ABSTRACT
Acinar cell carcinoma (ACC) is a rare pancreatic neoplasm with dismal prognosis. Insights into the molecular basis of ACC can pave the way for the application of more effective, personalized therapies and detection of patients with hereditary predisposition.
Molecular analysis revealed a germline BRCA2 (and CHEK2) mutation in a patient with a rare pancreatic ACC with extensive intraductal growth. Somatic loss of the wild-type BRCA2 allele in the tumor indicated the causal relationship of ACC with the germline defect. A thorough literature review identified another nine ACCs associated with germline BRCA2 mutation and two ACCs associated with germline BRCA1 mutation, resulting in a prevalence of BRCA1/2 germline mutations in almost 7% of ACCs. Moreover, somatic BRCA1/2 alterations are reported in 16% of sporadic ACCs. Overall, about one fifth (22%) of all pancreatic ACCs exhibit BRCA1/2 deficiency.
This study underscores the important role of BRCA1/2 mutations in pancreatic ACC. All ACC patients should undergo genetic testing for BRCA1/2 mutations to identify carriers of pathogenic variants. This will allow to select patients that can benefit from targeted therapies directed against BRCA1/2-deficient tumors and is also crucial as a referral to genetic screening for the relatives of affected individuals carrying germline BRCA1/2 alterations.
Abbreviations: ACC: acinar cell carcinoma; HBOC: Hereditary Breast and Ovarian Cancer; LOH: loss of heterozygosity; PARP: poly (ADP-ribose) polymerase; PDAC: pancreatic ductal adenocarcinoma; PP: pancreatic panniculitis; SD: standard deviation; WES: whole-exome sequencing.
KEYWORDS: Acinar cell carcinoma of the pancreas, germline mutation, somatic mutation, BRCA1, BRCA2
Introduction
Acinar cell carcinoma (ACC) is a rare pancreatic malignancy with poor prognosis accounting for <2% of all pancreatic tumors in adults and for about 15% in pediatric cases.1–3 At time of diagnosis, 50–60% of patients have distant metastasis and an advanced stage of disease leading to low survival rate and overall dismal prognosis.1,4 Nonetheless, 5-year survival of ACC patients is 45% which is higher compared to only 7% in conventional pancreatic ductal adenocarcinoma (PDAC).1 About 15% of ACC patients present with pancreatic panniculitis (PP), characterized by subcutaneous fat necrosis.1,4
The genomic landscape of ACC is distinct from other pancreatic tumors.3,5–8 Typical genetic alterations observed in PDAC are normally not detected in ACC or occur rarely, i.e., mutations in KRAS (~2% ACCs vs. >90% PDACs), TP53 (9–23% vs. 75%), CDKN2A (14% vs. 90%), SMAD4 (14–19% vs. 55%).6,9 Rare mutations in BRAF, GNAS and JAK1 and fusions in BRAF and RAF (detected in 23% of ACCs) indicate that a minority of ACCs can evolve due to driver events in oncogenes.6,9 Recent sequencing studies revealed that ACCs carry on average about 65 non-synonymous somatic mutations per tumor. Importantly, ACC appears to have few recurrent gene mutations since there were no genes mutated in more than 30% of ACC.6 Twenty to 25% of ACCs harbor abnormalities in Wnt/β-catenin pathway, including mutations in APC and CTNNB1 genes.8 The lack of highly recurrent mutations suggests that other genetic mechanisms drive tumor progression in ACC.3 Indeed, extensive chromosomal instability appears to be a defining feature of ACC distinguishing it from other pancreatic malignancies, potentially contributing to disease severity, progression and chemotherapy resistance.2,3,6,7,10 Amongst others loss of heterozygosity (LOH) of chromosomes 11p (~50% of ACCs), 17p (TP53 locus; 39%), and 18q (SMAD4 locus; 57%) is frequently detected.6–8 Importantly, despite the genetic heterogeneity, approximately 44% of ACCs harbor potentially targetable genetic abnormalities in DNA repair by homologous recombination (BRCA1/2, PALB2, BRIP1, BAP1, and ATM), JAK-STAT signaling cascade (JAK1), MAPK pathway (BRAF), and cell cycle control (CDKN2A, ID3, and APC).3,6,9
Although the association of BRCA1/2 mutations with familial and sporadic PDAC is established,11 there is only limited data on the role of BRCA1/2 genes in ACC.2,7 Since BRCA1/2 mutations are targets for therapy with platinum-based chemotherapeutics and poly (ADP-ribose) polymerase (PARP) inhibitors,12 it is important to determine the role of BRCA1/2 deficiency in the pathogenesis of pancreatic ACC. In addition, recognition of ACC as a phenotypic expression of a germline BRCA1/2 mutations is crucial for screening of patients and their families.
Here we describe a rare case of an ACC in a patient with a germline BRCA2 mutation, provide molecular evidence for a causal link between BRCA2 germline mutation and ACC, and review the literature on the role of germline and somatic BRCA1/2 mutations in ACC.
Case report
A 52-year-old man carrying a germline BRCA2 mutation presented with steatorrhea, abdominal pain and weight loss. His mother died at age 41 from breast cancer, and his sister was diagnosed with high grade serous ovarian adenocarcinoma. Abdominal CT scan revealed a tumor in the body and tail of the pancreas, suggestive of adenocarcinoma arising from the main-duct intraductal papillary mucinous neoplasm (IPMN). Endoscopic ultrasound with fine-needle aspiration cytology was performed and showed cytology consistent with ACC (Figure 1(a,b)). The patient underwent total pancreatectomy and histological examination confirmed an ACC with extensive intraductal spread (Figure 1(c,d)).13 One out of 11 lymph nodes showed metastasis. All surgical margins were free of tumor.
Figure 1.
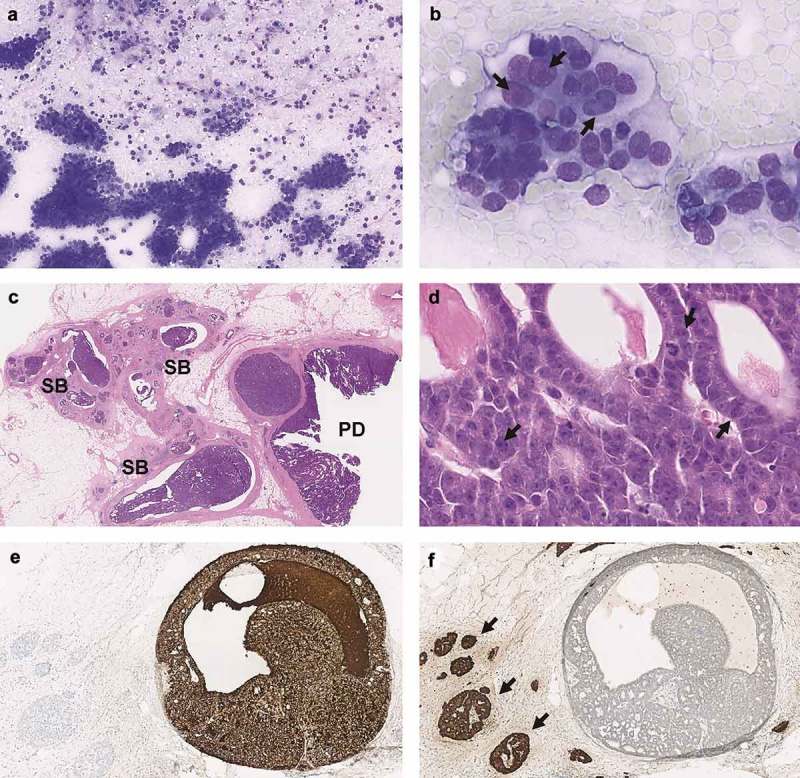
Fine needle aspiration cytology showed a highly cellular specimen consisting of a monotonous population of single cells and clusters of cells with a moderate amount of basophilic cytoplasm (a). The nuclei are round to oval with moderate anisonucleosis and a single prominent nucleolus (arrows) (b). Histologically the tumor showed extensive intraductal growth in the main pancreatic duct (PD) and side branches (SB) (c). The tumor was composed of uniform cells with granular cytoplasm and nucleoli with a single prominent nucleolus (arrows), forming small lumina (d). Immunohistochemically, the tumor cells were strongly positive for BCL10 (e) and negative for Chromogranin A (f). Note the opposite staining patterns in the adjacent islets of Langerhans (arrows). PD, pancreatic duct; SB, side branch of pancreatic duct.
Since the histopathological examination did not show adenocarcinoma, no adjuvant chemotherapy with gemcitabine was indicated. The patient recovered well, but six months postoperatively, multiple metastases appeared involving the lung, liver, peritoneum, and skin. Chemotherapy with oxaliplatin, 85 mg/m2 of body-surface area; irinotecan, 180 mg/m2; leucovorin, 400 mg/m2; and fluorouracil, 400 mg/m2 given as a bolus followed by 2400 mg/m2 given as a 46 hours continuous infusion, every 2 weeks (FOLFIRINOX) was initiated.14 The first evaluation CT scan showed partial response of liver metastases. Dose adjustments were made due to hematological toxicity. However, after more than one year of treatment (24 cycles), the neuropathy was too invalidating to perform his work and FOLFIRINOX was stopped. Unfortunately, the patient died three months later because of disease progression.
Materials and methods
Immunohistochemistry
Immunohistochemistry was performed using standard conditions in the Benchmark Ultra autostainer (Ventana Medical Systems, Inc. A Member of the Roche Group, Tucson, AZ, USA) with the following antibodies: Alpha-1-antitrypsin (1:20000; clone zmaat3, Zymed), β-catenin (1:40; clone 14, Cellmarque), BCL10 (1:400; clone 331.3, Santa Cruz), Chromogranin A (1:6400; clone LK2H10, Thermo Fisher Scientific), Cytokeratin 7 (1:6400; clone OV-TL 12/30, Biogenex), Cytokeratin 19 (1:100; clone B170, Novocastra), Cytokeratin 20 (1:200; clone Ks20.8, Dako), SMAD4 (1:800; clone EP6184, Abcam), MLH1 (1:20; clone G168-15, Pharmingen), MSH2 (1:50; clone G219-1129, Cellmarque), MSH6 (1:200; clone EPR3945, Abcam), PMS2 (1:25; clone EPR3947, Cellmarque), p53 (1:6000; clone DO-7, Dako), and Synaptophysin (1:100; clone 27G12, Novocastra).
DNA isolation
Genomic DNA was isolated from paraffin-embedded tissue. After deparaffinization, DNA was isolated using the Puregene DNA Isolation kit (Gentra Systems, Minneapolis, MN, USA). DNA concentrations were measured using the PicoGreen Double-Stranded DNA Quantitation kit (Molecular Probes, Leiden, The Netherlands).
DNA sequencing
Next-generation sequencing was performed using the Ion AmpliSeq Cancer Hotspot Panel v2, which includes 50 cancer-related genes and which was supplemented with five additional genes (Table 1), and the Oncomine BRCA Panel which includes the entire coding region of BRCA1 and BRCA2 (Thermo Fisher Scientific). Library preparation and sequencing using the Ion PGM System were performed as described previously.15
Table 1.
List of 50 target genes of the Ion AmpliSeq Cancer Hotspot Panel v2 supplemented with five additional genes.
| ABL1 | CSF1R | GNA11 | KRAS | PTEN |
| AKT1 | CTNNB1 | GNAS | MDM2 | PTPN11 |
| ALK | EGFR | GNAQ | MET | RB1 |
| APC | ERBB2 | HNF1A | MLH1 | RET |
| ARAF | ERBB4 | HRAS | MPL | SMAD4 |
| ATM | EZH2 | IDH1 | MYD88 | SMARCB1 |
| BRAF | FBXW7 | JAK2 | NOTCH1 | SMO |
| CALR | FGFR1 | JAK3 | NPM1 | SRC |
| CDH1 | FGFR2 | IDH2 | NRAS | STK11 |
| CDKN2A | FGFR3 | KDR | PDGFRA | TP53 |
| CRAF | FLT3 | KIT | PIK3CA | VHL |
In bold are indicated five additional genes that were added to the 50 target genes of the Ion AmpliSeq Cancer Hotspot Panel v2.
Multiplex ligation-dependent probe amplification (MLPA)
MLPA was performed to confirm LOH of BRCA2 using BRCA2/CHEK2 P045 probemix (MRC Holland, Amsterdam, The Netherlands) according to manufacturer’s instructions. Results were normalized on all control probes present in the kit and normal samples without copy number alterations. Deletions and duplications were defined as ratios of <0.55 and >1.45, respectively. The assay was performed in duplicate.
Results
Histopathology and immunohistochemistry
Histopathological examination of the tumor confirmed the diagnosis of pancreatic ACC. The tumor demonstrated strong positivity for pancreatic acinar cell marker BCL10 (Figure 1(e)). Cytokeratins 19 and 7 stained positive and Cytokeratin 20 was negative. Partial expression was observed for Alpha-1-antitrypsin. Chromogranin A (Figure 1(f)) and Synaptophysin were negative. Expression of p53 was wild-type, and β-catenin showed only membranous localization. No loss of SMAD4 or any of the mismatch repair proteins (MLH1, MSH2, MSH6, PMS2) was detected.
Molecular analysis
No mutations were found using the Cancer Hotspot Panel (Table 1). BRCA sequencing revealed an inactivating BRCA2 mutation (NM_000059.3: c.7974C>G or p.Tyr2658*, VAF 80% indicating the loss of the wild-type allele), which is considered pathogenic by ClinVar.16 Sequence analysis of DNA isolated from a blood sample at clinical genetics showed that this is a germline mutation. MLPA analysis revealed loss of one BRCA2 allele in the tumor tissue, further confirming biallelic inactivation of BRCA2. In addition, somatic LOH of BRCA1 was detected by BRCA sequencing and a germline CHEK2 mutation (c.1100delC or p.Thr367Metfs) was identified by MLPA.
Literature review of pancreatic ACCs associated with germline BRCA1/2 mutations
Literature review of ACC in patients with a germline BRCA2 mutation identified nine additional cases (Table 2).7,17–21 The mean age at diagnosis was 62 years (SD: 8.7; range: 50–78), which is similar to sporadic cases.4 Including the current patient, seven males (67%) and three females (33%) were identified, resulting in male-to-female ratio of 2.3:1, corresponding to the male predominance in sporadic ACC.1,2 All specified BRCA2 mutations were truncating.7,17,21 Noteworthy, in all six cases with known zygosity, loss of the wild-type BRCA2 allele was detected.7,17 Three out of 10 patients had a previous personal history of another BRCA2-associated malignancy, including breast, ovarian and prostate cancers.17,18,20 In addition, three patients, including the current, had a family history of BRCA2-associated malignancies and/or family member(s) harboring germline BRCA2 mutations.18,19 Two patients presented with PP as the initial manifestation of ACC.19,20 In addition, literature search identified two ACC patients with BRCA1 germline mutation.22–24 Among 46 ACCs tested by germline sequencing (Furukawa et al.7 (n = 7), Jakel et al.3 (n = 22), Lowery et al.24 (n = 17)), one patient (2.2%) with BRCA124 and two patients (4.3%) with BRCA27 mutations were detected.
Table 2.
Germline BRCA1/2 mutations in pancreatic ACCs.
| Reference, first author | Year | Sex | Age at diagnosis (y) | Gene | CDS Mutation | LOH | Protein | Personal history (y) | Family history (y) |
|---|---|---|---|---|---|---|---|---|---|
| Current case | 2018 | M | 52 | BRCA2 | c.7974C>G | Yes | p.Y2658* | No | Mother: breast cancer (died at 41); sister: serous ovarian adenocarcinoma |
| Li21 | 2018 | M | 59 | BRCA2 | NS | NS | p.I332fs | Hepatitis A for 10 years | Father: died from lung cancer; mother and brother: hepatic carcinoma |
| Lowery24 | 2018 | NS | NS | BRCA1 | NS | NS | NS | NS | NS |
| Naeyaert19a | 2016 | M | 59 | BRCA2 | NS | NS | NS | T2DM, hypercholesterolemia | BRCA2-mutation related breast cancer |
| Ploquin18 | 2015 | M | 50 | BRCA2 | NS | NS | NS | Localized prostatic adenocarcinoma (61) | Mother (60), sister (34): breast cancer; brother: bladder cancer. Patient’s cousin and two sons are carriers of BRCA2 mutation |
| Furukawa7 | 2015 | F | 78 | BRCA2 | c.7115C>G | Yes | p.S2372* | NS | NS |
| M | 59 | BRCA2 | c.4021del | Yes | p.S1341fs | NS | NS | ||
| Lowery22,23 | 2011 | M | NS | BRCA1 | NS | NS | NS | Acromegaly, colonic adenocarcinoma, papillary renal cell carcinoma | Multiple first- and second-degree relatives with early-onset breast cancer |
| Gandhi20a, b | 2010 | F | 63 | BRCA2 | NS | NS | NS | Ovarian cancer | NS |
| Skouldis17 | 2010 | M | 70 | BRCA2 | c.771_775delc | Yes | p.Asn257fs | NS | NS |
| F | 59 | BRCA2 | c.771_775delc | Yes | p.Asn257fs | Lobular breast cancer (58) | NS | ||
| M | 71 | BRCA2 | c.771_775delc | Yes | p.Asn257fs | NS | NS |
Y, year; LOH, loss of heterozygosity; M, male; F, female; NS, not stated; T2DM, type 2 diabetes mellitus.
aPatient presented with PP as an initial manifestation of pancreatic ACC.
bLikely pancreatic ACC and likely germline BRCA2 mutation.
cFormerly known as 999del5.
Literature review of somatic BRCA1/2 mutations in pancreatic ACC
Review of literature detected 12 ACCs with somatic BRCA2 mutations and four – with somatic BRCA1 mutations (Table 3).6,7,9,25,26 Of note, all four ACCs with somatic BRCA1 mutations were identified in a single study based on comprehensive genomic profiling of tumor series consisting of 44 ACCs,9 whereas 11 out of 12 ACCs containing somatic BRCA2 mutations were detected in genome sequencing studies6,7,9 and one additional ACC was described in a case study and patient-derived animal model.25,26
Table 3.
Somatic BRCA1/2 mutations in pancreatic ACCs.
| Reference, first author | Year | Type of ACC | Sex | Age at diagnosis (y) | Gene | CDS Mutation | Protein | Mutation reference |
|---|---|---|---|---|---|---|---|---|
| Furukawa7 | 2015 | Pure ACC | M | 67 | BRCA2 | c.8297delCa | p.T2766fs | COSM4972287 |
| Chmielecki9 | 2014 | Pure ACC | NS | NS | BRCA1 | NS | p.E1250fs | COSM4603639 |
| Pure ACC | NS | NS | BRCA1 | NS | p.E23fs | COSM1666624 | ||
| Mixed ACC/NE | NS | NS | BRCA1 | NS | splice | NS | ||
| Mixed ACC/DA | NS | NS | BRCA1 | NS | p.W1508* | COSM4603640 | ||
| Pure ACC | NS | NS | BRCA2 | NS | p.R3128* | COSM4603643 | ||
| Pure ACC | NS | NS | BRCA2 | NS | p.N1706fs | COSM4603646 | ||
| Pure ACC | NS | NS | BRCA2 | NS | p.S1951fs | COSM4603648 | ||
| Pure ACC | NS | NS | BRCA2 | NS | p.W563fs | COSM4603651 | ||
| Unknown ACCb | NS | NS | BRCA2 | NS | p.S1982fs | COSM166356 | ||
| NS | NS | BRCA2 | NS | p.Q1987fs | COSM4603645 | |||
| Mixed ACC/NE, Unknown ACCc | NS | NS | BRCA2 | NS | p.R645fs | COSM4603647 | ||
| Mixed ACC/NE | NS | NS | BRCA2 | NS | p.N433fs | COSM4603649 | ||
| Unknown ACCc | NS | NS | BRCA2 | NS | p.L659fs | COSM4603650 | ||
| Mixed ACC/DA/NE | NS | NS | BRCA2 | c.4535G>A | p.R1512Hd | COSM4603642 | ||
| Jiao6 | 2014 | Pure ACC | M | 66 | BRCA2 | c.1437C>G | p.D479Ed | COSM1734236 |
| Hall,26Armstrong25e | 2016, 2011 | Pure ACC | M | 61 | BRCA2 | c.1755_1759dela | p.Lys585fs | NS |
ACC, acinar cell carcinoma; Y, year; M, male; F, female; NS, not stated; NE, neuroendocrine; DA, ductal.
aLoss of heterozygosity.
bUnknown ACC stands for ACC with incomplete histological analysis.
cSame unknown ACC.
dLikely benign variants.
eHall et al. (2016) developed a xenograft murine model derived from the liver metastasis of the patient with ACC described in paper by Armstrong et al. (2011), therefore, data was collected from these two reports.
Among ACCs (n = 94) tested for the presence of somatic BRCA1/2 alterations with genome sequencing approaches (Chmielecki et al.9 (n = 44), Jiao et al.6 (n = 21), Furukawa et al.7 (n = 7), Jakel et al.3 (n = 22)),3,6,7,9 overall 15/94 ACCs (16%) harbored a somatic mutation in either BRCA1 or BRCA2. Specifically, 11/94 ACCs (11.7%) contained in total of 12 somatic BRCA2 mutations, and 4/94 ACCs (4.3%) exhibited one somatic BRCA1 mutation each. The majority of BRCA2 mutations were frameshifts introducing a premature stop codon.7,9 Two somatic BRCA2 variants were missense mutations leading to the change of corresponding amino acid.6,9 In silico analysis indicated that these are likely benign variants that should be considered as passenger mutations.
Discussion
Important lessons can be learned from tumors occurring in hereditary settings.27 We describe a novel case of a patient with a germline BRCA2 mutation and pancreatic ACC, and review the literature on the prevalence of germline and somatic BRCA1/2 alterations in ACC. Increasing evidence suggests that at least a subgroup of ACCs develops on basis of germline and somatic BRCA1/2 mutations, however their role in the onset of pancreatic ACC is not yet well recognized. Despite the rarity of ACC, hampering large studies, it is crucial to establish the link between ACC and BRCA1/2 mutations in view of the importance to recognize potentially hereditary tumors and identify patients that may benefit from targeted therapies.
Including the current case, a total of 10 ACC patients with germline BRCA2 mutations have now been reported (Table 2).7,17–21 Loss of the wild-type BRCA2 allele, observed in all cases with known zygosity,7,17 supports the causal relation between BRCA2 germline mutation and the onset of ACC. Moreover, literature review showed that somatic BRCA2 alterations occur in about 12% of ACCs.6,7,9 Involvement of BRCA1 in the onset of ACC remains elusive, although there are indications of this association. So far, only two patients with pancreatic ACC were reported to carry a germline BRCA1 mutation.22–24 Somatic BRCA1 alterations were observed in 4.3% of ACCs.3,6,7,9 However, even more pancreatic ACCs may lack functional BRCA1/2. An immunohistochemical analysis showed loss of BRCA2 expression in 5 out of 11 ACCs (45%)7 and a recent whole-exome sequencing (WES) study showed a mutational signature associated with BRCA1/2 deficiency in 10 out of 22 ACCs (45%) despite the absence of BRCA1/2 mutations.3 Methylation of the BRCA1 promoter was observed in 67% of ACCs in comparison to 28% of PDACs and 50% of islet carcinomas,28 potentially indicating that silencing of BRCA1 is a discriminative characteristic of ACC contributing to its pathogenesis. Importantly, sequencing studies revealed that about 7% of ACCs exhibited germline and up to 16% – somatic mutations in either BRCA1 or BRCA2 genes. Based on abovementioned mechanisms, up to 22% of pancreatic ACCs might exhibit BRCA1/2 deficiency (Table 4). In addition, loss of one BRCA2 allele due to monosomy of chromosome 13 was reported in two ACCs,29 and BRCA2 amplification – in one ACC.30 Further research is needed to define whether these alterations drive the pathogenesis of pancreatic ACC or occur mainly as passengers due to extensive chromosomal instability observed in this tumor type.
Table 4.
BRCA1/2 alterations observed in pancreatic ACC tumor series.
| in studied ACC tumor series |
||||
|---|---|---|---|---|
| Molecular alteration | Frequency | Percentage | Reference | Year |
| Germline BRCA1/2 mutations | 3/46a | 7% | Lowery24 | 2018 |
| Jäkel3 | 2017 | |||
| Furukawa7 | 2015 | |||
| Somatic BRCA1/2 mutations | 15/94b | 16% | Jäkel3 | 2017 |
| Furukawa7 | 2015 | |||
| Chmielecki9 | 2014 | |||
| Jiao6 | 2014 | |||
| IHC loss of BRCA2 expression | 5/11 | 45% | Furukawa7 | 2015 |
| Methylation of BRCA1 promoter | 8/12 | 67% | Guo28 | 2014 |
| Mutational signature associated with BRCA1/2 deficiency | 10/22 | 45% | Jäkel3 | 2017 |
| Overall | 41/185 | 22% | ||
ACC, acinar cell carcinoma; IHC, immunohistochemistry.
aTwo ACCs with BRCA2 and one with BRCA1 germline mutation.
b11 ACCs with BRCA2 and 4 with BRCA1 somatic mutations.
Of note, the actual prevalence of both germline and somatic BRCA1/2 mutations in pancreatic ACC can be under- or overestimated mainly due to the rarity of this tumor type, lack of large-scale genomic analyses and possible underrepresentation in literature. Patients with ACC and germline BRCA1/2 alterations were often described in case reports7,17–20,22,23 hindering the possibility to estimate real portion of ACCs emerged due to BRCA1/2 germline mutations. The heterogeneity observed between sequencing studies is also remarkable: only one out of four studies detected somatic BRCA1 mutations, and one WES study did not identify any BRCA1/2 mutation in 22 ACCs.3
Importantly, all cases of pancreatic ACC in patients with germline7,17 and somatic7,26 BRCA2 mutations with known zygosity demonstrated loss of the wild-type allele indicating a driver role in the tumorigenesis of pancreatic ACC. Moreover, among seven pancreatic cancer cases in the Icelandic Cancer Registry harboring germline Icelandic founder BRCA2999del5 mutation, LOH was observed in all three ACCs (included in this review), but only in one out of four PDACs.17 In the KrasG12D-driven murine model of pancreatic cancer harboring a heterozygous pathogenic germline Brca2 mutation, acinar tumors developed in 5 out of 28 mice (~18%) solely in the cohort with biallelic Brca2 inactivation. Authors hypothesized that LOH of BRCA2 plays a defining role in the triggering of pancreatic carcinogenesis towards acinar lineage,17 however, further research is needed to elucidate the exact role of zygosity status and loss of wild-type BRCA2 allele in the pathogenesis of ACC.
BRCA1 and 2 encode proteins that are crucial for DNA repair by homologous recombination.31 Due to their pivotal role in maintenance of genome integrity, BRCA1/2-deficient tumors are particularly sensitive to therapies introducing cross-linking and DNA damage, namely platinum-based chemotherapies and PARP inhibitors.32 Furukawa et al. reported complete remission in a patient with ACC and liver metastasis and somatic BRCA2 mutation after treatment with cisplatinum.7 Similarly, Ploquin et al. described a prolonged 14-year relapse-free survival of a male BRCA2 germline mutation carrier with ACC and multiple liver and spleen metastases.18 This patient was treated with the GEMOX regimen and demonstrated highest response rate to oxaliplatin chemotherapy.18 A xenograft murine model PA-018, derived from the ACC patient with somatic biallelic BRCA2 mutation,25 demonstrated the most pronounced and continuous response to oxaliplatin.26 Several studies further reported increased sensitivity to platinum chemotherapeutics in other BRCA1/2-deficient cancers,12,33,34 further emphasizing the relevance of platinum-based therapies in patients with BRCA1/2-related ACC. In our case, this advantageous effect was observed as well.
PARP inhibitors exploit the phenomenon of “synthetic lethality” when defects in certain genes are tolerated by cells if occur separately, but are lethal in case of co-occurrence.35 Clinical utility of PARP inhibitors is based on the “two-hit” paradigm for tumor suppressor genes, enabling them to selectively target BRCA1/2-deficient cells while having no effect on BRCA1/2 wild-type or heterozygous cells.17 Tumors of BRCA1/2 germline mutation carriers in approximately 80% of cases experience inactivation of wild-type allele by LOH.31 Moreover, a recent study by Lowery et al. detected LOH of BRCA1 (60%) and BRCA2 (60%) in germline BRCA mutation carriers with exocrine pancreatic malignancies.24 Since LOH of BRCA2 gene was observed in all reported cases of ACC with known zygosity, these tumors are expected to be sensitive to PARP inhibitors. Notably, in a recent case report, Li et al. described a first case of a patient with unresectable advanced pancreatic ACC and a germline BRCA2 mutation that demonstrated a partial response to the treatment with an oral PARP inhibitor olaparib.21
Overall, up to 22% of pancreatic ACCs exhibit various BRCA1/2 alterations (Table 4). This warrants genetic screening for the presence of BRCA1/2 deficiency in all patients diagnosed with pancreatic ACC. Notably, genetic testing of BRCA1/2 genes is widely utilized for patients with breast and ovarian cancers, whereas the prevalence of these mutations in unselected cohort of patients is reported to be only 3% and 10%, respectively.36 Germline mutations in BRCA1/2 predispose to a range of cancers associated with Hereditary Breast and Ovarian Cancer (HBOC) syndrome.37 Given that pancreatic ACC can be a phenotypical expression of germline BRCA1/2 mutations, screening of affected individuals for their carrier status will provide important insights on hereditary predisposition to HBOC also for their unaffected relatives, which can undergo genetic screening to identify their status and potentially benefit from preventive measures.
To conclude, occurrence of ACCs in carriers of BRCA2 germline mutations strongly suggests that at least a part of ACCs arise due to germline BRCA2 mutations. In addition, somatic BRCA1/2 alterations and mutational signature associated with BRCA1/2 deficiency are found in a significant subset of sporadic ACCs. ACC patients should be screened for the presence of BRCA1/2 deficiency, in order to apply personalized therapy and to identify patients with hereditary pancreatic cancer. Overall, this will promote early detection and may improve survival of ACC patients.
Funding Statement
This work was supported by the Dutch Cancer Society under Grant KWF 2016 10289.
Authors contributions
VK – drafting of the manuscript, acquisition of data, analysis and interpretation of data; NHM – acquisition of data, critical revision of the manuscript for important intellectual content; FHMM – acquisition of data, analysis and interpretation of data; MJLL – critical revision of the manuscript for important intellectual content; GJAO – critical revision of the manuscript for important intellectual content; IDN – critical revision of the manuscript for important intellectual content; WWJL – acquisition of data, analysis and interpretation of data, critical revision of the manuscript for important intellectual content; LAAB – study concept and design, analysis and interpretation of data, critical revision of the manuscript for important intellectual content, study supervision.
Disclosure of interest
The authors report no conflict of interest.
Ethical approval
This article was written with an approval of the patient.
References
- 1.Hackeng WM, Hruban RH, Offerhaus GJ, Brosens LA.. 2016. Surgical and molecular pathology of pancreatic neoplasms. Diagn Pathol. 11:47 DOI: 10.1186/s13000-016-0497-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.La Rosa S, Sessa F, Capella C.. 2015. Acinar Cell Carcinoma of the Pancreas: overview of Clinicopathologic Features and Insights into the Molecular Pathology. Front Med. 2 DOI: 10.3389/fmed.2015.00061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jakel C, Bergmann F, Toth R, Assenov Y, van der Duin D, Strobel O. 2017. Genome-wide genetic and epigenetic analyses of pancreatic acinar cell carcinomas reveal aberrations in genome stability. Nat Commun. 8:1323 DOI: 10.1038/s41467-017-01118-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Toll AD, Hruban RH, Ali SZ. 2013. Acinar cell carcinoma of the pancreas: clinical and cytomorphologic characteristics. J Pathol Transl Med. 47:93–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taruscio D, Paradisi S, Zamboni G, Rigaud G, Falconi M, Scarpa A. 2000. Pancreatic acinar carcinoma shows a distinct pattern of chromosomal imbalances by comparative genomic hybridization. Genes Chromosomes Cancer. 28:294–299. DOI: 10.1002/(ISSN)1098-2264. [DOI] [PubMed] [Google Scholar]
- 6.Jiao Y, Yonescu R, Offerhaus GJ, Klimstra DS, Maitra A, Eshleman JR. 2014. Whole-exome sequencing of pancreatic neoplasms with acinar differentiation. J Pathol. 232:428–435. DOI: 10.1002/path.4310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Furukawa T, Sakamoto H, Takeuchi S, Ameri M, Kuboki Y, Yamamoto T. 2015. Whole exome sequencing reveals recurrent mutations in BRCA2 and FAT genes in acinar cell carcinomas of the pancreas. Sci Rep. 5 DOI: 10.1038/srep08829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abraham SC, Wu TT, Hruban RH, Lee JH, Yeo CJ, Conlon K. 2002. Genetic and immunohistochemical analysis of pancreatic acinar cell carcinoma: frequent allelic loss on chromosome 11p and alterations in the APC/beta-catenin pathway. Am J Pathol. 160:953–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chmielecki J, Hutchinson KE, Frampton GM, Chalmers ZR, Johnson A, Shi C. 2014. Comprehensive genomic profiling of pancreatic acinar cell carcinomas identifies recurrent RAF fusions and frequent inactivation of DNA repair genes. Cancer Discov. 4:1398–1405. DOI: 10.1158/2159-8290.CD-14-0617. [DOI] [PubMed] [Google Scholar]
- 10.Bergmann F, Aulmann S, Sipos B, Kloor M, von Heydebreck A, Schweipert J. 2014. Acinar cell carcinomas of the pancreas: a molecular analysis in a series of 57 cases. Virchows Archiv. 465:661–672. DOI: 10.1007/s00428-014-1657-8. [DOI] [PubMed] [Google Scholar]
- 11.Shindo K, Yu J, Suenaga M, Fesharakizadeh S, Cho C, Macgregor-Das A. 2017. Deleterious germline mutations in patients with apparently sporadic pancreatic adenocarcinoma. J Clin Oncol. 35:3382–3390. DOI: 10.1200/JCO.2017.72.3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Imyanitov EN, Moiseyenko VM. 2011. Drug therapy for hereditary cancers. Hered Cancer Clin Pract. 9:5 DOI: 10.1186/1897-4287-9-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Basturk O, Zamboni G, Klimstra DS, Capelli P, Andea A, Kamel NS. 2007. Intraductal and papillary variants of acinar cell carcinomas: a new addition to the challenging differential diagnosis of intraductal neoplasms. Am J Surg Pathol. 31:363–370. DOI: 10.1097/01.pas.0000213376.09795.9f. [DOI] [PubMed] [Google Scholar]
- 14.Conroy T, Desseigne F, Ychou M, Bouche O, Guimbaud R, Becouarn Y. 2011. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 364:1817–1825. DOI: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 15.de Leng WWJ, Gadellaa-van Hooijdonk CG, Barendregt-Smouter FAS, Koudijs MJ, Nijman I, Hinrichs JWJ, et al. 2016. Targeted next generation sequencing as a reliable diagnostic assay for the detection of somatic mutations in tumours using minimal DNA amounts from formalin fixed paraffin embedded material. PLoS One. 11:e0149405 DOI: 10.1371/journal.pone.0149405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Landrum MJ, Lee JM, Benson M, Brown G, Chao C, Chitipiralla S. 2016. ClinVar: public archive of interpretations of clinically relevant variants. Nucleic Acids Res. 44:D862–8. DOI: 10.1093/nar/gkv1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Skoulidis F, Cassidy LD, Pisupati V, Jonasson JG, Bjarnason H, Eyfjord JE. 2010. Germline Brca2 heterozygosity promotes Kras(G12D) -driven carcinogenesis in a murine model of familial pancreatic cancer. Cancer Cell. 18:499–509. DOI: 10.1016/j.ccr.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 18.Ploquin A, Baldini C, Vuagnat P, Makhloufi S, Desauw C, Hebbar M. 2015. Prolonged survival in a patient with a pancreatic acinar cell carcinoma. Case Rep Oncol. 8:447–450. DOI: 10.1159/000441414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Naeyaert C, de Clerck F, De Wilde V. 2016. Pancreatic panniculitis as a paraneoplastic phenomenon of a pancreatic acinar cell carcinoma. Acta Clin Belg. 71:448–450. DOI: 10.1080/17843286.2016.1168065. [DOI] [PubMed] [Google Scholar]
- 20.Gandhi RK, Bechtel M, Peters S, Zirwas M, Darabi K. 2010. Pancreatic panniculitis in a patient with BRCA2 mutation and metastatic pancreatic adenocarcinoma. Int J Dermatol. 49:1419–1420. DOI: 10.1111/j.1365-4632.2009.04435.x. [DOI] [PubMed] [Google Scholar]
- 21.Li M, Mou Y, Hou S, Cao D, Li A. 2018. Response of germline BRCA2-mutated advanced pancreatic acinar cell carcinoma to olaparib: A case report. Medicine. 97:e13113 DOI: 10.1097/MD.0000000000013113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lowery MA, Kelsen DP, Stadler ZK, Yu KH, Janjigian YY, Ludwig E. 2011. An emerging entity: pancreatic adenocarcinoma associated with a known BRCA mutation: clinical descriptors, treatment implications, and future directions. Oncologist. 16:1397–1402. DOI: 10.1634/theoncologist.2011-0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lowery MA, Klimstra DS, Shia J, Yu KH, Allen PJ, Brennan MF. 2011. Acinar cell carcinoma of the pancreas: new genetic and treatment insights into a rare malignancy. Oncologist. 16:1714–1720. DOI: 10.1634/theoncologist.2011-0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lowery MA, Wong W, Jordan EJ, Lee JW, Kemel Y, Vijai J. 2018. Prospective evaluation of germline alterations in patients with exocrine pancreatic neoplasms. J Natl Cancer Inst. DOI: 10.1093/jnci/djy024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Armstrong MD, Von Hoff D, Barber B, Marlow LA, von Roemeling C, Cooper SJ. 2011. An effective personalized approach to a rare tumor: prolonged survival in metastatic pancreatic acinar cell carcinoma based on genetic analysis and cell line development. J Cancer. 2:142–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hall JC, Marlow LA, Mathias AC, Dawson LK, Durham WF, Meshaw KA. 2016. Novel patient-derived xenograft mouse model for pancreatic acinar cell carcinoma demonstrates single agent activity of oxaliplatin. J Transl Med. 14 DOI: 10.1186/s12967-016-0867-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kinzler KW, Vogelstein B. 1996. Lessons from hereditary coloractal cancer. Cell. 87:159–170. DOI: 10.1016/S0092-8674(00)81333-1 [DOI] [PubMed] [Google Scholar]
- 28.Guo MZ, Jia Y, Yu Z, House MG, Esteller M, Brock MV. 2014. Epigenetic changes associated with neoplasms of the exocrine and endocrine pancreas. Discov Med. 17:67–73. [PMC free article] [PubMed] [Google Scholar]
- 29.Dewald GW, Smyrk TC, Thorland EC, McWilliams RR, Van Dyke DL, Keefe JG. 2009. Fluorescence in situ hybridization to visualize genetic abnormalities in interphase cells of acinar cell carcinoma, ductal adenocarcinoma, and islet cell carcinoma of the pancreas. Mayo Clinic Proceedings. 84:801–810. DOI: 10.1016/S0025-6196(11)60490-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Wilde RF, Ottenhof NA, Jansen M, Morsink FH, de Leng WW, Offerhaus GJ. 2011. Analysis of LKB1 mutations and other molecular alterations in pancreatic acinar cell carcinoma. Modern Pathology. 24:1229–1236. DOI: 10.1038/modpathol.2011.83. [DOI] [PubMed] [Google Scholar]
- 31.Greer JB, Whitcomb DC. 2007. Role of BRCA1 and BRCA2 mutations in pancreatic cancer. Gut. 56:601–605. DOI: 10.1136/gut.2006.101220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Golan T, Kanji ZS, Epelbaum R, Devaud N, Dagan E, Holter S. 2014. Overall survival and clinical characteristics of pancreatic cancer in BRCA mutation carriers. Br J Cancer. 111:1132–1138. DOI: 10.1038/bjc.2014.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rigakos G, Razis E. 2012. BRCAness: finding the achilles heel in ovarian cancer. Oncologist. 17:956–962. DOI: 10.1634/theoncologist.2012-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Waddell N, Pajic M, Patch A-M, Chang DK, Kassahn KS, Bailey P. 2015. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature. 518:495–501. DOI: 10.1038/nature14169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lord CJ, Ashworth A. 2017. PARP inhibitors: synthetic lethality in the clinic. Science. 355:1152–1158. DOI: 10.1126/science.aam7344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Know:BRCA [Accessed 2018 August8]. https://www.knowbrca.org/Provider/FNA/.
- 37.Lux MP, Fasching PA, Beckmann MW. 2006. Hereditary breast and ovarian cancer: review and future perspectives. J Mol Med (Berl). 84:16–28. DOI: 10.1007/s00109-005-0696-7. [DOI] [PubMed] [Google Scholar]


