Abstract
Background
Many women who are at increased risk of breast cancer due to a mother or sister diagnosed with breast cancer aged under 40 do not currently qualify for surveillance before 40 years of age. There are almost no available data to assess whether mammography screening aged 35–39 years would be effective in this group, in terms of detection of breast cancer at an early stage or cost effective.
Methods
A cohort screening study (FH02) with annual mammography was devised for women aged 35–39 to assess the sensitivity and screening performance and potential survival of women with identified tumours.
Findings
2899 women were recruited from 12/2006–12/2015. These women underwent 12,086 annual screening mammograms and were followed for 13,365.8 years. A total of 55 breast cancers in 54 women occurred during the study period (one bilateral) with 50 cancers (49 women) (15 CIS) adherent to the screening. Eighty percent (28/35) of invasive cancers were ≤ 2 cm and 80% also lymph node negative. Invasive cancers diagnosed in FH02 were significantly smaller than the comparable (POSH-unscreened prospective) study group (45% (131/293) ≤ 2 cm in POSH vs 80% (28/35) in FH02 p < 0.0001), and were less likely to be lymph-node positive (54% (158/290, 3 unknown) in POSH vs 20% (7/35) in FH02: p = 0.0002. Projected and actual survival were also better than POSH. Overall radiation dose was not higher than in an older screened population at mean dose on study per standard sized breast of 1.5 mGy.
Interpretation
Mammography screening aged 35–39 years detects breast cancer at an early stage and is likely to be as effective in reducing mortality as in women at increased breast cancer risk aged 40–49 years.
Keywords: Mammography, Breast cancer, Young, BRCA1, BRCA2, Familial
Highlights
-
•
We show that mammography screening can detect the majority of breast tumours at small size and lymph node in familial breast cancer
-
•
Tumour characteristics & survival are better than in a comparison unscreened familial cohort
-
•
Radiation dose in 35–39 year olds is not higher than in older women
Research in Context
Evidence Before This Study
There is virtually no published evidence of efficacy of mammography in women aged less than 40 years of age. A number of randomised screening trials assessed screening 2-yearly in women aged 40–70 years of age and found more limited benefit in the 40–49 age group. However, the FH01 forerunner of FH02 showed that women with a family history of breast cancer had tumours detected earlier and with better prognosis aged 40–49 years than in two control unscreened populations: an age trial control group aged 40–49 and a family history control group. As a result of FH01 NICE guidelines in the UK recommended annual mammography in women at moderate and high risk of breast cancer aged 40–49 years. However, NICE recommended further research before mammography was offered aged less than 40 years. The authors undertook a full search of PubMed for all articles on mammography screening in young women. They used the search terms ‘mammography’ ‘screening’ ‘young’ 1980–2018. English language publications only.
Added Value of This Study
The present FH02 study shows that annual mammography aged 35–39 years in a multicentre study is as effective as it was in women aged 40–49 in FH01. Furthermore, tumours were detected earlier with high sensitivity and better prognostic features than an age matched comparison group from the POSH study. Breast cancer incidence was more than sufficient in the high risk group to justify screening, but further work may be needed in the moderate risk group to assess cost effectiveness.
Implications of All the Available Evidence
The results of FH02 are sufficient to consider annual screening in all high risk women and consider this for those at moderate risk with annual mammography.
Alt-text: Unlabelled Box
1. Introduction
Women younger than 50 years of age are not eligible for National Breast Screening Programmes, but women with significant breast cancer family histories are frequently referred for annual mammography as a secondary prevention strategy [1]. These women likely have breast cancer incidence substantially higher than the population risk of women in their fifties who qualify for the UK national screening programme. A study to evaluate annual mammography in women aged 40–49 (FH01 study) [2], [3] demonstrated a projected mortality advantage for annual mammography surveillance compared to age matched women with no screening based on tumour characteristics at diagnosis [4]. No prospective evaluation has yet been carried out in women aged < 40 who are currently invited for screening in many familial risk clinics. The design of mammographic surveillance evaluation studies is problematic. The feasibility study which was undertaken prior to FH01 included surveys of clinical staff managing women at familial risk. These surveys indicated that there was insufficient equipoise to carry out randomised controlled trials [2], [3]. As a result, FH01 was designed as a single-arm cohort study, estimating the likely benefit of annual surveillance on breast cancer mortality from both internal modelling and comparison with other contemporary cohorts not undergoing mammography [3].
In the UK the NICE guidelines [5] indicated a gap in knowledge of the potential benefit or otherwise of mammography in high-risk women aged < 40, and clear need for research in this area. NICE stated that any such screening in women aged < 40 should only be performed as part of research to evaluate this intervention. The current study aimed at providing such an evaluation. The NICE literature review in the 2013 guidance [5] did not reveal any significant evidence for screening with mammography without MRI < 40 years of age and our literature search in December 2017 still revealed no significant subsequent publications. Although a small proportion of women in the moderate/high-risk categories (~ 5%) are eligible for MRI screening in the UK in line with NICE guidance, mainly due to carrying BRCA1/2 pathogenic variants, the vast majority of those with familial risk < 40 years of age do not currently qualify for approved health service surveillance. Approximately 3% of women < 40 are at moderate to high-risk of breast cancer [5], [6] and at least half of these come forward with concerns about this [6]. The FH01 study established a likely beneficial effect on mortality from breast cancer in the 40–50 age group as estimated from reduced tumour size, absence of lymph-node involvement and grade of the breast tumours diagnosed in the women recruited [3], [4] and led to NICE recommendations supporting annual surveillance in this age group [5]. It was considered unlikely that a project in the 35–39 age group would be able to recruit sufficient numbers to give a precise estimate of these endpoints. We therefore proposed to base evaluation on the accuracy of the screening test. This approach was used in four UK breast screening trials in the last 20-years: the US one-view/two-view trial, the DMIST study of digital mammography, the MARIBS trial of MRI in very high-risk women and the CADET study of computer-aided detection in breast screening [7], [8], [9], [10]. Thus, there are precedents for projecting the effect on clinical outcomes from the observed results in terms of early detection. The rationale is that if the proof of principle of early detection is already established, screening regimens which achieve this early detection with high degrees of accuracy are likely to be effective in terms of subsequent clinical outcomes including mortality [3]. If this can be shown to be of a similar order as in the FH01 and UK age trials, which both studied the effects of annual mammography from age 40–49 [11], there would be some confidence that the surveillance would have similar effects as in those studies. A retrospective audit of annual mammography in three centres in the UK showed that results were similar to FH01 in terms of screening accuracy with similar tumour size and lymph node involvement in those undergoing annual mammography [1]. Here we present results of the multicentre prospective FH02 study.
2. Methods
Unaffected women with a family history of breast cancer (usually affecting at least one first degree relative) meeting the criteria in Table 1 and aged 35–39 years were eligible for trial entry. Women in the retrospective arm of FH02 [1] were only eligible if they were still aged 35–39 and without breast cancer. Women were screened with mammography annually.
Table 1.
Eligibility criteria for FH02.
| 1 first degree (FDR) female - breast cancer < 40 |
| 2) 1 FDR - bilateral breast cancer first cancer diagnosed < 50; |
| 3) 2 FDR or 1 FDR and 1-second degree (SDR) female - both with breast cancer < 60; |
| 4) 1 FDR/SDR female - breast and ovarian cancer first cancer diagnosed < 60 |
| 5) 2 FDR or 1 FDR and 1 SDR female - breast cancer < 60 and ovarian cancer at any age; |
| 6) 3 FDR/SDR degree female - breast or ovarian cancer at any age; |
| 7) 1 first degree male - breast cancer at any age |
| 8) paternal history of a minimum of 2 SDR (NB. father's first degree relatives) with breast cancer < 50 or breast < 50 and an ovarian cancer (any age), or paternal uncle/grandfather with breast cancer < 50 years; |
| 9) A BRCA1/2 mutation carrier or at least a 1 in 4 risk of carrying a known mutation in a family. |
Thirty four centres contributed prospective screening data as part of the FH02 study.
The aims and purpose of the study were:
-
1.
To estimate, in terms of screening accuracy (sensitivity/specificity and stage of cancers) the likely benefit of annual mammography in women at increased risk aged 35–39 by comparison with the accuracy and endpoints observed in the FH01 study and the UK Age Trial.
-
2.
To compare the screening accuracy and outcomes between the prospective study with strict entry criteria and interventions, to retrospective FH02 with no universal standard of eligibility or intervention.
-
3.
To compare the prognostic indicators of the detected tumours and long-term patient outcome with women with a family history who were not screened in the POSH study. The POSH study recruited incident breast cancers in women aged 40 or below in the UK from 2000 to 2007 [12], [13].
-
4.
To estimate the likely benefit, if any, of this mammographic activity - in order to inform policy as to whether it should continue in the UK.
The prospective study involved formal design and control, including risk criteria to ensure that the enrolled population undergoing surveillance was at sufficient breast cancer risk to achieve a worthwhile balance of benefit and harm of the intervention. Annual mammography was the study standard. Data were collected on screening episode outcomes, additional diagnostic activity in those recalled for further assessment, biopsy and detection rates, active follow-up to ascertain interval cancers, and full clinico-pathological data on the cancers diagnosed. The prospective study collected the same data items as in FH01 allowing a detailed comparison with FH01 [3]. The prospective collection of the pathology data allowed an informative comparison with the tumours in the POSH study which has complete tumour ascertainment [12]. Thus, the prospective study allowed formal evaluation addressing all 4 study aims.
The original protocol indicated recruitment of 2800 women between age 35–39, with a family history of breast cancer, and who fulfilled at least one of the criteria in Table 1.
Satisfying one of these inclusion criteria gives an individual a ten-year risk of developing breast cancer below age 40 of ≥ 1.5% [13], [14], [15], [16], [17]. This accords with information from the retrospective arm of FH02 in Manchester where 47 cancers in this age group occurred at an incidence of 4.77 per 1000 [1].
Exclusion criteria for FH02 included:
Inability or refusal to give written informed consent; pregnancy; women below the age of 35 or above the age of 39; a previous history of breast cancer or ductal carcinoma in situ or bilateral risk-reducing mastectomy; contraindication to annual X-rays and if a cancer is detected by MRI and not mammography.
Women already under surveillance, including those contributing to the retrospective study, were eligible, as were women newly presenting to genetic/family history clinics. Detailed information on the family history of each individual in the study was recorded at baseline. Standard outcomes included attendance; normal/abnormal outcome; investigations and outcome of subsequent assessment and any surgical intervention, for all those recruited. In addition, clinical and pathological data were collected on each cancer diagnosed at each screening episode and on interval cancers. The radiation dose from mammography was recorded, if available, in order to assess likely risks from additional radiation exposure. The value recorded was the mean glandular dose (MGD) per image, either as displayed by the mammography equipment or as calculated.
2.1. Endpoints and Analyses
The primary outcomes expected at the end of the study period were as follows:
-
1.
Detection rates by screening round (incidence or prevalence). This is the number of cancers detected per thousand women screened.
-
2.
Number of interval cancers by risk stratum.
-
3.
Programme and test sensitivity, the latter estimated taking mean sojourn time (the duration of the preclinical screen-detectable period) into account [18].
-
4.
Number of recalls, including benign, per cancer detected including bilateral.
-
5.
Number of surgery/surgical biopsy cases per cancer detected.
-
6.
Cancers diagnosed by size, node status, grade and receptor status.
As part of the data analysis, all of the above were compared with the corresponding outcomes in FH01, taking account of differences in underlying incidence in the two cohorts (see below). Likely future fatality from breast cancer as assessed by the numbers of screen-detected and interval cancers, was compared with that expected if there were no screening, taking into account lead time and length bias and potential overdiagnosis [19], [20], [21], [22], [23]. Tumour prognostic indicators and outcomes were compared with unscreened women with breast cancers aged 35–40 years in the UK POSH study with a similar family history diagnosed 2000–2008. The analyses were performed separately including and excluding ductal carcinoma in situ (DCIS) cases.
2.2. Study Size
For simplicity, we based our power/sample size calculations on the comparison of a measure of sensitivity between this study and the UK Age Trial. The sensitivity measure proposed was the programme sensitivity (S) in an annual screening regime. If CS is the number of screen-detected cancers and CI the number of interval cancers diagnosed within one year of a negative screen, we assume CS to be binomially distributed with denominator CS + CI. In the Age Trial, S was 65% [22]. To have 90% power detect a significant S in our population being 20% lower in absolute terms (i.e. 45%), we would need 65 cancers screen-detected plus interval cancers (those presenting symptomatically within one year) in total. This total would also give a 95% confidence interval on S of no more than 12% in either direction. To calculate the number of screens necessary to give 65 cancers, we note that in younger women incidence screens and prevalence screens have approximately the same detection rates [23]. We expect N × I × MST × ST cancers at screening, where N is the number screened, I the underlying incidence MST the mean sojourn time and ST the test sensitivity [24]. With S = 45%, we therefore need just over double the above product to be equal to 65. Assuming ST = 0.8, MST = 1.5, and an incidence of 2–4 per thousand per year (3–7 times the population incidence for this age group), we would need approximately 10,000 screens. We therefore aimed to collect data on this total number of screens. As a failsafe, we aimed to recruit 2800 subjects, with screening for five years.
2.2.1. Risk Categories
The risk thresholds for trial entry were those used in current NICE guidance which defines moderate risk as 17–29% lifetime risk of breast cancer or calculated 3% 10-year risk aged 40 years. The Tyrer-Cuzick algorithm was used as a single reproducible objective measure of 10-year risk. The high-risk category was defined as ≥ 8% 10-year risk with age set at 40 years regardless of age at entry. Similarly 3–7.99% 10-year risk was defined as moderate and < 3% near average risk.
2.2.2. Cancer Verification
Breast cancers were confirmed from each centre using hospital pathology reports. In addition a search was made in Dec 2017 on the National Cancer Registry to ensure that all interval cancers were identified.
2.2.3. Statistical Methods
Comparison of tumour attributes between FH02 and POSH and between FH02 and FH01 was done using chi-squared tests. Projected breast cancer deaths based on the tumour attributes in FH02 was compared with that expected in the absence of screening using a similar method as in FH01 [4], but simplified in that we select our comparison group as those cancers in POSH diagnosed in women satisfying the risk criteria of FH02. Thus, we estimated the projected relative mortality as:
Where d1 is the predicted fatality rate from the Nottingham Prognostic Index of the tumours in FH02, and d2 the corresponding rate for the POSH cases in this age group meeting the FH02 risk criteria. This is simpler than the formula used for FH01 as there is no necessity to adjust for different risk status of the two populations.
The expected number of breast cancers overall and in risk categories was obtained from the Tyrer-Cuzick model by summating each woman's expected breast cancer risk cumulative hazard from study entry mammogram to date of breast cancer diagnosis or 30/06/2016 whichever was earlier (unless censored for death or risk reducing mastectomy). Exact Poisson confidence intervals were calculated for incidence rate and to compare the observed with expected number of cancers. FH02 received research ethics approval in 2005 (MREC Reference No: 05/MRE02/67).
3. Results
3.1. Prospective Study
Thirty four UK centres recruited a total of 2899 women aged 35–39 years between Dec 2006 and Dec 2015. These women underwent 12,086 annual screening mammograms (9751 digital; 2335 analogue) and were followed for a total of 13,365.8 years to time of censoring. A total of 55 breast cancers (54 women) occurred during the study period (one bilateral) including 12-month follow-up until Dec 2016 to allow for potential interval cancers (cancer registration was checked in December 2017). Overall, 447 other women were withdrawn from the screening aspect of the study:, 24 due to change of genetic risk status, 193 failed to attend and were removed from local screening programme 4 had died (non-cancer), 40 moved away, 100 became pregnant, and 22 women underwent risk reducing mastectomy during the study period with two (9%) having occult breast cancers and 64 due to miscellaneous reasons.
Overall, approximately half of the women received their first ever mammogram in FH02 (n = 1410–2.1 per 1000 women) with 1438 having had previous mammography and 51 unknown. There were 3 prevalent cancers in the study (2.1 per 1000 women who had undergone previous mammography).
Table 2 shows breast cancer incidence by Tyrer-Cuzick risk category and prevalent/incident status, for all cancers and for those diagnosed up to age 41. There were 34 incident cancers detected at screening mammography (including two from a bilateral case) and 13 interval cancers (11 with previous digital mammogram) as such 72% (34/47) of women with post prevalent cancers were screen detected. Two of the interval cancers were found at risk reducing mastectomy as a 17 mm grade 2 DCIS and a 3 mm grade 1 invasive cancer.
Table 2.
Breast cancer incidence by Tyrer-Cuzick 10-year risk category in FH02.
| Tyrer-Cuzick Risks age 40 |
High risk 8% + 10-year |
Moderate risk 3–7.9% 10-year |
Average risk < 3% 10-year risk |
Total |
|---|---|---|---|---|
| Total years follow up | 1447.4 | 9220.0 | 2572.9 | 13,237.8 |
| Number of recruits | 344 | 1977 | 499 | 2820 |
| Breast cancers Prevalent |
1 | 2 | 0 | 3 |
| Breast cancers post prevalent | 13 | 33 | 6 | 52 |
| Expected T-C | 18.8 | 25 | 3.7 | 47.5 |
| Rate per 1000a | 9.0 (95%CI 5.2–15.5) | 3.6 (95%CI 2.5–5.1) | 2.3 (95%CI 1.0–5.2) | 3.9 (95%CI 2.9–5.2) |
| Recall mammograms | 90 | 481 | 142 | 713 |
| Screen detected cancers | 9 | 25 | 3 | 37 |
| Ratio of recalls to screen detected cancers | 10:1 | 19:1 | 47:1 | 19:1 |
| Recalls per year of follow up | 6.2% | 5.2% | 5.5% | 5.4% |
| Follow up to age 41 years only | ||||
| Years follow up | 1068.8 | 6197.3 | 1609.2 | 8875.3 |
| Number of recruits | 344 | 1977 | 499 | 2820 |
| Breast cancers Prevalent |
1 | 2 | 0 | 3 |
| Breast cancers Post prevalent |
11 | 17 | 2 | 30 |
| Expected T-C | 12.5 | 14.8 | 1.9 | 29.2 |
| Rate per 1000a | 10.3 (95%CI 5.7–18.6) | 2.7 (95%CI 1.6–4.4) | 1.2 (95%CI 0.2–4.9) | 3.4 (95%CI 2.3–4.9) |
| Numbers to be screened for each screen detected cancer 35–40 years | 31 | 95 | 322 | 85 |
| Intervals 35–40 | 4 | 6 | 1 | 11 |
Rates exclude prevalent cancers.
Four cancers occurred more than 18 months from the last screen in women who had defaulted on screening and a further large cancer was identified symptomatically in a woman consented to the study who had not received a baseline mammogram. These five cases were excluded from the estimation of screening parameters and comparison with POSH. Programme sensitivity is conservatively estimated as 34/(13 + 34) = 72% (95% CI 59—85%). Mean sojourn time was estimated as 1.8 years and test sensitivity as 93% (95% CI 0.68–0.99).
Tumour pathology at diagnosis compared with POSH study participants without surveillance who had similar family history to FH01 and FH02 diagnosed between 2000 and 2008 is shown in Table 3. The FH02 invasive cancers were significantly smaller than the POSH symptomatic controls (45% [131/293] ≤ 2 cm in POSH vs 80% [28/35] in FH02; Table 3). POSH controls were significantly more likely to be lymph node positive (54% [158/290, 3 unknown] in POSH vs 20% [7/35] in FH02). Women had a higher rate of breast cancer mortality in POSH than FH02 but a Kaplan Meier analysis (Fig. 1) did not confirm statistical significance and statistical power was limited (log rank chi square is 2.94, p = 0.086; HR 4.8 (95% CI 0.7–34.8). There was only one death in FH02 at 2.78 years with overall follow up of 2.7–11.2 years (mean = 5.35 years). There were similar rates of DCIS in prospective FH02 compared to retrospective FH02 and FH01 (Table 3).
Table 3.
Breast cancers identified in the prospective FH02 study compared to the POSH study and retrospective FH02 and FH01.
| Age at diagnosis | POSH FHpos Unscreened 35–40 (%) |
FH01 40-49 Prospective (%) |
Retrospective FH02 35–39 + (%) | Prospective FH02 35–40 (%) |
|---|---|---|---|---|
| N | 293 | 136 | 47 | 50 |
| Years of diagnosis | 2000–2008 | 2003–2010 | 1990–2008 | 2007–2016 |
| Years follow up | 1–12 years | 0–7 years | 1–12 years | 0–9 years |
| Histology | ||||
| Invasive (%) | 293 (100%) | 96 (74) | 35 (74) | 35 (70) |
| Grade 1 (% of invasive) | 18 (6) | 17 (19) | 3 (8.6) | 6 (17) |
| Grade 3 (% of invasive) | 177 (60.5) | 40 (45) | 17 (48.6) | 21 (60) |
| In-situ (%) | 0 not included in POSH | 34 (26) | 12 (26) | 15 (30) |
| Unknown | 6 | – | 0 | |
| P value compared to prospective FH02 | N/a as POSH only invasive | |||
| Size (in situ excluded) | N = 293 | N = 87 | N = 35 | N = 35 |
| ≤ 2 cm | 138 (47) | 61/87 (70) | 25 (74) | 28 (80) |
| 2–4.9 cm | 122 (41.5) | 8 (23) | 6 (18) | |
| ≥ 5 cm | 23 (8) | 1 (3) | 1 (3) | |
| Unknown | 10 | 9 | 1 | 0 |
| P value for ≤ 2 cm compared to prospective POSH | P < 0.0001 | |||
| Node involvement (in situ excluded) | N = 293 | N = 82 | N = 35 | N = 35 |
| 0 | 133 (45) | 56 (68) | 25 (77) | 28 (80) |
| 1–4 | 110 (38) | 7 (23) | 5 (15) | |
| > 4 | 48 (16) | – | 2 (6) | |
| Not sampled/[not known] | 3 (1) | [14] | 3 | 0 |
| P value for LN0 compared to POSH | P = 0.0002 | |||
| Stage 1 of invasive | 83 (28%) | – | 21 (60) | 24 (68.5%) |
| P value POSH ref | P < 0.0001 | |||
| Status | N = 289 | N = 47 | N = 49a | |
| Alive (no metastasis) | 204 (70) | NK | 43 (91) | 49 (98) |
| Alive (with metastasis) | 23 (8) | 0 (0) | 0 | |
| Died (of disease) | 63 (21.5) | 4 (9) | 1 (2) | |
| Died (other) | 10 (3.5) | 0 | 0 | |
| P value compared to POSH | P = 0.0009 | |||
| Annual incidence rate/1000 | 4.77 | 3.85 |
+ Retrospective FH02 was a survey of previous screening in the same age range prior to the launch of FH02 [1]. P value by chi square testing.
One case was bilateral at diagnosis.
Fig. 1.

Breast cancer specific survival in invasive cases versus POSH Family history positive cases.
3.1.1. Breast Cancer Incidence
The overall incidence including follow up to a maximum age of 49 years was 3.9 per 1000 excluding prevalent screen detected cancers (Table 2). Restricting follow up to the 41st birthday allowing for incident cancers in the 41st year (if screened at 40 women would only have a prevalent scan that year) incidence rates were a little below the NICE threshold for 10-year risk in the moderate-risk group at 2.7 per 1000, but above in the high-risk categories (10.3). Using a 3% 10-year threshold at entry for the moderate risk group this reduced the number eligible to 1188 women (60% of total) with a rate of 3.3% from 10 breast cancers to age 41 years with 9.9 expected. Those 499 women with average risk had overall rates of only 2.3 per 1000 and only 1.2 in the 35–40 age group. Overall, 47.5 breast cancers were expected using Tyrer-Cuzick with 52 occurring after prevalence (O/E 1.09, 95%CI 0.83–1.43).
3.1.2. Breast Biopsy and Recall
A total of 729/12086 mammograms (6%) resulted in a recall. The recall rate was 9.65% (278/2898) in first round and 4.9% (451/9188) subsequently. Overall, there were 191 breast biopsies (core or open) for 37 screen detected cancers, therefore 18.3% (35/191) of biopsies resulted in a cancer diagnosis. Thus, there were 20 recalls per cancer screen detected, 19 false positives per screen detected cancer, and 5 biopsies per cancer screen detected.
3.1.3. Radiation Dose
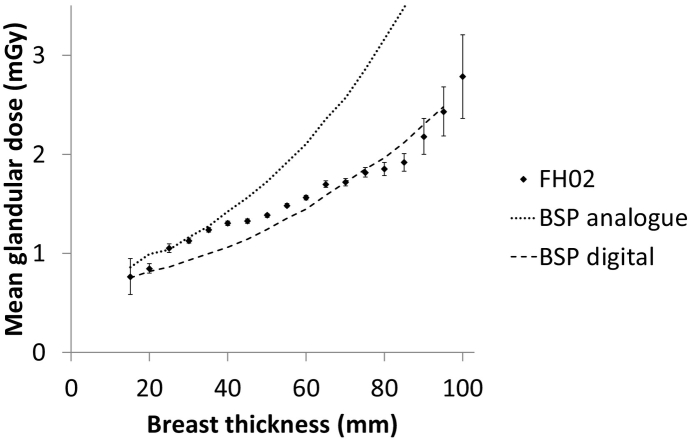
Radiation dose was available from 25/34 centres, with values recorded for a total of 8585/12086 examinations (71%). The distribution of dose values from two centres was inconsistent with real values, probably due to recording errors, and these were excluded from further analysis. Of the remaining 8278, a subset of 4666 examinations were of women in the age range 35–39 years. For this subset, the mean breast thickness was 54 mm and the mean MGD for a mixture of craniocaudal and mediolateral oblique views was 1.5 mGy. This dose is slightly lower than the average values of 1.6 to 2.0 mGy reported in the FH01 study [4]. It is consistent with the reported mean values of 2.0 mGy and 1.5 mGy for analogue and digital mammography equipment respectively used in the NHS Breast Screening Programme to screen women aged 47 years and older [25]. As expected, larger breasts received higher doses and this variation was similar to that reported for the older age group (Fig. 2). These results indicate that doses to women aged 35–39 in FH02 were comparable with those in the FH01 study and the NHS Breast Screening Programme.
Fig. 2.
Mean glandular dose per image as a function of breast thickness. FH02 refers to women aged 35–39 years in the present study and BSP refers to published data for older women in the NHS Breast Screening Programme [3]. Error bars show ± 2 standard errors.
3.1.4. Genetic Testing
Systematic genetic testing was only carried out in the Manchester cohort. Of 22 breast cancers 6 had a pathogenic BRCA1 variant, 4 in BRCA2 with 11 having no pathogenic variant identified and one remaining untested. Four BRCA1 carriers had been undergoing concurrent MRI but three of the lesions were also detected at mammography and the third was the 17mm DCIS found at risk reducing mastectomy. Only 19/854 (2.2%) in Manchester were known BRCA carriers at entry and an additional 16 at some point after entry. Of 23 other centres that submitted BRCA1/2 data only 28/1593 (1.76%) were know pathogenic variant carriers.
3.1.5. Projected Mortality
Of the breast cancer cases in POSH in the age group of FH02, 264 satisfied the FH02 risk criteria. Using the NPI formula for ten year survival as in FH01, we estimate that the FH02 cases will have 79% ten-year survival and the POSH cases will have 71% ten-year survival. Thus, we estimate a relative mortality of 21/29 = 0.72, with a 95% CI of (0.49–1.07), that is a 28% reduction in mortality associated with the surveillance activity in FH02. The study was not powered for this analysis, hence it does not reach statistical significance. As a sensitivity analysis, assuming that due to lead time, length bias etc. the hazard of death for the FH02 cases is underestimated by 10%, this would give an expected 77% survival in the FH02 cases, and a RR of 0.79, a 21% reduction in mortality.
4. Discussion
The present study shows that programme and test sensitivity (72% and 93%) of mammography screening in women aged 35–39 years of age at moderate and high-risk of breast cancer are similar to those in women aged 40–49 years in the FH01 study [4]. The overall and projected survival is substantially better than in comparable women aged 35–40 in the POSH study who did not undergo screening [13]. Overall 10-year survival in POSH was 73·4% (67·4–78·5) for BRCA1/2 pathogenic variant carriers versus 70·1% (67·7–72·3) for BRCA negative women [13]. This is very similar to our projected 71% survival from tumour characteristics. In contrast we estimate 10-year survival in invasive cases from FH02 to be 77–79%. Assessing only the invasive cases will underestimate the potential for saving lives from mammography screening. Whilst diagnosis of screen detected in situ disease is considered by many to be an ‘overdiagnosis’ this is very unlikely to be the case in women under 50 years of age. Long-term follow-up after cessation of screening in women < 50 showed no eventual excess of cancers [26], [27]. In particular most untreated high grade DCIS would relapse or transform to high grade invasive disease within 10 years if left untreated [28] and the majority of our DCIS were high grade (61.5%-8/13). Therefore successful identification of CIS in this age group is likely to prevent further deaths from invasive disease.
There are, of course, legitimate concerns regarding radiation exposure in young women that might induce a malignancy that may not otherwise occur. A meta-analysis revealed a non-significant increased risk of breast cancer among women at high-risk of breast cancer exposed to low-dose radiation OR = 1.3, (0.9–1.8) [29]. With exposure to a mean of ≥ 5 exposures giving an OR = 1.8 (1.1–3.0). The authors concluded that a careful approach was needed when using low-dose radiation in women at high-risk of breast cancer. We recently presented data showing no strong evidence for an increased risk in women receiving ≥ 5 mammograms before 40 years of age [30]. We have shown that radiation exposure in FH02 is not higher in this young aged group of women than in older women in routine screening in the UK. It is also reassuring that radiation doses are similar to older women in the UK national screening programme [25], and we do not appear to be doing excessive harm from overdiagnosis.
NICE guidelines in the UK currently recommend screening moderate and high-risk women aged 40–49 years of age with annual mammography [5]. A case could now be made to extend this to those aged 35–39 years. NICE did carry out a health economic evaluation to confirm that such screening aged 40–49 years with a 3% 10-year risk was cost effective based on the results of FH01 [4]. A large proportion of women who reach moderate risk because a single first degree relative (mother/sister) has been diagnosed with breast cancer aged < 40 years, would not meet a 3% 10-year risk until just before age 40 years using Tyrer-Cuzick v.8. Nevertheless, a life saved in a 38 year old would provide an extra 10 years of life expectancy compared to a similar woman aged 48 years and thus with similar potential gains in cure rates the cost effectiveness in 35–39 year olds may be met with a lower rate threshold. Otherwise women could become eligible once their 10-year risk reach 3% in the 35–39 year period, although this may mean that sisters with different reproductive risk factors gain entry to screening at different ages.
It could also be argued that testing all women at moderate or greater risk of breast cancer for BRCA1/2 pathogenic variants would have identified a large proportion of those who developed cancer in the 35–40 age group. The Manchester data suggest that perhaps half of the cancers could have occurred in BRCA1/2 carriers. However, none of these were from the moderate risk group. Currently only a small proportion of women at NICE defined high risk are covered by the NHS National Breast Screening Programme ‘Higher risk’ MRI screening. As such opening, mammography based screening to those at high and moderate risk based on just NICE algorithm would potentially benefit 3–4% of the population with this rising to 10–18% if risk algorithms incorporating mammographic density and single nucleotide polymorphism polygenic risk scores (SNP-PRS) were utilised [6], [31]. These may identify over 40% of the breast cancers in this age group if results can be replicated from the older screened population [31]. Screening of a multigene panel including high risk genes such as BRCA1 and BRCA2 will lift some women into an MRI screening category, but even with a negative BRCA1/2 test women with strong family histories still have high incidence rates aged < 50 years [32] Indeed testing of a SNP-PRS is likely to provide a better risk stratification in the familial population than panel testing [33].
There are some limitations to our study. The size is small, but was deliberately powered to assess whether the screening performance and sensitivity of the FH01 study could be replicated. Also, we have observed results on cancers diagnosed, but no results on survival or mortality, which instead was projected based on tumour attributes at diagnosis. The primary aim was therefore met in this exploratory study despite there being fewer cancers than predicted although greater numbers of cancers would be required to assess observed mortality. Further follow-up for actual mortality is a target for the future. A major strength is that the study involved 34 screening units showing that results can be replicated across many sites.
In conclusion we have shown that mammography screening in women at moderate and high risk of breast cancer aged 35–39 years has high sensitivity and detects the majority of cancer at low stage with good survival prospects. The Tyrer-Cuzick risk algorithm appears to predict cancer risk accurately and could be used as a ‘gatekeeper’ for entry to early mammography.
Acknowledgments
FH02 was funded by two grants from Breast Cancer Now (2013MayPR026). DGE and AH are supported by the NIHR Manchester Biomedical Research centre (IS-BRC-1215-20007).The POSH study was funded by CRUK grant C1275/A15956 The funders had no role in paper design, data collection, data analysis, interpretation or writing of the paper.
FH02 Study Group authors.
| Airedale NHS Foundation Trust | Claire Murphy | Helen Hothersall |
|---|---|---|
| Ardmillan Screening Centre, Edinburgh | Dr Lesley Smart | Lynda Luke |
| Belfast City Hospital | Dr Stuart McIntosh | Lesley McFaul |
| Birmingham | Mrs Simerjit Rai | Jenny Williamson |
| Bradford | Dr Richard Linforth | Helen Robertshaw |
| BTW Cardiff | Mr Dean Phillips and teams at screening centres across Wales | Dr Mark Rogers |
| BTW Llandudno | Dr Carrie Pottinger | |
| BTW Singleton | Dr Alex Murray | |
| Burton, Queen's Hospital | Dr Nick Luft | Jacqueline Elliott |
| Chester | Mrs Claudia Harding MacKean | Mary Aldous |
| Craigavon Area Hospital, Southern Health and Social Care Trust. Northern Ireland | Dr Cathy Farnon | Leanne McCourt |
| Derby | Mr Mark Sibbering | Wendy Chorley |
| Great Ormond Street/Barts | Dr Vian Salih | Dr Lucy Side |
| Grantham District Hospital | Mr Potdar | Lynn Osborne |
| Hope Hospital, Salford | Mr. S. Chatterjee | Kay Goulden |
| Kettering | Mr Rashed Margaret Turns |
Joanne Walsh |
| Leeds | Mr Philip Turton | Mrs Sue Hartup |
| Leicester | Dr Julian Barwell | Christine Masterson |
| Leighton Hospital, Crewe | Tracey Hale | Vanessa Adamson |
| Altnagelvin Hospital, Londonderry | Dr Michael Reilly | Celia Diver-Hall |
| Newcastle | Dr Alex Henderson | Irene Jobson |
| Northwick Park Hospital | Mr William Teh | |
| Nottinghamie Pottinger | Mr R.D Macmillan Lisa Brock |
Nicky Scott |
| Pilgrim Hospital Boston Lincs | Mr Potdar | Isobel Thomas |
| Portsmouth | Carmel Sheppard | Tracey Dobson |
| Royal Devon & Exeter | Di Cameron Pauline Sibley |
Mary Davies |
| Royal Liverpool University Hospital | Sue Holcombe | Laura Price |
| Royal Marsden Hospital, London | Mr Gerald Gui Dr. Janet Self |
Catherine Montgomery Suzanne England |
| Royal United Hospital, Bath | Dr Diana Dalgliesh | Katherine Knight |
| St Helen's & Knowsley NHS Trust. | Riccardo Audisio | Michelle Robinson |
| Southend University Hospital | Mr Neil Rothnie | Anne Mcpherson |
| Royal Cornwall Hospital, Truro. | Dr K Stepp | Helen Maguire |
| Ulster Hospital | Mr Stephen Kirk | Mrs Jennifer Foreman |
| Manchester Universities Hospital Foundation Trust | Rosemary Greenhalgh Jenny Affen Karen Tricker |
Sally Cole Julia Wiseman |
| University Hospitals Coventry & Warwickshire NHS Trust | Celia Lewis | |
| Wirral University Hospital Teaching Hospital | Ms M. Shaughnessy Alison McGenity |
Julie McEntee |
Footnotes
Outstanding questions: Longer term follow up of larger numbers is required to assess overall survival and any impact of radiation dose on future primary risk. Health Economic analysis should determine whether mammography screening in this age group should be limited to the high risk group or include those at moderate risk.
References
- 1.Evans D.G., Thomas S., Caunt J., Roberts L., Howell A., Wilson M. Mammographic surveillance in women aged 35–39 at enhanced familial risk of breast cancer (FH02) Familial Cancer. 2014;13(1):13–21. doi: 10.1007/s10689-013-9661-8. [DOI] [PubMed] [Google Scholar]
- 2.Mackay J., Rogers C., Fielder H. Development of a protocol for evaluation of mammographic surveillance services in women under 50 with a family history of breast cancer. J Epidemiol Biostat. 2001;6:365–369. [PubMed] [Google Scholar]
- 3.FH01 management committee, steering committee and collaborators The challenge of evaluating annual mammography screening for young women with a family history of breast cancer. J Med Screen. 2006;13:177–182. doi: 10.1177/096914130601300404. [DOI] [PubMed] [Google Scholar]
- 4.FH01 collaborative teams Mammographic surveillance in women younger than 50 years who have a family history of breast cancer: tumour characteristics and projected effect on mortality in the prospective, single-arm, FH01 study. Lancet Oncol. 2010;11:1127–1134. doi: 10.1016/S1470-2045(10)70263-1. [DOI] [PubMed] [Google Scholar]
- 5.McIntosh A., Shaw C., Evans G. Clinical guidelines and evidence review for the classification and care of women at risk of familial breast cancer, London: National Collaborating Centre for Primary Care/University of Sheffield. NICE guideline CG164. www.nice.org.uk [2013 updated 2017]
- 6.Evans D.G., Brentnall A.R., Harvie M., Dawe S., Sergeant J.C., Stavrinos P. Breast cancer risk in young women in the National Breast Screening Programme: implications for applying NICE guidelines for additional screening and chemoprevention. Cancer Prev Res. 2014;7(10):993–1001. doi: 10.1158/1940-6207.CAPR-14-0037. [DOI] [PubMed] [Google Scholar]
- 7.Wald N.J., Murphy P., Major P. UKCCCR multicentre randomised controlled trial of one and two view mammography in breast cancer screening. BMJ. 1995;311:1189–1193. doi: 10.1136/bmj.311.7014.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pisano E.D., Gatsonis C., Hendrick E. Diagnostic performance of digital versus film mammography for breast cancer screening. NEJM. 2005;353:1773–1783. doi: 10.1056/NEJMoa052911. [DOI] [PubMed] [Google Scholar]
- 9.Leach M.O., Boggis C.R., Dixon A.K. Screening with magnetic resonance imaging and mammography of a UK population at high familial risk of breast cancer: a prospective multicentre cohort study (MARIBS) Lancet. 2005;365:1769–1778. doi: 10.1016/S0140-6736(05)66481-1. [DOI] [PubMed] [Google Scholar]
- 10.Gilbert F.J., Astley S.M., Gillan M.G. Single reading with computer aided detection for screening mammography. NEJM. 2008;359:1675–1684. doi: 10.1056/NEJMoa0803545. [DOI] [PubMed] [Google Scholar]
- 11.Moss S.M., Cuckle H., Evans A., Johns L., Waller M., Bobrow L. Effect of mammographic screening from age 40 years on breast cancer mortality at 10 years' follow-up: a randomised controlled trial. Lancet. 2006;368:2053–2060. doi: 10.1016/S0140-6736(06)69834-6. [DOI] [PubMed] [Google Scholar]
- 12.Eccles D., Gerty S., Simmonds P., Hammond V., Ennis S., Altman D.G. POSH steering group. Prospective study of Outcomes in Sporadic versus Hereditary breast cancer (POSH): study protocol. BMC Cancer. 2007;7:160. doi: 10.1186/1471-2407-7-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Copson E., Maishman T.C., Tapper W.J., Cutress R.I., Greville-Heygate S., Altman D.G. The impact of germline BRCA mutation on outcome in young onset breast cancer - the POSH study. Lancet Oncol. 2018;19(2):169–180. doi: 10.1016/S1470-2045(17)30891-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Claus E., Risch N., Thompson W.D. Genetic analysis of breast cancer in the cancer and steroid hormone study. Am J Hum Genet. 1991;48:232–242. [PMC free article] [PubMed] [Google Scholar]
- 15.Tyrer J., Duffy S.W., Cuzick J. A breast cancer prediction model incorporating familial and personal risk factors. Stat Med. 2004;23:1111–1130. doi: 10.1002/sim.1668. [DOI] [PubMed] [Google Scholar]
- 16.Amir E., Evans D.G., Shenton A., Lalloo Evaluation of breast cancer risk assessment packages in the family history evaluation and screening programme. J Med Genet. 2003;40:807–814. doi: 10.1136/jmg.40.11.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eccles D.M., Evans D.G.R., Mackay J. Guidelines for a genetic risk based approach to advising women with a family history of breast cancer. J Med Genet. 2000;37:203–209. doi: 10.1136/jmg.37.3.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Day N.E., Walter S.D. Simplified models of screening for chronic disease: estimation procedures from mass screening programmes. Biometrics. 1984;43:1–13. [Pharoah P, Day NE, Duffy S et al Family history and the risk of breast cancer: a systematic review and meta-analysis Int J Cancer 1997;71:800-809] [PubMed] [Google Scholar]
- 19.Maurice A., Evans D.G.R., Shenton A., Boggis C., Wilson M., Duffy S. The screening of women aged less than 50 years at increased risk of breast cancer by virtue of their family history. Eur J Cancer. 2006;42:1385–1390. doi: 10.1016/j.ejca.2006.01.055. [DOI] [PubMed] [Google Scholar]
- 20.Maurice A., Evans D.G., Affen J., Greenhalgh R., Duffy S.W., Howell A. Surveillance of women at increased risk of breast cancer using mammography and clinical breast examination: further evidence of benefit. Int J Cancer. 2012;131:417–425. doi: 10.1002/ijc.26394. [DOI] [PubMed] [Google Scholar]
- 21.Day N., McCann J., Camilleri-Ferrante C. Vol. 2. Quality Assurance Management Group of the East Anglian Breast Screening Programme Med Screen; 1995. Monitoring interval cancers in breast screening programmes: the east Anglian experience; pp. 180–185. [DOI] [PubMed] [Google Scholar]
- 22.Moss S., Waller M., Anderson T.J., Cuckle H., Trial Management Group Randomised controlled trial of mammographic screening in women from age 40: predicted mortality based on surrogate outcome measures. Br J Cancer. 2005;92:955–960. doi: 10.1038/sj.bjc.6602395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bjurstam N., Bjorneld L., Duffy S.W. The Gothenburg breast screening trial: first results on mortality, incidence, and mode of detection for women ages 39–49 years at randomization. Cancer. 1997;80:2091–2099. [PubMed] [Google Scholar]
- 24.Paci E., Duffy S.W. Modelling the analysis of breast cancer screening programmes: sensitivity, lead time and predictive value in the Florence District Programme (1975–1986) Int J Epidemiol. 1991 Dec;20:852–858. doi: 10.1093/ije/20.4.852. [DOI] [PubMed] [Google Scholar]
- 25.Young K.C., Oduko J.M. Radiation doses received in the United Kingdom breast screening programme in 2010 to 2012. Br J Radiol. 2016;89 doi: 10.1259/bjr.20150831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hellquist B.N., Duffy S.W., Nyström L., Jonsson H. Overdiagnosis in the population-based service screening programme with mammography for women aged 40 to 49 years in Sweden. J Med Screen. 2012;19:14–19. doi: 10.1258/jms.2012.011104. [DOI] [PubMed] [Google Scholar]
- 27.Moss S.M., Wale C., Smith R., Evans A., Cuckle H., Duffy S.W. Effect of mammographic screening from age 40 years on breast cancer mortality in the UK age trial at 17 years' follow-up: a randomised controlled trial. Lancet Oncol. 2015 Sep;16(9):1123–1132. doi: 10.1016/S1470-2045(15)00128-X. [DOI] [PubMed] [Google Scholar]
- 28.Khan S., Epstein M., Lagios M.D., Silverstein M.J. Are we overtreating ductal carcinoma in situ (DCIS)? Ann Surg Oncol. 2017;24(1):59–63. doi: 10.1245/s10434-016-5501-z. [DOI] [PubMed] [Google Scholar]
- 29.Jansen-van der Weide M.C., Greuter M.J., Jansen L., Oosterwijk J.C., Pijnappel R.M., de Bock G.H. Exposure to low-dose radiation and the risk of breast cancer among women with a familial or genetic predisposition: a meta-analysis. Eur Radiol. 2010;20:2547–2556. doi: 10.1007/s00330-010-1839-y. [DOI] [PubMed] [Google Scholar]
- 30.Evans D.G., Kotre C.J., Harkness E., Wilson M., Maxwell A.J., Howell A. No strong evidence for increased risk of breast cancer 8–26 years after multiple mammograms in their 30 s in females at moderate and high familial risk. Br J Radiol. 2016;89(1059) doi: 10.1259/bjr.20150960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Veen E., Brentnall A.R., Byers H., Harkness E., Astley S., Sampson S. Improving classical breast cancer risk prediction with single nucleotide polymorphisms and mammographic density. JAMA Oncol. 2018;4(4):476–482. doi: 10.1001/jamaoncol.2017.4881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Evans D.G.R., Lennard F., Pointon L.J., Ramus S.J., Gayther S.A., Sodha N. Eligibility for MRI screening in the UK: effect of strict selection criteria and anonymous DNA testing on breast cancer incidence in the MARIBS study. Cancer Epidemiol Biomarkers Prev. 2009;18(7):2123–2131. doi: 10.1158/1055-9965.EPI-09-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Evans D.G., Brentnall A., Byers H., Harkness E., Stavrinos P., Howell A. The impact of a panel of 18 SNPs on breast cancer risk in women attending a UK familial screening clinic: a case-control study. J Med Genet. 2017;54(2):111–113. doi: 10.1136/jmedgenet-2016-104125. [DOI] [PubMed] [Google Scholar]