Abstract
Eggs are female germ cells that are required for producing offspring through sexual reproduction. In mammals, eggs are produced in the ovary and ovulated into the oviduct. It is well known that over 99% of eggs are degenerated without ovulation, so that many studies have attempted in vitro folliculogenesis to produce many eggs in different species for a few decades. Although many methods have been developed, a success of in vitro egg production with the resultant live birth of offspring has been limited, especially in livestock animals. More recently, we have succeeded in producing live pups derived from in vitro/ex vivo egg production in mice. This review aims to introduce our recent findings with a brief history of in vitro/ex vivo culture systems for follicles and ovaries.
Keywords: Folliculogenesis, In vitro egg production, Oogenesis
Introduction
Recently, the journal “Reproduction” has published a special issue entitled “the 40th Anniversary of human IVF” after the birth of the first baby, Louise Brown [1]. The technique of in vitro fertilization (IVF) was developed for embryo production, whereas it needed eggs and sperm, and was based on development of techniques for in vitro manipulation of the cells under a microscope. Such techniques are now called assisted reproductive technology (ART). In general, ART treats and needs eggs and sperm, because these cells are the germ cells that produce offspring. However, there is a difference between the geneses of the two germ cells; sperm can be produced from stem cells in the testis throughout most of the lifespan, whereas eggs are produced in the ovary throughout a part of the adult period only from within follicles primary oocytes that are differentiated from oogonia in the fetal or postpartum period. In addition, egg production in the adult ovary is limited, because of lack of stem cells, and over 99% of eggs are degenerated [2].
Many studies have been attempted to develop a technique for producing multiple eggs from the ovaries [3]. For example, superovulation by treatment with hormones is effective in calf production, but the number of ovulated eggs is limited to about 10 and more eggs per treatment. Furthermore, since around 1980, in vitro maturation, fertilization, and embryo culture (IVMFC) has been developed using ovaries obtained from animals in slaughterhouse. Unfortunately, eggs for in vitro maturation (IVM) are limited only from oocytes within antral follicles, so that its number is 10 to 20 full-grown oocytes in each ovary, meaning that a number of preantral follicles are unusable in the IVM technique. On the other hand, in vitro folliculogenesis from primordial and primary follicles has been studied to produce many eggs in several species [4]. Although many methods have been developed, successful in vitro egg production with the live birth of offspring has been limited, especially in livestock animals. More recently, we have succeeded in producing live pups derived from in vitro/ex vivo egg production in mice [5]. In this review, I will introduce our recent findings with a brief history of the in vitro/ex vivo culture system for follicles and ovaries.
Oogenesis and Folliculogenesis in General
In the course of fetal development, primordial germ cells (PGCs) move to the genital ridges, and in females, they differentiate to oogonia in the ovary. Depending on the species, oogonia increase their numbers up to several thousands, and finally all of them differentiate to primary oocytes with entry into meiosis at the diplotene stage of prophase I. The process of this oogenesis is a process of oocyte production and is quite different to spermatogenesis in the testis, where stem cells for spermatogonia are present throughout most of the lifespan. Except for the report by Tilly [6], the ovary has no stem cells after oogenesis.
After differentiation of oogonia to oocytes, each oocyte is enclosed in a single primordial follicle with a few flattened granulosa cells. Initiation of follicle development from primordial (non-grown oocyte) to antral follicle stages (full-grown oocyte), defined as folliculogenesis, is quite different among species; that is, from at the fetal stage to after birth. For example, in the cow, sheep, pigs, and humans, the ovary starts folliculogenesis in the fetus at around 140, 100, 70, and 110 to 150 days of pregnancy, respectively [7,8,9,10,11]. Therefore, if we could collect fetal ovaries in these animals, embryo production could be started before birth and get offspring in their childhood. On the other hand, in rats, mice, hamsters and rabbits, folliculogenesis starts after birth [2]. Once folliculogenesis starts, the selection occurs at each follicle developmental stage and only some dominant follicles can grow to the final stage. Full-grown oocytes within those follicles can be finally ovulated; the number of ovulated oocytes is dependent on the species. For example, in the cow and humans, one oocyte is ovulated in each cycle, while in pigs, more than ten oocytes are ovulated. In mammals, it is known that during folliculogenesis, only 1% or less of matured oocytes are ovulated during their reproductive period, whereas the remaining enter the atretic process and are degenerated.
In Vitro/Ex Vivo Oogenesis and Folliculogenesis
To release and change the fate of oocytes from the course of the follicle atretic event, the strategy of in vitro growth is valuable for application to human ART as well as for preservation of germ cells in endangered animals, and in accelerating breeding improvement for livestock animals by supplying a large number of eggs. For example, when progeny tests in cattle are planned, several years are needed for the candidate to reach reproductive age and a further several years are needed to analyze its ability; in contrast, by somatic cloning and embryo transfer with many eggs a sib test or individual performance test of the sire can be performed and the duration for analyzing the performance and characteristics of the candidate can be shortened. In addition, collecting immature oocytes from newborn offspring, maintaining them in vitro, and then performing IVMFC and embryo transfer could shorten the duration of producing progeny with high performance by half [3].
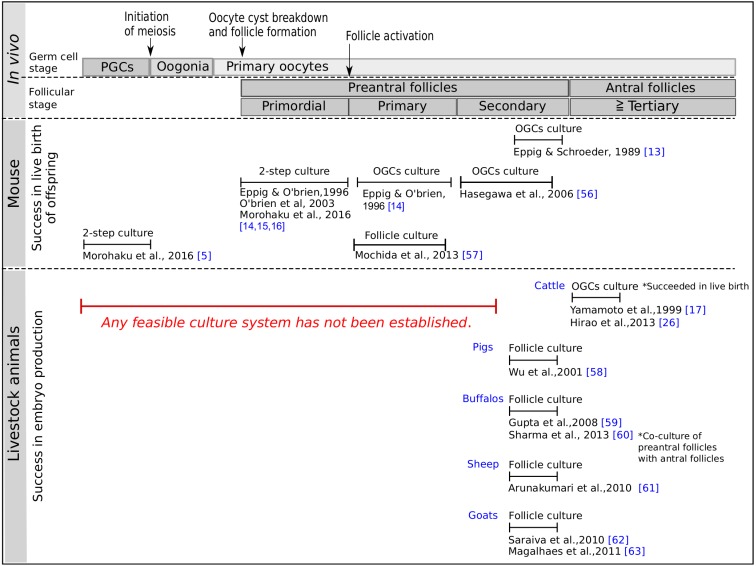
In the in vitro experiment with the oocytes of mammalian ovaries, the first successful production of matured eggs with proven ontogenic potential was reported by Cross and Brinster [12]. They collected and cultured full-grown oocytes from antral follicles of the mouse ovary, resulting in resumption of meiosis of the oocytes cultured in 199 medium. They used bovine calf serum to obtain matured eggs. In general, it is known that serum for cell culture, especially fetal bovine serum (FBS), has a key effect when added in a basal medium, because it contains a large number of growth factors and other unknown factors for cell proliferation. In 1989, Eppig and Schroeder [13] developed a follicular culture system that enables the growth of oocytes. They cultured mouse oocyte-granulosa cell complexes (OGCs) from preantral follicles in a medium supplemented with FBS for 10 days. Furthermore, in 1996, they developed a 2-step method; that is, in the first step, neonatal mouse ovaries with primitive follicles were ex vivo cultured for 8 days, and in the second step, after isolated secondary follicles grown from the cultured ovaries they reached the mature follicle stage after in vitro culture for 14 days [14]. Finally, they obtained live pups for the first time by in vitro/ex vivo egg production. Considering that it is extremely difficult to isolate and cultivate primitive follicles intact from the ovaries, the 2-step method developed by Eppig’s group must be considered a commendable protocol to pave the way for in vitro/ex vivo egg production. Unfortunately, although they improved their culture condition in 2003 with an increment in live pups [15], their method has shown a professional problem with difficulties faced by other researchers to reproduce the protocol for a long time (Fig. 1).
Fig. 1.
A schematic overview of in vitro/ex vivo egg production in the mouse and livestock animals [5, 13,14,15,16,17, 26, 56,57,58,59,60,61,62,63]. In the mouse, the culture methods have been developed in all follicular stages as well as in primordial germ cells with successful live birth of offspring, whereas in livestock animals, the methods have been developed only in the late secondary and antral follicles with a low yield of embryo production. The 2-step protocol consists of ex vivo organ culture and in vitro follicle culture. PGCs; primordial germ cells, OGCs; oocyte-granulosa cell complexes.
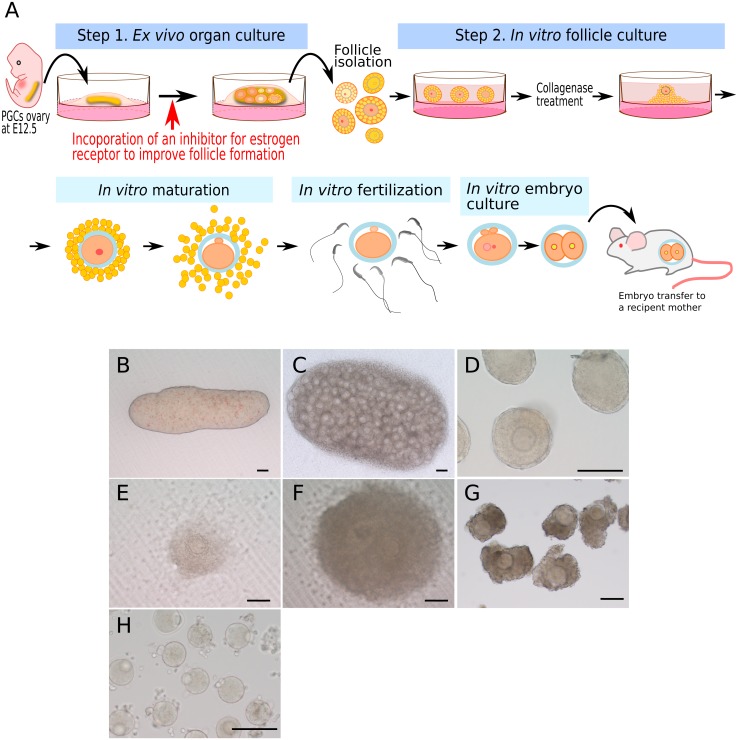
Under such circumstances, in 2016, Morohaku and colleagues promoted in vitro follicle development in modified culture medium by introducing polyvinylpyrrolidone (PVP) that is a high molecular weight compound [16]. After our modification to the 2-step method, the production of matured oocytes has been shown to be highly reproducible. In addition, we applied this 2-step method to cultivate mouse fetal ovaries that contain PGCs, resulting in isolation of follicles developed after ex vivo culture for 17 days followed by further culture for 14 days, and successful production of matured eggs (Fig. 2). This achievement in in vitro/ex vivo egg production from PGCs shows complete a representation of the following process: 1) oogenesis and folliculogenesis (meiotic entry and formation of primordial follicles), 2) establishment of oocyte-specific genome imprinting, and 3) growth and maturation of the cytoplasm and karyoplast in the oocyte.
Fig. 2.
An overview of the 2-step culture of mouse PGCs ovaries collected at 12.5 embryonic day (E12.5). In step 1, the ovaries are subjected to ex vivo organ culture on a Transwell-COL membrane for 17 days in α-MEM medium supplemented with 10% FBS. Follicle formation is assisted by the addition of an inhibitor for estrogen receptors using ICI182,780 at day 5 to 11 after culture to facilitate oocyte cyst breakdown. In step 2, secondary follicles isolated after 17 days of culture are cultured on the membrane with 5% FBS, 0.1 IU/ml follicle stimulating hormone (FSH), and 2% PVP supplemented α-MEM. In the course of culture, the follicles are treated with collagenase on day 20 after culture to remove the theca cell layers and basement membrane, both of which suppress in vitro follicle growth, and further cultured to day 31, after culture followed by IVMFC and embryo transfer to produce offspring. (A) Illustration of the 2-step culture. (B)–(H) Micro photos of cultures; PGCs ovary before culture (B), PGCs ovary after 17 days of culture (C), secondary follicles after 20 days of culture (D), in vitro follicle growth at day 26 (E) and day 30 (F) after culture, respectively, cumulus cell-oocyte complexes (G) collected from in vitro grown follicles and matured oocytes (H) produced after IVM. Each bar represents 100 µm.
Considerable Key Points for In Vitro/Ex Vivo Culture
Origin/kind of tissues
When in vitro culture of follicles is performed, the follicle developmental stage used is important for successful egg production, especially in livestock animals. In cattle, pigs, sheep, goats, and horses, in vitro culture of OGCs from antral follicles has yield viable matured eggs followed by production of embryos after IVF [17,18,19,20,21,22,23,24,25], whereas the protocols for culture of preantral follicles is very limited in these animals.
In case of OGCs culture from bovine and porcine follicles in most studies, the reason for using antral follicles is that oocytes within these follicles are already fully grown in ovaries derived from slaughterhouses, which are easy to be collected by aspirating with a syringe attached to a needle or by dissection. On the other hand, since the oocytes included in preantral follicles are not fully grown, follicle culture is needed before IVM to help oocytes grow fully. Therefore, a protocol for in vitro culture must have the ability to maintain follicle morphology, and several methods for collecting preantral follicles have been developed including mechanical isolation by dissecting ovarian tissues with needles, enzymatic isolation with collagenase, or a combination of both. In the bovine, when early antral follicles were cultured in the medium supplemented with 4% PVP for 14 days, the recovery rate of cumulus cell-oocyte complexes with almost entire morphology was increased, resulting in the birth of a live calf [26]. However, it is very hard to isolate preantral follicles, because of well-developed abundant interstitial cells in the ovaries of livestock animals examined [27,28,29]. In addition, considering that primordial follicles are reserved within the cortical area in adult livestock animals such as cattle, pigs, and sheep, any protocol for isolating of these follicles may have little feasibility so far. As an alternative protocol, ex vivo or ex situ culture of ovarian cortical tissues has been performed, resulting in the yield of viable eggs [30,31,32].
Nutrients in culture medium
For in vitro culture in most studies, FBS is used as a key element to supply various growth factors and nutrients for various kinds of cells as well as for oocytes and follicles. However, it has been reported that FBS contains estrogen, due to which follicular dysplasia in vitro can be possibly induced in the mouse [5]. Further, oocyte development and susceptibility to estrogen differ among mouse strains in ex vivo culture of the neonatal ovary [33]. Until now, research groups in the United States have shown that the number and types of follicles show variation in mouse ovaries in the neonatal period, and that the ratio of primordial follicles at the initiation of follicular development and the rate of regressed follicles already differ in that period [34]. Considering these findings, we introduced estrogen inhibitors into the culture medium, resulting in promotion of follicle formation [5]. Further studies are required to establish a chemically defined medium to understand the detail of the mechanisms of oogenesis. Some predisposing factor(s) from follicle development to induction of ovulation may be possibly involved in the success of in vitro oocyte growth.
Induction of follicle activation
At initiation of follicle development, it is revealed that primordial follicles must be activated for starting their development, which is called as follicle activation. The phosphoinositide 3-kinase (PI3K)/Akt pathway is known to play a central role in follicle activation. This pathway is fundamental for cell proliferation, survival, and metabolism. In several studies, PI3K/Akt signaling is shown to be related to follicle activation in livestock animals; for example, in mare ovaries, PI3K/Akt and Janus kinase/signal transducer and activator of transcription (JAK/STAT) signaling are conserved in follicles [35]. In fact, several regulating factors of the PI3K/Akt pathway have been applied to induce in vitro follicle activation in rodents and livestock animals.
Phosphatase homologue of chromosome-10 (PTEN) is known as a negative regulator of the PI3K/Akt signaling pathway. In PTEN deficient mice, depletion of primordial follicles from ovarian stocks is observed due to accelerated follicle activation after birth [36]. Inhibition of PTEN using bpV(HOpic) promotes activation of mouse primordial follicles [37, 38]. When culturing the pieces of bovine ovarian tissue or cortical strips with bpV(HOpic), the transition from primordial to primary follicles increases, whereas DNA damage in oocytes and granulosa cells of the primary and secondary follicles increases as well [39]. More recently, unlike bpV(HOpic), flavanol kaempferol that is known to be one of activators of PI3K/Akt signaling has been shown to exert a positive effect on increasing follicle activation with less DNA damage in culture of sheep cortical tissues [40].
A forkhead transcription factor, FOXO3, is also known to be a negative regulator of follicle activation by acting downstream of Akt by binding to a specific promoter in the nucleus [41]. In FOXO3 deficient mice, the ovaries start follicle activation simultaneously, and show early exhaustion of the oocytes [42]. After in vitro knockdown of FOXO3 in porcine ovaries, their xenograft into severe combined immunodeficiency mice promotes follicle activation [43].
As a positive regulator of PI3K/Akt signaling pathway in the follicle, kit ligand and testosterone have been reported in some species. Kit ligand is produced from granulosa cells in the follicle, and is a ligand of the tyrosine kinase receptor, KIT, on the oocyte. Interaction between kit ligand and KIT activates mouse primordial follicles and promotes oocyte growth [44, 45]. Supplementation of kit ligand in culture medium increases oocyte diameter and mRNA expression in somatic cell differentiation of lamb primordial follicles isolated from frozen ovaries. Unfortunately, it remains to be clarified whether to induce follicle activation [46], while follicle survival is not affected by kit ligand. In the rabbit, kit ligand has no effect in vitro to stimulate follicle activation, despite oocytes growth [47]. On the other hand, testosterone stimulates follicle activation in cultured porcine ovarian cortical tissues, at a rate of around 20% [48].
Anti-Mullerian hormone (AMH), is a member of the transforming growth factor beta family, inhibits follicle activation in rodents [49, 50]. Research by Fortune’s group demonstrated the effect of AMH in a culture of bovine ovaries obtained at a mid-gestation stage, which showed that follicle activation is inhibited under culture condition with addition of insulin as a stimulator after culture for 12 days [51]. This is contradictory to their previous studies [52, 53], where AMH addition did not affect follicle activation under insulin. This inconsistency may be due to the duration of culture period; 12 vs. 2 days. Possibly, as AMH has less or slow ability to penetrate into cortical tissues under in vitro conditions, a short-term culture may be insufficient for inducing follicle activation [51].
As described above, in case of in vitro culture of primordial follicles, follicle activation is the first key event for further success to produce viable eggs. Interestingly, despite multiple pathways committed in follicle activation, in vitro culture of ovarian pieces shows spontaneous follicle activation without addition of any specific factors such as kit ligand and AMH [47, 51]. As a possible explanation, when cortical tissues or primordial follicles are cultured in vitro/ex vivo, they may be free from the ovary’s in- situ niche of inhibitory factor(s) in the ovary.
Conclusion
For almost a half century, although many scientists have tried to develop in vitro growth and development of mammalian primary oocytes in order to produce viable eggs, a feasible and effective protocol for egg production in livestock animals and humans still remains to be established (Fig. 1). Currently, to differentiate primordial germ cells to mature oocytes, assisted technology developed has been reported, but its application is limited to mice [5, 16]. Further studies are needed to unravel the mechanisms of oogenesis and folliculogenesis in order to establish effectively in vitro usage of oocytes, most of which will degenerate in the ovary in vivo, and also in order to facilitate in vitro/ex vivo egg production in endangered species and genetically superior livestock animals beyond/outside of their reproductive period.
In in vitro/ex vivo condition, it is unlikely to completely mimic in vivo physiological status, in other words, not necessary to mimic that. In fact, some successful reports including ours have shown follicle development in a limited condition. Recently, metabolism of different follicular stages has been defined directly by measuring the nicotinamide adenine dinucleotide hydride (NADH) with the technique of fluorescence lifetime imaging [54], which seems contrary to measuring metabolites under in vitro culture conditions. This report has shown that oocytes of mouse primordial follicles have free NADH compared to those of primary and secondary follicles, meaning that the oocytes can produce more NADH through glycolysis and Krebs cycle. Thus, we expect the development of a new technology that would help us better understand the in vivo systems related to oogenesis and folliculogenesis.
Perspective
Our report, in which viable eggs are produced in vitro/ex vivo from primordial germ cells, could facilitate breakthrough in egg production i.e. the production of mature oocytes from pluripotent stem cells. Immediately after our report, Hikabe and colleagues produced primordial germ cell-like cells by differentiating mouse induced pluripotent stem cells (iPSCs) using our technique; eventually they acquired offspring from matured eggs [55]. In the future, with the establishment of iPSCs in livestock animals, a new technology with artificial intelligence will enable us to efficiently perform in vitro/ex vivo egg production.
Acknowledgments
I would like to extend special thanks to Professors; Dr Hiroshi Sasada (Kitasato University), Dr Yayoi Obata (Tokyo University of Agriculture) and Dr Vimal Selvaraj (Cornell University, USA) for encouraging and supporting my research.
References
- 1.Winston RML. The 40th anniversary of human IVF: time to celebrate and time to reflect. Reproduction 2018; 156: E1–E3. [DOI] [PubMed] [Google Scholar]
- 2.Plant TM, Zeleznik AJ, Albertini DF, Goodman RL, Herbison AE, McCarthy MM, Muglia LJ, Richards JS, Knobil E, Neill JD. Knobil and Neill’s Physiology of reproduction. Oxford: Elsevier/Academic Press; 2015: 2 v. [Google Scholar]
- 3.Moore SG, Hasler JF. A 100-Year Review: Reproductive technologies in dairy science. J Dairy Sci 2017; 100: 10314–10331. [DOI] [PubMed] [Google Scholar]
- 4.Herta AC, Lolicato F, Smitz JEJ. In vitro follicle culture in the context of IVF. Reproduction 2018; 156: F59–F73. [DOI] [PubMed] [Google Scholar]
- 5.Morohaku K, Tanimoto R, Sasaki K, Kawahara-Miki R, Kono T, Hayashi K, Hirao Y, Obata Y. Complete in vitro generation of fertile oocytes from mouse primordial germ cells. Proc Natl Acad Sci USA 2016; 113: 9021–9026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tilly JL, Niikura Y, Rueda BR. The current status of evidence for and against postnatal oogenesis in mammals: a case of ovarian optimism versus pessimism? Biol Reprod 2009; 80: 2–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang MY, Fortune JE. The capacity of primordial follicles in fetal bovine ovaries to initiate growth in vitro develops during mid-gestation and is associated with meiotic arrest of oocytes. Biol Reprod 2008; 78: 1153–1161. [DOI] [PubMed] [Google Scholar]
- 8.McNatty KP, Smith P, Hudson NL, Heath DA, Tisdall DJ, O WS, Braw-Tal R. Development of the sheep ovary during fetal and early neonatal life and the effect of fecundity genes. J Reprod Fertil Suppl 1995; 49: 123–135. [PubMed] [Google Scholar]
- 9.Oxender WD, Colenbrander B, van deWiel DF, Wensing CJ. Ovarian development in fetal and prepubertal pigs. Biol Reprod 1979; 21: 715–721. [DOI] [PubMed] [Google Scholar]
- 10.Kurilo LF. Oogenesis in antenatal development in man. Hum Genet 1981; 57: 86–92. [DOI] [PubMed] [Google Scholar]
- 11.Reynaud K, Cortvrindt R, Verlinde F, De Schepper J, Bourgain C, Smitz J. Number of ovarian follicles in human fetuses with the 45,X karyotype. Fertil Steril 2004; 81: 1112–1119. [DOI] [PubMed] [Google Scholar]
- 12.Cross PC, Brinster RL. In vitro development of mouse oocytes. Biol Reprod 1970; 3: 298–307. [DOI] [PubMed] [Google Scholar]
- 13.Eppig JJ, Schroeder AC. Capacity of mouse oocytes from preantral follicles to undergo embryogenesis and development to live young after growth, maturation, and fertilization in vitro. Biol Reprod 1989; 41: 268–276. [DOI] [PubMed] [Google Scholar]
- 14.Eppig JJ, O’Brien MJ. Development in vitro of mouse oocytes from primordial follicles. Biol Reprod 1996; 54: 197–207. [DOI] [PubMed] [Google Scholar]
- 15.O’Brien MJ, Pendola JK, Eppig JJ. A revised protocol for in vitro development of mouse oocytes from primordial follicles dramatically improves their developmental competence. Biol Reprod 2003; 68: 1682–1686. [DOI] [PubMed] [Google Scholar]
- 16.Morohaku K, Hirao Y, Obata Y. Developmental competence of oocytes grown in vitro: Has it peaked already? J Reprod Dev 2016; 62: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamamoto K, Otoi T, Koyama N, Horikita N, Tachikawa S, Miyano T. Development to live young from bovine small oocytes after growth, maturation and fertilization in vitro. Theriogenology 1999; 52: 81–89. [DOI] [PubMed] [Google Scholar]
- 18.Mattioli M, Bacci ML, Galeati G, Seren E. Developmental competence of pig oocytes matured and fertilized in vitro. Theriogenology 1989; 31: 1201–1207. [DOI] [PubMed] [Google Scholar]
- 19.Keskintepe L, Darwish GM, Kenimer AT, Brackett BG. Term development of caprine embryos derived from immature oocytes in vitro. Theriogenology 1994; 42: 527–535. [DOI] [PubMed] [Google Scholar]
- 20.Blondin P, Sirard MA. Oocyte and follicular morphology as determining characteristics for developmental competence in bovine oocytes. Mol Reprod Dev 1995; 41: 54–62. [DOI] [PubMed] [Google Scholar]
- 21.Crozet N, Ahmed-Ali M, Dubos MP. Developmental competence of goat oocytes from follicles of different size categories following maturation, fertilization and culture in vitro. J Reprod Fertil 1995; 103: 293–298. [DOI] [PubMed] [Google Scholar]
- 22.O’Brien JK, Catt SL, Ireland KA, Maxwell WM, Evans G. In vitro and in vivo developmental capacity of oocytes from prepubertal and adult sheep. Theriogenology 1997; 47: 1433–1443. [DOI] [PubMed] [Google Scholar]
- 23.Hirao Y, Itoh T, Shimizu M, Iga K, Aoyagi K, Kobayashi M, Kacchi M, Hoshi H, Takenouchi N. In vitro growth and development of bovine oocyte-granulosa cell complexes on the flat substratum: effects of high polyvinylpyrrolidone concentration in culture medium. Biol Reprod 2004; 70: 83–91. [DOI] [PubMed] [Google Scholar]
- 24.Hinrichs K, Choi YH, Love LB, Varner DD, Love CC, Walckenaer BE. Chromatin configuration within the germinal vesicle of horse oocytes: changes post mortem and relationship to meiotic and developmental competence. Biol Reprod 2005; 72: 1142–1150. [DOI] [PubMed] [Google Scholar]
- 25.Choi YH, Love CC, Varner DD, Hinrichs K. Equine blastocyst development after intracytoplasmic injection of sperm subjected to two freeze-thaw cycles. Theriogenology 2006; 65: 808–819. [DOI] [PubMed] [Google Scholar]
- 26.Hirao Y, Naruse K, Kaneda M, Somfai T, Iga K, Shimizu M, Akagi S, Cao F, Kono T, Nagai T, Takenouchi N. Production of fertile offspring from oocytes grown in vitro by nuclear transfer in cattle. Biol Reprod 2013; 89: 57. [DOI] [PubMed] [Google Scholar]
- 27.Figueiredo JR, Hulshof SC, Van den Hurk R, Ectors FJ, Fontes RS, Nusgens B, Bevers MM, Beckers JF. Development of a combined new mechanical and enzymatic method for the isolation of intact preantral follicles from fetal, calf and adult bovine ovaries. Theriogenology 1993; 40: 789–799. [DOI] [PubMed] [Google Scholar]
- 28.Carámbula SF, Gonçalves PB, Costa LF, Figueiredo JR, Wheeler MB, Neves JP, Mondadori RG. Effect of fetal age and method of recovery on isolation of preantral follicles from bovine ovaries. Theriogenology 1999; 52: 563–571. [DOI] [PubMed] [Google Scholar]
- 29.Cortvrindt R, Smitz J. In vitro follicle growth: achievements in mammalian species. Reprod Domest Anim 2001; 36: 3–9. [DOI] [PubMed] [Google Scholar]
- 30.Kagawa N, Kuwayama M, Miyano T, Manabe N. Growth and maturation of follicles and oocytes following xenotransplantation of porcine ovarian tissues and in vitro maturation. J Reprod Dev 2005; 51: 741–748. [DOI] [PubMed] [Google Scholar]
- 31.Moniruzzaman M, Bao RM, Taketsuru H, Miyano T. Development of vitrified porcine primordial follicles in xenografts. Theriogenology 2009; 72: 280–288. [DOI] [PubMed] [Google Scholar]
- 32.Bao RM, Yamasaka E, Moniruzzaman M, Hamawaki A, Yoshikawa M, Miyano T. Development of vitrified bovine secondary and primordial follicles in xenografts. Theriogenology 2010; 74: 817–827. [DOI] [PubMed] [Google Scholar]
- 33.Pepling ME, Sundman EA, Patterson NL, Gephardt GW, Medico L, Jr, Wilson KI. Differences in oocyte development and estradiol sensitivity among mouse strains. Reproduction 2010; 139: 349–357. [DOI] [PubMed] [Google Scholar]
- 34.Canning J, Takai Y, Tilly JL. Evidence for genetic modifiers of ovarian follicular endowment and development from studies of five inbred mouse strains. Endocrinology 2003; 144: 9–12. [DOI] [PubMed] [Google Scholar]
- 35.Hall SE, Upton RMO, McLaughlin EA, Sutherland JM. Phosphoinositide 3-kinase/protein kinase B (PI3K/AKT) and Janus kinase/signal transducer and activator of transcription (JAK/STAT) follicular signalling is conserved in the mare ovary. Reprod Fertil Dev 2018; 30: 624–633. [DOI] [PubMed] [Google Scholar]
- 36.Reddy P, Liu L, Adhikari D, Jagarlamudi K, Rajareddy S, Shen Y, Du C, Tang W, Hämäläinen T, Peng SL, Lan ZJ, Cooney AJ, Huhtaniemi I, Liu K. Oocyte-specific deletion of Pten causes premature activation of the primordial follicle pool. Science 2008; 319: 611–613. [DOI] [PubMed] [Google Scholar]
- 37.Li J, Kawamura K, Cheng Y, Liu S, Klein C, Liu S, Duan EK, Hsueh AJ. Activation of dormant ovarian follicles to generate mature eggs. Proc Natl Acad Sci USA 2010; 107: 10280–10284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morohaku K, Hoshino Y, Sasada H, Sato E. Incorporation of phosphatase inhibitor in culture prompts growth initiation of isolated non-growing oocytes. PLoS One 2013; 8: e77533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maidarti M, Clarkson YL, McLaughlin M, Anderson RA, Telfer EE. Inhibition of PTEN activates bovine non-growing follicles in vitro but increases DNA damage and reduces DNA repair response. Hum Reprod 2019; 34: 297–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Santos JMS, Lins T, Barberino RS, Menezes VG, Gouveia BB, Matos MHT. Kaempferol promotes primordial follicle activation through the PI3K/AKT signaling pathway and reduces DNA fragmentation of sheep preantral follicles cultured in vitro. Mol Reprod Dev 2019. (in press). [DOI] [PubMed] [Google Scholar]
- 41.Brenkman AB, Burgering BM. FoxO3a eggs on fertility and aging. Trends Mol Med 2003; 9: 464–467. [DOI] [PubMed] [Google Scholar]
- 42.Castrillon DH, Miao L, Kollipara R, Horner JW, DePinho RA. Suppression of ovarian follicle activation in mice by the transcription factor Foxo3a. Science 2003; 301: 215–218. [DOI] [PubMed] [Google Scholar]
- 43.Moniruzzaman M, Lee J, Zengyo M, Miyano T. Knockdown of FOXO3 induces primordial oocyte activation in pigs. Reproduction 2010; 139: 337–348. [DOI] [PubMed] [Google Scholar]
- 44.Yoshida H, Takakura N, Kataoka H, Kunisada T, Okamura H, Nishikawa SI. Stepwise requirement of c-kit tyrosine kinase in mouse ovarian follicle development. Dev Biol 1997; 184: 122–137. [DOI] [PubMed] [Google Scholar]
- 45.Parrott JA, Skinner MK. Kit-ligand/stem cell factor induces primordial follicle development and initiates folliculogenesis. Endocrinology 1999; 140: 4262–4271. [DOI] [PubMed] [Google Scholar]
- 46.Muruvi W, Picton HM, Rodway RG, Joyce IM. In vitro growth of oocytes from primordial follicles isolated from frozen-thawed lamb ovaries. Theriogenology 2005; 64: 1357–1370. [DOI] [PubMed] [Google Scholar]
- 47.Hutt KJ, McLaughlin EA, Holland MK. KIT/KIT ligand in mammalian oogenesis and folliculogenesis: roles in rabbit and murine ovarian follicle activation and oocyte growth. Biol Reprod 2006; 75: 421–433. [DOI] [PubMed] [Google Scholar]
- 48.Magamage MPS, Zengyo M, Moniruzzaman M, Miyano T. Testosterone induces activation of porcine primordial follicles in vitro. Reprod Med Biol 2010; 10: 21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Durlinger AL, Kramer P, Karels B, de Jong FH, Uilenbroek JT, Grootegoed JA, Themmen AP. Control of primordial follicle recruitment by anti-Müllerian hormone in the mouse ovary. Endocrinology 1999; 140: 5789–5796. [DOI] [PubMed] [Google Scholar]
- 50.Durlinger AL, Gruijters MJ, Kramer P, Karels B, Ingraham HA, Nachtigal MW, Uilenbroek JT, Grootegoed JA, Themmen AP. Anti-Müllerian hormone inhibits initiation of primordial follicle growth in the mouse ovary. Endocrinology 2002; 143: 1076–1084. [DOI] [PubMed] [Google Scholar]
- 51.Yang MY, Cushman RA, Fortune JE. Anti-Müllerian hormone inhibits activation and growth of bovine ovarian follicles in vitro and is localized to growing follicles. Mol Hum Reprod 2017; 23: 282–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wandji SA, Sršeň V, Voss AK, Eppig JJ, Fortune JE. Initiation in vitro of growth of bovine primordial follicles. Biol Reprod 1996; 55: 942–948. [DOI] [PubMed] [Google Scholar]
- 53.Fortune JE, Cushman RA, Wahl CM, Kito S. The primordial to primary follicle transition. Mol Cell Endocrinol 2000; 163: 53–60. [DOI] [PubMed] [Google Scholar]
- 54.Cinco R, Digman MA, Gratton E, Luderer U. Spatial haracterization of bioenergetics and metabolism of primordial to preovulatory follicles in whole ex vivo murine ovary. Biol Reprod 2016; 95: 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hikabe O, Hamazaki N, Nagamatsu G, Obata Y, Hirao Y, Hamada N, Shimamoto S, Imamura T, Nakashima K, Saitou M, Hayashi K. Reconstitution in vitro of the entire cycle of the mouse female germ line. Nature 2016; 539: 299–303. [DOI] [PubMed] [Google Scholar]
- 56.Hasegawa A, Mochida N, Ogasawara T, Koyama K. Pup birth from mouse oocytes in preantral follicles derived from vitrified and warmed ovaries followed by in vitro growth, in vitro maturation, and in vitro fertilization. Fertil Steril 2006; 86 (Suppl): 1182–1192. [DOI] [PubMed] [Google Scholar]
- 57.Mochida N, Akatani-Hasegawa A, Saka K, Ogino M, Hosoda Y, Wada R, Sawai H, Shibahara H. Live births from isolated primary/early secondary follicles following a multistep culture without organ culture in mice. Reproduction 2013; 146: 37–47. [DOI] [PubMed] [Google Scholar]
- 58.Wu J, Emery BR, Carrell DT. In vitro growth, maturation, fertilization, and embryonic development of oocytes from porcine preantral follicles. Biol Reprod 2001; 64: 375–381. [DOI] [PubMed] [Google Scholar]
- 59.Gupta PS, Ramesh HS, Manjunatha BM, Nandi S, Ravindra JP. Production of buffalo embryos using oocytes from in vitro grown preantral follicles. Zygote 2008; 16: 57–63. [DOI] [PubMed] [Google Scholar]
- 60.Sharma GT, Dubey PK, Nath A, Saikumar G. Co-culture of buffalo (Bubalus bubalis) preantral follicles with antral follicles: a comparative study of developmental competence of oocytes derived from in vivo developed and in vitro cultured antral follicles. Zygote 2013; 21: 286–294. [DOI] [PubMed] [Google Scholar]
- 61.Arunakumari G, Shanmugasundaram N, Rao VH. Development of morulae from the oocytes of cultured sheep preantral follicles. Theriogenology 2010; 74: 884–894. [DOI] [PubMed] [Google Scholar]
- 62.Saraiva MV, Rossetto R, Brito IR, Celestino JJ, Silva CM, Faustino LR, Almeida AP, Bruno JB, Magalhães DM, Matos MH, Campello CC, Figueiredo JR. Dynamic medium produces caprine embryo from preantral follicles grown in vitro. Reprod Sci 2010; 17: 1135–1143. [DOI] [PubMed] [Google Scholar]
- 63.Magalhães DM, Duarte AB, Araújo VR, Brito IR, Soares TG, Lima IM, Lopes CA, Campello CC, Rodrigues AP, Figueiredo JR. In vitro production of a caprine embryo from a preantral follicle cultured in media supplemented with growth hormone. Theriogenology 2011; 75: 182–188. [DOI] [PubMed] [Google Scholar]