Abstract
Long-acting bronchodilators are the cornerstone of pharmacologic treatment of chronic obstructive pulmonary disease (COPD). Spiolto® or Stiolto® is a fixed-dose combination (FDC) containing two long-acting bronchodilators, the long-acting muscarinic receptor antagonist tiotropium (TIO) and the long-acting β2-adrenoceptor agonist olodaterol (OLO), formulated in the Respimat® Soft Mist™ inhaler. A total of 13 large, multicentre studies of up to 52 weeks’ duration have documented its efficacy in more than 15,000 patients with COPD. TIO/OLO 5/5 µg FDC significantly increases pulmonary function compared with placebo and its respective constituent mono-components TIO 5 µg and OLO 5 µg. TIO/OLO 5/5 µg also results in statistically and clinically significant improvements in patient-reported outcomes, such as dyspnoea, use of rescue medication, and health status. Addition of OLO 5 µg to TIO 5 µg reduces the rate of moderate-to-severe exacerbations by approximately 10%. Compared with placebo and TIO 5 µg, TIO/OLO 5/5 µg significantly improves exercise capacity (e.g. endurance time) and physical activity, the latter increase being reached by a unique combination behavioural modification intervention, dual bronchodilatation and exercise training. Overall, the likelihood for patients to experience a clinically significant benefit is higher with TIO/OLO 5/5 µg than with its constituent mono-components, which usually yield smaller improvements which do not always reach statistical significance, compared with baseline or placebo. This supports the early introduction of TIO/OLO 5/5 µg in the management of patients with symptomatic COPD.
Keywords: bronchodilatation, COPD, dyspnoea, exacerbation, exercise tolerance, hyperinflation, inspiratory capacity, LABA, LAMA, physical activity, spirometry
Introduction
Chronic obstructive pulmonary disease (COPD) is characterized by persistent airflow limitation caused by smoking or exposure to noxious gases. Typical symptoms are reduced exercise tolerance, breathlessness that progresses over the years, cough and sputum production, which worsen acutely during an exacerbation and require additional treatment, unscheduled medical visits and even hospital care. COPD eventually leads to reduced activities of daily living, poor quality of life and premature death.1
Data provided by the Global Burden of Disease study indicate that more than 300 million people have COPD worldwide. Of these, 4.7 million will die from COPD in 2020, corresponding to the third place in the world ranking of causes of death, just behind ischemic heart disease and stroke. COPD also contributes to 29.4 million years lost to disability worldwide.2
In Europe, COPD was responsible in 2014 for €38.7 billion of direct medical costs. In the United States (US), direct medical costs burden the healthcare budget with US$31.2 billion and a further US$3.9 billion because of absenteeism costs.2,3
COPD is not curable, but strategies to reduce its global burden have been developed, comprising smoking cessation, avoidance of exposure to occupational risk factors, prevention and management of comorbidities and promotion of physical activity. The management of the individual patient with COPD aims to alleviate symptoms, improve health status and exercise tolerance, and reduce the frequency and severity of exacerbations. It basically consists of influenza and pneumococcal vaccination, smoking cessation, pulmonary rehabilitation as well as treatment with bronchodilating and anti-inflammatory drugs.1
The 2019 Global Initiative for Chronic Obstructive Lung Disease (GOLD) strategy recommends primarily the use of one long-acting bronchodilator [either a long-acting β2-agonist (LABA) or a long-acting muscarinic antagonist (LAMA)] as the first-line therapy for patients with symptomatic COPD, classified as GOLD B patients, and proposes to escalate this to dual long-acting bronchodilatation in the case of persistent symptoms. Likewise, dual long-acting bronchodilatation is the preferred option in COPD GOLD C patients, the oligosymptomatic patients who experience recurrent exacerbations, in cases of an insufficient response to a LAMA as first-line treatment. For GOLD group D patients, dual bronchodilatation is one among several proposed treatment options.1
In the light of these recommendations and the available scientific evidence, several formulations containing both a LAMA and a LABA have been developed as fixed-dose combination (FDC) and approved for marketing in the treatment of COPD. Tiotropium-olodaterol 5/5 µg (TIO/OLO 5/5 µg) once daily (Spiolto® or Stiolto® formulated in the Respimat® Soft Mist™ inhaler; Boehringer Ingelheim, Ingelheim, Germany) and umeclidinium-vilanterol 55/22 μg (UMEC/VIL 55/22 µg) once daily (Laventair-Anoro® Ellipta®; GlaxoSmithKline PLC, London, United Kingdom) are approved worldwide. Aclidinium-formoterol 340/12 µg twice daily (Duaklir® Genuair®; AstraZeneca PLC, London, United Kingdom) and glycopyrronium-indacaterol 85/43 µg (GLY/IND 85/43 µg) once daily (QVA149; Ultibro® Breezhaler®, Novartis International AG, Basel, Switzerland) are approved in Europe. In the US, however, the GLY/IND 27.5/12.5 µg formulation is only approved at a twice daily dose and marketed as Utibron™ Neohaler®, as are two doses twice daily of glycopyrrolate-formoterol 18/9.6 µg (Bevespi, Aerosphere®).
We have previously extensively reviewed the therapeutic efficacy of TIO/OLO 5/5 µg FDC at a once-daily dose in patients with COPD. We described in detail its effects on lung function, dyspnoea and health-related quality of life (HR-QoL) compared with placebo, the respective mono-components tiotropium (TIO) 5 µg and olodaterol (OLO) 5 µg, and the LABA-inhaled corticosteroid (ICS) FDC salmeterol-fluticasone (SALM/FLU).4 The present review will primarily focus on the phase III studies published as abstract (MORACTO, TORRACTO5,6) or unpublished (VESUTO, DYNAGITO, PHYSACTO, OTIVATO7–10) at the time of our previous review: effects of TIO/OLO 5/5 μg on lung volumes, exercise tolerance, physical activity and exacerbations. This publication will also assess some of the post hoc analyses of previously published material (OTEMTO, TONADO11,12) dealing with cost-effectiveness and safety. It will also focus on the effects in Japanese patients. In the first paragraphs, we shall, however, briefly reassess the effects of TIO/OLO 5/5 μg on spirometry, HR-QoL and dyspnoea.
Most of the data, analysed in the present review, are derived from 13 phase III, multicentre, randomized, double-blind, controlled studies conducted in over 16,500 patients with COPD. All studies (six of which were replicate studies) are published as full papers in peer-reviewed journals and briefly summarised in Table 1. Overall, six studies (VIVACITO,13 ENERGITO,14 MORACTO I and II,5 VESUTO,9 OTIVATO10), used a crossover design, seven studies (OTEMTO I and II,11 VIVACITO,13 TORRACTO,6 MORACTO I + II,5 PHYSACTO8) were placebo controlled, and five studies (TONADO 1 + 2,12 VESUTO,9 OTIVATO10 and DYNAGITO7) compared TIO/OLO with TIO or OLO as monotherapy. In one trial, the ENERGITO study, TIO/OLO 5/5 µg was compared with SALM/FLU.14
Table 1.
Key design details of 13 completed phase III studies in patients with COPD comparing clinical effects of tiotropium/olodaterol fixed-dose combinations (TIO/OLO 5/5 µg) with mono-components or placebo in patients with COPD.
| Study name | First author (year of publication) | Daily treatments (n of patients randomized) | Primary endpoint | Key secondary endpoints |
|---|---|---|---|---|
| TONADO 1+212 | Buhl (2015) |
TIO/OLO 5/5 μg (522/507) TIO/OLO 2.5/5 μg (522/508) OLO 5 μg (528/510) TIO 5 μg (527/506) TIO 2.5 µg (525/507) |
FEV1 AUC0–3 at wk 24 Trough FEV1 at wk 24 SGRQ at wk 24 |
TDI at wk 24 |
| VIVACITO13 | Beeh (2015) |
TIO/OLO 5/5 μg (139) TIO/OLO 2.5/5 μg (136) OLO 5 μg (138) TIO 5 μg (138) TIO 2.5 µg (137) PLA (138) |
FEV1 AUC0–24 at wk 6 | FEV1 AUC0–12 at wk 6 FEV1 AUC12–24 at wk 6 Peak FEV1 AUC0–3 at wk 6 Trough FEV1 at wk 6 ΔRV and ΔFRC at wk 6 |
| OTEMTO 1+211 | Singh (2015) | TIO/OLO 5/5 μg (204/202) TIO/OLO 2.5/5 μg (202/202) TIO 5 μg (204/203) PLA (204/202) |
SGRQ at wk 12 FEV1AUC0–3 at wk 12 Trough FEV1 at wk 12 |
TDI at wk 12 |
| ENERGITO14 | Beeh (2016) |
TIO/OLO 5/5 μg (221) TIO/OLO 2.5/5 μg (215) SALM/FLU 50/500 µg bid (215) SALM/FLU 50/250 µg bid (212) |
FEV1 AUC0–12 at wk 6 | FEV1 AUC0–24
FEV1 AUC12–24 at wk 6 Peak FEV1 AUC0–3 at wk 6 Trough FEV1 at wk 6 |
| TORRACTO6 | Maltais (2018) | TIO/OLO 5/5 μg (139) TIO/OLO 2.5/5 μg (133) PLA (132) |
CWRCE at wk 12 | ESWT at wk 6 and 12, CWRCE at wk 6, pre-exercise IC and IC during exercise at wk 12 |
| MORACTO 1+25 | O’Donnell (2017) | TIO/OLO 5/5 μg (226/224) TIO/OLO 2.5/5 μg (223/219) TIO 5 μg (227/218) OLO 5 µg (217/219) PLA (222/216) |
Pre-exercise IC at wk 6 CWRCE at wk 6 |
Borg–time slope/endurance time, FVC 1 h post-dose, isotime and exercise values for IC |
| VESUTO9 | Ichinose (2018) | TIO/OLO 5/5 μg (184) TIO 5 μg (184) |
Pre-exercise IC at wk 6 | FEV1, FVC 30 min post-dose, 6MWD, physical activity at wk 6 |
| PHYSACTO8 | Troosters (2018) | SMBM + TIO/OLO + exercise (70) SMBM + TIO/OLO 5/5 μg (72) SMBM + TIO 5 μg (67) SMBM + PLA (65) |
ESWT at wk 8 | ESWT at wk 12, 6MWD, spirometry, IC, CRQ-SAS, mMRC, rescue medication, physical activity |
| DYNAGITO7 | Calverley (2018) | TIO/OLO 5/5 µg (3939) TIO 5 µg (3941) |
Moderate and severe exacerbations over 52 wk | Time to first moderate or severe exacerbation, rate of exacerbations leading to hospitalization, time to first exacerbation leading to hospitalization, time to all-cause mortality, rate of exacerbations treated with corticosteroids or antibiotics |
| OTIVATO10 | Maltais (2019) | TIO/OLO 5/5 μg (106) TIO 5 μg (106) |
Borg Scale at the end of the 3-min CSST at wk 6 | IC, FEV1 post-dose, Borg scale at 1, 2 and 2.5 min during the 3-min CSST, TDI at wk 6 |
6MWD, 6-minute walking distance; AUC, area under the curve; COPD, chronic obstructive pulmonary disease; CRQ-SAS, chronic respiratory disease questionnaire-self-administered standardized; CSST, constant speed shuttle test; CWRCE, constant work-rate cycle endurance time at 75% of peak work rate; ESWT, endurance time during shuttle walk test; FEV1, forced expiratory volume in one second; FVC, forced vital capacity; IC, inspiratory capacity; mMRC, modified Medical Research Council; OLO, olodaterol; PLA, placebo; SALM/FLU, salmeterol/fluticasone; SGRQ, St. George’s respiratory questionnaire score; TDI, transitional dyspnoea index; TIO, tiotropium; TIO/OLO, tiotropium/olodaterol fixed-dose combination, wk, week.
The present review focuses on TIO/OLO 5/5 μg, the only recommended dosage formulated in a Soft Mist™ inhaler and given as two inhalations of TIO/OLO 2.5/2.5 μg once daily. It will not analyse data obtained at a lower dose of 2.5 µg published in some studies, nor compare the efficacy and safety of TIO/OLO 5/5 µg with other LABA/LAMA FDCs. Different network analyses and systematic reviews have addressed this topic recently, showing that if differences between FDCs exist, these are often small and of unclear clinical significance.15–24 Though limited in number, well-designed head-to-head comparisons show that differences between FDCs may exist.25 The only head-to-head comparison between TIO/OLO FDC and UMEC/VIL FDC published so far showed a 52 ml difference in trough forced expiratory volume in 1 second (FEV1) in favour of UMEC/VIL.26 In that study, however, methodological differences such as unsupervised inhalation of the study medication and the assessment of the bronchodilating effect at only one point in time might have interfered with eventual results. The pharmacological properties of TIO/OLO 5/5 μg FDC, which have been reviewed in detail elsewhere27–29 also fall beyond the scope of the present review.
Therapeutic efficacy on spirometric variables
The VIVACITO study as well as the replicate OTEMTO and TONADO studies have assessed the effects of TIO/OLO 5/5 µg on spirometry over 6, 12, and 52 weeks, respectively. Statistically significant and clinically meaningful improvements in FEV1, defined as an increase exceeding 100–140 ml (Table 2), were observed for peak FEV1 and the FEV1 area under the curve (AUC) from 0 to 3 h (FEV1 AUC0–3), FEV1 AUC0–12, FEV1 AUC12–24 and FEV1 AUC0–24, compared with placebo,11,13 the mono-components TIO 5 µg11–13 and OLO 5 µg12,13 and SALM/FLU FDC.14 For example, the increase in FEV1 3 h after inhalation of TIO/OLO 5/5 µg exceeded 300 ml compared with placebo and 100 ml compared with its mono-component TIO 5 µg. Even for trough FEV1, the increase of 162 ml and 166 ml relative to placebo reached statistical and clinical significance.11 In the six randomized controlled clinical trials (RCTs) in which TIO/OLO 5/5 µg was compared with TIO, OLO or SALM/FLU FDC, differences between dual bronchodilatation and single bronchodilatation were statistically significant, but just failed to reach the minimal clinically important difference (MCID) of 100 ml.4
Table 2.
Differences between TIO/OLO 5/5 μg and PLA, TIO 5 µg monotherapy, and OLO 5 µg monotherapy for different spirometric endpoints, expressed as change from baseline.
| Study | AUC0–24 FEV1 (ml) | AUC0–3 FEV1 (ml) | Trough FEV1 (ml) |
|---|---|---|---|
| TIO/OLO 5/5 µg versus PLA | |||
| Beeh13 | Δ = 280 | 201 versus −6 | |
| Singh 1+211 | Δ = 331 and Δ = 299 | Δ = 162 and Δ = 166 | |
| TIO/OLO 5/5 µg versus TIO 5 µg | |||
| Beeh13 | Δ = 110 | 201 versus 122 | |
| Singh11 | Δ = 111 and Δ = 105 | Δ = 28 and Δ = 39 | |
| Buhl 1+212 | Δ = 117 and Δ = 103 | Δ = 71 and Δ = 50 | |
| TIO/OLO 5/5 µg versus OLO 5 µg | |||
| Beeh13 | Δ = 115 | 201 versus 109 | |
| Buhl 1+212 | Δ = 123 and Δ = 132 | Δ = 82 and Δ = 88 | |
AUC, area under the curve; FEV1, forced expiratory volume in one second; MCID, minimal clinically important difference; OLO, olodaterol; PLA, placebo; TIO, tiotropium; TIO/OLO, tiotropium/olodaterol fixed-dose combination.
MCID for FEV1 equals 100–140 ml.30
Breathlessness
The Mahler Transition Dyspnea Index (TDI) was assessed in the OTEMTO and TONADO studies.11,12 In OTEMTO, TDI focal scores significantly improved after 12 weeks by 1.62 units with TIO/OLO 5/5 μg compared with placebo and 0.59 units compared with TIO 5 μg. In TONADO, TDI scores significantly improved after 24 weeks by 0.36 and 0.42 units with TIO/OLO 5/5 μg compared with TIO 5 µg and OLO 5 µg, respectively. A response analysis of the OTEMTO and TONADO studies showed that 54% of the patients treated with TIO/OLO 5/5 μg achieved MCID of one unit improvement in TDI, which contrasts with the 41–50% of the patients on TIO 5 µg, 48% of those on OLO 5 µg and 26% of those on placebo.4 Overall, these two studies indicate that more patients receiving TIO/OLO 5/5 µg were likely to achieve clinically important improvements in TDI than with the mono-components (Table 3). Indeed, the risk ratio (RR) was 1.17 [95% confidence interval (CI): 1.07, 1.28] for TIO/OLO 5/5 µg versus TIO 5 µg and 1.14 (95% CI: 1.01, 1.28) versus OLO 5 µg.31
Table 3.
Differences between TIO/OLO 5/5 μg and PLA, TIO 5 µg monotherapy, and OLO 5 µg monotherapy for two patient-centred outcomes with response rates and numbers needed to treat.
| Study | TDI | TDI responders (%) | NNT (95% CI) | SGRQ | SGRQ responders (%) | NNT (95% CI) |
|---|---|---|---|---|---|---|
| TIO/OLO 5/5 µg versus PLA | ||||||
| Singh 1+211 | 2.1 and 1.2 | 53.9 versus 26.2 | 4 (3.1–4.2) | −4.89 and −4.59 | 53.1 versus 31.2 and 51.8 versus 32.6 |
5 (3.8–5.7) and 6 (4.2–6.6) |
| TIO/OLO 5/5 µg versus TIO 5 µg | ||||||
| Singh 1+211 | 0.6 and 0.6 | 53.9 versus 41.0 | 8 (5.8–11.7) | −2.49 and −1.72 | 53.1 versus 41.7 and 51.8 versus 41.1 |
9 (6.3–14.2) and 10 (6.6–15.7) |
| Buhl 1+212 | 0.4 | 54.9 versus 50.6 (NS) | 24 (NS) | −1.23 | 57.5 versus 48.7 | 12 (7.6–22.5) |
| TIO/OLO 5/5 µg versus OLO 5 µg | ||||||
| Buhl 1+212 | 0.4 | 54.9 versus 48.2 | 15 (9.0–42.9) | −1.69 | 57.5 versus 44.8 | 8 (5.9–12.0) |
CI, confidence interval; HR-QoL, health-related quality of life; NNT, number needed to treat; NS, nonsignificant; OLO, olodaterol; PLA, placebo; SGRQ, St. George’s respiratory questionnaire score; TDI, Transitional Dyspnea Index; TIO, tiotropium; TIO/OLO, tiotropium/olodaterol fixed-dose combination.
The number of patients deteriorating over time in terms of dyspnoea under a given treatment represents another approach to compare different interventions. In OTEMTO, less than 11% of patients on any active treatment worsened compared with placebo (23%) at week 12.34 In TONADO, patients on TIO/OLO 5/5 µg showed a delayed onset to deteriorate in dyspnoea over 52 weeks, compared with either monotherapy, with hazard ratios (HRs) for TDI of 0.84 (95% CI: 0.72, 0.98) for TIO/OLO 5/5 µg versus TIO 5 µg and 0.82 (95% CI: 0.71, 0.96) for TIO/OLO 5/5 µg versus OLO 5 µg.34
In the PHYSACTO study, the dyspnoea domain of the Chronic Respiratory Questionnaire-Self Administered Standardized (CRQ-SAS) at week 12 increased significantly in the placebo-treated patients to whom a 12-week self-management behaviour modification (SMBM) was offered, compared with baseline (5.59 versus 5.35 points per question). Further improvements in the CRQ-SAS dyspnoea domain score were observed at week 12 with increasingly potent additional interventions, reaching statistical significance and largely exceeding the MCID of 0.5 units for the average score on each domain35 with TIO/OLO 5/5 µg with or without exercise training, compared with placebo. The modified Medical Research Council (mMRC) scale of dyspnoea data were consistent with the CRQ-SAS dyspnoea domain results.8
In the two-period crossover OTIVATO study,10 the effects of TIO/OLO 5/5 µg were compared with those of TIO 5 µg in GOLD stage II–III COPD patients with lung hyperinflation at rest [functional residual capacity (FRC) at baseline: >155% predicted] and a significant degree of breathlessness during every day activities. After 6 weeks, Borg dyspnoea score had decreased at the end of the 3-min constant speed shuttle test (CSST) from baseline with both TIO 5 µg (mean −0.968; 95% CI: −1.238,−0.698) and TIO/OLO 5/5 µg (mean: −1.325; 95% CI: −1.594,−1.056), with a treatment difference of −0.357 (95% CI: −0.661, −0.053). The number needed to treat (NNT) was seven to achieve the MCID for activity-related breathlessness for TIO/OLO 5/5 µg compared with TIO 5 µg.10
Use of rescue medication
In COPD, increased use of rescue medication is usually a sign of increased respiratory symptoms, whereas a decrease in its use is a marker of clinical efficacy and reduced risk of exacerbations.36,37 Moreover, night-time rescue medication usage may be an important surrogate for night-time symptom control and sleep disturbance, which is known to impact on HR-QoL in COPD.
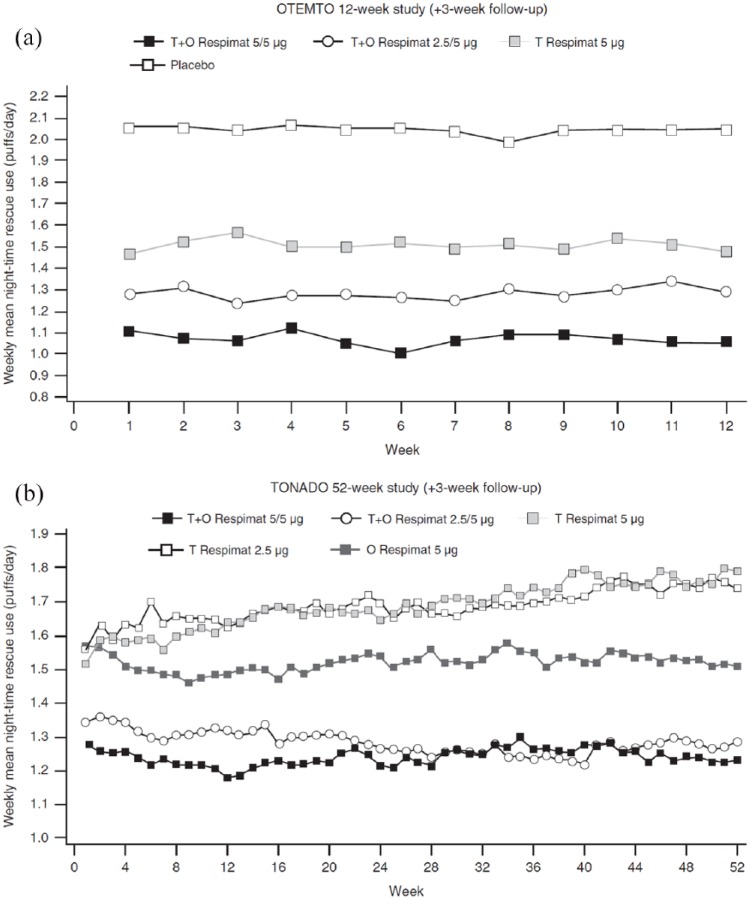
Rescue medication use or days and nights free of rescue medication were assessed in 5 of the 11 trials in which the effects of TIO/OLO 5/5 µg were assessed. In the 12-week OTEMTO study, overall use of rescue salbutamol diminished from 3.0 puffs/day in placebo recipients to 2.4 puffs/day in TIO 5 µg-treated patients and to 1.8 puffs/day in the TIO/OLO 5/5 µg group.11 At night, patients on TIO/OLO 5/5 μg needed less rescue medication, as they reduced their use at week 12 by 0.42 (95% CI: −0.64, −0.20) puff/night compared with TIO 5 µg and by 1.00 (95% CI: −1.22, −0.78) puff/night compared with placebo [Figure 1(a)].
Figure 1.
Adjusted weekly mean night-time rescue medication use over 12 weeks in the OTEMTO studies (a) and over 52 weeks in the TONADO studies (b).
Reproduced from Ferguson and colleagues, 2017.34
O, olodaterol; T, tiotropium.
Throughout the 52-week follow up in the TONADO studies, TIO/OLO 5/5 µg significantly reduced the use of salbutamol rescue medication, its daytime use falling from 0.97 puff/day with TIO 5 µg to 0.76 puff/day with TIO/OLO 5/5 μg.4,12 In the same trial, the common baseline use of rescue medication was 2.2 puffs/night. Rescue medication use reduced significantly for patients receiving TIO/OLO 5 µg by 0.55 (95% CI: −0.72, −0.39) puff/night compared with TIO 5 µg and 0.28 (95% CI: −0.44, −0.11) puff/night compared with OLO 5 µg at 52 weeks (Figure 1, panel B).
The recently published PHYSACTO study investigated the use of rescue medication during physical activity. A reduction in rescue salbutamol use was observed in all actively treated groups compared with placebo at week 9 and 12, the effect tending to be more pronounced in patients treated with dual bronchodilatation.8
Health-related quality of life
HR-QoL of patients with COPD is only loosely related to the degree of spirometric impairment.38 For that reason, measuring the effects of therapeutic interventions on HR-QoL using the St. George’s Respiratory Questionnaire (SGRQ) represents an important complement of spirometric data.
The SGRQ total scores increased in all studies with TIO/OLO 5/5 µg compared with the respective monotherapies or placebo. In the OTEMTO 1 and 2 studies, SGRQ improved statistically at 12 weeks by 4.89 and 4.56 points with TIO/OLO 5/5 μg versus placebo,11 respectively, an increase that exceeded the MCID of 4 points (Table 2). Compared with TIO 5 µg, the improvement with TIO/OLO 5/5 µg ranged between 2.49 points and 1.72 points, which was also statistically significant, but did not reach the MCID. Response rates (the percentage of patients in whom the MCID was reached) were 41% in the TIO 5 µg recipients and 52% in the TIO/OLO 5/5 μg group (Table 3). This indicates that many patients do well with monotherapy and only a restricted number of patients (11%) reach the MCID with dual bronchodilatation, with a NNT to reach the MCID of 10.4 In the OTEMTO study, an important Hawthorne effect was present, as 32% of the placebo recipients were ranked as SGRQ responders,11 rendering the interpretation of these response rates difficult.
In the TONADO studies, 58% of patients who were on TIO/OLO 5/5 µg were classified as SGRQ responders after 24 weeks, compared with 49% on TIO 5 µg and 45% on OLO 5 µg.12 More participants receiving TIO/OLO 5/5 µg had a clinically meaningful difference in SGRQ in favour of dual treatment compared with TIO 5 µg (RR: 1.21; 95% CI: 1.12, 1.30) or OLO 5 µg (RR: 1.28; 95% CI: 1.18, 1.40).31
A post hoc analysis of the OTEMTO studies reported that the magnitude of the improvement in SGRQ with TIO/OLO 5/5 µg, TIO 5 µg and placebo was dependent on GOLD COPD stage, with greater effects in symptomatic patients.39 When analysed by baseline breathlessness, the difference between SGRQ scores achieved by TIO/OLO 5/5 μg compared with TIO 5 µg was greatest in patients with a mMRC of 2 or more compared with those with an mMRC <2.40 This indicates that the greatest benefit of combination bronchodilator therapy can be expected in the more symptomatic patients.
Assessing the preventive effect of an intervention on change over time of the SGRQ is another way to establish the validity of a treatment. In the OTEMTO studies, the likelihood to experience a deterioration in HR-QoL at week 12 was significantly lower in recipients of TIO/OLO 5/5 μg (18%) compared with those in the TIO 5 μg (23%) or placebo group (30%), with no statistically significant difference between the latter two groups. Likewise, significantly fewer patients deteriorated in terms of SGRQ with TIO/OLO 5/5 μg (14%) than with either monotherapy (19%) in the TONADO studies at week 24. In a further analysis, the time to SGRQ deterioration over 52 weeks in TONADO was significantly delayed for TIO/OLO 5/5 μg with HR of 0.82 (95% CI : 0.70, 0.96) versus TIO 5 μg and 0.70 (95% CI: 0.60, 0.82) versus OLO.34
Lung volumes
One of the characteristic clinical features of COPD is airflow limitation, caused by the increased resistance of conducting airways and the heterogeneous pathological alterations of the elastic properties of the lung. Both mechanisms are responsible for the ineffective gas emptying during expiration, the latter only for the increase in end-expiratory lung volume (EELV) at rest (static hyperinflation). A temporary and variable increase in EELV above the resting volume of the respiratory system often develops in patients with COPD during exercise as a consequence of the shortening of expiratory time during exercise, a phenomenon termed dynamic hyperinflation.41 Increases in EELV are mirrored by decreases in inspiratory capacity (IC) and vice versa.42
Dynamic hyperinflation is in part reversible: physiological studies have demonstrated that inhalation of bronchodilators reduces exertional dyspnoea and improves exercise endurance through a sustained reduction of EELV and increase in IC at rest and during exercise,43 illustrating the interdependency between these variables.
End-expiratory lung volume
In the explorative part of the VIVACITO study, the effects of TIO/OLO 5/5 µg and its mono-components TIO 5 µg and OLO 5 µg on hyperinflation were investigated in a subset of the 143 patients. After 6 weeks of a treatment, TIO/OLO 5/5 µg yielded significantly larger reductions in EELV at 2 h 30 min post-dose than placebo and the mono-components TIO 5 µg or OLO 5 µg. Changes in EELV from baseline were −52 ml with placebo, −435 ml and −431 ml with the mono-components OLO 5 µg and TIO 5 µg, respectively and −547 ml with TIO/OLO 5/5. At 22 h 30 min post-dose, only TIO/OLO 5/5 µg resulted in a significant decrease in EELV of −250 ml versus baseline, contrasting with the modest and nonsignificant changes in EELV (less than −100 ml) observed with the two monotherapies.13
Inspiratory capacity
The reductions in severity of hyperinflation after TIO/OLO 5/5 µg reported in the previous paragraph were mirrored by significant improvements in IC, which increased by 351 ml at 2 h 30 min and by 99 ml at 22 h 30 min post-dose, compared with baseline (Table 4).13 Compared with placebo, these increases exceeded the MCID, which has been estimated to range between 140–200 ml.30,44 A more systematic assessment of the effects of dual bronchodilatation on indices of hyperinflation was performed in the TORRACTO, PHYSACTO, OTIVATO and both MORACTO studies.5,6,8,10
Table 4.
Differences between TIO/OLO 5/5 μg and PLA, TIO 5 µg monotherapy, and OLO 5 µg monotherapy for IC and endurance time during exercise, expressed as change from baseline.
| Study | IC at rest (ml) | CWRCE (s) | ESWT (s) |
|---|---|---|---|
| TIO/OLO 5/5 µg versus PLA | |||
| Beeh13 | 351 versus 16 | ||
| Maltais6 | Δ ≈ 220 | 85 versus 23 | 65 |
| O’Donnell 1+25 | Δ = 244 and Δ = 265 | Δ = 79 and Δ = 55 | |
| Troosters8 | 360 versus 50 | 71 | |
| TIO/OLO 5/5 µg versus TIO 5 µg | |||
| Beeh13 | 351 versus 251 | ||
| O’Donnell 1+25 | Δ = 114 and Δ = 88 | Δ = −3 and Δ = 19 | |
| Troosters8 | 360 versus 230 | 61 | |
| Maltais10 | 489 versus 271 | ||
| TIO/OLO 5/5 µg versus OLO 5 µg | |||
| Beeh13 | 351 versus 242 | ||
| O’Donnell 1+25 | Δ = 119 and Δ = 80 | Δ = 1 and Δ = 47 | |
IC, inspiratory capacity; CWRCE, endurance time during constant work rate at 70–80% of VO2 max; ESWT, endurance time during shuttle walk test; IC, inspiratory capacity; MCID, minimal clinically important difference; PLA, placebo; TIO, tiotropium; OLO, olodaterol; TIO/OLO, tiotropium/olodaterol fixed-dose combination.
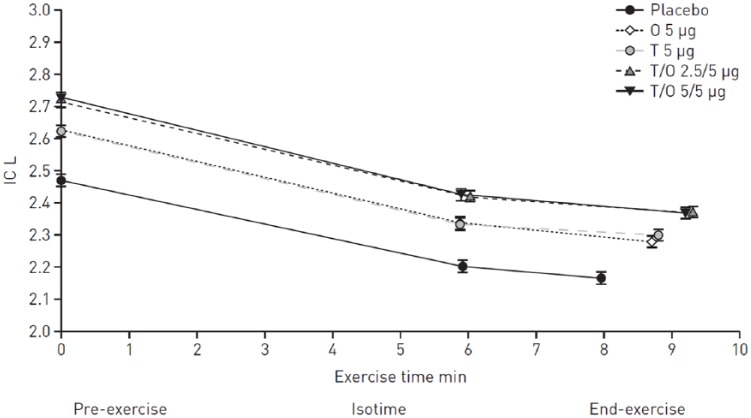
In the two replicate MORACTO studies,5 with their incomplete crossover design, TIO/OLO 5/5 μg significantly increased IC (2 h post-dose) after 6 weeks of treatment by approximately 250 ml compared with placebo (Figure 2). These significant improvements exceeded the MCID. These increases contrast with the statistically significant but clinically insignificant increase with TIO 5 µg and OLO 5 µg, which fell just below 100 ml compared with placebo. Here, the effect of dual bronchodilatation was slightly more than additive in nature. A response analysis showed that a significantly greater proportion of patients were classified as IC responders when on TIO/OLO 5/5 µg than on monotherapies or placebo. For example, 55% of the patients exhibited a 150 ml increase in IC with dual therapy, whereas the percentage of patients reaching that threshold was 45% when treated with monotherapy and about 20% when on placebo. Improvements in IC with TIO/OLO 5/5 μg were sustained during the endurance test at both isotime and end exercise (Figure 2).
Figure 2.
Adjusted means (±SE) inspiratory capacity (IC) at pre-exercise, at isotime and at end exercise after 6 weeks (combined studies).
Reproduced from O’Donnell and colleagues, 2017.5
As each patient only received four out of five possible treatments, isotimes differ between treatments.
O, olodaterol; SE, standard error; T, tiotropium.
Similar effects were observed in the TORRACTO and PHYSACTO studies. In the TORRACTO study, mean IC significantly increased by approximately 200 ml in the TIO/OLO 5/5 µg group at pre-exercise, compared with placebo. Here again, the gain was sustained during exercise both at isotime and at the end of exercise after 6 and 12 weeks of treatment.6 In the PHYSACTO study, IC increased from 2.43 l in the placebo group to 2.54 l in the TIO 5 µg treated group to reach 2.74 and 2.81 l in the two TIO/OLO 5/5 µg-treated groups. Differences between TIO/OLO 5/5 µg and placebo were statistically significant and averaged 310 ml (95% CI: 170, 450) and 390 ml (95% CI: 250, 530), an effect that exceeded twice the MCID.8
In the OTIVATO study,10 resting IC increased by 271 ml with TIO 5 µg and 489 ml with TIO/OLO 5/5 µg with a treatment difference of 218 ml (95% CI: 121, 314) . That difference vastly exceeded that found in MORACTO (101 ml).5 Apparently, by selecting patients with COPD with a severe degree of hyperinflation (FRC: >120% predicted), more room for improvement of IC was available in the OTIVATO than in the TORRACTO, PHYSACTO, and both MORACTO studies, which did not exclude patients with COPD without hyperinflation.5,6,8
Exercise capacity
In patients with COPD, bronchodilators enhance tidal expiratory flow rate, promote lung emptying and reduce hyperinflation at rest and during exercise. These beneficial effects eventually alleviate the degree of exertional dyspnoea and delay the time point at which mechanical limitation to ventilation develops.42,46,47 This has been documented with the mono-components TIO46,48–51 and OLO,52 which both beneficially impact on EET. Whether maximisation of bronchodilatation with TIO/OLO 5/5 µg may further improve exercise endurance was addressed in the TORRACTO, MORACTO and PHYSACTO studies.5,6,8 In these studies, constant work-rate cycle ergometry (CWRCE) to symptom limitation at 75% of peak incremental work rate44 and the more recently developed endurance shuttle walk test (ESWT) at 85% of maximum oxygen consumption were selected as main outcomes, since these tests are among the most sensitive ones to detect changes in exercise capacity following pharmacologic and nonpharmacologic interventions.53 Well-defined thresholds of MCID have been established for the CWRCE30,44 and the ESWT.45 Possibly, the ESWT is more reflective of activities performed by patients in everyday life.54
The TORRACTO study
The TORRACTO study was primarily designed to compare the effects of TIO/OLO 5/5 µg with placebo on EET during CWRCE in patients with COPD in a parallel-group design after 12 weeks of treatment. It also evaluated EET during the ESWT in a subset of patients.6 A total of 404 patients were randomized and received treatment: 385 were included in the full analysis set. Overall, 73% were GOLD stage 2, with mean predicted FEV1 of 59%.
Data obtained at 12 weeks were similar to those at week 6. A statistically significant 14% improvement in endurance time during CWRCE was observed after 12 weeks with TIO/OLO 5/5 µg versus placebo: baseline EET (geometric means) during CWRCE was 443 ± 12 s, reached 465 ± 19 s in the placebo recipients and 528 ± 20 s in the TIO/OLO 5/5 μg group, the 63 s difference reaching both statistical and clinical significance. The increase in dyspnoea (Borg scale) during exercise [(end of exercise Borg score minus pre-exercise Borg score)/per time unit] was numerically lower for TIO/OLO 5/5 µg compared with placebo at weeks 6 and 12 but failed to reach statistical significance.6
In the ESWT substudy, the common baseline mean EET was 311 ± 14 s. ESWT remained unaffected by placebo (311 ± 23 s) but increased to 376 ± 26 s with TIO/OLO 5/5 µg. That 21% improvement in endurance time with TIO/OLO 5/5 µg after 12 weeks just failed to reach statistical significance versus placebo (p = 0.055), although it numerically reached clinical significance.6
The MORACTO studies
With their double-blind, incomplete crossover design, the replicate MORACTO studies compared the effects of TIO/OLO 5/5 μg with those of TIO 5 μg, OLO 5 μg and placebo on EET during CWRCE after 6 weeks.5 A total of 586 patients were randomized and 571 were included in the full analysis. 73% of the included patients were GOLD stage 2 and 21% GOLD stage 3, with a mean FEV1 of 58%. The presence of lung hyperinflation (with FRC exceeding more than 120% predicted) was not an inclusion criterion in these studies.
TIO/OLO 5/5 μg enhanced endurance time during CWRCE significantly versus placebo by 55 s (+13%) and 79 s (+22%) after 6 weeks in the two MORACTO studies (Figure 3 for the combined studies). These increases exceeded the MCID.30 For a 100 s increase, approximately 35% of patients were classified as EET responders with TIO/OLO 5/5 µg compared with 20% of placebo-treated patients. Moreover, TIO/OLO 5/5 µg reduced dyspnoea significantly more than placebo.5
Figure 3.
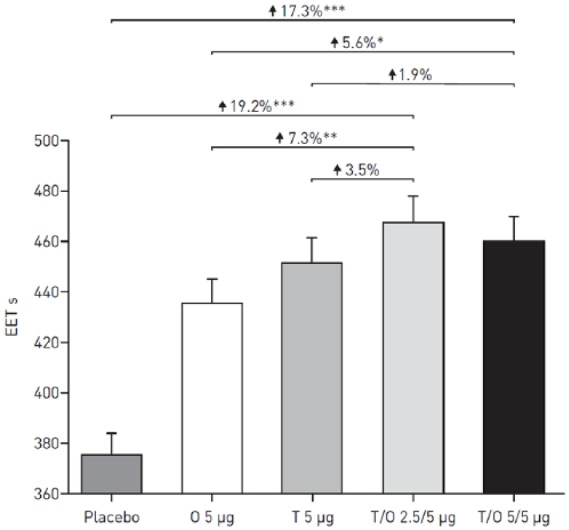
Adjusted geometric mean (± SE) EET in s during constant work-rate cycle ergometry after 6 weeks of treatment (combined studies).
*p < 0.05; **p < 0.01; ***p < 0.0001. Reproduced from O’Donnell and colleagues, 2017.5
EET, exercise endurance time; O, olodaterol; SE, standard error; T, tiotropium.
Differences between TIO/OLO 5/5 µg and the mono-components TIO 5 µg or OLO 5 µg were inconsistent in terms of statistical significance and results slightly differed between the individual studies. Indeed, increases in endurance time were only observed in MORACTO 2 with TIO/OLO 5/5 µg versus OLO 5 µg but not versus TIO 5 µg. Post hoc analyses did not identify any specific subgroup of patients exhibiting greater improvements in EET with TIO/OLO 5/5 µg versus monotherapies.5
The PHYSACTO study
The effects on exercise endurance and on functional exercise capacity of TIO 5 µg and TIO/OLO 5/5 µg alone or in conjunction with exercise training were addressed in the PHYSACTO study, a 12-week, randomized, partially double-blind, placebo-controlled, parallel-group trial.8 In that study, all participating patients with COPD attended a 12-week lasting program aimed at modifying their behaviour. That SMBM program consisted of a total of six individual and group sessions and focused on improving patient engagement in, and maintenance of, exercise and physical activity. During individual sessions, the case manager dealt with the patient’s current level of physical activity, functional limitations, clinical barriers to physical activity (including motivation) and the ultimate physical activity goal at the end of the program. Group sessions concentrated on the benefits of physical activity, set physical activity goals and educated the patients on their disease, healthy life habits, stress management, breathing techniques, exercise planning and how to distinguish normal from abnormal physical symptoms.55
In a short time window of 3 months such SMBM programs typically have only a limited impact on exercise tolerance.56,57 Endpoints were EET during an ESWT at 85% of VO2 max after 8 weeks (end of the exercise training program) and 12 weeks. The six-minute walking distance (6MWD) captured functional exercise tolerance. A total of 304 patients were randomized, with 303 receiving study treatment. A total of 88% of patients completed the 12-week trial.
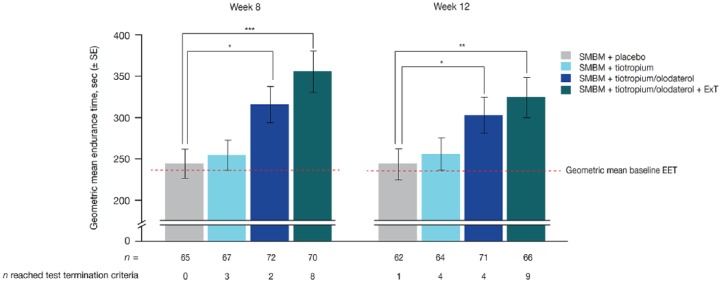
Endurance time at week 8 did not change from baseline in the placebo group (244 versus 243 s), but increased with TIO 5 µg by 10 s, with TIO/OLO 5/5 µg without exercise training by 71 s and with TIO/OLO 5/5 µg with exercise training by 111 s, the MCID being reached in the latter two treatment groups (Figure 4). Treatment ratios versus placebo were 1.29 (95% CI: 1.06, 1.57) for TIO/OLO 5/5 µg without and 1.46 (95% CI: 1.20, 1.78) for TIO/OLO 5/5 µg with exercise training. The overall results may not reflect the true benefit as 13 patients exceeded the endurance time termination criteria of 20 min after 8 weeks: none for placebo, 3 for TIO 5 µg, 2 for TIO/OLO 5/5 µg without and 8 for TIO/OLO 5/5 µg with exercise training. The 6MWD followed the same profile and significant changes compared with placebo were observed in both TIO/OLO 5/5 µg groups. Improvements in endurance time were accompanied by a significant reduction in dyspnoea at isotime at week 8 in the TIO/OLO 5/5 µg groups without and with exercise training. Comparable results were observed at week 12.
Figure 4.
Geometric mean EET during endurance shuttle walk test at weeks 8 and 12. Exercise training was stopped after 8 weeks in the SMBM + TIO/OLO 5/5 µg + exercise training arm; all treatment arms continued study medication until week 12. Geometric means baseline exercise endurance test: 243 ± 9 s (week 8); 242 ± 9 s (week 12). Test termination time limit was 20 mins.
Reproduced from Troosters and colleagues, 2018.8
*p < 0.05; **p < 0.01; ***p < 0.001.
EET, exercise endurance time; OLO, olodaterol; SE, standard error; SMBM, self-management behaviour modification; TIO, tiotropium.
Intensity of dyspnoea using the modified Borg scale at isotime during the ESWT or at the 6MWT decreased with all the active treatments compared with the placebo + SMBM group week 8. The 6MWD at week 8 did not change from baseline (464 versus 457 m) in the placebo group, but TIO/OLO 5/5 µg without and with exercise training provided small but statistically significant improvements in 6MWD compared with placebo (21 m and 27 m, respectively). A reduction in dyspnoea intensity during the 6MWT at week 8 was seen in the TIO/OLO 5/5 µg group with exercise training.
Physical activity
The physiologic benefits of enhanced exercise capacity do not automatically translate into the behavioural change of engaging in a more active lifestyle.58–60 Therefore, the PHYSACTO study also investigated whether the improvements in endurance time and the reductions in breathing discomfort resulting from a multimodal approach consisting of behavioural intervention, maximal bronchodilatation and exercise training may complementary affect physical activity. At week 12, the SMBM program had significantly increased the number of steps per day by 1.098 in the placebo group compared with baseline. Further increases in number of steps per day were not observed with any of the additional interventions.8
A significant improvement in Functional Performance Inventory Short Form total score versus baseline was observed for SMBM + TIO/OLO 5/5 µg without exercise training compared with SMBM + placebo at week 12, with the most notable improvements seen in the ‘body care’, ‘physical exercise’, and ‘recreation’ subscales.
The PHYSACTO trial is one of the first to evaluate the patient’s experience with physical activity in the domain of amount and difficulty using the PROactive scale.61 Significant improvements in daily PROactive Physical Activity (measuring a combination of ‘amount’ and ‘difficulty’) in COPD total score at week 12 were observed for SMBM TIO/OLO 5/5 µg with (4.10 points) or without (3.66 points) exercise training compared with placebo (1.96 points). The overall impression of the PHYSACTO study is that improvements in physical activity experience and dyspnoea symptoms during daily life are best served by the addition of dual bronchodilatation and exercise training to the SMBM program. Indeed, although the SMBM program increased substantially physical activity on a daily basis, improvements for several other outcomes reached only statistical significance in combination with maximal bronchodilatation and exercise training.8
Exacerbations
The DYNAGITO study, with its double-blind, parallel-group, active-controlled, randomized design, was conducted as a 52-week study to investigate whether TIO/OLO 5/5 µg reduced the rate of COPD exacerbations compared with TIO 5 µg alone.7 Only patients with COPD were included with an FEV1 < 60% predicted and at least one moderate or severe exacerbation in the preceding year requiring treatment with systemic corticosteroids, or antibiotics. Patients on ICSs at inclusion continued their treatment. The primary endpoint was the rate of moderate-to-severe COPD exacerbations from the first dose of medication until one day after last drug administration. Secondary endpoints were the time to first moderate or severe COPD exacerbation during the treatment period, the rate of exacerbations leading to hospitalization, time to first exacerbation leading to hospitalization, and time to all-cause mortality.
More than 7800 patients with a mean percent predicted FEV1 of 44% were randomized. A total of 70% of patients were on ICSs. Patients on TIO/OLO 5/5 µg were more likely to complete the study than those on TIO 5 µg alone [3451 (88%) versus 3939 (84%), with a discontinuation HR of 0.73; 95% CI: 0.65, 0.82]. The rate of moderate-to-severe exacerbations was numerically lower with TIO/OLO 5/5 µg (0.90; 99% CI: 0.84, 0.96 per year) than with TIO 5 µg (0.97; 99% CI: 0.93, 1.03 per year) with a RR of 0.93 (99% CI: 0.85, 1.02). As the p value equalled 0.0498, the targeted 0.01 significance level, needed to meet regulatory requirements, was not met.7
The HR for time to first moderate-to-severe COPD exacerbation with TIO/OLO 5/5 µg versus TIO 5 µg was 0.95 (99% CI: 0.87, 1.03). The HR for time to first COPD exacerbation leading to hospitalization was 0.93 (95% CI: 0.82, 1.06). For severe exacerbations, the RR for TIO/OLO 5/5 µg compared with TIO 5 µg was 0.89 (95% CI: 0.78, 1.02) and for exacerbations leading to hospitalization the RR was 0.89 (95% CI: 0.76, 1.03). Interestingly, a post hoc analysis of the subgroup of patients receiving ICSs at baseline demonstrated that the RR for exacerbations was 0.91 (95% CI: 0.84, 0.99), whereas there was no difference between TIO/OLO 5/5 µg and TIO 5 µg in the group not receiving any corticosteroid at baseline (RR: 1.00, 95% CI: 0.86, 1.15).7
The authors concluded that TIO/OLO 5/5 µg did not reduce exacerbation rate as much as expected compared with TIO 5 µg alone.
Effects and baseline ß-blocker use
Cardiovascular disease is linked closely with COPD and physicians may be reluctant to use ß-blocking agents in patients with COPD, despite their proven effectiveness in preventing cardiovascular events. In the TONADO 1 and 2 studies, 557 of 5162 patients (11%) received ß-blockers at baseline, among whom at least 80% were on cardioselective ß-blockers. These patients had more frequent a history of cardiovascular comorbidities and medications. The observation that baseline post-bronchodilator FEV1 was higher in the ß-blocker group (1.470 l) compared with that in the no ß-blocker group (1.362 l) may suggest that ß-blockers were less often prescribed in more diseased patients with COPD. No differences were observed in trough FEV1, trough forced vital capacity (FVC), SGRQ and TDI between patients treated with and without ß-blockers, supporting the cautious and appropriate use of ß-blockers in patients with COPD and cardiovascular comorbidity.62
Effects in Asian patients with COPD
Asian patients with COPD, and particularly Japanese patients, appear to differ from patients of European or American descent in terms of physiology and use of medication. Such differences may potentially affect the outcome of medical interventions. This issue might have easily been missed, since European and American patients with COPD numerically dominate most large multicentre trials. As a consequence, subanalyses of the Asian and Japanese patients with COPD participating in the TONADO® and DYNAGITO® studies have been recently performed.
Of the 5163 patients randomized in the TONADO study, 1152 (22%) were of east Asian and 413 (8%) of Japanese origin.63,64 The east Asian population showed slightly greater FEV1 AUC0–3 and trough FEV1 treatment differences between TIO/OLO 5/5 μg and TIO 5 μg, when compared with the overall population.63
The Japanese patients randomized in the TONADO study were slightly older and contained more men and fewer current smokers, but with higher pack-year smoking history. Body mass index was lower, as were baseline SGRQ and TDI. A lower proportion of Japanese patients used ICSs (27 versus 47%) at baseline but the use of LAMAs was higher (65 versus 36%).64
Improvements in pulmonary function were similar to those seen in the overall population. Relative to baseline, SGRQ total scores improved in Japanese patients at week 24 of treatment in all groups, with the larger reduction in symptom scores seen in the group receiving TIO/OLO 5/5 μg and relatively modest changes with the mono-components compared with the overall population. Compared with the overall population, improvements in total SGRQ scores with TIO/OLO 5/5 µg versus the mono-components were greater in the Japanese population. For example, mean treatment difference between TIO/OLO 5/5 µg and TIO 5 µg was −3.60 (95% CI: −6.71, −0.50) in the Japanese and only −1.23 (95% CI: −2.31, −0.15) in the overall population. The number of responders with TIO/OLO 5/5 µg was, however, similar for the Japanese and the overall population.64
TDI at baseline was slightly higher (i.e. fewer symptoms) in Japanese patients compared with the overall population (7.75 versus 6.54, respectively). That score improved to >1 for TIO/OLO 5/5 µg versus baseline compared with only 0.40–0.85 for the mono-components. Compared with the monotherapies, TIO/OLO 5/5 μg improved TDI by >0.7 units in Japanese patients, whereas these increases were much smaller in the overall population. Hence, dual bronchodilatation yielded greater benefits for patient-centred outcomes than monotherapies in Japanese patients compared with the overall population, whereas such differences were not observed for pulmonary function.64
A total of 461 (5%) of the 7880 treated patients in the DYNAGITO study were of Japanese origin.65 As in the TONODO study, Japanese patients were older, with longer smoking histories, a higher proportion of men and ex-smokers, and a lower body mass index. LAMA use at baseline was more widespread (86 versus 63%), whereas ICS use at baseline was less common (48 versus 70%). More patients in the Japanese subgroup received LAMA/LABAs (24% versus 12%) and fewer received ICS/LABA combination therapy (5% versus 25%) at baseline. Overall, 40% of the Japanese patients used triple ICS/LAMA/LABA combination therapy, which was similar to the overall study population.
A total of 21% of the Japanese patients randomized in the TIO 5 µg discontinued the study versus 10% in the TIO/OLO 5/5 µg arm (discontinuation HR: 0.45; 95% CI: 0.27, 0.73), contrasting with the 16% versus 12% in the overall population (discontinuation HR: 0.73, 95% CI 0.65, 0.82).65
In Japanese patients, TIO/OLO 5/5 µg resulted in a 29% lower rate of annualized moderate-to-severe COPD exacerbations relative to TIO 5 µg monotherapy, a difference that did not reach the p < 0.01 target (0.94 versus 1.32/years, RR: 0.71; 99% CI: 0.46, 1.10; p = 0.0434). This ratio numerically exceeded the nonsignificant 7% reduction reported in the overall population (RR: 0.93, 99% CI: 0.85, 1.02). Likewise, the annualized rate of severe exacerbations was 19% lower in the TIO/OLO 5/5 µg arm than TIO 5 µg arm (0.35 versus 0.43/year), contrasting with the 11% reduction seen in the overall population. Within the limitations of this exploratory analysis, it thus appears that TIO/OLO 5/5 µg exerted numerically larger effects on moderate-to-severe exacerbations in the Japanese subgroup, compared with the overall study population, an observation potentially related to differences in clinical characteristics between the two populations.65
The aim of the VESUTO study, a randomized, double-blind, active-controlled, crossover trial, was to assess the efficacy of TIO/OLO 5/5 µg and TIO 5 µg on lung volumes, exercise capacity, and physical activity in 184 Japanese COPD patients.65 All patients exhibited an mMRC dyspnoea scale ⩾1, a 6MWD <400 m and a score ⩾4 on the modified Borg scale of dyspnoea at the end of the 6MWT. The difference in IC between TIO/OLO 5/5 µg and TIO 5 µg was 115 ml (95% CI: 77, 153), which is similar to what was seen in the MORACTO, TORRACTO and PHYSICATO studies.5,6,8 Dual bronchodilatation did not affect the 6MWD compared with TIO 5 µg. In the combined GOLD 3 and 4 subgroups, however, a small, but statistically significant increase in 6MWD was seen with TIO/OLO 5/5 µg versus TIO 5 µg (18 m; 95% CI: 2, 34). No significant differences were found for physical activity between the two treatments in terms of mean number of steps per day or daily duration of physical activity.9
Cost-effectiveness
Overall, three studies have attempted to assess the cost-effectiveness and cost utility of TIO/OLO 5/5 µg, using a new cost-effectiveness model, developed by Carl Selya-Hammer.66 This model is based on an individual patient-level approach and takes the heterogeneity of the individual patient into account. More specifically, each patient is tracked over time and individual changes in pulmonary function (improvement and decline), disease severity, exacerbation risk, mortality, costs and quality of life are monitored over a certain time span and used to estimate and predict cost-effectiveness and cost utility over the following years. That new model represents an improvement over the older Markov model, which uses a cohort-like approach whereby patients are distributed in different cohorts in accordance with their health states based on the GOLD COPD classification.
The economic studies were run over a time horizon of 15 years while using the data of patients participating in the two 52-weeks TONADO studies.12 Pulmonary function was assumed to decline at a constant rate based on disease stage, even after patients had left the study. Exacerbation risk was estimated based on a random-effects logistic regression analysis of exacerbations in UPLIFT.67 Mortality by age and disease stage was estimated from an analysis of TIOSPIR68 trial data or data from existing COPD cohorts. Medical expenditures were calculated from the perspective of the health care payer, and took direct medical expenditures associated with bronchodilator therapy and other COPD treatments, such as exacerbations, into account.66
In the Italian study, incremental cost-effectiveness ratio was €7518 per additional quality adjusted life year (QALY) with TIO/OLO 5/5 µg versus TIO 5 µg, which is far below the thresholds currently in use to evaluate the clinical value of a treatment.66 A Dutch study confirmed these data, as it estimated that TIO/OLO 5/5 µg had an incremental cost-effectiveness ratio of €7004 per QALY, compared with TIO 5 µg alone.69 In the United Kingdom, the free-dose combination of TIO 5 µg and salmeterol was associated with the same QALYs as TIO/OLO 5/5 µg, but with higher costs.70
Safety
The safety profile of TIO, given as mono-component, has been investigated in 11,626 patients with COPD treated with placebo and 12,929 with TIO (totalling 35 studies with 14,909 patient-years of TIO exposure). A numerically lower risk of adverse events (AEs) with a RR of 0.90 (95% CI: 0.87, 0.93) and serious AEs (SAEs) with a RR of 0.94 (95% CI: 0.89, 0.99)) was reported in favour of the TIO recipients.71 Cardiac AEs were not increased in TIO-treated patients, with a RR of 0.93 (95% CI: 0.85, 1.02)).71
Safety of OLO was assessed in 16,591 patients (among whom 14,915 had COPD GOLD stage 2–4) who participated in 26 studies, among which 17 placebo-controlled trials and 9 trials in which TIO/OLO was compared with TIO. A total of 9855 patients were in the OLO arm and 6736 were in the control arm. In the placebo-controlled studies, the OLO arm had a numerically higher odds ratio (OR) for death, a finding that, however, did not reach statistical significance (OR: 1.31; 95% CI: 0.90, 1.89). Heterogeneity was not observed and subgroup analyses according to the treatment regimen, type of respiratory disease, and use of an ICS or a methylxanthine did not reveal any specific risk factors or risk groups. The risk of nonfatal SAEs in the OLO arm was not significantly higher compared with the control arm (OR: 1.03; 95% CI: 0.91, 1.15).72
The cardiovascular and respiratory safety profile of TIO/OLO 5/5 µg has been compared with that of TIO 5 µg in more than 9900 patients who participated in the 52-week DYNAGITO and TONADO studies.7,11,73 Total exposure was 4579 person-years for TIO/OLO 5/5 µg, and 4438 person-years for TIO 5 µg. The proportion of patients leaving the trials prematurely was lower with TIO/OLO 5/5 µg (12.5%) than with TIO 5 µg (16.4%). This was mainly attributed to a lower proportion of patients with respiratory AEs, such as COPD exacerbations and dyspnoea in the TIO/OLO 5/5 µg arm (5.9%) than in the TIO 5 µg arm (7.9%; RR: 0.72; 95% CI 0.62, 0.84). Significant differences in the incidence of AEs and SAEs, cardiovascular AEs or central nervous system vascular AEs between treatments were not observed. The incidence of major adverse cardiovascular events (MACEs) was 2.11 per 100 patient-years with TIO/OLO 5/5 µg and 2.22 with TIO 5 µg (RR: 0.95; 95% CI 0.72, 1.25). The incidence of fatal MACE (including death with undetermined cause) was 0.91 and 1.00 per 100 patient-years with TIO/OLO 5/5 µg and TIO 5 µg, respectively (RR: 0.91; 95% CI 0.60, 1.37).
Similar conclusions were made in specific subgroups, such as the 1742 patients with renal failure enrolled in the TONADO studies.74 Despite the presence of mild (44%), moderate (13%) and severe (0.2%) renal impairment, and a history of hypertension (46%), cardiac disorders (23%) or a disturbed glucose metabolism (13%), side effects were distributed equally regardless of the severity of renal impairment. Approximately 70% of patients reported one or more AEs. Interestingly, the proportion of patients experiencing an AE was independent of the severity of renal impairment, and the proportion of patients with an AE or SAE was not related to the arm in which they were randomized. These recent studies confirm previous data, showing the absence of any signal of differences in frequency of AEs and SAEs between TIO/OLO 5/5 µg and its respective mono-components.31
When investigating the safety of TIO/OLO in patients with COPD, it is important to highlight that in the classical RCTs a well-selected patient population is treated and examined, since many exclusion criteria are applied. Most importantly, patients with asthma or asthma COPD overlap have been excluded, since monotherapy with LABAs in asthma has been associated with an increased risk of severe exacerbations and mortality.75 Therefore, the safety of TIO/OLO as well as other LAMA/LABA FDCs will also need to be investigated in real life studies (encompassing observational pharmaco-vigilance studies and pragmatic trials, enrolling broader patient populations in primary and secondary care settings). Indeed, older asthma patients (such as smoking asthmatics or patients with late-onset asthma) might be misdiagnosed as COPD, putting them at risk of treatments with long-acting bronchodilators (LABAs or LAMAs) without ICSs.
Perspective: dual bronchodilatation in patients with COPD: does it really matter?
Large studies in patients with COPD have unequivocally demonstrated that long-acting bronchodilator monotherapy not only improves FEV1, lung volumes and exercise endurance, but also reduces the risk of exacerbations and improves patient-reported outcomes, such as breathlessness, health status or use of rescue medication.10,43,76–79 The key question is whether a switch from long-acting bronchodilator monotherapy to a LAMA/LAMA FDC such as TIO/OLO 5/5 µg leads to additional clinically significant benefits and should be introduced early in the management of patients with COPD.
LAMAs and LABAs are primarily bronchodilators, but their mechanism of action on airway smooth muscle is complex. Studies characterizing the pharmacological interactions between TIO and OLO in vitro showed that TIO and OLO induce a submaximal concentration-dependent inhibition of bronchial contractility, when administered separately. Low concentrations of a fixed 5:5 concentration ratio of a TIO/OLO combination elicited an effect that exceeded the expected additive effect of the drugs given separately, suggesting the existence of a strong synergistic interaction.80 The same authors reported similar in vitro findings with other LAMA/LABA combinations.81–83
As observed in vitro, the improvement in FEV1 with TIO/OLO 5/5 µg or another LAMA/LAMA, seen in patients with COPD, slightly exceeds the sum of the bronchodilating action of its individual components, as it ranges between 20 and 66 ml in parallel-group studies and 29 ml in the only crossover study published so far.84
In an attempt to understand the working mechanism of short-acting muscarinic antagonists (SAMAs) and short-acting ß2-agonists (SABAs) in patients with COPD, Donohue reported 15 years ago that some patients with COPD reversed to salbutamol only, others to ipratropium only, a third group to both drugs, whereas some were not reversible at all.85 This eventually supports the use of SABA/SAMA formulations as rescue therapy in patients with COPD. More recently, the same author investigated in a crossover study the ability of individual patients with COPD to increase their FEV1 after inhalation of either UMEC, VIL or UMEC/VIL.86 This allowed the investigators to classify patients with COPD into four groups: responders to UMEC only, responders to VIL only, responders to both UMEC and VIL and nonresponders to either of the two drugs. Small improvements with UMEC/VIL over monotherapy were observed in nonresponders to both UMEC and VIL, which tended to be more than additive in nature. Less than additive effects to UMEC/VIL were observed in dual responders, suggesting that in the latter patients the effects of UMEC/VIL were near the plateau of the dose–response curve. In patients responding to only one drug, full additive effects were noted. Although these findings require confirmation with other LABA/LAMA FDCs, this analysis strongly supports that beneficial effects of dual bronchodilatation may be expected in most patients with COPD, with quantitatively greater improvements in patients identified as responders to either a LAMA or a LABA.
The GOLD 2019 document proposes to start with one bronchodilator in symptomatic patients with COPD, currently classified as GOLD stage B and upgrade to dual bronchodilator therapy, if symptoms persist.1 This recommendation is based on the response analysis of the effects of long-acting bronchodilators on patient-reported outcomes, which indicates that already 40–50% of the patients with COPD experience a clinically significant improvement with one long-acting bronchodilator, whereas the addition of a second bronchodilator enhances the number of responders by no more than 10%,4 restricting its use to patients with persistent daytime dyspnoea or reduced HR-QoL on monotherapy.39
This approach can be questioned, since data now support that an earlier introduction of dual bronchodilatation slows down the decline over time of dyspnoea and HR-QoL34 and leads to a substantial reduction in rescue medication (Figure 1). Moreover, doubts exist whether traditional thresholds may be used to distinguish responders from nonresponders to compare two active treatments, since these thresholds have only been validated for placebo-controlled trials.87
It is well established that bronchodilators reduce dynamic hyperinflation, even in the absence of any change in FEV1.88 In this regard, the effects of TIO/OLO 5/5 µg on hyperinflation, exercise endurance and activity-related breathlessness represent undoubtedly the most convincing plea for the early initiation of dual bronchodilatation in the treatment of patients with COPD. Indeed, only dual bronchodilatation yielded statistically significant increases in EELV and residual volume (RV)which persist for more than 22 h.13 These data were confirmed in six other studies which unequivocally showed that TIO/OLO 5/5 µg yielded increases in IC at rest and during exercise, that were twice as large as after monotherapy and vastly exceeded the MCID, compared with placebo.5,6,8–10
These increases in IC observed with mono- and dual bronchodilator therapy were not always transferred into statistically or clinically significant improvements in EET.
In some studies, TIO/OLO 5/5 µg yielded numerically larger improvements in exercise endurance than a single bronchodilator,8 whereas in other studies the additional increase in IC was not or barely transferred into a gain in exercise endurance.5 Such inconsistencies have also been observed with other LAMA/LABA FDCs89–92 and might be attributed to at least two factors. First, the presence of hyperinflation was not an inclusion criterion in exercise endurance studies with TIO/OLO 5/5 µg and the absence of lung hyperinflation in some of the included patients might have minimized the improvement in IC even after maximal bronchodilatation. Second, it is unlikely to expect an exercise benefit of similar magnitude in patients with COPD when adding a second bronchodilator to an existing one than when adding one bronchodilator to placebo, since exercise limitation is often multifactorial. If providing additional bronchodilatation with a second bronchodilator prolongs exercise duration in some patients with COPD, in others it shifts the mechanism of symptom limitation from dyspnoea to leg fatigue.93,94 In the latter situation, only a combination of exercise training and maximum bronchodilatation could enhance exercise endurance.
The relevance of the latter mechanism was illustrated in the PHYSACTO study, which showed that the gain in exercise endurance was greater in TIO/OLO 5/5 µg recipients treated with than without exercise training.8 Although the numerical difference in EET between these two groups was not significant, it should be underlined that the magnitude of the effect of patients receiving training, reported in the paper, was underestimated, since the endurance test was terminated after 20 min.
Whereas TIO/OLO 5/5 µg is superior to a mono-component in the context of endurance time if combined with exercise training (and therefore should be prescribed to patients with COPD in order to maximise the effect of a pulmonary rehabilitation program8), the incremental benefit of adding OLO 5 µg to TIO 5 µg to reduce COPD exacerbations is apparently less convincing7: a decrease by only 7% in moderate-to-severe exacerbations (p = 0.498) was seen with dual bronchodilatation versus TIO 5 µg in the DYNAGITO study. That the targeted 0.01 significance level was not met may be attributed to inappropriate choices made in its statistical analysis. Indeed, confounding factors were not taken into account. If multiple covariates models similar to those used in previous COPD trials, such as SPARK95 or FLAME96 had been used, the targeted p value <0.001 would have been reached for differences in annualized rate of moderate-to-severe exacerbations between TIO/OLO 5/5 µg and TIO 5 µg, with reductions in exacerbation rate ranging between 9 and 11%.7 These results are consistent with the reduction by 10–12% reported in the SPARK study,95 the only other study in which the effects of GLY/IND and LAMAs on exacerbation rates were compared.
The modest reduction in the risk to exacerbate when adding a LABA to LAMA monotherapy7,95 does not invalidate the role of dual bronchodilatation in the reduction of COPD exacerbations. Indeed, several studies have documented that adding a LAMA to a LABA/ICS leads to a significant reduction in COPD exacerbations.97–100 Currently, the main mechanism by which LAMAs and LABAs reduce exacerbation rates is attributed to the reduction of the baseline threshold for symptoms through a lowering the degree of hyperinflation. That beneficial effect may be amplified by a decrease of symptoms via other mechanisms, such as decreased mucus secretion (by LAMAs) and enhanced mucociliary clearance (by LABAs).101 Conversely, ICSs reduce exacerbation rate in patients with COPD by its anti-inflammatory properties and particularly by reducing the number of recurrent exacerbations, with a substantial treatment benefit that is higher in patients with an elevated blood eosinophil count.102,103
Data from the IMPACT study, which addressed the effects of UMEC/VIL, a LAMA/LABA FDC, VIL/FLU, a LABA/ICS and UMEC/VIL/FLU, a LAMA/LABA/ICS on exacerbation rate in patients with COPD with moderate-to-severe and often recurrent exacerbations, allows the clinician to position LAMA/LABA and ICS-containing formulations in the maintenance treatment of COPD.97 Indeed, a detailed analysis of the IMPACT study demonstrates that the ability of the ICS-containing treatments to reduce exacerbation rates is highly dependent on blood eosinophil count: LABA/ICS and LAMA/LABA/ICS unequivocally reduce exacerbation rate compared with LAMA/LABA in patients whose blood eosinophil count exceeds 150/μl, with a greater effect at blood eosinophil counts >300/µl and a virtually absent effect below a blood eosinophil count of 100–150/μl. In the latter patients, the effect of LAMA/LABA equalled that of triple therapy and was superior to that of a LABA/ICS FDC.104 Similar findings were made in the KRONOS study.100 These prospectively collected data are in line with previously published retrospective data on the role of blood eosinophil levels in predicting the therapeutic response to ICS in patients with COPD with frequent exacerbation phenotype98,105–109 and eventually confirms its role as a theragnostic masker. Hence, maximum bronchodilatation might be the treatment of choice in patients with COPD who exacerbate and exhibit low blood eosinophil counts, as GOLD 2019 suggests.1
Conclusion
TIO/OLO 5/5 µg is a LAMA/LABA FDC which has been thoroughly investigated in over 15,000 patients with COPD. Its effects on a variety of relevant clinical endpoints demonstrate superiority over its mono-components, with improvements in spirometric variables and lung volumes that exceed the minimal clinically important difference. These physiological improvements are translated into gains in patient-reported outcomes (such as dyspnoea, HR-QoL, and use of rescue medication), exercise endurance and physical activity, which generally exceed the effects seen with monotherapies. Addition of OLO 5 μg to TIO 5 μg reduces exacerbation rate in patients with COPD by 10%, a finding which is consistent with those seen with other LAMA/LABA combinations. In patients with COPD with low blood eosinophil counts, there is increasing evidence that dual bronchodilatation is as good in preventing COPD exacerbations as LAMA/LABA/ICSs and even better than LABA/ICSs. TIO/OLO 5/5 µg is well tolerated, provided that patients with asthma are excluded.
Acknowledgments
The authors thank Thierry Troosters for valuable suggestions, and Annie Wittevrongel and Anny Mattelaer for expert technical help and writing assistance.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: ED’s clinical department has received financial support from Boehringer Ingelheim, Chiesi, GSK and Novartis to perform clinical studies. He has participated in advisory boards for Boehringer Ingelheim, Chiesi, Cipla, GSK, Novartis and Astra Zeneca, for which a fee was given to the clinical department. He has received travel grants from Boehringer Ingelheim, Chiesi, GSK and AstraZeneca to attend international congresses. He has received speaker’s fees (given to the clinical department) from Boehringer Ingelheim, GSK, Chiesi, Astra Zeneca and Novartis to give scientific presentations to local general practitioner groups and pulmonologists.
ORCID iD: Eric Derom  https://orcid.org/0000-0003-1110-4940
https://orcid.org/0000-0003-1110-4940
Contributor Information
Eric Derom, Department of Respiratory Medicine, Ghent University Hospital, Ingang 12, Route 1404, Corneel Heymanslaan 10, B-9000 Ghent, Belgium.
Guy G. Brusselle, Department of Respiratory Medicine, Ghent University Hospital, Ghent, Belgium
Guy F. Joos, Department of Respiratory Medicine, Ghent University Hospital, Ghent, Belgium
References
- 1. Agusti A, Vogelmeier C. Global initiative for chronic obstructive lung disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease; (updated 2019). Report 2019. [Google Scholar]
- 2. Lopez-Campos JL, Tan W, Soriano JB. Global burden of COPD. Respirology (Carlton, Vic) 2016; 21: 14–23. [DOI] [PubMed] [Google Scholar]
- 3. Ford ES, Murphy LB, Khavjou O, et al. Total and state-specific medical and absenteeism costs of COPD among adults aged ⩾18 years in the United States for 2010 and projections through 2020. Chest 2015; 147: 31–45. [DOI] [PubMed] [Google Scholar]
- 4. Derom E, Brusselle GG, Joos GF. Efficacy of tiotropium-olodaterol fixed-dose combination in COPD. Int J Chron Obstruct Pulmon Dis 2016; 11: 3163–3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. O’Donnell DE, Casaburi R, Frith P, et al. Effects of combined tiotropium/olodaterol on inspiratory capacity and exercise endurance in COPD. Eur Respir J 2017; 49: 1601348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Maltais F, O’Donnell D, Galdiz Iturri JB, et al. Effect of 12 weeks of once-daily tiotropium/olodaterol on exercise endurance during constant work-rate cycling and endurance shuttle walking in chronic obstructive pulmonary disease. Ther Adv Respir Dis 2018; 12: 1753465818755091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Calverley PMA, Anzueto AR, Carter K, et al. Tiotropium and olodaterol in the prevention of chronic obstructive pulmonary disease exacerbations (DYNAGITO): a double-blind, randomised, parallel-group, active-controlled trial. Lancet Respir Med 2018; 6: 337–344. [DOI] [PubMed] [Google Scholar]
- 8. Troosters T, Maltais F, Leidy N, et al. Effect of bronchodilation, exercise training, and behavior modification on symptoms and physical activity in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2018; 198: 1021–1032. [DOI] [PubMed] [Google Scholar]
- 9. Ichinose M, Minakata Y, Motegi T, et al. Efficacy of tiotropium/olodaterol on lung volume, exercise capacity, and physical activity. Int J Chron Obstruct Pulmon Dis 2018; 13: 1407–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Maltais F, Aumann JL, Kirsten AM, et al. Dual bronchodilation with tiotropium/olodaterol further reduces activity-related breathlessness versus tiotropium alone in COPD. Eur Respir J 2019. 53: 1802049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Singh D, Ferguson GT, Bolitschek J, et al. Tiotropium + olodaterol shows clinically meaningful improvements in quality of life. Respir Med 2015; 109: 1312–1319. [DOI] [PubMed] [Google Scholar]
- 12. Buhl R, Maltais F, Abrahams R, et al. Tiotropium and olodaterol fixed-dose combination versus mono-components in COPD (GOLD 2-4). Eur Respir J 2015; 45: 969–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Beeh KM, Westerman J, Kirsten AM, et al. The 24-h lung-function profile of once-daily tiotropium and olodaterol fixed-dose combination in chronic obstructive pulmonary disease. Pulm Pharmacol Ther 2015; 32: 53–59. [DOI] [PubMed] [Google Scholar]
- 14. Beeh KM, Derom E, Echave-Sustaeta J, et al. The lung function profile of once-daily tiotropium and olodaterol via Respimat® is superior to that of twice-daily salmeterol and fluticasone propionate via Accuhaler® (ENERGITO®) study. Int J Chron Obstruct Pulmon Dis 2016; 11: 193–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Calzetta L, Rogliani P, Matera MG, et al. A systematic review with meta-analysis of dual bronchodilation with LAMA/LABA for the treatment of stable COPD. Chest 2016; 149: 1181–1196. [DOI] [PubMed] [Google Scholar]
- 16. Oba Y, Sarva ST, Dias S. Efficacy and safety of long-acting beta-agonist/long-acting muscarinic antagonist combinations in COPD: a network meta-analysis. Thorax 2016; 71: 15–25. [DOI] [PubMed] [Google Scholar]
- 17. Ismaila AS, Huisman EL, Punekar YS, et al. Comparative efficacy of long-acting muscarinic antagonist monotherapies in COPD: a systematic review and network meta-analysis. Int J Chron Obstruct Pulmon Dis 2015; 10: 2495–2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rodrigo GJ, Price D, Anzueto A, et al. LABA/LAMA combinations versus LAMA monotherapy or LABA/ICS in COPD: a systematic review and meta-analysis. Int J Chron Obstruct Pulmon Dis 2017; 12: 907–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Calzetta L, Rogliani P, Ora J, et al. LABA/LAMA combination in COPD: a meta-analysis on the duration of treatment. Eur Respir Rev 2017; 26: 160043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schlueter M, Gonzalez-Rojas N, Baldwin M, et al. Comparative efficacy of fixed-dose combinations of long-acting muscarinic antagonists and long-acting beta2-agonists: a systematic review and network meta-analysis. Ther Adv Respir Dis 2016; 10: 89–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cazzola M, Rogliani P, Calzetta L, et al. Triple therapy versus single and dual long-acting bronchodilator therapy in chronic obstructive pulmonary disease: a systematic review and meta-analysis. Eur Respir J 2018; 52: 1801586. [DOI] [PubMed] [Google Scholar]
- 22. Miravitlles M, Baek S, Vithlani V, et al. Optimal bronchodilation for COPD patients: are all long-acting beta2-agonist/long-acting muscarinic antagonists the same? Tuberc Respir Dis (Seoul) 2018; 81: 198–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rogliani P, Calzetta L, Braido F, et al. LABA/LAMA fixed-dose combinations in patients with COPD: a systematic review. Int J Chron Obstruct Pulmon Dis 2018; 13: 3115–3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sion KYJ, Huisman EL, Punekar YS, et al. A network meta-analysis of Long-Acting Muscarinic Antagonist (LAMA) and Long-Acting ß2-Agonist (LABA) Combinations in COPD. Pulm Ther 2017; 3: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kerwin E, Ferguson GT, Sanjar S, et al. Dual bronchodilation with indacaterol maleate/glycopyrronium bromide compared with umeclidinium bromide/vilanterol in patients with moderate-to-severe COPD: results from two randomized, controlled, cross-over studies. Lung 2017; 195: 739–747. [DOI] [PubMed] [Google Scholar]
- 26. Feldman GJ, Sousa AR, Lipson DA, et al. Comparative Efficacy of Once-Daily Umeclidinium/Vilanterol and Tiotropium/Olodaterol Therapy in Symptomatic Chronic Obstructive Pulmonary Disease: A Randomized Study. Adv Ther 2017; 34: 2518–2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dhillon S. Tiotropium/Olodaterol: a review in COPD. Drugs 2016; 76: 135–146. [DOI] [PubMed] [Google Scholar]
- 28. Mosley JF, Smith LL, Dutton BN. Tiotropium Bromide/Olodaterol (Stiolto Respimat): once-daily combination therapy for the maintenance of COPD. Pharmacy & Therapeutics 2016; 41: 97–102. [PMC free article] [PubMed] [Google Scholar]
- 29. Deeks ED. Olodaterol: a review of its use in chronic obstructive pulmonary disease. Drugs 2015; 75: 665–673. [DOI] [PubMed] [Google Scholar]
- 30. Cazzola M, MacNee W, Martinez FJ, et al. Outcomes for COPD pharmacological trials: from lung function to biomarkers. Eur Respir J 2008; 31: 416–469. [DOI] [PubMed] [Google Scholar]
- 31. Miravitlles M, Urrutia G, Mathioudakis AG, et al. Efficacy and safety of tiotropium and olodaterol in COPD: a systematic review and meta-analysis. Respir Res 2017; 18: 196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mahler DA, Witek TJ., Jr. The MCID of the transition dyspnea index is a total score of one unit. COPD 2005; 2: 99–103. [DOI] [PubMed] [Google Scholar]
- 33. Jones PW. St. George’s respiratory questionnaire: MCID. COPD 2005; 2: 75–79. [DOI] [PubMed] [Google Scholar]
- 34. Ferguson GT, Karpel J, Bennett N, et al. Effect of tiotropium and olodaterol on symptoms and patient-reported outcomes in patients with COPD: results from four randomised, double-blind studies. NPJ Prim Care Respir Med 2017; 27: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schunemann HJ, Puhan M, Goldstein R, et al. Measurement properties and interpretability of the Chronic respiratory disease questionnaire (CRQ). COPD 2005; 2: 81–89. [DOI] [PubMed] [Google Scholar]
- 36. Jenkins CR, Postma DS, Anzueto AR, et al. Reliever salbutamol use as a measure of exacerbation risk in chronic obstructive pulmonary disease. BMC Pulm Med 2015; 15: 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Punekar YS, Sharma S, Pahwa A, et al. Rescue medication use as a patient-reported outcome in COPD: a systematic review and regression analysis. Respir Res 2017; 18: 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Spruit MA, Watkins ML, Edwards LD, et al. Determinants of poor 6-min walking distance in patients with COPD: the ECLIPSE cohort. Respir Med 2010; 104: 849–857. [DOI] [PubMed] [Google Scholar]
- 39. Singh D, Gaga M, Schmidt O, et al. Effects of tiotropium + olodaterol versus tiotropium or placebo by COPD disease severity and previous treatment history in the OTEMTO® studies. Respir Res 2016; 17: 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Halpin DMG. The Role of Tiotropium+Olodaterol dual bronchodilator therapy in the management of chronic obstructive pulmonary disease. Tuberc Respir Dis (Seoul) 2018; 81: 13–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. O’Donnell DE. Hyperinflation, dyspnea, and exercise intolerance in chronic obstructive pulmonary disease. Proc Am Thorac Soc 2006; 3: 180–184. [DOI] [PubMed] [Google Scholar]
- 42. O’Donnell DE, Elbehairy AF, Webb KA, et al. The link between reduced inspiratory capacity and exercise intolerance in chronic obstructive pulmonary disease. Ann Am Thorac Soc 2017; 14: S30–S39. [DOI] [PubMed] [Google Scholar]
- 43. O’Donnell DE, Laveneziana P. Lung hyperinflation in COPD: the impact of pharmacotherapy. Eur Respir Review 2006; 15: 85–89. [Google Scholar]
- 44. Puente-Maestu L, Palange P, Casaburi R, et al. Use of exercise testing in the evaluation of interventional efficacy: an official ERS statement. Eur Respir J 2016; 47: 429–460. [DOI] [PubMed] [Google Scholar]
- 45. Borel B, Pepin V, Mahler DA, et al. Prospective validation of the endurance shuttle walking test in the context of bronchodilation in COPD. Eur Respir J 2014; 44: 1166–1176. [DOI] [PubMed] [Google Scholar]
- 46. O’Donnell DE, Flüge T, Gerken F, et al. Effects of tiotropium on lung hyperinflation, dyspnoea and exercise tolerance in COPD. Eur Respir J 2004; 23: 832–840. [DOI] [PubMed] [Google Scholar]
- 47. O’Donnell DE, Casaburi R, Vincken W, et al. Effect of indacaterol on exercise endurance and lung hyperinflation in COPD. Resp Med 2011; 105: 1030–1036. [DOI] [PubMed] [Google Scholar]
- 48. Maltais F, Hamilton A, Marciniuk D, et al. Improvements in symptom-limited exercise performance over 8 h with once-daily tiotropium in patients with COPD. Chest 2005; 128: 1168–1178. [DOI] [PubMed] [Google Scholar]
- 49. Bedard ME, Brouillard C, Pepin V, et al. Tiotropium improves walking endurance in COPD. Eur Respir J 2012; 39: 265–271. [DOI] [PubMed] [Google Scholar]
- 50. Cooper CB, Celli BR, Jardim JR, et al. Treadmill endurance during 2-year treatment with tiotropium in patients with COPD: a randomized trial. Chest 2013; 144: 490–497. [DOI] [PubMed] [Google Scholar]
- 51. Casaburi R, Maltais F, Porszasz J, et al. Effects of tiotropium on hyperinflation and treadmill exercise tolerance in mild to moderate chronic obstructive pulmonary disease. Ann Am Thorac Soc 2014; 11: 1351–1361. [DOI] [PubMed] [Google Scholar]
- 52. Maltais F, Kirsten AM, Hamilton A, et al. Evaluation of the effects of olodaterol on exercise endurance in patients with chronic obstructive pulmonary disease: results from two 6-week crossover studies. Respir Res 2016; 17: 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Borel B, Provencher S, Saey D, et al. Responsiveness of various exercise-testing protocols to therapeutic interventions in COPD. Pulm Med 2013; 2013: 410748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Pepin V, Laviolette L, Brouillard C, et al. Significance of changes in endurance shuttle walking performance. Thorax 2011; 66: 115–120. [DOI] [PubMed] [Google Scholar]
- 55. Bourbeau J, Lavoie KL, Sedeno M, et al. Behaviour-change intervention in a multicentre, randomised, placebo-controlled COPD study: methodological considerations and implementation. BMJ Open 2016; 6: e010109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mendoza L, Horta P, Espinoza J, et al. Pedometers to enhance physical activity in COPD: a randomised controlled trial. Eur Respir J 2015; 45: 347–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Demeyer H, Louvaris Z, Frei A, et al. Physical activity is increased by a 12-week semiautomated telecoaching programme in patients with COPD: a multicentre randomised controlled trial. Thorax 2017; 72: 415–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Thorpe O, Johnston K, Kumar S. Barriers and enablers to physical activity participation in patients with COPD: a systematic review. J Cardiopulm Rehabil Prev 2012; 32: 359–369. [DOI] [PubMed] [Google Scholar]
- 59. Thorpe O, Kumar S, Johnston K. Barriers to and enablers of physical activity in patients with COPD following a hospital admission: a qualitative study. Int J Chron Obstruct Pulmon Dis 2014; 9: 115–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Nolan CM, Maddocks M, Canavan JL, et al. Pedometer step count targets during pulmonary rehabilitation in chronic obstructive pulmonary disease. A randomized controlled trial. Am J Respir Crit Care Med 2017; 195: 1344–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Gimeno-Santos E, Raste Y, Demeyer H, et al. The PROactive instruments to measure physical activity in patients with chronic obstructive pulmonary disease. Eur Respir J 2015; 46: 988–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Maltais F, Buhl R, Koch A, et al. β-blockers in COPD: a cohort study from the TONADO research program. Chest 2018; 153: 1315–1325. [DOI] [PubMed] [Google Scholar]
- 63. Bai C, Ichinose M, Lee SH, et al. Lung function and long-term safety of tiotropium/olodaterol in East Asian patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 2017; 12: 3329–3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ichinose M, Taniguchi H, Takizawa A, et al. The efficacy and safety of combined tiotropium and olodaterol via the Respimat® inhaler in patients with COPD: results from the Japanese sub-population of the Tonado® studies. Int J Chron Obstruct Pulmon Dis 2016; 11: 2017–2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ichinose M, Nishimura M, Akimoto M, et al. Tiotropium/olodaterol versus tiotropium in Japanese patients with COPD: results from the DYNAGITO study. Int J Chron Obstruct Pulmon Dis 2018; 13: 2147–2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Selya-Hammer C, Gonzalez-Rojas Guix N, Baldwin M, et al. Development of an enhanced health-economic model and cost-effectiveness analysis of tiotropium + olodaterol Respimat® fixed-dose combination for chronic obstructive pulmonary disease patients in Italy. Therap Adv Respir Dis 2016; 10: 391–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Tashkin DP, Celli B, Senn S, et al. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med 2008; 359: 1543–1554. [DOI] [PubMed] [Google Scholar]
- 68. Wise RA, Anzueto A, Cotton D, et al. Tiotropium Respimat inhaler and the risk of death in COPD. N Engl J Med 2013; 369: 1491–1501. [DOI] [PubMed] [Google Scholar]
- 69. van Boven JF, Kocks JW, Postma MJ. Cost-effectiveness and budget impact of the fixed-dose dual bronchodilator combination tiotropium-olodaterol for patients with COPD in the Netherlands. Int J Chron Obstruct Pulmon Dis 2016; 11: 2191–2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Tebboth A, Ternouth A, Gonzalez-Rojas N. UK-specific cost-effectiveness of tiotropium + olodaterol fixed-dose combination versus other LAMA + LABA combinations in patients with COPD. Clinicoecon Outcomes Res 2016; 8: 667–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Halpin DM, Dahl R, Hallmann C, et al. Tiotropium HandiHaler® and Respimat® in COPD: a pooled safety analysis. Int J Chron Obstruct Pulmon Dis 2015; 10: 239–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Lee HW, Kim HJ, Lee CH. The impact of olodaterol on the risk of mortality and serious adverse events: a systematic review and meta-analysis. Br J Clin Pharmacol 2017; 83: 1166–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ferguson GT, Buhl R, Bothner U, et al. Safety of tiotropium/olodaterol in chronic obstructive pulmonary disease: pooled analysis of three large, 52-week, randomized clinical trials. Respir Med 2018; 143: 67–73. [DOI] [PubMed] [Google Scholar]
- 74. LaForce C, Derom E, Bothner U, et al. Long-term safety of tiotropium/olodaterol Respimat® in patients with moderate-to-very severe COPD and renal impairment in the TONADO® studies. Int J Chron Obstruct Pulmon Dis 2018; 13: 1819–1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Nelson HS, Weiss ST, Bleecker ER, et al. The salmeterol multicenter asthma research trial: a comparison of usual pharmacotherapy for asthma or usual pharmacotherapy plus salmeterol. Chest 2006; 129: 15–26. [DOI] [PubMed] [Google Scholar]
- 76. Halpin DM, Vogelmeier C, Pieper MP, et al. Effect of tiotropium on COPD exacerbations: a systematic review. Respir Med 2016; 114: 1–8. [DOI] [PubMed] [Google Scholar]
- 77. Chapman KR, Rennard SI, Dogra A, et al. Long-term safety and efficacy of indacaterol, a long-acting beta2-agonist, in subjects with COPD: a randomized, placebo-controlled study. Chest 2011; 140: 68–75. [DOI] [PubMed] [Google Scholar]
- 78. Calverley PM, Anderson JA, Celli B, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med 2007; 356: 775–789. [DOI] [PubMed] [Google Scholar]
- 79. Jones PW, Donohue JF, Nedelman J, et al. Correlating changes in lung function with patient outcomes in chronic obstructive pulmonary disease: a pooled analysis. Respir Res 2011; 12: 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Calzetta L, Rogliani P, Mattei M, et al. Pharmacological characterization of the interaction between tiotropium and olodaterol administered at 5:5 concentration-ratio in equine bronchi. COPD 2017; 14: 526–532. [DOI] [PubMed] [Google Scholar]
- 81. Calzetta L, Rogliani P, Facciolo F, et al. Pharmacological characterization of the interaction between umeclidinium and vilanterol in human bronchi. Eur J Pharmacol 2017; 812: 147–154. [DOI] [PubMed] [Google Scholar]
- 82. Cazzola M, Calzetta L, Page CP, et al. Pharmacological characterization of the interaction between aclidinium bromide and formoterol fumarate on human isolated bronchi. Eur J Pharmacol 2014; 745: 135–143. [DOI] [PubMed] [Google Scholar]
- 83. Cazzola M, Calzetta L, Puxeddu E, et al. Pharmacological characterisation of the interaction between glycopyrronium bromide and indacaterol fumarate in human isolated bronchi, small airways and bronchial epithelial cells. Respir Res 2016; 17: 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Price D, Ostrem A, Thomas M, et al. Dual bronchodilation in COPD: lung function and patient-reported outcomes - a review. Int J Chron Obstruct Pulmon Dis 2017; 12: 141–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Donohue JF. Therapeutic responses in asthma and COPD. Bronchodilators. Chest 2004; 126: 125S–137S. [DOI] [PubMed] [Google Scholar]
- 86. Donohue JF, Singh D, Munzu C, et al. Magnitude of umeclidinium/vilanterol lung function effect depends on monotherapy responses: results from two randomised controlled trials. Respir Med 2016; 112: 65–74. [DOI] [PubMed] [Google Scholar]
- 87. Jones PW, Beeh KM, Chapman KR, et al. Minimal clinically important differences in pharmacological trials. Am J Respir Crit Care Med 2014; 189: 250–255. [DOI] [PubMed] [Google Scholar]
- 88. Tantucci C, Duguet A, Similowski T, et al. Effect of salbutamol on dynamic hyperinflation in chronic obstructive pulmonary disease patients. Eur Respir J 1998; 12: 799–804. [DOI] [PubMed] [Google Scholar]
- 89. Beeh KM, Korn S, Beier J, et al. Effect of QVA149 on lung volumes and exercise tolerance in COPD patients: the BRIGHT study. Respir Med 2014; 108: 584–592. [DOI] [PubMed] [Google Scholar]
- 90. Maltais F, Singh S, Donald AC, et al. Effects of a combination of umeclidinium/vilanterol on exercise endurance in patients with chronic obstructive pulmonary disease: two randomized, double-blind clinical trials. Ther Adv Respir Dis 2014; 8: 169–181. [DOI] [PubMed] [Google Scholar]
- 91. Singh S, Maltais F, Tombs L, et al. Relationship between exercise endurance and static hyperinflation in a post hoc analysis of two clinical trials in patients with COPD. Int J Chron Obstruct Pulmon Dis 2018; 13: 203–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Watz H, Troosters T, Beeh KM, et al. ACTIVATE: the effect of aclidinium/formoterol on hyperinflation, exercise capacity, and physical activity in patients with COPD. Int J Chron Obstruct Pulmon Dis 2017; 12: 2545–2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Deschenes D, Pepin V, Saey D, et al. Locus of symptom limitation and exercise response to bronchodilation in chronic obstructive pulmonary disease. J Cardiopulm Rehabil Prev 2008; 28: 208–214. [DOI] [PubMed] [Google Scholar]
- 94. Magnussen H, Paggiaro P, Schmidt H, et al. Effect of combination treatment on lung volumes and exercise endurance time in COPD. Respir Med 2012; 106: 1413–1420. [DOI] [PubMed] [Google Scholar]
- 95. Wedzicha JA, Decramer M, Ficker JH, et al. Analysis of chronic obstructive pulmonary disease exacerbations with the dual bronchodilator QVA149 compared with glycopyrronium and tiotropium (SPARK): a randomised, double-blind, parallel-group study. Lancet Respir Med 2013; 1: 199–209. [DOI] [PubMed] [Google Scholar]
- 96. Wedzicha JA, Banerji D, Chapman KR, et al. Indacaterol-Glycopyrronium versus Salmeterol-Fluticasone for COPD. N Engl J Med 2016; 374: 2222–2234. [DOI] [PubMed] [Google Scholar]
- 97. Lipson DA, Barnhart F, Brealey N, et al. Once-daily single-inhaler triple versus dual therapy in patients with COPD. N Engl J Med 2018; 378: 1671–1680. [DOI] [PubMed] [Google Scholar]
- 98. Singh D, Papi A, Corradi M, et al. Single inhaler triple therapy versus inhaled corticosteroid plus long-acting beta2-agonist therapy for chronic obstructive pulmonary disease (TRILOGY): a double-blind, parallel group, randomised controlled trial. Lancet (London, England) 2016; 388: 963–973. [DOI] [PubMed] [Google Scholar]
- 99. Lipson DA, Barnacle H, Birk R, et al. FULFIL trial: once-daily triple therapy for patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2017; 196: 438–446. [DOI] [PubMed] [Google Scholar]
- 100. Ferguson GT, Rabe KF, Martinez FJ, et al. Triple therapy with budesonide/glycopyrrolate/formoterol fumarate with co-suspension delivery technology versus dual therapies in chronic obstructive pulmonary disease (KRONOS): a double-blind, parallel-group, multicentre, phase 3 randomised controlled trial. Lancet Respir Med 2018; 6: 747–758. [DOI] [PubMed] [Google Scholar]
- 101. Beeh KM, Burgel PR, Franssen FME, et al. How do dual long-acting bronchodilators prevent exacerbations of chronic obstructive pulmonary disease? Am J Respir Crit Care Med 2017; 196: 139–149. [DOI] [PubMed] [Google Scholar]
- 102. Brusselle GG, Bracke K, Lahousse L. Targeted therapy with inhaled corticosteroids in COPD according to blood eosinophil counts. Lancet Respir Med 2015; 3: 416–417. [DOI] [PubMed] [Google Scholar]
- 103. Brusselle G, Pavord ID, Landis S, et al. Blood eosinophil levels as a biomarker in COPD. Respir Med 2018; 138: 21–31. [DOI] [PubMed] [Google Scholar]
- 104. Pascoe S, Barnes N, Brusselle G, et al. Blood eosinophil counts and treatment response in COPD: analyses of IMPACT. Eur Respir J 2018; 52: OA2127. DOI: 10.1183/13993003.congress-2018.OA2127. Abstract book. [DOI] [Google Scholar]
- 105. Pascoe S, Locantore N, Dransfield MT, et al. Blood eosinophil counts, exacerbations, and response to the addition of inhaled fluticasone furoate to vilanterol in patients with chronic obstructive pulmonary disease: a secondary analysis of data from two parallel randomised controlled trials. Lancet Respir Med 2015; 3: 435–442. [DOI] [PubMed] [Google Scholar]
- 106. Bafadhel M, Peterson S, De Blas MA, et al. Predictors of exacerbation risk and response to budesonide in patients with chronic obstructive pulmonary disease: a post-hoc analysis of three randomised trials. Lancet Respir Med 2018; 6: 117–126. [DOI] [PubMed] [Google Scholar]
- 107. Chapman KR, Hurst JR, Frent SM, et al. Long-term triple therapy de-escalation to indacaterol/glycopyrronium in patients with chronic obstructive pulmonary disease (SUNSET): a randomized, double-blind, triple-dummy clinical trial. Am J Respir Crit Care Med 2018; 198: 329–339. [DOI] [PubMed] [Google Scholar]
- 108. Calverley PMA, Tetzlaff K, Vogelmeier C, et al. Eosinophilia, frequent exacerbations, and steroid response in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2017; 196: 1219–1221. [DOI] [PubMed] [Google Scholar]
- 109. Papi A, Vestbo J, Fabbri L, et al. Extrafine inhaled triple therapy versus dual bronchodilator therapy in chronic obstructive pulmonary disease (TRIBUTE): a double-blind, parallel group, randomised controlled trial. Lancet (London, England) 2018; 391: 1076–1084. [DOI] [PubMed] [Google Scholar]