3+CD8+ (GOF, n = 19; LOF, n = 6), Tregs (GOF, n = 11; LOF, n = 3) and iNKT cells (GOF, n = 5; LOF, n = 3); *P < 0.05, ***P < 0.001 compared to corresponding LOF donors; ††P < 0.01, †††P < 0.001, compared to corresponding CD3+ T cells. k Data represents group means ± SEM (n = 3), ****P < 0.0001 compared to hPBMCs incubated with ATP and JNJ. GOF, gain-of-function; iNKT cell, invariant natural killer T cell; LOF, loss-of-function; Tregs, regulatory T cells
Human T cells and Tregs are the main mediators [37, 38] and regulators of GVHD [39] in humanised NSG mice, respectively. Therefore the role of P2X7 in ATP-induced YO-PRO-12+ uptake was assessed in these cells using the antagonist JNJ-47965567, which blocks P2X7 but not other P2X subtypes [40] present on T cells [41, 42]. P2X7 blockade with JNJ-47965567 caused a significant reduction in ATP-induced YO-PRO-12+ uptake in CD3+ T cells (98.7% inhibition), CD4+ T cells (99.4% inhibition), CD8+ T cells (98.4% inhibition) and Tregs (94.69% inhibition) (P < 0.0001 for all T cell subsets) (Fig. 1 k). These results demonstrate that P2X7 is the major P2X subtype mediating ATP-induced YO-PRO-12+ uptake in these T cell subsets.
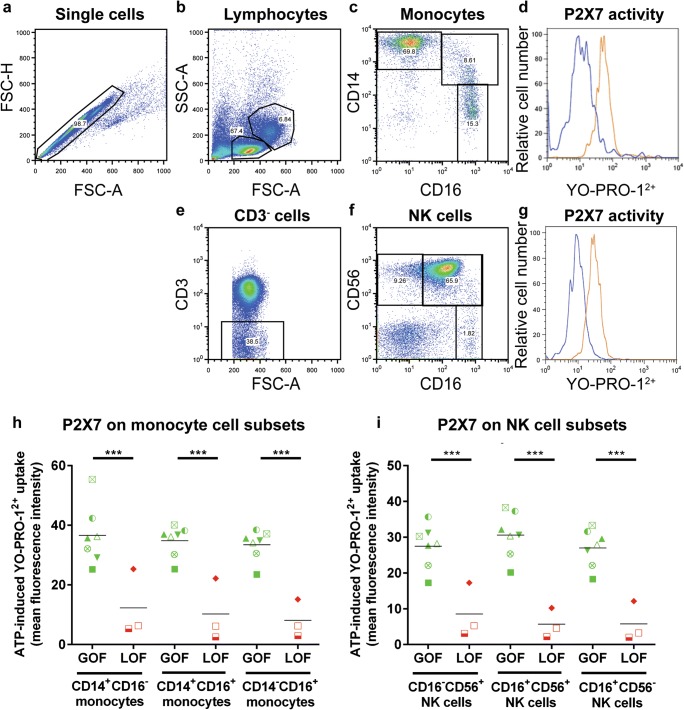
P2X7 activity was then measured on classical (CD14+CD16−), non-classical (CD14−CD16+) and intermediate (CD14+CD16+) monocytes using a defined gating strategy (Fig. 2 a–d). In each donor, all monocyte subsets demonstrated similar P2X7 activity (P > 0.05). This activity corresponded to donor P2RX7 genotype, with GOF donors demonstrating significantly greater ATP-induced YO-PRO-12+ uptake than corresponding monocyte subsets from LOF donors (P < 0.0001) (Fig. 2 h).
Fig. 2.
Donor P2X7 activity correlates with P2RX7 genotype on human monocyte subsets and NK cell subsets. a–i hPBMCs, from donors of either GOF or LOF P2RX7 genotype, in NaCl medium were incubated with 1 μM YO-PRO-12+ in the absence or presence of 1 mM ATP for 5 min at 37 °C. YO-PRO-12+ uptake into human monocyte and NK cell subsets was analysed by flow cytometry using the gating strategy shown. a Single cells were gated by forward scatter area (FSC-A) and height (FSC-H) and b viable cells gated using FSC-A and side scatter (SSC-A). Single viable cells were then used to identify c CD14+CD16−, CD14+CD16+ or CD14−CD16+ monocyte subsets. Single, viable cells were used to gate e CD3− cells to subsequently identify f CD16+CD56−, CD16+CD56+ or CD16−CD56+ NK cell subsets. d, g P2X7 activity on these cells was determined by comparing YO-PRO-12+ uptake in the absence (blue histograms) and presence (orange histogram) of ATP; histograms for d CD14+CD16− monocytes and g CD16−CD56+ NK cells are shown as examples. h, i P2X7 activity in h monocyte subsets and i NK cell subsets was determined in multiple donors. Symbols correlate to Table 2 and represent mean values of duplicate ATP-induced YO-PRO-12+ uptake tests from different donors (GOF, n = 7; LOF, n = 3); ***P < 0.001 compared to corresponding LOF donors. GVHD, graft-versus-host disease; GOF, gain-of-function; NK cell, natural killer cell; LOF, loss-of-function
Finally, P2X7 activity was measured on CD56+CD16−, CD56−CD16+ and CD56+CD16+ NK cell subsets using a defined gating strategy (Fig. 2 a, b, e–g). Again, no difference in P2X7 activity was observed between any subset in each donor (P > 0.05) (Fig. 2 i). P2X7 activity also corresponded with donor P2RX7 genotype on these cells, with GOF donors demonstrating significantly higher ATP-induced YO-PRO-12+ uptake than corresponding NK cell subsets from LOF donors (P < 0.001) (Fig. 2 i).
Notably, one LOF donor of the H2/H16 haplotype demonstrated P2X7 activity comparable to GOF donors in all immune cell subsets analysed. This donor was heterozygous for the T357S SNP which is a partial LOF allele [43], and as such may be less dominant than the wild-type allele, thus accounting for their higher than expected P2X7 activity. Alternatively, the effect of this LOF SNP may have been confounded by the presence of a GOF SNP outside of the P2RX7 coding region.
hP2X7GOF and hP2X7LOF mice demonstrate similar engraftment of human leukocytes
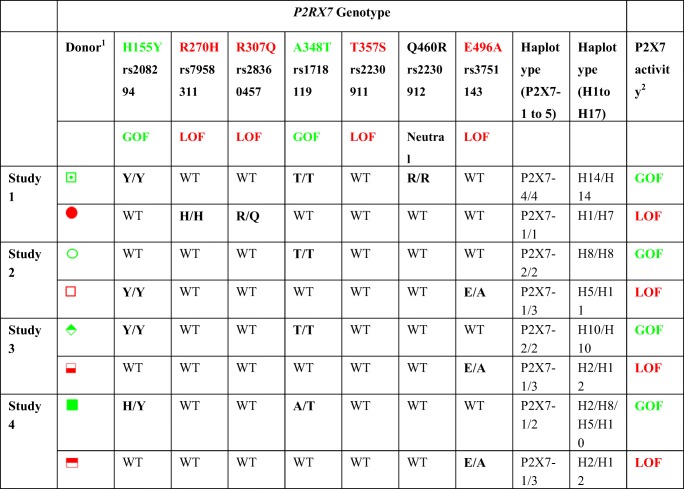
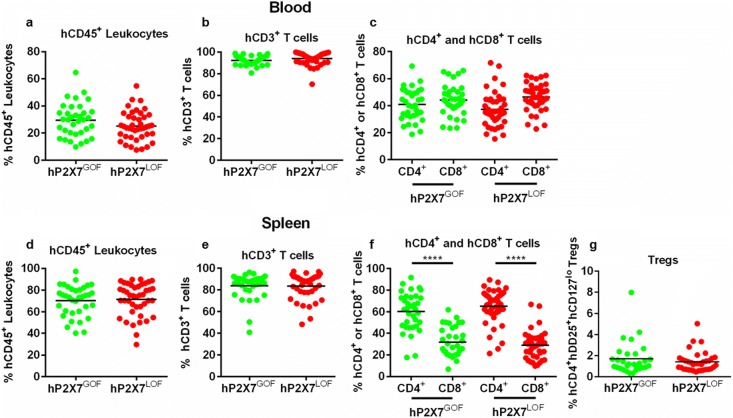
Injection (i.p.) of hPBMCs into NSG mice is a well-established pre-clinical model of GVHD [37]. To determine if human donor P2RX7 genotype effects the development of GVHD in this model, NSG mice were injected with hPBMCs isolated from either GOF (hP2X7GOF mice) or LOF (hP2X7LOF mice) P2RX7 genotype donors (n = 4 donors per genotype) (Table 3) and monitored for GVHD for up to 10 weeks. These eight donors were part of the original donor cohort (Table 2). Donor P2RX7 genotype did not affect the engraftment of human leukocytes or T cell subsets in NSG mice (Fig. 3 a–g). At 3 weeks post-hPBMC injection, comparable frequencies of human leukocytes were observed between hP2X7GOF mice (28.67 ± 2.30% hCD45+ mCD45− cells, n = 30) and hP2X7LOF mice (24.49 ± 1.78% hCD45+ mCD45− cells, n = 37) (P = 0.1495) (Fig. 3 a). The majority of these human leukocytes were T cells, the frequency of which was similar between the two groups (hP2X7GOF 92.31 ± 0.73% hCD3+ hCD19− cells, n = 30; hP2X7LOF 94.6 ± 0.90% hCD3+ hCD19− cells, n = 37) (P = 0.0776) (Fig. 3 b). Both hP2X7GOF mice and hP2X7LOF mice were also engrafted with similar frequencies of hCD4+ T cells (40.71 ± 2.14%, n = 30 vs. 41.36 ± 1.68%, n = 37, respectively) (P = 0.8102) and hCD8+ T cells (43.43 ± 2.20%, n = 30 vs. 45.83 ± 1.67%, respectively, n = 37) (P = 0.3792). There were no significant differences between the percentages of hCD4+ and hCD8+ T cells in hP2X7GOF mice (P = 0.3803) or hP2X7LOF mice (P = 0.0625) (Fig. 3 c).
Table 3.
P2RX7 genotype and P2X7 activity of paired human donors for a humanised mouse model of GVHD. Each donor pair with opposing P2RX7 genotypes were matched for sex and age. Donor P2RX7 genotype was sequenced from genomic DNA by sequencing 13 exons in the human P2RX7 gene. Donors were classed into P2RX7 haplotypes based on the presence or absence of different P2RX7 SNPs according to Stokes et al. [25] (P2X7–1 to 5) and Jørgensen et al. [27] (H1-H17). Loss or gain of P2X7 activity in CD3+, CD4+ and CD8+ T cells was confirmed by flow cytometric measurements of ATP-induced YO-PRO-12+ uptake prior to mouse studies
1Symbols represent individual donors in Figs. 1 and 2
2P2X7 activity was confirmed in T cells by flow cytometry of ATP-induced YO-PRO-12+ uptake
GOF, gain-of-function; LOF, loss-of-function
Colours represent P2X7 activity: green, GOF; red, LOF
All donors were wild type for unlisted non-synonymous P2RX7 SNPs
Fig. 3.
Engraftment of human leukocytes is similar in hP2X7GOF and hP2X7LOF mice. NSG mice were injected (i.p.) with 10 × 106 hPBMCs isolated from donor of either GOF (hP2X7GOF mice) or LOF (hP2X7LOF) P2RX7 genotype (four donors per group). hP2X7GOF and hP2X7LOF mice were checked for the engraftment of human leukocytes in a–c the blood at 3 weeks post-injection and d–g spleen at endpoint. a, d hCD45+ leukocytes are expressed as a percentage of total mCD45+ and hCD45+ leukocytes. b, e hCD3+ cells are expressed as a percentage of total hCD45+ leukocytes. c, f hCD4+ and hCD8+ cells are expressed as a percentage of hCD3+ cells. g hCD4+hCD25+ hCD127lo Tregs are expressed as a percentage of CD4+ T cells. Data is presented as a group mean ± SEM; symbols represent individual mice; ****P < 0.0001 compared to hCD8+ T cells. GOF, gain-of-function; LOF, loss-of-function
At endpoint, human leukocytes constituted the bulk of the total leukocyte population in spleens of both hP2X7GOF mice (69.73 ± 2.59%, hCD45+ mCD45− cells, n = 30) and hP2X7LOF mice (71.36 ± 2.51%, hCD45+ mCD45− cells, n = 37) with comparable levels of engraftment (P = 0.6550) (Fig. 3 d). T cells comprised the majority of the splenic human leukocyte population in both hP2X7GOF mice (83.32 ± 2.22% hCD3+ hCD19− cells, n = 30) and hP2X7LOF mice (82.82 ± 1.97% hCD3+ hCD19− cells, n = 37). Both mouse groups demonstrated similar frequencies of human T cells (P = 0.8654) (Fig. 3 e). hCD4+ and hCD8+ T cell subsets were also identified at endpoint, with significantly higher frequencies of hCD4+ T cells than hCD8+ T cells in both hP2X7GOF mice (62.77 ± 3.35% vs 28.47 ± 2.33%, respectively, n = 30) (P < 0.0001) and hP2X7LOF mice (64.98 ± 2.76% vs. 28.93 ± 2.22%, respectively, n = 37) (P < 0.0001). However, frequencies of hCD4+ and hCD8+ T cells were similar between hP2X7GOF and hP2X7LOF mice (hCD4+ T cells, P = 0.6093; hCD8+ T cells, P = 0.8894) (Fig. 3 f). Analysis of hCD4+ T cells revealed that hP2X7GOF mice and hP2X7LOF mice contained similar frequencies of hCD4+hCD25+hCD127lo Tregs (1.68 ± 0.27%, n = 30 vs. 1.395 ± 0.16%, n = 37, respectively) (P = 0.3490) (Fig. 3 g).
hP2X7GOF and hP2X7LOF mice demonstrate similar development of GVHD
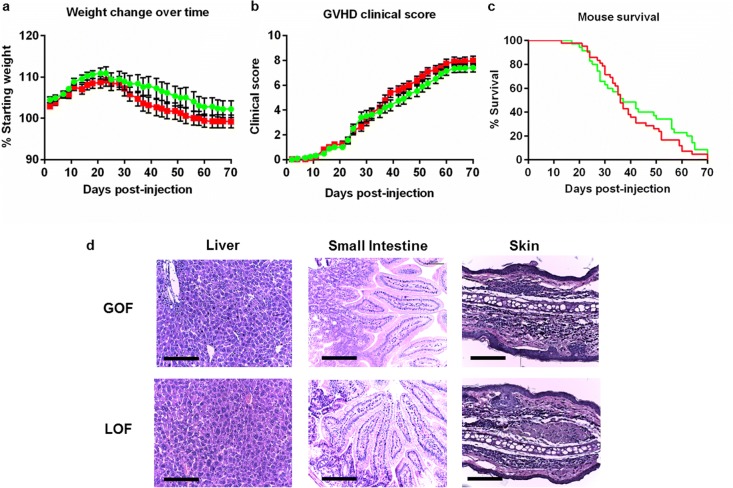
GVHD development in humanised mice was analysed by monitoring weight loss and scoring clinical symptoms. Donor P2X7 activity did not affect GVHD development in humanised mice (Fig. 4 a–c). Weight loss was similar between hP2X7GOF and hP2X7LOF mice (P = 0.3398), which was first observed from day 25 in both groups (Fig. 4 a). Both hP2X7GOF and hP2X7LOF mice exhibited clinical symptoms from day 16, with comparable mean clinical scores over 10 weeks (P = 0.6093) and at endpoint (7.26 ± 0.35, n = 30 vs. 7.53 ± 0.26, n = 37, respectively) (P = 0.6512) (Fig. 4 b). Finally, all mice in both groups succumbed to the ethical endpoint by 10 weeks, with no significant difference in median survival time (MST) between hP2X7GOF mice (MST of 34 days, n = 30) and hP2X7LOF mice (MST of 36 days, n = 37) (P = 0.8107) (Fig. 4 c).
Fig. 4.
Clinical and histological GVHD is similar in hP2X7GOF and hP2X7LOF mice. NSG mice were injected (i.p.) with 10 × 106 hPBMCs isolated from either a GOF (hP2X7GOF mice) or LOF (hP2X7LOF) P2RX7 genotype donor (four donors per group). a–c Mice were monitored for up to 10 weeks post-hPBMC injection for clinical GVHD development including a weight loss, b GVHD clinical score and c survival. Data represents a, b group means ± SEM or j percentage survival (GOF, n = 30; LOF, n = 37). d At endpoint, tissue sections (liver, small intestine and skin) from hP2X7GOF mice (top panel) and hP2X7LOF mice (bottom panel) were stained with haematoxylin and eosin, and viewed by microscopy. Representative images from four mice per group are shown; bars represent 100 μm. GOF, gain-of-function; LOF, loss-of-function
hP2X7GOF and hP2X7LOF mice demonstrate similar histological evidence of GVHD
NSG mice injected with hPBMCs demonstrate histological signs of GVHD damage in the liver, small intestine and skin [14, 37, 44]. In the current study, leukocyte infiltration was observed in the livers of hP2X7GOF and hP2X7LOF mice with similar amounts of tissue fibrosis between the two mouse groups (Fig. 4 d). Both groups also demonstrated similar leukocyte infiltration and overall tissue damage in the small intestine, and also showed mild signs of skin GVHD with comparable amounts of epidermal and dermal thickening alongside detachment of the epidermis from the dermis (Fig. 4 d).
Relative hIFNγ expression is significantly increased in the livers of hP2X7GOF compared to hP2X7LOF mice, but serum hIFNγ concentrations are similar
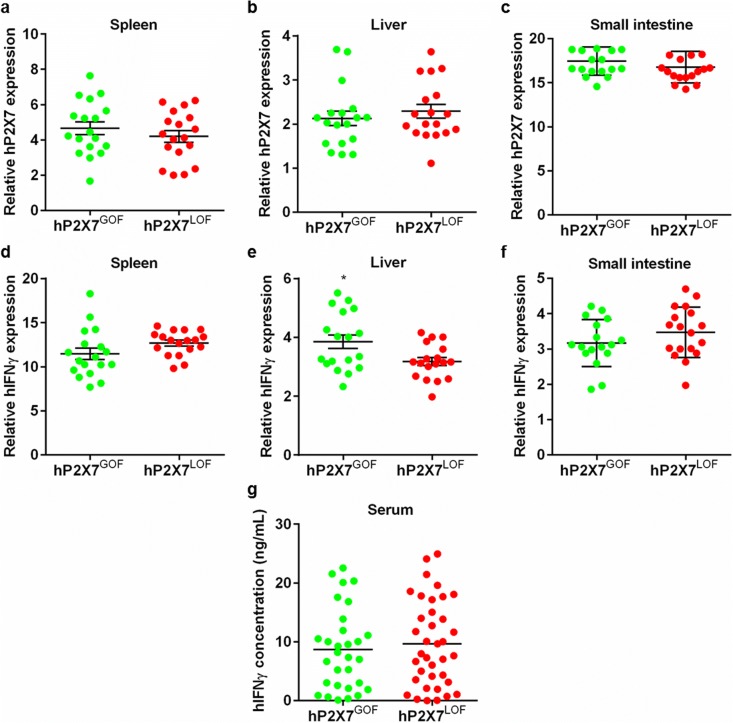
P2X7 expression is upregulated in allogeneic models of GVHD [11], whilst P2X7 blockade reduces serum IFNγ in both allogeneic [11, 12] and humanised mouse models of GVHD [14]. Therefore, qPCR was used to measure the relative expression of hP2X7 and hIFNγ in spleens and GVHD target organs. hP2X7GOF and hP2X7LOF mice demonstrated similar relative hP2X7 expression in the spleen (4.87 ± 0.38, n = 18 vs. 4.19 ± 0.33, n = 18, respectively) (P = 0.1912) (Fig. 5 a), liver (2.16 ± 0.19, n = 18 vs. 2.29 ± 0.16, n = 18, respectively) (P = 0.6005) (Fig. 5 b) and small intestine (17.62 ± 0.37, n = 18 vs. 16.77 ± 0.42, n = 18, respectively) (P = 0.1444) (Fig. 5 c). Relative hIFNγ expression was also comparable in the spleens of hP2X7GOF mice (12.46 ± 0.52, n = 18) and hP2X7LOF mice (13.11 ± 0.27, n = 18) (P = 0.3363) (Fig. 5 d). In contrast, a significant but small (1.3-fold) increase in relative hIFNγ expression was observed in livers from hP2X7GOF mice (3.82 ± 0.24, n = 18) compared to hP2X7LOF mice (3.12 ± 0.14, n = 18) (P = 0.0154) (Fig. 5 e). Similar relative hIFNγ expression was observed in the small intestines of hP2X7LOF mice (3.66 ± 0.15, n = 18) and hP2X7GOF mice (3.25 ± 0.15, n = 18) (P = 0.0623) (Fig. 5 f).
Fig. 5.
Relative hIFNγ expression is significantly increased in the livers of hP2X7GOF mice compared to hP2X7LOF mice. a–g NSG mice were injected (i.p.) with 10 × 106 hPBMCs isolated from either a GOF or LOF P2RX7 genotype donor (four donors per group). a–f Relative expression of a–c hP2X7 and d–f hIFNγ in a, d spleen, b, e liver and c, f small intestine at endpoint was examined by qPCR. g Concentrations of hIFNγ in serum of humanised mice were analysed by ELISA. a–g Data represents group mean ± SEM (a–f, n = 12–18; g GOF, n = 30; LOF, n = 36); symbols represent individual mice; *P < 0.05 compared to hP2X7LOF mice. hIFNγ, human interferon-γ; GOF, gain-of-function; LOF, loss-of-function
Finally, the concentrations of serum hIFNγ in hP2X7GOF and hP2X7LOF mice were analysed by ELISA (Fig. 5 g). hIFNγ was present in the serum of all mice; however, there was no significant difference in the concentration of serum hIFNγ between hP2X7GOF mice (8.68 ± 1.26 pg/mL, n = 30) and hP2X7LOF mice (9.67 ± 1.20 pg/mL, n = 37) (P = 0.6662).
Discussion
This study demonstrated that P2X7 activity correlates with GOF and LOF P2RX7 genotypes on human T cell, NK cell and monocyte subsets, and showed this correlation for the first time on human CD4+CD25+CD127lo Tregs and iNKT cells. Despite the impact of P2RX7 genotype on P2X7 activity on human immune subsets, altered donor P2X7 did not affect the development of clinical or histological disease in a pre-clinical humanised mouse model of GVHD. Our results in humanised mice are contrary to previous findings in allogeneic mouse models that have shown that genetic ablation of P2X7 [11] or P2X7 blockade with KN-62 [11], stavudine [12] or BBG [13] can reduce GVHD development. However, it must be noted that whilst P2X7 activity is implicated in exacerbating GVHD, it is P2X7 activation on host, not donor, antigen-presenting cells that is believed to worsen disease [11]. Consequently, the current study is one of the few to examine the importance of human donor P2X7 activity on GVHD development. Despite the current results suggesting that donor P2X7 activity does not affect GVHD development in this humanised mouse model, individual P2RX7 SNPs may still have important effects on GVHD since they can influence survival outcomes in allo-HSCT patients [30]. Additionally, during the course of the current study, a conference abstract was published highlighting the potential role of P2RX7 genotype in predicting GVHD onset [45]. This preliminary data indicated that the presence of the LOF SNPs rs3751143 (E496A) or rs16536624 (I568N) in recipients reduces GVHD post-HSCT. Lastly, given that P2RX7 genotypes are associated with a number of inflammatory diseases such as multiple sclerosis [28] and rheumatoid arthritis [29], further investigation into the role of host P2RX7 genotype on the development of GVHD in humans is warranted.
The current study also demonstrated that human donor P2RX7 genotype does not affect the engraftment of human leukocytes (predominantly T cells) in this humanised mouse model of GVHD. Moreover, the engraftment of hCD4+ and hCD8+ T cells in these humanised mice was comparable to the proportions of hCD4+ and hCD8+ T cell engraftment observed by Geraghty et al. [14]. This is consistent with these T cell subsets being the main effectors of GVHD in this model [37, 46, 47], and the similar engraftment of T cell subsets between hP2X7GOF or hP2X7LOF mice parallels the similarity in GVHD development between the two mouse groups. The current study also demonstrated no difference in the engraftment of Tregs between hP2X7GOF and hP2X7LOF mice, which have protective roles against GVHD in humanised NSG mice [39, 48] and in allo-HSCT patients [49, 50]. Since P2X7 is present on human Tregs [36] and can influence the proliferation and suppressive capabilities of these cells in vitro [51], it is possible that GOF and LOF P2RX7 genotypes may influence GVHD development by enhancing and reducing Treg numbers and function, respectively. However, the similar engraftment of Tregs, and severity of GVHD in both groups of mice, indicates that this is not the case in this study.
Donor P2RX7 genotype did not alter relative expression of hP2X7 in the spleen, liver or small intestine in this humanised mouse model. Although P2X7 is upregulated in allogeneic mice and humans with GVHD [11], in humanised mice, the P2X7 expression observed indirectly reflects human donor cell engraftment and supports human leukocyte engraftment being similar between P2RX7 genotypes. In contrast, hIFNγ RNA expression was significantly higher in the livers of hP2X7GOF than in hP2X7LOF mice. Although this difference was small, and assuming IFNγ expression correlates with GVHD severity [52], the possibility exists that molecular GVHD is worse in the livers of mice injected with GOF P2RX7 genotype donors. Nonetheless, such differences were not readily observed by histology, and serum hIFNγ was similar between donor P2RX7 genotypes. This latter finding contrasts previous data in allogeneic mouse models of GVHD where genetic deficiency of P2RX7 reduced serum IFNγ [11, 12], and in this humanised mouse model, where short-term P2X7 blockade with BBG reduced serum hIFNγ and tissue leukocyte infiltration and damage [14]. This suggests that BBG may be acting on host (murine) rather than donor (human) P2X7 to reduce GVHD development in humanised mice.
This study demonstrated for the first time the presence of functional P2X7 on CD4+CD25+CD127lo Tregs and iNKT cells, as well as on CD16+CD56−, CD16+CD56+ and CD16+CD56− NK cell subsets. Furthermore, this study confirms the presence of functional P2X7 on CD4+ and CD8+ T cells [34] and non-classical (CD14−CD16+), intermediate (CD14+CD16+) and classical (CD14+CD16−) monocytes [35, 53, 54]. Notably, P2RX7 genotype corresponded to P2X7 activity on each of these subsets, validating the functional predictions of the respective haplotypes made by both Stokes et al. [25] and Jørgensen et al. [27]. The possibility remains that some P2RX7 haplotypes may reduce or increase cell-surface P2X7 expression; however, previous attempts using an anti-P2X7 mAb have repeatedly been unable to reveal this in primary leukocytes [25, 33, 43, 55, 56]. Whilst mean P2X7 activity was similar between all leukocyte subsets in the current study, iNKT cells demonstrated significantly higher activity than all other cell subsets but whether this reflects differences in cell-surface P2X7 expression remains to be determined. The current results contrast previous studies which have shown higher P2X7 activity in monocytes compared to NK cells, B cells and T cells [15, 16]. The reasons for the differences between the current and past studies remain unclear, but most likely reflect the assessment of dye uptake in fixed-time and real-time measurements, respectively. Although the identity of the P2X7 pore remains elusive [57], ethidium+ is thought to pass through the same pore as YO-PRO-12+ [58]. Thus, differences between studies due to the use of different dyes are unlikely.
In conclusion, the current study demonstrates that donor P2RX7 genotype affects P2X7 activity on human T cell, monocyte and NK cell subsets. This study also demonstrated that altered donor P2X7 activity does not affect the development of GVHD in this humanised mouse model. This indicates that hPBMCs, regardless of P2RX7 genotype, can be used to establish GVHD in NSG mice. However, whether certain P2RX7 genotypes can predict GVHD and other outcomes in allo-HSCT recipients will need to be confirmed by larger, adequately powered studies.
Acknowledgements
The authors thank all blood donors who contributed to this study, Margaret Phillips (University of Wollongong) for sequencing DNA, Sandra Burrell for assistance with DNA sequencing, the technical staff of the Illawarra Health and Medical Research Institute (Wollongong, Australia) and the animal staff of the University of Wollongong Faculty of Science, Medicine and Health (University of Wollongong) for technical support.
Funding information
This project was funded by the Faculty of Science, Medicine and Health, University of Wollongong. SR Adhikary, NJ Geraghty and P Cuthbertson are supported through Australian Government Research Training Program Scholarships. D Watson is supported by AMP’s Tomorrow Fund. D Watson and R Sluyter receive additional support from Molecular Horizons (University of Wollongong).
Compliance with ethical standards
Conflict of interest
Sam R Adhikary declares that he has no conflict of interest.
Nicholas J Geraghty declares that he has no conflict of interest.
Peter Cuthbertson declares that he has no conflict of interest.
Ronald Sluyter declares that he has no conflict of interest.
Debbie Watson declares that she has no conflict of interest.
Ethical approval
All animal experiments were approved under protocol AE16/03 by the University of Wollongong Animal Ethics Committee. All human experiments were approved under protocol HE12/290 by the University of Wollongong Human Ethics Committee.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
R Sluyter and D Watson are co-senior authors.
Contributor Information
R. Sluyter, Email: rsluyter@uow.edu.au
D. Watson, Email: dwatson@uow.edu.au
References
- 1.Markey KA, MacDonald KP, Hill GR. The biology of graft-versus-host disease: experimental systems instructing clinical practice. Blood. 2014;124:354–362. doi: 10.1182/blood-2014-02-514745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fowler DH. Shared biology of GVHD and GVT effects: potential methods of separation. Crit Rev Oncol Hematol. 2006;57:225–244. doi: 10.1016/j.critrevonc.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 3.Hill GR, Crawford JM, Cooke KR, Brinson YS, Pan L, Ferrara JL. Total body irradiation and acute graft-versus-host disease: the role of gastrointestinal damage and inflammatory cytokines. Blood. 1997;90:3204–3213. [PubMed] [Google Scholar]
- 4.Coghill JM, Sarantopoulos S, Moran TP, Murphy WJ, Blazar BR, Serody JS. Effector CD4(+) T cells, the cytokines they generate, and GVHD: something old and something new. Blood. 2011;117:3268–3276. doi: 10.1182/blood-2010-12-290403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Storb R, Gyurkocza B, Storer BE, Sorror ML, Blume K, Niederwieser D, Chauncey TR, Pulsipher MA, Petersen FB, Sahebi F, Agura ED, Hari P, Bruno B, McSweeney PA, Maris MB, Maziarz RT, Langston AA, Bethge W, Vindelov L, Franke GN, Laport GG, Yeager AM, Hubel K, Deeg HJ, Georges GE, Flowers ME, Martin PJ, Mielcarek M, Woolfrey AE, Maloney DG, Sandmaier BM. Graft-versus-host disease and graft-versus-tumor effects after allogeneic hematopoietic cell transplantation. J Clin Oncol. 2013;31:1530–1538. doi: 10.1200/JCO.2012.45.0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paczesny S. Discovery and validation of graft-versus-host disease biomarkers. Blood. 2013;121:585–594. doi: 10.1182/blood-2012-08-355990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Masetti R, Zama D, Urbini M, Astolfi A, Libri V, Vendemini F, Morello W, Rondelli R, Prete A, Pession A. Impact of inflammatory cytokine gene polymorphisms on developing acute graft-versus-host disease in children undergoing allogeneic hematopoietic stem cell transplantation. J Immunol Res. 2015;2015:248264–248265. doi: 10.1155/2015/248264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chien JW, Zhang XC, Fan W, Wang H, Zhao LP, Martin PJ, Storer BE, Boeckh M, Warren EH, Hansen JA. Evaluation of published single nucleotide polymorphisms associated with acute GVHD. Blood. 2012;119:5311–5319. doi: 10.1182/blood-2011-09-371153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Castillo-Leon E, Dellepiane S, Fiorina P. ATP and T-cell-mediated rejection. Curr Opin Organ Tran. 2018;23:34–43. doi: 10.1097/MOT.0000000000000484. [DOI] [PubMed] [Google Scholar]
- 10.Di Virgilio F, Dal Ben D, Sarti AC, Giuliani AL, Falzoni S. The P2X7 receptor in infection and inflammation. Immunity. 2017;47:15–31. doi: 10.1016/j.immuni.2017.06.020. [DOI] [PubMed] [Google Scholar]
- 11.Wilhelm K, Ganesan J, Muller T, Durr C, Grimm M, Beilhack A, Krempl CD, Sorichter S, Gerlach UV, Juttner E, Zerweck A, Gartner F, Pellegatti P, Di Virgilio F, Ferrari D, Kambham N, Fisch P, Finke J, Idzko M, Zeiser R. Graft-versus-host disease is enhanced by extracellular ATP activating P2X7R. Nat Med. 2010;16:1434–1438. doi: 10.1038/nm.2242. [DOI] [PubMed] [Google Scholar]
- 12.Fowler BJ, Gelfand BD, Kim Y, Kerur N, Tarallo V, Hirano Y, Amarnath S, Fowler DH, Radwan M, Young MT, Pittman K, Kubes P, Agarwal HK, Parang KA, Hinton DR, Bastos-Carvalho A, Li S, Yasuma T, Mizutani T, Yasuma R, Wright C, Ambati J. Nucleoside reverse transcriptase inhibitors possess intrinsic anti-inflammatory activity. Science. 2014;346:1000–1003. doi: 10.1126/science.1261754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhong X, Zhu F, Qiao J, Zhao K, Zhu S, Zeng L, Chen X, Xu K. The impact of P2X7 receptor antagonist, brilliant blue G on graft-versus-host disease in mice after allogeneic hematopoietic stem cell transplantation. Cell Immunol. 2016;310:71–77. doi: 10.1016/j.cellimm.2016.07.014. [DOI] [PubMed] [Google Scholar]
- 14.Geraghty NJ, Belfiore L, Ly D, Adhikary SR, Fuller SJ, Varikatt W, Sanderson-Smith ML, Sluyter V, Alexander SI, Sluyter R, Watson D. The P2X7 receptor antagonist Brilliant Blue G reduces serum human interferon-γ in a humanized mouse model of graft-versus-host disease. Clin Exp Immunol. 2017;190:79–95. doi: 10.1111/cei.13005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gu BJ, Zhang WY, Bendall LJ, Chessell IP, Buell GN, Wiley JS. Expression of P2X(7) purinoceptors on human lymphocytes and monocytes: evidence for nonfunctional P2X(7) receptors. Am J Phys Cell Phys. 2000;279:C1189–C1197. doi: 10.1152/ajpcell.2000.279.4.C1189. [DOI] [PubMed] [Google Scholar]
- 16.Stevenson RO, Taylor RM, Wiley JS, Sluyter R. The P2X(7) receptor mediates the uptake of organic cations in canine erythrocytes and mononuclear leukocytes: comparison to equivalent human cell types. Purinergic Signal. 2009;5:385–394. doi: 10.1007/s11302-009-9163-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jursik C, Sluyter R, Georgiou JG, Fuller SJ, Wiley JS, Gu BJ. A quantitative method for routine measurement of cell surface P2X7 receptor function in leucocyte subsets by two-colour time-resolved flow cytometry. J Immunol Methods. 2007;325:67–77. doi: 10.1016/j.jim.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 18.Korpi-Steiner NL, Sheerar D, Puffer EB, Urben C, Boyd J, Guadarrama A, Schell K, Denlinger LC. Standardized method to minimize variability in a functional P2X(7) flow cytometric assay for a multi-center clinical trial. Cytom B-Clin Cytom. 2008;74:319–329. doi: 10.1002/cyto.b.20421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pandolfi JB, Ferraro AA, Sananez I, Gancedo MC, Baz P, Billordo LA, Fainboim L, Arruvito L. ATP-induced inflammation drives tissue-resident Th17 cells in metabolically unhealthy obesity. J Immunol. 2016;196:3287–3296. doi: 10.4049/jimmunol.1502506. [DOI] [PubMed] [Google Scholar]
- 20.Kappel LW, Goldberg GL, King CG, Suh DY, Smith OM, Ligh C, Holland AM, Grubin J, Mark NM, Liu C, Iwakura Y, Heller G, van den Brink MR. IL-17 contributes to CD4-mediated graft-versus-host disease. Blood. 2009;113:945–952. doi: 10.1182/blood-2008-08-172155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Di Ianni M, Falzetti F, Carotti A, Terenzi A, Castellino F, Bonifacio E, Del Papa B, Zei T, Ostini RI, Cecchini D, Aloisi T, Perruccio K, Ruggeri L, Balucani C, Pierini A, Sportoletti P, Aristei C, Falini B, Reisner Y, Velardi A, Aversa F, Martelli MF. Tregs prevent GVHD and promote immune reconstitution in HLA-haploidentical transplantation. Blood. 2011;117:3921–3928. doi: 10.1182/blood-2010-10-311894. [DOI] [PubMed] [Google Scholar]
- 22.Hubert S, Rissiek B, Klages K, Huehn J, Sparwasser T, Haag F, Koch-Nolte F, Boyer O, Seman M, Adriouch S. Extracellular NAD(+) shapes the Foxp3(+) regulatory T cell compartment through the ART2–P2X7 pathway. J Exp Med. 2010;207:2561–2568. doi: 10.1084/jem.20091154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schenk U, Frascoli M, Proietti M, Geffers R, Traggiai E, Buer J, Ricordi C, Westendorf AM, Grassi F. ATP inhibits the generation and function of regulatory T cells through the activation of purinergic P2X receptors. Sci Signal. 2011;4:ra12. doi: 10.1126/scisignal.2001270. [DOI] [PubMed] [Google Scholar]
- 24.Sluyter R. The P2X7 receptor. Adv Exp Med Biol. 2017;1051:17–53. doi: 10.1007/5584_2017_59. [DOI] [PubMed] [Google Scholar]
- 25.Stokes L, Fuller SJ, Sluyter R, Skarratt KK, Gu BJ, Wiley JS. Two haplotypes of the P2X(7) receptor containing the Ala-348 to Thr polymorphism exhibit a gain-of-function effect and enhanced interleukin-1beta secretion. FASEB J. 2010;24:2916–2927. doi: 10.1096/fj.09-150862. [DOI] [PubMed] [Google Scholar]
- 26.Fuller SJ, Stokes L, Skarratt KK, Gu BJ, Wiley JS. Genetics of the P2X7 receptor and human disease. Purinergic Signal. 2009;5:257–262. doi: 10.1007/s11302-009-9136-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jørgensen NR, Husted LB, Skarratt KK, Stokes L, Tofteng CL, Kvist T, Jensen J-EB, Eiken P, Brixen K, Fuller S, Clifton-Bligh R, Gartland A, Schwarz P, Langdahl BL, Wiley JS. Single-nucleotide polymorphisms in the P2X7 receptor gene are associated with post-menopausal bone loss and vertebral fractures. Eur J Hum Genet. 2012;20:675–681. doi: 10.1038/ejhg.2011.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oyanguren-Desez O, Rodriguez-Antiguedad A, Villoslada P, Domercq M, Alberdi E, Matute C. Gain-of-function of P2X7 receptor gene variants in multiple sclerosis. Cell Calcium. 2011;50:468–472. doi: 10.1016/j.ceca.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 29.Al-Shukaili A, Al-Kaabi J, Hassan B, Al-Araimi T, Al-Tobi M, Al-Kindi M, Al-Maniri A, Al-Gheilani A, Al-Ansari A. P2X7 receptor gene polymorphism analysis in rheumatoid arthritis. Int J Immunogenet. 2011;38:389–396. doi: 10.1111/j.1744-313X.2011.01019.x. [DOI] [PubMed] [Google Scholar]
- 30.Lee KH, Park SS, Kim I, Kim JH, Ra EK, Yoon SS, Hong YC, Park S, Kim BK. P2X7 receptor polymorphism and clinical outcomes in HLA-matched sibling allogeneic hematopoietic stem cell transplantation. Haematologica. 2007;92:651–657. doi: 10.3324/haematol.10810. [DOI] [PubMed] [Google Scholar]
- 31.Karaesmen E, Rizvi AA, Preus LM, McCarthy PL, Pasquini MC, Onel K, Zhu X, Spellman S, Haiman CA, Stram DO, Pooler L, Sheng X, Zhu Q, Yan L, Liu Q, Hu Q, Webb A, Brock G, Clay-Gilmour AI, Battaglia S, Tritchler D, Liu S, Hahn T, Sucheston-Campbell LE. Replication and validation of genetic polymorphisms associated with survival after allogeneic blood or marrow transplant. Blood. 2017;130:1585–1596. doi: 10.1182/blood-2017-05-784637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spildrejorde M, Bartlett R, Stokes L, Jalilian I, Peranec M, Sluyter V, Curtis BL, Skarratt KK, Skora A, Bakhsh T, Seavers A, McArthur JD, Dowton M, Sluyter R. R270C polymorphism leads to loss of function of the canine P2X7 receptor. Physiol Genomics. 2014;46:512–522. doi: 10.1152/physiolgenomics.00195.2013. [DOI] [PubMed] [Google Scholar]
- 33.Gu BJ, Sluyter R, Skarratt KK, Shemon AN, Dao-Ung LP, Fuller SJ, Barden JA, Clarke AL, Petrou S, Wiley JS. An Arg307 to Gln polymorphism within the ATP-binding site causes loss of function of the human P2X7 receptor. J Biol Chem. 2004;279:31287–31295. doi: 10.1074/jbc.M313902200. [DOI] [PubMed] [Google Scholar]
- 34.Sluyter R, Wiley JS. P2X7 receptor activation induces CD62L shedding from human CD4+ and CD8+ T cells. Inflamm Cell Sig. 2014;1:44–49. [Google Scholar]
- 35.Hadadi E, Zhang B, Baidžajevas K, Yusof N, Puan KJ, Ong SM, Yeap WH, Rotzschke O, Kiss-Toth E, Wilson H, Wong SC. Differential IL-1β secretion by monocyte subsets is regulated by Hsp27 through modulating mRNA stability. Sci Rep. 2016;6:39035. doi: 10.1038/srep39035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cortes-Garcia JD, Lopez-Lopez C, Cortez-Espinosa N, Garcia-Hernandez MH, Guzman-Flores JM, Layseca-Espinosa E, Portales-Cervantes L, Portales-Perez DP. Evaluation of the expression and function of the P2X7 receptor and ART1 in human regulatory T-cell subsets. Immunobiology. 2016;221:84–93. doi: 10.1016/j.imbio.2015.07.018. [DOI] [PubMed] [Google Scholar]
- 37.King MA, Covassin L, Brehm MA, Racki W, Pearson T, Leif J, Laning J, Fodor W, Foreman O, Burzenski L, Chase TH, Gott B, Rossini AA, Bortell R, Shultz LD, Greiner DL. Human peripheral blood leucocyte non-obese diabetic-severe combined immunodeficiency interleukin-2 receptor gamma chain gene mouse model of xenogeneic graft-versus-host-like disease and the role of host major histocompatibility complex. Clin Exp Immunol. 2009;157:104–118. doi: 10.1111/j.1365-2249.2009.03933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kawasaki Y, Sato K, Hayakawa H, Takayama N, Nakano H, Ito R, Mashima K, Oh I, Minakata D, Yamasaki R, Morita K, Ashizawa M, Yamamoto C, Hatano K, Fujiwara SI, Ohmine K, Muroi K, Kanda Y. Comprehensive analysis of the activation and proliferation kinetics and effector functions of human lymphocytes, and antigen presentation capacity of antigen-presenting cells in xenogeneic graft-versus-host disease. Biol Blood Marrow Transplant. 2018;24:1563–1574. doi: 10.1016/j.bbmt.2018.04.016. [DOI] [PubMed] [Google Scholar]
- 39.Ruggeri L, Di Ianni M, Urbani E, Mancusi A, Falzetti F, Carotti A, Terenzi A, Massei MS, Amico L, Zei T, Iacucci R, Martelli MF, Velardi A. Tregs suppress GvHD at the periphery and unleash the Gvl effect in the bone marrow. Blood. 2014;124:842. doi: 10.1182/blood-2014-04-567743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bhattacharya A, Wang Q, Ao H, Shoblock JR, Lord B, Aluisio L, Fraser I, Nepomuceno D, Neff RA, Welty N, Lovenberg TW, Bonaventure P, Wickenden AD, Letavic MA. Pharmacological characterization of a novel centrally permeable P2X7 receptor antagonist: JNJ-47965567. Br J Pharmacol. 2013;170:624–640. doi: 10.1111/bph.12314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Woehrle T, Yip L, Elkhal A, Sumi Y, Chen Y, Yao Y, Insel PA, Junger WG. Pannexin-1 hemichannel-mediated ATP release together with P2X1 and P2X4 receptors regulate T-cell activation at the immune synapse. Blood. 2010;116:3475–3484. doi: 10.1182/blood-2010-04-277707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ledderose C, Liu K, Kondo Y, Slubowski CJ, Dertnig T, Denicolo S, Arbab M, Hubner J, Konrad K, Fakhari M, Lederer JA, Robson SC, Visner GA, Junger WG. Purinergic P2X4 receptors and mitochondrial ATP production regulate T cell migration. J Clin Invest. 2018;128:3583–3594. doi: 10.1172/JCI120972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shemon AN, Sluyter R, Fernando SL, Clarke AL, Dao-Ung LP, Skarratt KK, Saunders BM, Tan KS, Gu BJ, Fuller SJ, Britton WJ, Petrou S, Wiley JS. A Thr357 to Ser polymorphism in homozygous and compound heterozygous subjects causes absent or reduced P2X7 function and impairs ATP-induced mycobacterial killing by macrophages. J Biol Chem. 2006;281:2079–2086. doi: 10.1074/jbc.M507816200. [DOI] [PubMed] [Google Scholar]
- 44.Geraghty NJ, Belfiore L, Adhikary SR, Alexander SI, Sluyter R, Watson D (2019) Increased splenic human CD4(+):CD8(+) T cell ratios, serum human interferon-gamma and intestinal human interleukin-17 are associated with clinical graft-versus-host disease in humanized mice. Transpl Immunol 10.1016/j.trim.2019.02.003 [DOI] [PubMed]
- 45.Koldej R, Perera T, Ritchie DS. Polymorphisms in donor and recipient p2x7 receptor predict patient outcome in allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2018;24:S42. doi: 10.1016/j.bbmt.2017.12.596. [DOI] [Google Scholar]
- 46.Abraham S, Choi J-G, Ye C, Manjunath N, Shankar P. IL-10 exacerbates xenogeneic GVHD by inducing massive human T cell expansion. Clin Immunol. 2015;156:58–64. doi: 10.1016/j.clim.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Covassin L, Jangalwe S, Jouvet N, Laning J, Burzenski L, Shultz LD, Brehm MA. Human immune system development and survival of non-obese diabetic (NOD)-scid IL2rgamma(null) (NSG) mice engrafted with human thymus and autologous haematopoietic stem cells. Clin Exp Immunol. 2013;174:372–388. doi: 10.1111/cei.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hannon M, Lechanteur C, Lucas S, Somja J, Seidel L, Belle L, Bruck F, Baudoux E, Giet O, Chantillon AM, Delvenne P, Drion P, Beguin Y, Humblet-Baron S, Baron F. Infusion of clinical-grade enriched regulatory T cells delays experimental xenogeneic graft-versus-host disease. Transfusion. 2014;54:353–363. doi: 10.1111/trf.12666. [DOI] [PubMed] [Google Scholar]
- 49.Rezvani K, Mielke S, Ahmadzadeh M, Kilical Y, Savani BN, Zeilah J, Keyvanfar K, Montero A, Hensel N, Kurlander R, Barrett AJ. High donor FOXP3-positive regulatory T-cell (Treg) content is associated with a low risk of GVHD following HLA-matched allogeneic SCT. Blood. 2006;108:1291–1297. doi: 10.1182/blood-2006-02-003996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kennedy-Nasser AA, Ku S, Castillo-Caro P, Hazrat Y, Wu MF, Liu H, Melenhorst J, Barrett AJ, Ito S, Foster A, Savoldo B, Yvon E, Carrum G, Ramos CA, Krance RA, Leung K, Heslop HE, Brenner MK, Bollard CM. Ultra low-dose IL-2 for GVHD prophylaxis after allogeneic hematopoietic stem cell transplantation mediates expansion of regulatory T cells without diminishing antiviral and antileukemic activity. Clin Cancer Res. 2014;20:2215–2225. doi: 10.1158/1078-0432.CCR-13-3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Trabanelli S, Ocadlikova D, Gulinelli S, Curti A, Salvestrini V, Vieira RP, Idzko M, Di Virgilio F, Ferrari D, Lemoli RM. Extracellular ATP exerts opposite effects on activated and regulatory CD4+ T cells via purinergic P2 receptor activation. J Immunol. 2012;189:1303–1310. doi: 10.4049/jimmunol.1103800. [DOI] [PubMed] [Google Scholar]
- 52.Yi T, Chen Y, Wang L, Du G, Huang D, Zhao D, Johnston H, Young J, Todorov I, Umetsu DT, Chen L, Iwakura Y, Kandeel F, Forman S, Zeng D. Reciprocal differentiation and tissue-specific pathogenesis of Th1, Th2, and Th17 cells in graft-versus-host disease. Blood. 2009;114:3101–3112. doi: 10.1182/blood-2009-05-219402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gu BJ, Huang X, Ou A, Rembach A, Fowler C, Avula PK, Horton A, Doecke JD, Villemagne VL, Macaulay SL, Maruff P, Fletcher EL, Guymer R, Wiley JS, Masters CL. Innate phagocytosis by peripheral blood monocytes is altered in Alzheimer’s disease. Acta Neuropathol. 2016;132:377–389. doi: 10.1007/s00401-016-1596-3. [DOI] [PubMed] [Google Scholar]
- 54.Amores-Iniesta J, Barberà-Cremades M, Martínez CM, Pons JA, Revilla-Nuin B, Martínez-Alarcón L, Di Virgilio F, Parrilla P, Baroja-Mazo A, Pelegrín P. Extracellular ATP activates the NLRP3 inflammasome and is an early danger signal of skin allograft rejection. Cell Rep. 2017;21:3414–3426. doi: 10.1016/j.celrep.2017.11.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gu BJ, Zhang W, Worthington RA, Sluyter R, Dao-Ung P, Petrou S, Barden JA, Wiley JS. A Glu-496 to Ala polymorphism leads to loss of function of the human P2X7 receptor. J Biol Chem. 2001;276:11135–11142. doi: 10.1074/jbc.M010353200. [DOI] [PubMed] [Google Scholar]
- 56.Wiley JS, Dao-Ung LP, Li C, Shemon AN, Gu BJ, Smart ML, Fuller SJ, Barden JA, Petrou S, Sluyter R. An Ile-568 to Asn polymorphism prevents normal trafficking and function of the human P2X7 receptor. J Biol Chem. 2003;278:17108–17113. doi: 10.1074/jbc.M212759200. [DOI] [PubMed] [Google Scholar]
- 57.Di Virgilio F, Schmalzing G, Markwardt F. The elusive P2X7 macropore. Trends Cell Biol. 2018;28:392–404. doi: 10.1016/j.tcb.2018.01.005. [DOI] [PubMed] [Google Scholar]
- 58.Cankurtaran-Sayar S, Sayar K, Ugur M. P2X7 receptor activates multiple selective dye-permeation pathways in RAW 264.7 and human embryonic kidney 293 cells. Mol Pharmacol. 2009;76:1323–1332. doi: 10.1124/mol.109.059923. [DOI] [PubMed] [Google Scholar]