Abstract
Objective
This study aimed to examine whether brown adipose tissue (BAT) or skeletal muscle activity mediates the relationship between personal level of environmental temperature (Personal‐ET) and wrist skin temperature (WT). Moreover, we examined whether BAT and skeletal muscle have a mediating role between Personal‐ET and WT (as a proxy of peripheral vasoconstriction/vasodilation).
Methods
The levels of BAT were quantified by cold‐induced 18F‐fluorodeoxyglucose–positron emission tomography/computed tomography scan and measured the Personal‐ET and WT by using iButtons (Maxim Integrated, Dallas, Texas) in 75 participants (74.6% women).
Results
The study found that BAT volume and metabolic activity played a positive and significant role (up to 25.4%) in the association between Personal‐ET and WT. In addition, at the coldest temperatures, the participants with lower levels of WT (inducing higher peripheral vasoconstriction) had higher levels of BAT outcomes, whereas in warm temperatures, participants with higher levels of WT (inducing higher peripheral vasodilation) had lower levels of BAT outcomes. The study did not find any mediating role of skeletal muscle activity.
Conclusions
BAT volume and metabolic activity play a role in the relationship between Personal‐ET and WT. Moreover, the data suggest that there are two distinct phenotypes: individuals who respond better to the cold, both through nonshivering thermogenesis and peripheral vasoconstriction, and individuals who respond better to the heat.
Introduction
The regulation of core body temperature is one of the most critical functions of the human body 1. Core body temperature is regulated by behavioral and physiological mechanisms 1, 2. Behavioral strategies are voluntary and oriented responses that help to maintain core body temperature, such as modifying posture, wearing clothing in winter, or using cold air conditioning in summer 2. On the other hand, physiological mechanisms are involuntary responses that generate or dissipate heat. In mammals, four physiological mechanisms are particularly involved in thermoregulation 1: (i) water evaporation (sweating), (ii) control of the skin blood flow, (iii) nonshivering thermogenesis (NST), and (iv) shivering thermogenesis. These mechanisms constantly interact, and their main aim is to keep the core body temperature in a normal range.
Skin temperature is a feed‐forward mechanism of the thermoregulatory system 1. When a change in the ambient temperature is detected by skin thermoreceptors, these trigger thermoregulatory responses that prevent any change in core body temperature 3. When humans are exposed to warm environments, peripheral blood vessels are dilated in order to promote heat loss (vasodilation), whereas in cold environments, peripheral blood vessels are constricted to prevent heat loss (vasoconstriction) 1. In animals, the engagement of specific thermoregulatory strategies is hierarchical 4. For instance, vasoconstriction occurs before NST because vasoconstriction energy efficiency is higher than NST activation, at least in mouse models 4, 5. However, whether skin blood flow regulation mechanisms work hierarchically or concomitantly with NST activation or inhibition has not yet been studied in humans.
Both brown adipose tissue (BAT) and some skeletal muscles were shown to play a role in NST 6. BAT is a specialized tissue for the rapid production of heat when the body is exposed to cold temperatures, which is mediated by the action of the uncoupling protein 1 7. In humans, BAT is mainly metabolically active upon cold exposure 8, 9, 10. However, BAT consumes large quantities of energy in small mammals, although its contribution to NST in humans seems to be negligible, given that skeletal muscle is the main effector of NST 6, 11, 12 and shivering (muscle contractions) during cold exposure 13, 14 . However, the contribution of BAT and skeletal muscle in the regulation of thermogenesis is largely unknown 6, 15. Moreover, BAT has been associated with the personal level of environmental temperature (Personal‐ET) and wrist skin temperature (WT) in humans 16 as surrogate markers of ambient exposure and skin blood flow mechanisms 17. Because BAT is a tissue that generates heat, the activation of this tissue upon cold exposure could be reflected in body skin temperature, which in turn can be measured by WT.
Based on the aforementioned data, we studied the mediating role of BAT and skeletal muscle activity (assessed by cold‐induced 18F‐fluorodeoxyglucose [18F‐FDG] uptake) between Personal‐ET and WT in young healthy adults for 7 days (24 h/d). In order to understand the physiological mechanisms, we examined whether the association of the number of hours exposed to a certain Personal‐ET with BAT and skeletal muscle 18F‐FDG uptake is mediated by WT as a surrogate marker of skin blood flow mechanisms 17, 18.
Methods
A total of 90 (n = 65 women) healthy adults aged 21.9 (SD 2.3) years participated in the present study (Table 1). The participants were enrolled in the Activating Brown Adipose Tissue Through Exercise (ACTIBATE) study 19, an exercise‐based randomized controlled trial (ClinicalTrials.gov identifier NCT02365129). All participants were nonsmokers, were not enrolled in a weight loss program, had a stable body weight (body weight changes < 3 kg) over the previous 3 months, were not physically active (< 20 minutes on < 3 d/wk), did not take any medication, had no acute or chronic illness, and reported not being regularly exposed to cold. We included healthy participants from 18 to 25 years of age with a body mass index (BMI) range from 18 to 35 kg/m2. The study was conducted in Granada (southern Spain) between October and November in 2015 and 2016. The study protocol and informed consent procedure were conducted in accordance with the Declaration of Helsinki (revision of 2013), and they were approved by the Human Research Ethics Committee of both the University of Granada (number 924) and the Servicio Andaluz de Salud (Centro de Granada, CEI‐Granada). Written informed consent was obtained from all of the participants.
Table 1.
Characteristics of study participants
| n = 75 | Min. | Max. | |
|---|---|---|---|
| Sex (% women) | 74.6 | ||
| Age (y) | 21.9 ± 2.3 | 18.2 | 26.1 |
| BMI (kg/m2) | 25.2 ± 4.8 | 17.2 | 38.4 |
| Lean mass (kg) | 41.3 ± 9.6 | 28.2 | 68.7 |
| Fat mass (kg) | 26.9 ± 9.5 | 10.1 | 51.9 |
| Fat mass (%) | 37.6 ± 7.0 | 15.6 | 52.2 |
| BAT volume (mL) | 69.0 ± 61.3 | 0 | 232.1 |
| BAT activity (SUVmean) | 3.7 ± 1.9 | 0 | 8.6 |
| BAT activity (SUVpeak) | 11.0 ± 8.5 | 0 | 43.6 |
Data presented as mean and standard deviation, unless otherwise stated.
BAT, brown adipose tissue; Min., minimum; Max., maximum; SUV, standardized uptake value.
WT and Personal‐ET measurements
All participants wore two iButtons at the same time (DS1922L, Thermochron; resolution: 0.0625°C; frequency: 10‐minute intervals; Maxim Integrated, Dallas, Texas) for 7 days and 24 h/d. One iButton was placed on the ventral side of the wrist of the nondominant hand over the radial artery with a wristband in order to determine WT. We instructed the participants to wear the iButton on the wrist for the whole day (even when asleep) and to take it off only when bathing or swimming. WT is a representative marker of distal skin temperature and skin blood flow mechanisms 17, 18. A second iButton was attached to a plastic fob and was used to quantify the Personal‐ET. This iButton remained with the participant at all times but was never in direct contact with the body 20 or under clothing. Therefore, Personal‐ET represents which temperature the participants were exposed to and the duration of this exposure. We recently showed that WT and Personal‐ET are strongly related to human BAT 16. All iButtons were programmed and analyzed with Temperatus software (Promoting Fitness & Health Through Physical Activity Research Group, University of Granada; http://profith.ugr.es/temperatus?lang=en). We calculated an average of the valid recordings for the 7 days for both WT and Personal‐ET separately 16.
Personalized cooling protocol
The personalized cooling protocol has been explained in detail elsewhere 21. Briefly, the participants entered a mildly cold room (around 19.5°C), and they were asked to wear a water‐perfused cooling vest (Polar Products Inc., Stow, Ohio). We determined the participants’ shivering threshold, reducing the water temperature gradually until shivering occurred. Shivering was determined both visually by researchers and by self‐report of the participants. After 48 to 72 hours, we exposed the participants to 2 hours at their personalized temperature to induce maximum NST (above ~4°C) 22. After 1 hour of cold exposure, we injected a bolus of 18F‐FDG (~185 MBq), and we increased the water temperature 1°C in order to prevent shivering. After 2 hours of cold exposure, we performed the positron emission tomography (PET)/computed tomography (CT) scan from the atlas vertebra to the thoracic vertebra 6. The evaluations were performed in four different weeks during 2 months (from October to November 2016) in Granada.
Quantification of 18F‐FDG uptake by BAT and skeletal muscle
We quantified BAT volume and activity following the recently published recommendations 23. PET/CT images were analyzed using the Beth Israel plugin (http://sourceforge.net/projects/bifijiplugins/) for FIJI 21 software (ImageJ, NIH, Bethesda, Maryland) by author BMT with the supervision of a nuclear medicine physician. We applied an individualized standardized uptake value (SUV) threshold (1.2 / [lean body mass / body mass]) 23 with a fixed range of Hounsfield units (−190 to −10). We quantified BAT volume and activity (i.e., SUVmean and SUVpeak). We computed BAT metabolic activity as BAT volume × SUVmean 21 and computed 18F‐FDG uptake by using a reference tissue (descending aorta). We quantified 18F‐FDG uptake (SUVpeak) of several skeletal muscles between the atlas vertebra and the thoracic vertebra 4. We drew a single region of interest from one slice in the paracervical, sternocleidomastoid, scalene, Longus colli, trapezius, parathoracic, supraspinatus, subscapular, deltoid, pectoralis major, and triceps brachii muscles from both the left and right sides of the body 11, 24. An average of both sides, including all skeletal muscles, was calculated in order to obtain a single representative value of the skeletal muscle glucose uptake of the upper part of the body. Our protocol has shown a high interobserver reliability, regardless of the threshold applied to quantify BAT 25.
Body composition
Body composition was assessed on a separate day by dual‐energy x‐ray absorptiometry (Discovery Wi; Hologic, Inc., Marlborough, Massachusetts) 19. The participants’ weight and height were measured without shoes and while wearing a T‐shirt and shorts using a SECA scale and stadiometer (model 799; Electronic Column Scale, Hamburg, Germany), and we calculated BMI (weight in kilograms divided by height in meters squared).
Statistical analysis
The descriptive characteristics of the study sample are presented as the mean and standard deviation unless otherwise stated. There was no sex interaction (all P > 0.10) in any of the study variables; thus, we conducted the analyses in men and women together.
To quantify the mediating role of BAT volume, activity (i.e., SUVmean and SUVpeak), metabolic activity, and skeletal muscle activity in the relationship between Personal‐ET and WT, we conducted mediation analyses 26. In addition, we tested the mediating role of WT on the association of the number of hours per day exposed to a certain Personal‐ET with BAT volume and activity and with skeletal muscle activity. We used the PROCESS macro version 3.0, model 4 with 5,000 bias‐corrected bootstrap samples and 95% confidence intervals. Bootstrapping is a nonparametric resampling procedure that does not require the assumption of normality of the sampling distribution 27. The mediation was estimated using the indirect effect, which indicates the change on the effect of the independent variable on the outcome that can be endorsed to the proposed mediator. Indirect effects (a × b paths) with confidence intervals not including zero are interpreted as statistically significant 28, which could occur regardless of the significance of the total effect (c path, effect of the independent variable on the dependent variable) and the direct effect (c’ path, effect on the dependent variable when both the independent and the mediator variables are included as independent variables) 26. To quantify how much of the total effect was due to the mediation, we calculated the percentage of mediation ([indirect effect / total effect] × 100) provided when the total effect was larger than the indirect effect with the same direction 26. All the analyses were performed using SPSS Statistics for Windows version 22.0 (IBM Corp., Armonk, New York), and the level of significance was set at P < 0.05.
Results
Table 1 shows the characteristics of the participants. A total of 15 out of 90 participants were excluded because less than five valid days of temperatures had been recorded. A total of 75 participants (74.6% women) were finally included in the analyses, with 6.3 ± 0.5 valid days.
Mediating role of BAT
Figure 1 shows the mediating effect of BAT volume, activity (i.e., SUVmean and SUVpeak), BAT metabolic activity, and skeletal muscle activity (SUVpeak) in the relationship between Personal‐ET and WT. Personal‐ET was positively associated with WT (c path = 0.0763; P = 0.0014) and negatively associated with BAT‐related outcomes (volume, SUVmean, SUVpeak, and metabolic activity, a path; all P < 0.001; Figure 1A‐1D) and skeletal muscle activity (a path; P = 0.0023; Figure 1E). BAT‐related outcomes and skeletal muscle activity were not significantly associated with WT (b path). After including the mediator variables in the model (Figure 1, c’ path; all P < 0.05), the direct effect of Personal‐ET on WT remained statistically significant. The percentages of mediation of BAT volume and metabolic activity in the relationship between Personal‐ET and WT were 25.4% and 23.9%, respectively. However, we did not observe any mediating effect of BAT activity (i.e., SUVmean and SUVpeak) and skeletal muscle activity in the relationship between Personal‐ET and WT (Figure 1B‐1C and 1E). These results persisted after controlling for sex, BMI, fat mass index (FMI), lean mass index (LMI), or level of objectively measured physical activity by accelerometry (data not shown). Furthermore, we repeated the analyses using BAT‐related outcomes as well as skeletal muscle activity multiplied by lean body mass percentage 29, and the results remained unchanged (data not shown).
Figure 1.
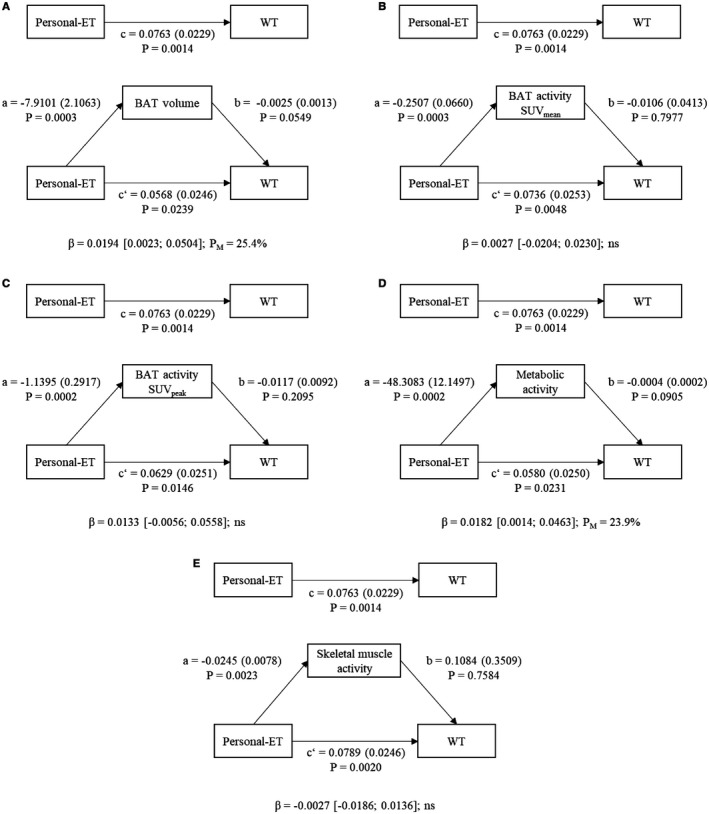
Mediation models of the relationship between Personal‐ET and WT with (A) BAT volume (milliliters), (B) SUVmean, (C) SUVpeak, (D) metabolic activity (calculated as BAT volume × BAT SUVmean), and (E) skeletal muscle activity included as mediator variables. Paths a, b, c, and c’ are presented as unstandardized coefficients (SE). β = indirect effect (a × b paths) [lower‐limit CI; upper‐limit CI], lower and upper levels for bias‐corrected 95% CIs of the indirect effect based on 5,000 bootstraps. BAT, brown adipose tissue; CI, confidence interval; ns, nonsignificant; Personal‐ET, personal level of environmental temperature; SUV, standardized uptake value; WT, wrist skin temperature.
Mediating role of WT
Figure 2A shows the mediating effect of WT in the relationship between the number of hours exposed to a certain Personal‐ET and BAT‐related outcomes (volume, SUVpeak, and metabolic activity). The number of hours per day exposed to a warm Personal‐ET was negatively associated with BAT volume (from 25°C to 28°C; c path; all P < 0.05) and positively associated with WT (from 24°C to 27°C; a path; all P < 0.05) (Supporting Information Table S1). WT was also negatively associated with BAT volume at this temperature range (b path; all P < 0.05). The direct effect was significant only when examining the number of hours per day exposed to temperatures ≥ 26°C (c’ path; all P < 0.05) (Supporting Information Table S1). WT showed the highest percentage of mediation (57%) in the relationship between the number of hours exposed to 24°C and BAT volume in comparison with other ranges of warm temperatures (Figure 2E). In addition, we observed that the number of hours per day exposed to a cold Personal‐ET was positively related to BAT volume (from 14°C to 20°C; c path; all P < 0.05) and negatively associated with WT (from 16°C to 20°C; a path; all P < 0.05) (Supporting Information Table S2). WT was negatively associated with BAT volume (b path; P < 0.05), and the association between the number of hours exposed to cold temperatures (from 16°C to 19°C) and BAT volume persisted after including WT as a mediator (c’ path; both; all P < 0.05). The sign of the indirect effect changed during the ambient exposure, being positive during cold ambient exposure and negative during warm ambient exposure (Figure 2B‐2G). Moreover, when the participants were exposed to a certain range of temperatures in the thermoneutral zone, WT did not play a mediating role in BAT volume (from 21°C to 23°C, Figure 2B and 2E). The mediation analyses were performed for the number of hours exposed to each degree of Personal‐ET, showing that the mediating effect disappeared at temperatures ≥ 28°C or ≤ 14°C, probably because of a lack of statistical power at these ranges (small number of participants exposed to these extreme temperatures). The mediating role of WT was also observed in the relationship between Personal‐ET and BAT activity (i.e., SUVpeak and metabolic activity; indirect effect in Figure 2C‐D and percentage of mediation in Figure 2F‐G; see Supporting Information Tables S2‐S3 for further details). Furthermore, we did not find a mediating effect of WT on the association of the number of hours exposed to a certain Personal‐ET with SUVmean and skeletal muscle activity (data not shown), nor did we find a mediating effect in upper (> 29°C) and lower (< 13°C) ranges of temperatures because of the lack of statistical power in these ranges (data not shown). The results persisted after controlling by sex, BMI, LMI, FMI, or date when the evaluation was performed (data not shown). Overall, the results persisted when we repeated all the analyses excluding data regarding the temperature ranges, for both WT and Personal‐ET, when the participants were asleep (data not shown). Moreover, we repeated the analyses using other classifications of skeletal muscle 11 activity (SUVpeak), and the absence of a mediating role of this tissue persisted (data not shown).
Figure 2.

(A) WT mediation models of the relationship between the number of hours exposed to a certain Personal‐ET and BAT‐related outcomes in young adults. Path c shows the association between independent and dependent variables. Arrow a multiplied by arrow b shows the natural indirect effect (β) pathway, and the arrow for c’ shows the natural direct effect pathway. (B) Indirect effects (β) of the simple mediation analyses of WT on the association between the number of hours exposed to each degree of Personal‐ET (from 14°C to 28°C) and BAT volume. (C,D) Indirect effect for BAT SUVpeak and metabolic activity, respectively. (E) PM of the simple mediation analyses of WT on the association between the number of hours exposed to each degree of Personal‐ET (from 14°C to 28°C) and BAT volume. (F,G) PM for BAT SUVpeak and metabolic activity, respectively. Black dots indicate that zero was in the 95% confidence interval of the indirect effect, and therefore, the mediation was considered not statistically significant (P > 0.05). Red and blue dots mean that the mediation analysis was statistically significant but with a different direction. BAT, brown adipose tissue; Personal‐ET, personal level of environmental temperature; PM, percentage of mediation; SUV, standardized uptake value; WT, wrist skin temperature. [Colour figure can be viewed at wileyonlinelibrary.com]
Discussion
The present study quantifies for the first time, to our knowledge, the mediating role of human BAT and cold‐induced skeletal muscle activity in the relationship between Personal‐ET and WT as an indirect proxy of skin blood flow 17. Intriguingly, the results show that BAT volume and metabolic activity mediate up to 25.4% of the association between Personal‐ET and WT. Moreover, the results indicate that the association of the number of hours exposed to a certain Personal‐ET with BAT volume, SUVpeak, and metabolic activity is mediated by WT at temperatures from 14°C to 20°C and from 24°C to 28°C, but not in the thermoneutral zone, as expected. We did not find a mediating role of human skeletal muscle or a relationship between WT and skeletal muscle. We also found that the participants with lower WT (inducing higher peripheral vasoconstriction) at the coldest temperatures had higher levels of BAT volume, SUVpeak and metabolic activity, whereas the participants with higher WT (inducing higher peripheral vasodilation) at the warmest temperatures had lower levels of BAT volume, SUVpeak, and metabolic activity. These findings show how WT (as a proxy of blood flow 17) is related to BAT volume and activity (SUVpeak) in young adults. However, further studies are needed to elucidate the possible mechanisms behind these relationships.
Mediating role of BAT
We show that both BAT volume and metabolic activity have a mediating role in the relationship between Personal‐ET and WT measured in daily living conditions independent of sex, BMI, LMI, FMI, and level of physical activity. This indicates that participants exposed to the same Personal‐ET over the 7 days have different WTs, which is explained, at least in part, by different levels of BAT volume or metabolic activity. Therefore, for every 1°C that the Personal‐ET is decreased, BAT volume would explain an increase of approximately 0.0194°C in WT. The relationship between Personal‐ET and the WT daily pattern has been widely used in the field of chronobiology 20, 30. Several studies comparing WT daily patterns in women with obesity versus women with normal weight 31, young versus older men and women 18, 32, and men versus women 19 have shown worse patterns (higher variability and higher daytime values) of WT in older participants and participants with obesity 33. These findings are also in accordance with those of human BAT studies, which showed that people with obesity, older people, and men had lower BAT volume and activity 34. Therefore, we postulate that BAT volume and metabolic activity should be taken into account in further chronobiological studies using WT, especially in those studies that measured WT only as a proxy of the circadian pattern without the inclusion of the Personal‐ET. We established this based on the following: (i) the observed mediating role of human BAT volume (and metabolic activity) in the relationship between Personal‐ET and WT, (ii) the activation of BAT in cold ambient temperatures (Personal‐ET ≤ 20°C), (iii) the fact that those with obesity, older people, and men have lower BAT volume and activity as well as worse patterns of WT, and (iv) the fact that the circadian rhythms and, specifically, the core body temperature rhythms are all controlled by specific neural pathways in the anterior hypothalamus 1. For instance, Martinez‐Nicolas et al. 20 studied the mediation role of WT in the relationship between Personal‐ET and mean arterial blood pressure in summer and winter, and they postulated that BAT could mediate this relationship. In this study, we show that this hypothesis might be true, although further studies are needed to fully understand the possible mechanisms behind these assumptions.
Mediating role of WT
All the physiological mechanisms of the thermoregulatory system seem to be orchestrated in the preoptic area of the hypothalamus 1. In addition to the peripheral tissues, the temperature of the brain is an input into the thermoregulatory system 35. One of the hypotheses explaining why human BAT is placed in the cervical and supraclavicular zone is that, as a thermogenic tissue, its main function is to regulate the temperature of the blood going to the brain 36, 37. Several studies have shown that human BAT activation is related to an increase in the blood flow in BAT 15, 38. Based on these results, the present study postulates that the increase in BAT activation (blood flow) could result in a redistribution of the blood in the peripheral part of the body during a cold stimulus in order to generate heat, as BAT is highly irrigated 39.
In warm ambient environments (from 24°C to 28°C), we observed that the higher the Personal‐ET is, the higher the WT is, which is associated with lower BAT volume and activity. Therefore, for every hour exposed at 27°C (Personal‐ET), WT would increase and explain a decrease of approximately 3.2 mL of BAT volume. The skin has warmth‐sensitive neurons specially allocated to perceive warmth exposure 1. However, there is controversy as to which main transient receptor potential (TRP) channel is involved as a warmth sensor, the candidates being TRPV1, TPRV3, TPRV4, and TRPM2 1. Therefore, there might be participants with higher or greater numbers of TRP channels than others, and this fact could explain why there are different responses to the same stimulus, although further studies are needed. Regardless of the main TRP channel involved, our results suggest that when Personal‐ET is high (hot), the body initiates some physiological response in order to preserve core temperature. Thus, the main physiological mechanism involved is induction of a peripheral vasodilation with an inhibition of human BAT (redistribution of blood flow to peripheral regions to dissipate heat) 12. We also showed that the higher the WT is, the lower the levels of BAT volume and activity (inhibition of this tissue) are. On the other hand, in cold ambient exposure (from 14°C to 19°C), we showed that the lower the Personal‐ET is, the lower the WT is, which is associated with higher BAT volume and activity. Therefore, for every hour exposed at 15°C (Personal‐ET), WT would decrease and explain an increase of approximately 2.5 mL of BAT volume. In the skin of the peripheral parts of the body, there are also cold‐sensitive neurons. These cold‐sensitive neurons highly expressed levels of TRP cation channel subfamily M member 8 (TRPM8), which is the primary peripheral cold sensor in the thermoregulatory system 40. Animal models showed that the inhibition of this sensor inhibited the behavioral and physiological responses to cooling 40. Taking this into consideration, we can postulate that there are individuals with a more efficient thermoregulatory system against cold stimuli, inducing a higher peripheral vasoconstriction and BAT activation in order to keep the core body temperature constant, which could be explained by a higher sympathetic tone. According to this, it might be possible for people with higher levels of human BAT to have a higher concentration of TRMP8, and it may be that a different polymorphism of the TRMP8 gene is associated with a better response to cold stimuli; however, these hypotheses have not been studied so far.
Mediating role of skeletal muscle
Skeletal muscles are involved in the thermoregulatory responses during cold exposure 6, 11. Interestingly, we did not observe an effect of skeletal muscle activity (as measured by 18F‐FDG uptake) in the relationship between Personal‐ET and WT. This lack of mediating effect does not necessarily mean that skeletal muscle is not involved in cold‐induced thermogenesis. This lack of effect might be due to the fact that the cold ambient temperatures were not cold enough to induce skeletal muscle activation or because the 18F‐FDG tracer is not a good marker of skeletal muscle metabolism 6.
This cross‐sectional and observational study should be replicated in older participants and using other nuclear tracers such as 15O‐O2 and [11C]acetate 6 or adenosine perfusion, a vasodilator that seems to active human BAT 25. Moreover, we know that during sleep phases, humans can lose at least 25% of their total thermoregulatory capacity. Because our aim was to study the mediating role of human BAT over 7 days (even in sleep phases), we kept these analyses as main results, although excluding the sleep phase did not alter the results (data not shown). Moreover, in this study, the levels of clothing during the measurements were not evaluated. Future experimental studies are warranted to elucidate the possible mechanisms behind this efficiency in the thermoregulatory system and new therapies that could be developed to improve this physiological system.
Conclusion
We showed that BAT volume and metabolic activity mediate the relationship between Personal‐ET and WT. Moreover, our data support the idea that the individuals who were exposed to lower environmental temperatures, and at the same time had lower WT, concomitantly had higher BAT volume. We also observed the opposite effect when the participants were exposed to warmer temperatures, which indicates a redistribution of the blood flow between the peripheral part of the body and BAT activation/inhibition in order to keep the core body temperature constant. Future interventional studies should try to find strategies to improve the thermoregulatory system and its relationship with metabolic diseases.
Supporting information
Acknowledgments
The authors would like to thank all the participants who took part in this investigation. This study is part of a PhD thesis conducted in the Biomedicine Doctoral Studies of the University of Granada, Spain. We are grateful to Alberto Quesada‐Aranda for helping with the development of the Temperatus software (free trial at http://profith.ugr.es/temperatus?lang=en). We are grateful to Ms Carmen Sainz‐Quinn for assistance with English‐language editing.
Funding agencies: This study was supported by the Spanish Ministry of Economy and Competitiveness, Fondo de Investigación Sanitaria del Instituto de Salud Carlos III (PI13/01393), Retos de la Sociedad (DEP2016‐79512‐R), and Fondos Estructurales de la Unión Europea (FEDER); by the Spanish Ministry of Education (FPU 13/04365); by the Fundación Iberoamericana de Nutrición; by the Redes Temáticas de Investigación Cooperativa RETIC (Red SAMID RD16/0022); by AstraZeneca HealthCare Foundation; by the University of Granada, Plan Propio de Investigación 2016, Excellence actions: Units of Excellence; Unit of Excellence on Exercise and Health (UCEES); and by the Junta de Andalucía, Consejería de Conocimiento, Investigación y Universidades and European Regional Development Fund (ERDF), ref. SOMM17/6107/UGR, Programa Contratos‐Puente. MAR is supported by a predoctoral research grant from University Jaume I (PREDOC/2015/13). AMN was supported by the Ministry of Economy and Competitiveness, the Instituto de Salud Carlos III through the Centro de Investigación Biomédica en Red Fragilidad y Envejecimiento Saludable (CB16/10/00239), and grant 19899/GERM/15 (cofinanced by FEDER).
Disclosure: The authors declared no conflict of interest.
Author contributions: BMT and JRR conceived and designed the research. BMT, FMA, GSD, and JMLE performed the experiments; MAR, BMT, VMV, and JRR analyzed the data; MAR and BMT prepared the figures and drafted the manuscript; BMT, MAR, FMA, GSD, AMN, MRB, JMLE, VMV, and JRR interpreted the results, revised the manuscript, and approved the final version.
References
- 1. Tan CL, Knight ZA. Regulation of body temperature by the nervous system. Neuron 2018;98:31‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Batchelder P, Kinney RO, Demlow L, Lynch CB. Effects of temperature and social interactions on huddling behavior in Mus musculus . Physiol Behav 1983;31:97‐102. [DOI] [PubMed] [Google Scholar]
- 3. Romanovsky AA. Skin temperature: its role in thermoregulation. Acta Physiol 2014;210:498‐507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Satinoff E. Neural organization and evolution of thermal regulation in mammals. Science 1978;201:16‐22. [DOI] [PubMed] [Google Scholar]
- 5. McAllen RM, Tanaka M, Ootsuka Y, McKinley MJ. Multiple thermoregulatory effectors with independent central controls. Eur J Appl Physiol 2010;109:27‐33. [DOI] [PubMed] [Google Scholar]
- 6. U Din M, Raiko J, Saari T, et al. Human brown adipose tissue [15O]O2 PET imaging in the presence and absence of cold stimulus. Eur J Nucl Med Mol Imaging 2016;43:1878‐1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev 2004;84:277‐359. [DOI] [PubMed] [Google Scholar]
- 8. van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, et al. Cold‐activated brown adipose tissue in healthy men. N Engl J Med 2009;360:1500‐1508. [DOI] [PubMed] [Google Scholar]
- 9. Virtanen KA, Lidell ME, Orava J, et al. Functional brown adipose tissue in healthy adults. N Engl J Med 2009;360:1518‐1525. [DOI] [PubMed] [Google Scholar]
- 10. Cypess AM, Lehman S, Williams G, et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med 2009;360:1509‐1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Blondin DP, Labbé SM, Phoenix S, et al. Contributions of white and brown adipose tissues and skeletal muscles to acute cold‐induced metabolic responses in healthy men. J Physiol 2015;593:701‐714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Muzik O, Mangner TJ, Leonard WR, Kumar A, Janisse J, Granneman JG. 15O PET measurement of blood flow and oxygen consumption in cold‐activated human brown fat. J Nucl Med 2013;54:523‐531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Haman F, Blondin DP. Shivering thermogenesis in humans: origin, contribution and metabolic requirement. Temperature (Austin) 2017;4:217‐226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Acosta FM, Martinez‐Tellez B, Sanchez‐Delgado G, et al. Physiological responses to acute cold exposure in young lean men. PLoS One 2018;13:e0196543. doi: 10.1371/journal.pone.0196543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Muzik O, Mangner TJ, Leonard WR, Kumar A, Janisse J, Granneman JG. 15O PET measurement of blood flow and oxygen consumption in cold‐activated human brown fat. J Nucl Med 2013;54:523‐531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Martinez‐Tellez B, Xu H, Sanchez‐Delgado G, et al. Association of wrist and ambient temperature with cold‐induced brown adipose tissue and skeletal muscle [18F]FDG uptake in young adults. Am J Physiol Integr Comp Physiol 2018;315:R1281‐R1288. [DOI] [PubMed] [Google Scholar]
- 17. Rubinstein EH, Sessler DI. Skin‐surface temperature gradients correlate with fingertip blood flow in humans. Anesthesiology 1990;73:541‐545. [PubMed] [Google Scholar]
- 18. Kräuchi K, Gompper B, Hauenstein D, et al. Diurnal blood pressure variations are associated with changes in distal‐proximal skin temperature gradient. Chronobiol Int 2012;29:1273‐1283. [DOI] [PubMed] [Google Scholar]
- 19. Sanchez‐Delgado G, Martinez‐Tellez B, Olza J, et al. Activating Brown Adipose Tissue Through Exercise (ACTIBATE) in young adults: rationale, design and methodology. Contemp Clin Trials 2015;45:416‐425. [DOI] [PubMed] [Google Scholar]
- 20. Martinez‐Nicolas A, Meyer M, Hunkler S, et al. Daytime variation in ambient temperature affects skin temperatures and blood pressure: ambulatory winter/summer comparison in healthy young women. Physiol Behav 2015;149:203‐211. [DOI] [PubMed] [Google Scholar]
- 21. Martinez‐Tellez B, Sanchez‐Delgado G, Garcia‐Rivero Y, et al. A new personalized cooling protocol to activate brown adipose tissue in young adults. Front Physiol 2017;8:863. doi: 10.3389/fphys.2017.00863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. van der Lans AAJJ, Wierts R, Vosselman MJ, Schrauwen P, Brans B, van Marken Lichtenbelt WD. Cold‐activated brown adipose tissue in human adults: methodological issues. Am J Physiol Regul Integr Comp Physiol 2014;307:R103‐R113. [DOI] [PubMed] [Google Scholar]
- 23. Chen KY, Cypess AM, Laughlin MR, et al. Adipose reporting criteria in imaging studies (BARCIST 1.0): recommendations for standardized FDG‐PET/CT experiments in humans. Cell Metab 2016;24:210‐222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hanssen MJW, van der Lans AAJJ, Brans B, et al. Short‐term cold acclimation recruits brown adipose tissue in obese humans. Diabetes 2016;65:1179‐1189. [DOI] [PubMed] [Google Scholar]
- 25. Martinez‐Tellez B, Nahon KJ, Sanchez‐Delgado G, et al. The impact of using BARCIST 1.0 criteria on quantification of BAT volume and activity in three independent cohorts of adults. Sci Rep 2018;8:8567. doi: 10.1038/s41598-018-26878-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hayes AF. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression‐Based Approach. New York, NY: Guildford Press; 2013. [Google Scholar]
- 27. Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods 2008;40:879‐891. [DOI] [PubMed] [Google Scholar]
- 28. Hayes AF. Beyond Baron and Kenny: statistical mediation analysis in the new millennium. Commun Monogr 2009;76:408‐420. [Google Scholar]
- 29. Leitner BP, Huang S, Brychta RJ, et al. Mapping of human brown adipose tissue in lean and obese young men. Proc Natl Acad Sci U S A 2017;114:8649‐8654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Martinez‐Nicolas A, Ortiz‐Tudela E, Rol MA, Madrid JA. Uncovering different masking factors on wrist skin temperature rhythm in free‐living subjects. PLoS One 2013;8:e61142. doi: 10.1371/journal.pone.0061142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Corbalán‐Tutau MD, Madrid JA, Ordovás JM, Smith CE, Nicolás F, Garaulet M. Differences in daily rhythms of wrist temperature between obese and normal‐weight women: associations with metabolic syndrome features. Chronobiol Int 2011;28:425‐433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Batinga H. Ontogeny and aging of the distal skin temperature rhythm in humans. Age (Dordr) 2015;37:29. doi: 10.1007/s11357-015-9768-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Martinez‐Nicolas A, Guaita M, Santamaría J, Montserrat JM, Rol MÁ, Madrid JA. Circadian impairment of distal skin temperature rhythm in patients with sleep‐disordered breathing: the effect of CPAP. Sleep 2017;40:31‐37. [DOI] [PubMed] [Google Scholar]
- 34. Ouellet V, Routhier‐Labadie A, Bellemare W, et al. Outdoor temperature, age, sex, body mass index, and diabetic status determine the prevalence, mass, and glucose‐uptake activity of 18F‐FDG‐detected BAT in humans. J Clin Endocrinol Metab 2011;96:192‐199. [DOI] [PubMed] [Google Scholar]
- 35. Hammel HT, Pierce JB. Regulation of internal body temperature. Annu Rev Physiol 1968;30:641‐710. [DOI] [PubMed] [Google Scholar]
- 36. Bahler L, Holleman F, Booij J, Hoekstra JB, Verberne HJ. Hot heads & cool bodies: the conundrums of human brown adipose tissue (BAT) activity research. Eur J Intern Med 2017;40:26‐29. [DOI] [PubMed] [Google Scholar]
- 37. Yoneshiro T, Matsushita M, Nakae S, et al. Brown adipose tissue is involved in the seasonal variation of cold‐induced thermogenesis in humans. Am J Physiol Regul Integr Comp Physiol 2016;310:R999‐R1009. [DOI] [PubMed] [Google Scholar]
- 38. Orava J, Nuutila P, Lidell ME, et al. Different metabolic responses of human brown adipose tissue to activation by cold and insulin. Cell Metab 2011;14:272‐279. [DOI] [PubMed] [Google Scholar]
- 39. Muzik O, Mangner TJ, Leonard WR, Kumar A, Granneman JG. Sympathetic innervation of cold‐activated brown and white fat in lean young adults. J Nucl Med 2017;58:799‐806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dhaka A, Murray AN, Mathur J, Earley TJ, Petrus MJ, Patapoutian A. TRPM8 is required for cold sensation in mice. Neuron 2007;54:371‐378. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


