Abstract
Effective monitoring of Salmonella contamination in seafood processing to conform the requirements of HACCP is a great challenge today. Such challenges can be effectively addressed, if the conventional detection methods are replaced with DNA-based molecular methods. Accordingly, it was aimed to develop a robust PCR protocol for specific detection of Salmonella spp. Out of the different primers screened, one pair of primers developed in this study targeting invA gene demonstrated 100% inclusivity for a wide range of Salmonella serotypes and 100% exclusivity for wide range of non-target species. The in silico analysis of the nucleotide sequence obtained from the PCR product suggests its potential as a hybridization probe for genus specific detection of Salmonella spp. contamination. The PCR protocol was sensitive enough to detect 15 cells per reaction using crude DNA prepared within a short time directly from artificially contaminated shrimp tissue. The study demonstrated that the result of PCR reaction can come out on the same day of sample arrival. Incorporation of this pair of primers in a multiplex PCR designed for simultaneous detection of four common seafood-borne human pathogens yielded 147 bp, 302 bp, 403 bp, and 450 bp distinct DNA bands specifically targeting E. coli, toxigenic Vibrio cholerae, Salmonella spp., and V. parahaemolyticus, respectively in a single PCR tube. The PCR methods developed in this study has the potential to be used in the seafood processing plants for effective monitoring of CCPs required for implementation of HACCP-based quality assurance system.
Keywords: Salmonella spp., invA gene, Multiplex PCR, CCP, HACCP, Seafood quality
Introduction
Seafood products have carved their niche over the years as a billion-dollar export-oriented industry in India. However, in recent years, due to the increased rate of incidence of food-borne diseases, many regulatory bodies around the globe have fixed zero tolerance level to some of the food-borne pathogens like Salmonella spp., Vibrio cholerae, V. parahaemolyticus, and V. vulnificus in any seafood export consignment [1]. Among all, Salmonella spp. can be singled out, as its outbreaks are frequently reported from both developed and developing countries. Salmonella spp. contamination occurs through the consumption of contaminated foods including fish and fishery products, and the infection can also be transferred through open as well as tap water [2, 3].Salmonella, the Gram-negative bacillus of family Enterobacteriaceae, is widely distributed in nature and can cause diseases ranging from gastroenteritis to typhoid fever [4].More than 2500 serovars of Salmonella are known so far [4, 5], and most of them are considered pathogenic to animals and humans [6].Therefore, USFDA has strict guidelines that the seafood products entering the US market should be have zero Salmonella contamination. Similar guidelines have also been laid down in the European Union Directive (94/65/EC) [7].
The conventional methods for detection of Salmonella contamination involves cultures on selective media and characterization of suspicious colonies by biochemical tests followed by serotyping, which are not only laborious but also time consuming and may require up to 7 to 10 days. Further, the occurrence of typical and atypical colony characteristics on selective agar plates [8] increases the bulkiness of manual labor before inferring on the contamination status. Hence, these conventional techniques for monitoring of critical control points (CCPs) in hazard analysis critical control point (HACCP) format are cumbersome, where huge number of samples are to be analyzed, and the result is required within a very short time. It is in this context that there is an utmost need of molecular methods like PCR-based diagnostic tools for fast, accurate and sensitive detection of Salmonella spp. in seafood products.
Although a number of PCR-based protocols have been developed throughout the world [9], no protocol has demonstrated to cover all the Salmonella serovars. It was in this context that the present study was aimed to develop and standardize a specific PCR protocol that can detect accurately all the strains of Salmonella spp. prevalent in Indian seafood industry within a very short period of time. Further, attempt was also made to explore the possibility of its incorporation in a multiplex-PCR format for simultaneous detection of few important seafood pathogens quickly and accurately.
Materials and methods
Bacterial strains
In this study, 26 Salmonella spp. strains isolated from seafoods and seafood handling environments, characterized previously in our laboratory and confirmed and serotyped at a referral laboratory, National Salmonella Centre, Indian Veterinary Research Institute (IVRI), Izatnagar, Bareilly, India, were used. Besides, 21 Salmonella strains and 12 different non-Salmonella strains obtained from various culture collection centers and prominent laboratories of India were used as standard reference. The characteristics of the strains used in the present study have been described in Table 1.
Table 1.
List of Salmonella and non-Salmonella strains used for PCR screening reaction
| Sl. No. | Name of the strain | Source code no.# |
|---|---|---|
| A | Standard reference Salmonella strain | |
| 1 | Salmonella Typhi | MTCC 734 |
| 2 | S. Paratyphi | CRI 1 |
| 3 | S. ParatyphiA | MTCC 735 |
| 4 | S. ParatyphiB | NSC/E 120 |
| 5 | S. Enteritidis | CRI 2 |
| 6 | S. Typhimurium | NCIM 2501 |
| 7 | S. Newport | NSC/E 112 b |
| 8 | S. Abony | NCIM, 2257 |
| 9 | S. Virchow | MTCC 1163 |
| 10 | S. Infantis | MTCC 1167 |
| 11 | S. Brunei | MTCC 1168 |
| 12 | S. enterica subsp. arizonae | MTCC 660 |
| 13 | S. Weltevreden | MTCC 1169 |
| 14 | S. Kentucky | NSC, IVRI 1 |
| 15 | S. Paratyphi | NSC, IVRI 2 |
| 16 | S. Typhimurium | NSC, IVRI 3 |
| 17 | S. Gallinarum | NSC, 9R/83 |
| 18 | S. Typhimurium | MTCC 1253 |
| 19 | S. Kirkee | NSC 3642 |
| 20 | S. Monophasic | NSC 3643 |
| 21 | S. Javiana | NSC 3646 |
| B | Standard reference non-Salmonella strain | |
| 1 | Vibrio cholerae O1 | NICED 01 |
| 2 | V. cholerae - non pathogenic | UAS COF 01 |
| 3 | V.parahaemolyticus | MTCC 451 |
| 4 | V.vulnificus | MTCC 1145 |
| 5 | Proteus mirabilis | NCIM 2241 |
| 6 | P. morganii | NCIM 2860 |
| 7 | Staphylococcus aureus | NCIM 5021 |
| 8 | Aeromonas hydrophila | NCIM 2319 |
| 9 | Klebsiella pneumonia | NCIM 2719 |
| 10 | Hafnia alvei | NCIM 2351 |
| 11 | Escherichia coli | MTCC 1687 |
| 12 | E. coli O157:H7 | NICED 02 |
| C | Lab isolated and serotyped Salmonella spp.* | |
| 1 | Salmonella Senftenberg | 2 isolates |
| 2 | S. Saintpaul | 4 isolates |
| 3 | S. Reading | 3 isolates |
| 4 | S. Stanley | 4 isolates |
| 5 | S. Sarajane | 1 isolate |
| 6 | S. Sandiego | 1 isolate |
| 7 | S. Heidelberg | 1 isolate |
| 8 | S. Reinickendorf | 1 isolate |
| 9 | S. Bradford | 1 isolate |
| 10 | S. Typhimurium | 1 isolate |
| 11 | S. Ohio | 7 isolates |
#CRI, Central Research Institute (Govt. of India), Kasauli, HP, India
NSC National Salmonella Centre (Vet.), IVRI (ICAR), Izatnagar, UP, India
MTCC, Microbial Type Culture Collection, Institute of Microbial Technology (CSIR), Chandigarh, Punjab, India.
NCIM, National Collection of Industrial Microorganisms, National Chemical Laboratory (CSIR), Pune, Maharastra, India.
NICED, National Institute of Cholera and Enteric Diseases, Kolkata, WB, India.
UAS (M), College of Fisheries (UAS), Mangalore, Karnataka, India.
Extraction of purified and crude template DNA
The purified genomic DNA from standard reference Salmonella and non-Salmonella strains was extracted following the CTAB-NaCl method described previously [10]. The extracted genomic DNA in Tris- EDTA buffer (pH 8.0) (TE buffer) were analyzed for their purity and concentration following standard protocol [10].
Secondly, crude template DNA was prepared from fresh bacterial culture by rapid-boiling lysis method described earlier [11] with little modification. Briefly, 1500 μl of young culture was pelleted down by centrifugation at 8000×g for 5 min. The pellet was then washed and re-suspended in 100 μl TE buffer. The suspension was then incubated for 10 min at 95 °C and immediately chilled on ice. After centrifugation at 12,000×g for 5 min at 4 °C, the supernatant was harvested and an aliquot of 5 μl was used for PCR reaction in place of 50 ng purified genomic DNA.
Crude template DNA was also prepared directly from artificially contaminated seafood products. For this, young Salmonella culture suspensions in Luria Bertani (LB) medium, Hi-media, India, were adjusted to a concentration of 107 cells/ml using sterile physiological saline, considering OD600 nm = 0.1 is equivalent to 107 cells/ml [12]. Processed shrimp (processed and ready for export) sample homogenates were prepared in sterile physiological saline at 1:9 ratio. One milliliter each of the above culture suspensions was added to 99 ml of the shrimp homogenate and mixed thoroughly. These homogenates were then briefly centrifuged, and an aliquot of 1500 μl from each of the above suspensions was used for preparation of crude template DNA following the methods delineated above.
Selection of a Salmonella spp. specific PCR primer
In order to shortlist a Salmonella spp. specific PCR primer, few pairs of primers were selected from the published literature. Besides, two pairs of primers were designed in the present study using invA gene of Salmonella spp. in one case and S. Typhi specific probe (NCBI accession number- U78640) in the other. The conserved regions of the gene sequences were used for designing PCR primers using Primer3Plus software [13]. The particulars of the primers used in the present study have been given in Table 2. The primers were screened one by one by performing PCR reaction for a set of Salmonella and non-Salmonella strains until finalizing the best set of primers.
Table 2.
Particulars of the primers used for PCR screening reaction
| Primer set | Primer sequence (5′ – 3′) | Melting temp. Tm (°C) | Target sequence | Amplicon size (bp) | Reference | |
|---|---|---|---|---|---|---|
| A |
L-INVA R-INVA |
CTC TAC TTA ACA GTG CTC GTT TAC TTG ATA AAC TTC ATC GCA CCG TCA |
61 61 |
inv A gene | 571 | Brasher et al. (1998) |
| B |
BS1-F BS1-R |
TGA CGC AAA AGA GGA AGG AT ATA ACC GTG CGG AAG TTG AC |
58 60 |
S. Typhi-specific probe (U78640) | 201 | Designed in this study |
| C |
BS2-F BS2-R |
GTA TTG TTG ATT AAT GAG ATC CG ATA TTA CGC ACG GAA ACA C |
57 56 |
inv A gene | 403 | Designed in the present study |
Optimisation of PCR protocol
PCR protocol was optimized for each pair of primers [14] in a heated lid gradient thermocycler (Effendorf, Germany) using S Typhi (MTCC 734) and E. coli (MTCC 1687) as positive and negative controls, respectively. Seventeen Salmonella spp. and ten non-Salmonella strains were taken for preliminary screening of the primers (Table 2) one by one following above standardized protocol. The best performing pair of primers was then used for further screening using the other Salmonella spp. and non-Salmonella strains (Table 1).
For the finally selected set of primers, PCR reaction was carried out; using chemicals procured from MBI Fermentas, Thermo Fisher Scientific, 168 Third Avenue Waltham, MA, USA 02451; in a 25 μl reaction mixture containing 2.5 μl of 10X PCR buffer, 1 U of Taq DNA polymerase (Sigma-Aldrich, US), 5 mM of each dNTPs, 37.5 mM of MgCl2, 10 pmol of each primers, and 50 ng of purified template DNA. The optimized thermal cycling conditions comprised of initial denaturation at 94 °C for 5 min, followed by 35 cycles of denaturation at 94 °C for 1 min, annealing at 56 °C for 1.5 min and extension at 72 °C for 2 min followed by final extension at 72 °C for 10 min. The PCR products (10 μl) were then analyzed by electrophoresis using 1.8% Tris-Borate EDTA Agarose (low EEO, Sigma-Aldrich, USA) gel stained with Ethidium bromide. The DNA bands were viewed under UV light and documented using Gel documentation system (M/s syngene (A division of the synoptic group), Beacon House, Nuffield Road, Cambridge, CB4 1TF, UK) and Thermal printer (M/s SonyCorporation, Asahi-cho, Atsugi, Kanagawa, Japan).
The strength of the best performing pair of primers was further analyzed in silico by sequencing the PCR amplicon of one of the randomly selected strain and performing NCBI-BLAST of the resulting sequence. The PCR amplicon was directly cloned to T/A cloning vector (pTZ57R/T) and then transformed into E. coli (DH5α) using “InsT/Aclone™ PCR product cloning kit” (MBI Fermentas, Thermo Fisher Scientific, 168 Third Avenue Waltham, MA, USA 02451) followed by sequencing using BigDye® Terminator v 3.1 cycle sequencing kit (PE Applied Biosystems, Waltham, MA, USA). The resulting unambiguous sequence was used for NCBI-BLAST analysis.
Evaluation of PCR protocol using crude template DNA
The robust nature of the PCR protocol developed as above was evaluated using crude template DNA prepared from pure culture of all the standard reference as well as lab isolated strains (Table 1). Secondly, crude template DNA prepared directly from artificially contaminated shrimp homogenates was used for PCR reaction following above protocol. The above PCR reactions were repeated in a heated lid gradient thermocycler (PCR Express, Thermo Hybrid, Thermofisher, USA) with tube sensor in a different laboratory to study its reproducibility.
Evaluation of sensitivity of PCR protocol
The sensitivity of the above PCR protocol was found out using crude DNA prepared directly from artificially inoculated shrimp homogenate. For this 10 ml shrimp tissue homogenate prepared as above was taken in each of the nine test tubes marked as 0, 1, 2, 3, 4, 5, 6,.7, and B. Young culture of Salmonella sp. was adjusted to a concentration of 107cells/ml as described above. It was then serially diluted to get 107(neat), 106 , and 105 up to 100 cells/ml. The homogenate taken in test tubes were then inoculated with 10 μl of above serially diluted Salmonella cells, so that each test tube down the line of inoculation receives 10 times less inoculation (i.e., 104, 103, 102, up to 10−2, 10−3cells/ml in tube No. 0, 1, 2, up to 7). The test tube “B” was kept un-inoculated to serve as blank. An aliquot of 1500 μl from each tube was used to prepare crude template DNA followed by PCR reaction.
Evaluation of PCR protocol in a multiplex PCR format
The performance of the Salmonella spp. specific PCR primer shortlisted in this study was evaluated in a multiplex PCR protocol for the simultaneous detection of three common seafood pathogens and E. coli. It involved four pairs of primers (Table 3), each one specifically targeting uidA gene of E. coli, a common background microflora; invA gene of Salmonella spp., one of the leading agent of food-borne infections; ctx gene of V. cholerae, the causative agent of enteric cholera and the tlh gene of V. parahaemolyticus, a leading cause of seafood-borne gastroenteritis.
Table 3.
Details of the primers used in multiplex PCR along with their source and the target pathogen
| Target pathogen | Primer set | Primer sequence (5′ – 3′) | Target gene | Amplicon size (bp) | References |
|---|---|---|---|---|---|
| Salmonella spp. |
BS2-F BS2-R |
GTA TTG TTG ATT AAT GAG ATC CG ATA TTA CGC ACG GAA ACA C |
inv A | 403 | Designed in the present study |
| Escherichia coli |
L-uid A R-uid A |
TGG TAA TTA CCG ACG AAA ACG GC ACG CGT GGT TAC AGT CTT GCG |
uidA | 147 |
Bej et al. (1991b) Bej et al. (1991c) |
| Vibrio cholerae |
L – ctx R – ctx |
CTC AGA CGG GAT TTG TTA GGC ACG TCT ATC TCT GTA GCC CCT ATT ACG |
ctx | 302 |
Shirai et al. (1991) Bej et al. (1996) |
| V. parahaemolyticus |
L – tlh R – tlh |
AAA GCG GAT TAT GCA GAA GCA CTG GCT ACT TTC TAG CAT TTT CTC TGC |
tlh | 450 |
Taniguchi (1986) Bej et al. 1999 |
All the above primers were standardized to work in a common reaction condition. Purified genomic DNA of the above strains were taken along with the optimized reaction components in 3 sets of 0.2 ml PCR tubes. In one tube, all the four template DNA with all the four pairs of primers; in the second set of four tubes, one pair of primers at a time with all the four DNAs; and in the thirdset of four tubes, one DNA at a time with four pairs of primers. The common thermocycling schedule comprised of initial denaturation at 95 °C for 3 min followed by 30 cycles at 94 °C for 1 min, 56 °C for 1.5 min and 72 °C for 2 min, and a final extension at 72 °C for 7 min. A 10 μl aliquot of amplified DNA was examined by electrophoresis as described earlier.
Specificity of the participating pair of primers was then established by performing cross-reactions of each pair of the primers with about ten non-target strains while using the target strains as positive control.
Results
Screening of PCR primers to test their specificity
The selected set of PCR primers was evaluated for their specificity one by one, and the results have been presented in Table 4. As evident from the results, the primer set C (BS2-F, BS2-R), designed in the present study based on invA gene of Salmonella, gave positive reaction for all the reference Salmonella strains tested in the preliminary screening producing 403 bp Salmonella-specific DNA bands, while there was no false positive DNA bands with any of the non-Salmonella strain tested. Hence, this set of primers was used for further screening using other reference Salmonella strains, reference non-Salmonella strains, and laboratory-isolated Salmonella strains. Based on the results (Table 4), the primer set C was short-listed as the best performing pair of primers. Accordingly, further tests were confined to this pair of primers only.
Table 4.
Result of screening of PCR primers for specific detection of Salmonella spp.
| Sl. No. | Name of the strain | Source code no.# | Result of PCR test | ||
|---|---|---|---|---|---|
| Primer set A* | Primer set B* | Primer set C$* | |||
| invA gene | GeneBank Acc no U78640 | invA gene | |||
| A | Standard reference Salmonella strain | ||||
| 1 | Salmonella Typhi | MTCC 734 | + | + | + |
| 2 | S. Paratyphi | CRI 1 | + | + | + |
| 3 | S. Paratyphi A | MTCC 735 | + | – | + |
| 4 | S. Paratyphi B | NSC/ E 120 | + | + | + |
| 5 | S. Enteritidis | CRI 2 | + | + | + |
| 6 | S. Typhimurium | NCIM 2501 | + | + | + |
| 7 | S. Newport | NSC / E 112 b | + | + | + |
| 8 | S. Abony | NCIM, 2257 | + | + | + |
| 9 | S. Virchow | MTCC 1163 | + | + | + |
| 10 | S. Infantis | MTCC 1167 | + | + | + |
| 11 | S. Brunei | MTCC 1168 | + | + | + |
| 12 | S. enterica subsp. arizonae | MTCC 660 | + | – | + |
| 13 | S. Weltevreden | MTCC 1169 | + | + | + |
| 14 | S. Kentucky | NSC, IVRI 1 | + | – | + |
| 15 | S. Paratyphi | NSC, IVRI 2 | ND | ND | + |
| 16 | S. Typhimurium | NSC, IVRI 3 | ND | ND | + |
| 17 | S. Gallinarum | NSC, 9R/83 | + | + | + |
| 18 | S. Typhimurium | MTCC 1253 | ND | ND | + |
| 19 | S. Kirkee | NSC 3642 | ND | ND | + |
| 20 | S. Monophasic | NSC 3643 | – | – | + |
| 21 | S. Javiana | NSC 3646 | + | + | + |
| B | Standard reference non-Salmonella strain | ||||
| 1 | Vibrio cholerae O1 | NICED 01 | – | – | – |
| 2 | V. cholerae - non pathogenic | UAS COF 01 | – | – | – |
| 3 | V. parahaemolyticus | MTCC 451 | + | – | – |
| 4 | V. vulnificus | MTCC 1145 | + | – | – |
| 5 | Proteus mirabilis | NCIM 2241 | ND | ND | – |
| 6 | P. morganii | NCIM 2860 | + | + | – |
| 7 | Staphylococcus aureus | NCIM 5021 | + | – | – |
| 8 | Aeromonas hydrophila | NCIM 2319 | – | – | – |
| 9 | Klebsiella pneumoniae | NCIM 2719 | – | – | – |
| 10 | Hafnia alvei | NCIM 2351 | – | + | – |
| 11 | Escherichia coli | MTCC 1687 | – | – | – |
| 12 | E. coli O157:H7 | NICED 02 | ND | ND | – |
| C | Lab isolated and serotyped Salmonella spp. | ||||
| 1 | Salmonella Senftenberg | 2 isolates | ND | ND | + |
| 2 | S. Saintpaul | 4 isolates | ND | ND | + |
| 3 | S. Reading | 3 isolates | ND | ND | + |
| 4 | S. Stanley | 4 isolates | ND | ND | + |
| 5 | S. Sarajane | 1 isolate | ND | ND | + |
| 6 | S. Sandiego | 1 isolate | ND | ND | + |
| 7 | S. Heidelberg | 1 isolate | ND | ND | + |
| 8 | S. Reinickendorf | 1 isolate | ND | ND | + |
| 9 | S. Bradford | 1 isolate | ND | ND | + |
| 10 | S. Typhimurium | 1 isolate | ND | ND | + |
| 11 | S. Ohio | 7 isolates | ND | ND | + |
ND not done. $Test performed using both purified and crude template DNA
*Details of the primersets used is explained in Table 2
Cloning and sequencing of a random PCR product of primer set C resulted a 311 bp unambiguous sequence (GenBank Accession no. AY593967.1). NCBI-BLAST analysis of this sequence shows that it has 100% homology with all the serovars of Salmonella genus and 0% with all non-Salmonella strains.
Evaluation of PCR protocol using crude template DNA
Salmonella spp. specific DNA bands of 403 bp were obtained with primer set C using crude template DNA prepared both from pure bacterial culture and also from artificially inoculated shrimp tissue homogenate repeatedly and reproducibility. The results of these screening tests were same as that using purified template DNA (Table 4).
Evaluation of sensitivity of PCR protocol
The result of sensitivity test for the primer set C using crude DNA prepared directly from artificially inoculated shrimp homogenate showed that the protocol is sensitive enough to detect 101 cells per ml, as tube No 3, or 15 cells per reaction when1500 μl of aliquot was taken for crude DNA extraction.
Evaluation of multiplex PCR for detection of seafood pathogens
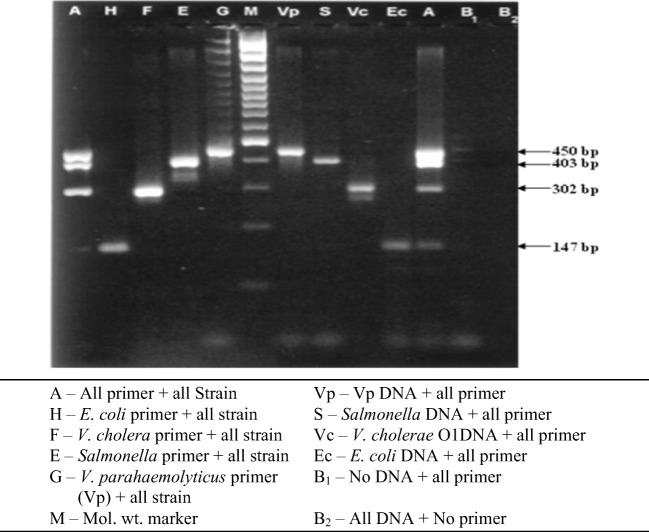
The result of multiplex PCR shows that it can specifically detect the target pathogen in the two way cross reaction performed (Fig. 1).
Fig. 1.
Simultaneous detection of few common pathogens of seafood by multiplex PCR and study of cross-reaction among the primers and the template DNA. A, all primer + all strain; H, E. coli primer + all strain; F, V. cholera primer + all strain; E, Salmonella primer + all strain; G, V. parahaemolyticus primer (Vp) + all strain; Vp, Vp DNA + all primer; S, Salmonella DNA + all primer; Vc, V. cholerae O1DNA + all primer; Ec, E. coli DNA + all primer; B1, no DNA + all primer; B2, all DNA + no primer
Further, all the four pairs primers taken together with all the four template DNA in a single reaction tube produced the four specific bands (Fig. 1). Cross reaction studies of the above four pairs of primers with about ten other non-target strains have shown that these primers are specific for the target strain (Table 5).
Table 5.
Result of the test of specificity of primers used in multiplex PCR
| Sl. No. | Name of the strain | Source code no.# | Result of PCR screening test | ||
|---|---|---|---|---|---|
| ctx gene of V. cholera | tlh gene of V. parahaemolyticus | uidA gene of E. coli | |||
| 1 | Salmonella Typhi | MTCC 734 | – | – | –* |
| 2 | Vibrio cholerae O1 | NICED 01 | + | – | – |
| 3 | V. cholerae - non pathogenic | UAS (M) 01 | – | – | – |
| 4 | V. parahaemolyticus | MTCC 451 | – | + | – |
| 5 | V. vulnificus | MTCC 1145 | – | – | – |
| 6 | Proteus mirabilis | NCIM 2241 | – | – | – |
| 7 | Staphylococcus aureus | NCIM 5021 | – | – | – |
| 8 | Aeromonas hydrophila | NCIM 2319 | – | – | – |
| 9 | Klebsiella pneumoniae | NCIM 2719 | – | – | – |
| 10 | Escherichia coli | MTCC 1687 | – | – | + |
| 11 | E. coli EHEC O157:H7 | NICED 02 | – | – | + |
*False positive band of about 300 bp
#The details of the source code is same as Table 1.
Discussion
Screening of PCR primers to test their specificity
The need for timely results has led to the development of many rapid methods of detection with high specificity and sensitivity among which, the in vitro amplification of DNA by PCR is a powerful technique that offers rapid, sensitive, and specific detection of food-borne pathogens [15]. A number of PCR methods have been developed targeting specific gene sequence for the detection of Salmonella [13]. However, the development of PCR primers specific for the detection of salmonellae with all the serotypes possesses some difficulties [16]. It is in this context that a search for a robust PCR protocol was undertaken, and one set of primers were carefully selected from the published literature, and two sets of primers were designed in this study (Table 2). Out of the three set of PCR primers used in this study, the primer set C, designed in the present study using invA gene sequence of Salmonella, was short-listed as the best set of primers screened in this study (Table 4).
In Salmonella, neither virulence factor nor toxin gene specific for all serovars have been reported [17, 18], and thus the search for a genus specific DNA sequence is more complicated. Even though the recent nomenclature based on DNA homology has classified all the 2500 serovars of Salmonella known so far [4, 5] into two species, S. enterica and S. bongori [19–21], the data available is insufficient to establish a specific gene or nucleotide sequence that is universal in the Salmonella genus. Only the invA gene has been described as essential for the invasion into epithelial cells by Salmonella. The invA gene appears to be present in all serovars of S. enterica [22, 23]. Since the invA gene sequence is strongly conserved but unique to Salmonella spp. [13, 24], a robust PCR primer based on it has the capability to detect all the serovars of Salmonella spp. The primer set C designed in the present study based on invA gene demonstrated 100% inclusivity (percentage of target DNA samples that gave a positive signal) for a wide range of Salmonella serotypes and 100% exclusivity (percentage of non-target samples that gave a negative signal). The results of this set of PCR primers are even encouraging than that of the multicenter validation of few Salmonella specific PCR [13] who observed 99.6% inclusivity and could not detect serotype Salmonella Saintpaul by the best performing pair of primers. Further, this pair of primers had not detected two serotype Salmonella Litchfield and Salmonella Senftenberg [25]. Such finding had led [13] to speculate that the invA gene is absent in Salmonella Saintpaul. However, result of the present study proves otherwise, as it could repeatedly detect Salmonella Saintpaul and Salmonella Senftenberg even by use of crude template DNA. These findings strengthen the uniqueness status of the “primer set C” for specific detection of Salmonella spp.
NCBI-BLAST analysis of the nucleotide sequence obtained from the PCR amplicon of primer set C (GenBank Accession No. AY593967.1) showed that it has 100% homology with all the serovars of Salmonella genus and 0% with all non-Salmonella strains. This in silico analysis (data not given) infers that the primer set C designed in this study can detect the presence of any member of Salmonella genus. Such analysis further affirms the uniqueness of the primer set for specific detection of Salmonella spp. Further, this sequence can conveniently be used as a hybridization probe for genus specific detection of Salmonella spp. However, there is huge scope for its international validation physically involving more number of strains of both target and non-target species in more than one laboratory.
Evaluation of PCR protocol using crude template DNA
One of the prerequisite of a successful PCR assay for food-borne pathogens is the use of an effective DNA extraction and purification procedure to remove the contaminating substances from the food that may inhibit the activity of DNA polymerase [26]. Partial or total inhibition of PCR reaction may also be encountered due to compounds in selective media or chemicals from DNA extraction procedures [27]. Several workers have investigated on the DNA extraction methods and came out with varying results [27–29].
The rapid DNA preparation method to get crude template DNA in the present study was based on the earlier findings [30], in which they have tried to develop a rapid, reproducible, and robust method of DNA extraction and purification for use in real-time PCR. Similar method has been adopted as the faster, economical, and sensitive method of DNA preparation by several researchers [13, 31]. The present method of template DNA preparation is very fast, takes only half an hour to prepare the DNA in contrast to 6 to 8 h required for preparing DNA by standard CTAB/NaCl method [10]. It did not incorporate any chemical and thus reduced the chances of PCR inhibition [29]. Besides, it demonstrates the effectiveness of PCR reaction to detect Salmonella spp. present in the complex food matrix. The dependable performance of crude template DNA prepared by rapid boiling lysis of artificially inoculated shrimp homogenates demonstrated that the result of PCR reaction can come out on the same day of receipt of the sample. Therefore, PCR method developed based on primer set C has lot of potential in the seafood processing industry of India to keep track of Salmonella contamination, determination of CCPs, and effectiveness of the corrective action taken.
Evaluation of sensitivity of PCR protocol
The result of sensitivity test for the primer set C using crude DNA prepared directly from artificially inoculated shrimp homogenate shows that the protocol is sensitive enough to detect 101 cells per ml. This method seems to be more sensitive than that of earlier method of Salmonella detection [6] in which S. Typhimurium was detected in ground beef sample after 4–6 h of enrichment at an initial inoculum of 100 bacteria. In comparison to 50 CFU per reaction [13], in the present method, only 15 cells were used for Salmonella detection. Though, such a level of contamination seems to be too high in a neat sample; a brief enrichment of the naturally contaminated seafood sample for 1–2 h at 37 °C in a shaker incubator would be sufficient to yield a successful detection in this PCR protocol. However, further study is required to establish the level of contamination in the naturally contaminated neat sample that can be tested with brief enrichment.
Evaluation of multiplex PCR for detection of seafood pathogens
Fish and seafood products are implicated with several pathogenic bacteria comprising of the indigenous microbiota of the natural environment, contaminants of sewage or fecal waste, or contaminants during processing, storage, and distribution. Among all, Salmonella spp. and other pathogenic microbes like V. cholera and V. parahaemolyticus are the important ones. Molecular detection using PCR is the rapid procedure with high sensitivity and specificity for the immediate detection and identification of specific pathogenic bacterial contamination. Several PCR methods are available for the specific detection of Salmonella spp. [32, 33], V. cholerae [34, 35], and V. parahaemolyticus [35, 36]. However, identification of individual pathogens by PCR becomes time-consuming and costly. It is in this context, simultaneous detection of all the important pathogens of fish and seafood products in a multiplex PCR format is a valued proposition. Such a method would be relatively rapid and also cost effective. A number of multiplex PCR methods have been reported for different food borne pathogens [37, 38].
The multiplex PCR protocol for simultaneous detection of three important seafood pathogens namely, V. cholera (toxigenic), Salmonella spp. and V. parahaemolyticus and one indicator organism, E. coli showed that each of the four target DNAs could be co-amplified with the other in a single reaction tube (Fig. 1). The test of specificity of the participating pair of primers has also gave encouraging result (Table 5). The specificity of the pair of primers based on invA gene of Salmonella, designed in the present study, has been vividly discussed above. The pair of primers targeting ctx gene of V. cholera was found to be highly specific towards the toxigenic V. cholera and did not produce any false positives with the any other strains including non-toxigenic V. cholerae. Cholera toxin is the only major virulence factor of toxigenic V. cholerae, encoded by ctx gene [39], and the objective of seafood quality control laboratory is to differentiate these pathogenic strains from the non-pathogenic ones. Hence, the PCR reaction targeting the ctx gene has lot of potential for rapid and specific monitoring of pathogenic V. cholerae contamination in fish and seafood products. Similarly, the PCR method targeting tlh gene was also found to be specific towards all the strains of V. parahaemolyticus. There was no false positive reaction or cross reaction with any other non-target strain. The thermolabile hemolysin (tlh) gene is present in all the V. parahaemolyticus strains irrespective of its virulence [40]. Therefore, the PCR method targeting tlh gene can serve as the species specific detection of V. parahaemolyticus. The pair of primers targeting uidA gene was also specific for E. coli and both the non-toxigenic E. coli and toxigenic E. coli (O157:H7) produced the specific 147 bp PCR product. There was no false positive result with any of the non-E. coli strains except of a non-specific product of about 300 bp produced by Salmonella typhi. This non-specific band is of no significance when only E. coli is targeted. However, in multiplex PCR, this band may interfere with the 302 bp V. cholerae specific band using ctx gene based primer. Therefore, in such a situation, a separate PCR reaction targeting specifically V. cholerae can be set up along with the multiplex PCR for further confirmation. Alternatively, the multiplex PCR may be performed for simultaneously detection of only three pathogenic strains namely, Salmonella spp., V. cholera, and V. parahaemolyticus, by excluding the indicator organism, E. coli from the targeted list. However, further study is required to validate the finding in multicenter analysis and to study the feasibility of using crude template DNA in multiplex PCR analysis.
Conclusion
The new pair of primers developed in this study has vast potential for genus specific detection of Salmonella spp. contamination in seafood products. The quick preparation of template DNA by rapid boiling lysis of artificially contaminated shrimp homogenates stresses that the result of PCR test can be obtained on the same day of receipt of the test sample. Further, the multiplex PCR standardized in this study can be used for rapid detection of the three important pathogenic bacterial contamination namely, Salmonella spp., V. cholera, and V. parahaemolyticus, simultaneously in any seafood product. On further simplification, this protocol can be used by ordinary technician in the Quality Control Laboratory of any seafood processing industry in particular and food processing industry in general.
Acknowledgements
The authors are highly grateful to Indian Council of Agricultural Research (ICAR), New Delhi, for providing fellowship to undertake the research program. The authors are also thankful to the Director, CIFE, Mumbai, India, for providing necessary facility to conduct the research program.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.USFDA (2011) FDA and EPA safety levels in regulations and guidance. In: Fish and fishery products hazards and controls guidance, fourth edition, appendix 5. FDA, CFSAN, pp. 439–442
- 2.Bailey JL. Detection of Salmonella cells within 24 to 26 hours in poultry samples with polymerase chain reaction BAX system. J Food Prot. 1998;61(7):792–795. doi: 10.4315/0362-028X-61.7.792. [DOI] [PubMed] [Google Scholar]
- 3.Hendriksen RS, Vieira AR, Karlsmose S, Fo L, Wong DM, Jensen AB, Wegener HC, Aarestrup FM. Global monitoring of Salmonella serovar distribution from the WHO global foodborne infections network country data bank: results of quality assured laboratories from 2001 to 2007. Foodborne Pathog Dis. 2011;8(8):887–900. doi: 10.1089/fpd.2010.0787. [DOI] [PubMed] [Google Scholar]
- 4.Choudhury M, Borah P, Sarma HK, Barkalita LM, Deka NK, Hussain I, Hussain M (2016) Multiplex-PCR assay for detection of some major virulence genes of Salmonella enterica serovars from diverse sources. Curr Sci (00113891), 111(7)
- 5.Popoff MY, Bockemühl J, Brenner FW. Supplement (no. 42) to the Kauffmann-white scheme. Res Microbiol. 1998;2000151(1):63–65. doi: 10.1016/S0923-2508(00)00126-1. [DOI] [PubMed] [Google Scholar]
- 6.Kwang J, Littledike ET, Keen JE. Use of the polymerase chain reaction for Salmonella detection. Lett Appl Microbiol. 1996;22(1):46–51. doi: 10.1111/j.1472-765X.1996.tb01106.x. [DOI] [PubMed] [Google Scholar]
- 7.Abu-Salem FM, Arab EA. Chemical properties, microbiological quality and sensory evaluation of chicken and duck liver paste (foiegras) Grasas Aceites. 2010;61(2):126–135. doi: 10.3989/gya.074908. [DOI] [Google Scholar]
- 8.Andrews WH, Hammack TS (2001) Salmonella. In: Bacteriological analytical manual online, official methods of analysis of AOAC international chapter 5. Center for food safety and applied nutrition, U.S.FDA (http://www.cfsan.fda.gov/~ebam/ bam-toc.Html)
- 9.Malorny B, Hoorfar J, Bunge C, Helmuth R. Multicenter validation of the analytical accuracy of Salmonella PCR: towards an international standard. Appl Environ Microbiol. 2003;69(1):290–296. doi: 10.1128/AEM.69.1.290-296.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ausubel FM, Brent R, Kingstone RE, Moore DD, Seidman JG, Smith JA, Struhl K. Curr Protoc Mol Biol. USA: John Wiley & Sons, Inc.; 1987. [Google Scholar]
- 11.Rodriguez-Lazaro D, Gonzalez-García P, Delibato E, De Medici D, García-Gimeno RM, Valero A, Hernandez M. Next day Salmonella spp. detection method based on real-time PCR for meat, dairy and vegetable food products. Int J Food Microbiol. 2014;184:113–120. doi: 10.1016/j.ijfoodmicro.2014.03.021. [DOI] [PubMed] [Google Scholar]
- 12.Cocolin L, Manzano M, Cantoni C, Comi G. Use of polymerase chain reaction and restriction enzyme analysis to directly detect and identify Salmonella Typhimurium in food. J Appl Microbiol. 1998;85(4):673–677. doi: 10.1111/j.1365-2672.1998.00575.x. [DOI] [PubMed] [Google Scholar]
- 13.Untergasser A, Nijveen H, Rao X, Bisseling T, Geurts R, Leunissen JA. Primer3Plus, an enhanced web interface to Primer3. Nucleic Acids Res. 2007;35(suppl_2):W71–W74. doi: 10.1093/nar/gkm306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sambrook J, Russel DW. Molecular cloning: a laboratory manual, 3rd ed., Vol. 1 and 2. Cold Spring Harbour: Cold Spring Harbour Laboratory Press; 2001. pp. 1.1–14.53. [Google Scholar]
- 15.Olsen JE. DNA-based methods for detection of food-borne bacterial pathogens. Food Res Int. 2000;33(3):257–266. doi: 10.1016/S0963-9969(00)00045-4. [DOI] [Google Scholar]
- 16.Tsen HY, Liou JW, Lin CK. Possible use of a polymerase chain reaction method for specific detection of Salmonella in beef. J Ferment Bioeng. 1994;77(2):137–143. doi: 10.1016/0922-338X(94)90312-3. [DOI] [Google Scholar]
- 17.Fitts R, Diamond M, Hamilton C, Neri M. DNA-DNA hybridization assay for detection of Salmonella spp. in foods. Appl Environ Microbiol. 1983;46(5):1146–1151. doi: 10.1128/aem.46.5.1146-1151.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De la Cruz MA, Zhao W, Farenc C, Gimenez G, Raoult D, Cambillau C, Gorvel JP, Méresse SA. Toxin-antitoxin module of Salmonella promotes virulence in mice. PLoS Pathog. 2013;9(12):e1003827. doi: 10.1371/journal.ppat.1003827. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19.Le ML, Popoff MY. Designation of Salmonella enterica sp. nov., nom. Rev., as the type and only species of the genus Salmonella: request for an opinion. Int J Syst Evol Microbiol. 1987;37(4):465–468. [Google Scholar]
- 20.Boyd EF, Wang FS, Whittam TS, Selander RK. Molecular genetic relationships of the salmonellae. Appl Environ Microbiol. 1996;62(3):804–808. doi: 10.1128/aem.62.3.804-808.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fàbrega A, Vila J. Salmonella enteric serovar typhimurium skills to succeed in the host: virulence and regulation. Clin Microbiol Rev. 2013;26(2):308–341. doi: 10.1128/CMR.00066-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chiu CH, Ou JT. Rapid identification of Salmonella serovars in feces by specific detection of virulence genes, invA and spvC, by an enrichment broth culture-multiplex PCR combination assay. J Clin Microbiol. 1996;34(10):2619–2622. doi: 10.1128/jcm.34.10.2619-2622.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pavlova B, Volf J, Ondrackova P, Matiasovic J, Stepanova H, Crhanova M, Karasova D, Faldyna M, Rychlik I. SPI-1-encoded type III secretion system of Salmonella enterica is required for the suppression of porcine alveolar macrophage cytokine expression. Vet Res. 2011;42(1):16. doi: 10.1186/1297-9716-42-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galan JE, Ginocchio C, Costeas P. Molecular and functional characterization of the Salmonella invasion gene invA: homology of InvA to members of a new protein family. J Bacteriol. 1992;174:4338–4349. doi: 10.1128/jb.174.13.4338-4349.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rahn K, De Grandis SA, Clarke RC, McEwen SA, Galan JE, Ginocchio C, Curtiss RI, Gyles CL. Amplification of an invA gene sequence of Salmonella typhimurium by polymerase chain reaction as a specific method of detection of Salmonella. Mol Cell Probes. 1992;6(4):271. doi: 10.1016/0890-8508(92)90002-F. [DOI] [PubMed] [Google Scholar]
- 26.Umesha S, Manukumar HM, Raghava SA (2016) Rapid method for isolation of genomic DNA from food-borne fungal pathogens. 3 Biotech; 6(2) [DOI] [PMC free article] [PubMed]
- 27.Rossen L, Nørskov P, Holmstrøm K, Rasmussen OF. Inhibition of PCR by components of food samples, microbial diagnostic assays and DNA-extraction solutions. Int J Food Microbiol. 1992;17(1):37–45. doi: 10.1016/0168-1605(92)90017-W. [DOI] [PubMed] [Google Scholar]
- 28.Widjojoatmodjo MN, Fluit AC, Verhoef J. Molecular identification of bacteria by fluorescence-based PCR-single-strand conformation polymorphism analysis of the 16S rRNA gene. J Clin Microbiol. 1995;33(10):2601–2606. doi: 10.1128/jcm.33.10.2601-2606.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roewer L (2013) DNA fingerprinting in forensics: past, present, future. Investig Genet.; 4 [DOI] [PMC free article] [PubMed]
- 30.De Medici D, Croci L, Delibato E, Di Pasquale S, Filetici E, Toti L. Evaluation of DNA extraction methods for use in combination with SYBR green I real-time PCR to detect Salmonella enterica serotype enteritidis in poultry. AEM. 2003;69(6):3456–3461. doi: 10.1128/AEM.69.6.3456-3461.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mukhopadhyay A, Mukhopadhyay UK. Novel multiplex PCR approaches for the simultaneous detection of human pathogens: Escherichia coli 0157: H7 and Listeria monocytogenes. J Microbiol Methods. 2007;68(1):193–200. doi: 10.1016/j.mimet.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 32.Zhai L, Yu Q, Bie X, Lu Z, Lv F, Zhang C, Kong X, Zhao H. Development of a PCR test system for specific detection of Salmonella Paratyphi B in foods. FEMS Microbiol Lett. 2014;355(1):83–89. doi: 10.1111/1574-6968.12443. [DOI] [PubMed] [Google Scholar]
- 33.Ogunremi D, Nadin-Davis S, Dupras AA, Márquez IG, Omidi K, Pope L, Devenish J, Burke T, Allain R, Leclair D. Evaluation of a multiplex PCR assay for the identification of Salmonella serovars Enteritidis and Typhimurium using retail and Abattoir samples. J Food Prot. 2017;80(2):295–301. doi: 10.4315/0362-028X.JFP-16-167. [DOI] [PubMed] [Google Scholar]
- 34.Yamasaki E, Sakamoto R, Matsumoto T, Maiti B, Okumura K, Morimatsu F, Balakrish Nair G, Kurazono H (2017) Detection of Cholera Toxin by an Immuno-chromatographic Test Strip. Microbial Toxins: Methods and Protocols :1–7 [DOI] [PubMed]
- 35.Eschbach E, Martin A, Huhn J, Seidel C, Heuer R, Schumacher JH, Ulrich S, Axe JO, Konietzny A, Strauch E, Oberheitmann B (2017) Detection of enteropathogenic Vibrio parahaemolyticus, Vibrio cholerae and Vibrio vulnificus: performance of real-time PCR kits in an interlaboratory study. Eur Food Res Technol :1–8
- 36.Li, R, Chiou J, Chan EW, Chen S (2016) A novel PCR-based approach for accurate identification of Vibrio parahaemolyticus. Front Microbiol.;7 [DOI] [PMC free article] [PubMed]
- 37.Karus A, Ceciliani F, Bonastre AS, Karus V. Development of simple multiplex real-time PCR assays for foodborne pathogens detection and identification on lightcycler. Mac Vet Rev. 2017;40(1):53–58. doi: 10.1515/macvetrev-2017-0010. [DOI] [Google Scholar]
- 38.Shimada T, Arakawa E, Itoh K, Okitsu T, Matsushima A, Asai Y, Takeda Y. Extended serotyping scheme for Vibrio cholerae. Curr Microbiol. 1994;28(3):175–178. doi: 10.1007/BF01571061. [DOI] [Google Scholar]
- 39.Weynberg KD,Voolstra CR, Neave MJ, Buerger P, Van Oppen MJ (2015) From cholera to corals: viruses as drivers of virulence in a major coral bacterial pathogen. Sci Rep;5 [DOI] [PMC free article] [PubMed]
- 40.Taniguchi H, Hirano H, Kubomura S, Higashi K, Mizuguchi Y. Comparison of the nucleotide sequences of the genes for the thermostable direct hemolysin and the thermolabile hemolysin from Vibrio parahaemolyticus. Microb Pathog. 1986;1(5):425–432. doi: 10.1016/0882-4010(86)90004-5. [DOI] [PubMed] [Google Scholar]