Supplemental Digital Content is available in the text
Keywords: artificial insemination, birth outcome, donor semen, pregnancy, sperm parameters
Abstract
Artificial insemination with donor sperm (AID) is a widely used procedure, but its success rate in China remains uncharacterized. This study investigated the factors associated with occurrence of clinical pregnancy and live birth and evaluated the birth outcomes in the offspring after AID in Northwest China.
We retrospectively reviewed the results of 1805 AID courses in 1046 couples during 2006–2015. We analyzed whether the number of AID cycles, age of the female patient, and number of sperm with progressive motility were associated with the occurrence of clinical pregnancy and live birth. We also evaluated the birth outcomes in the offspring.
Among the 1805 cycles, 447 (24.8%) resulted in clinical pregnancy and 384 (21.3%) resulted in a live birth. Miscarriage occurred in 57 of the 447 cases of clinical pregnancy (12.8%). The proportion of cycles resulting in a live birth decreased significantly with age (P < .001). The proportion of clinical pregnancies that resulted in miscarriage increased with age (P < .001). Cumulative pregnancy rate (the proportion of patients achieving a clinical pregnancy) increased progressively from 23.0% after 1 cycle to 42.7% after ≥5 cycles. The proportion of cycles resulting in clinical pregnancy did not vary with the total number of sperm with progressive motility administered per cycle. Multivariate logistic regression analysis revealed that superovulation treatment and number of cycles were factors associated with clinical pregnancy, while superovulation treatment, number of cycles, and patient age were factors associated with live birth. Among the 384 live births, only one case (0.3%) of birth defect (hexadactyly) was observed.
In patients undergoing AID, clinical pregnancy is associated with superovulation treatment and number of cycles, and live birth is associated with superovulation treatment, number of cycles, and patient age. The risk of birth defects in the offspring after AID is low.
1. Introduction
Male factors are responsible for nearly 40% of cases of infertility.[1] Artificial insemination with donor sperm (AID) is an assisted reproductive technology[2] used in many countries. AID involves the injection of donor sperm into the uterine cavity or cervix of the female to achieve in vivo fertilization.[3] In China, the use of AID is allowed in couples in whom the male partner has azoospermia, severe oligozoospermia, asthenospermia, teratozoospermia, or serious hereditary disease.[4] However, artificial insemination is not offered to women in China who do not have a male partner.[4]
The availability of AID has brought hope to many couples unable to conceive due to male factor infertility. The AID technique has undergone multiple improvements since its use in humans was first reported by William Pancoast in 1884,[5] and medical costs are lower for AID than for in vitro fertilization-embryo transfer (IVF-ET).[6] The success rate of AID is influenced by various factors, including female age, smoking, secondary infertility, progesterone level at the start of the cycle, and use of ovarian stimulation.[6] In one study, the factors associated with higher pregnancy rates after AID were age <35 years, ovarian stimulation, and the insemination of ≥5 million sperm with progressive motility.[7] Genetic screening of the donor and evaluation of his family history can also help to ensure the well-being of future offspring.[8] Previous studies have reported that neonates conceived using donor sperm are not at higher risk of preterm birth, low birth weight, or birth defects than neonates conceived spontaneously.[9] However, the parameters affecting pregnancy outcomes after AID remain largely uncharacterized in China. Therefore, the aim of this study was to identify factors associated with pregnancy and parturition in patients who received AID in Northwest China and evaluate the birth outcomes in the offspring.
2. Patients and methods
2.1. Patients
This was a retrospective analysis of all patients who underwent AID at the Reproductive Medicine Department, First Hospital Affiliated to Lanzhou University between June 2006 and December 2015. The patients were from five provinces in Northwest China (Gansu, Qinghai, Ningxia, Tibet, and Xinjiang), and the analysis included 1805 courses of AID in 1046 couples. All the included cases conformed to the requirements of Document No. 176 issued by the China National Health and Family Planning Commission in 2003.
The ethics committee of the First Hospital Affiliated to Lanzhou University approved this study (ethical approval number: LDYYLL2018-123). The ethics committee waived consent for inclusion in the analysis because the study was retrospective. However, all involved couples provided informed written consent before AID. Furthermore, each male partner among the couples seeking assisted conception provided additional written confirmation that he understood that intracytoplasmic sperm injection (ICSI) was an alternative technique (except in the case of irreversible azoospermia) that, if successful, would result in the birth of his biological child but had nonetheless decided to proceed using AID rather than ICSI.
The inclusion criteria for women receiving AID were as follows: 1) the uterus was well developed; 2) at least one fallopian tube was patent; and 3) the number of basal follicles in the bilateral ovaries was ≥3. The exclusion criteria were 1) polycystic ovary syndrome; 2) acute infection of the genitourinary system or sexually transmitted infection; 3) serious hereditary, physical, or psychiatric disorder; 4) prior exposure to potentially teratogenic radiation, toxins, or drugs; and 5) substance abuse.
The inclusion criteria for the male counterparts of the couples using AID were 1) irreversible azoospermia, severe oligozoospermia, asthenospermia, or teratozoospermia; 2) failed vasectomy; 3) ejaculatory disorder; 4) serious hereditary disease and/or family history of serious hereditary disease; and 5) genotype that would lead to maternal–fetal blood group incompatibility, precluding the birth of live neonates.
The inclusion criteria for the donors were 1) Chinese resident; 2) 20–45 years of age; 3) height ≥165 cm; 4) no infectious diseases; 5) no abnormalities detected on routine physical and male genital examinations; 6) no family history of genetic disorders; 7) normal chromosomes; 8) semen volume >2 mL/time; and 9) sperm concentration ≥60 × 106/mL and progressive motility ≥60% before freezing.
The Department of Public Health in China requires that all AID outcomes must be followed up. Measures for preventing loss to follow-up included: 1) recording of the patients’ addresses and contact phone numbers to allow regular communication and 2) follow-up of some patients by the community family planning service center (China has tight birth control measures, and a one-child policy was implemented from 1979 to 2015). In this study, the patients were followed-up throughout pregnancy, during delivery and for up to 1 year after delivery. No patients were lost to follow-up.
2.2. Female age, follicle monitoring, and time of artificial insemination
The natural ovulation cycle was determined according to previous menstrual cycles and ovulations. Superovulation treatment was used in patients with reduced ovarian reserve or follicular dysplasia. Superovulation treatment was used in 591 cycles and involved the administration of letrozole (Jiangsu Hengrui Pharmaceutical Co. Ltd, Lianyungang, China; n = 371, 62.8%), clomiphene citrate (Shanghai Hengshan Pharmaceutical Co. Ltd, Shanghai, China; n = 34, 5.8%), human menopausal gonadotropins (HMG; Lizhu Pharmaceutical Trading Co. Ltd, Zhuhai, China; n = 72, 12.2%), or HMG in combination with either clomiphene citrate or letrozole (n = 114, 19.3%). In most patients who attempted more than one cycle of AID, superovulation treatment involved the use of more than one of these methods.
Follicular development and endometrial thickness were monitored by transvaginal ultrasound at 8–12 days after menstruation. Human chorionic gonadotropin (HCG; 5000 IU; Lizhu Pharmaceutical Trading Co. Ltd, Zhuhai, China) was injected if the following conditions were met: 1) dominant follicle diameter ≥16 mm, positive result for the luteinizing hormone (LH) test, and an increase in blood LH level of 2–3 times the basal level or 2) dominant follicle diameter ≥18 mm, serum estradiol (E2) >200 pg/mL, no increase in blood LH level, or luteinized unruptured follicle (LUF) syndrome in previous cycles. Conventional artificial insemination was performed 24 h later. B-ultrasonography was performed the following day in patients without follicular discharge. Artificial insemination was repeated if the dominant follicle had been discharged.
2.3. Sperm sources and method of preparation
AID was achieved by intrauterine insemination of sperm. Frozen sperm were provided by three Chinese human sperm banks, namely, the Human Sperm Bank of the Reproductive and Genetic Hospital of CITIC-Xiangya, Zhejiang Provincial Human Sperm Bank, and Human Sperm Bank of the First Hospital of Lanzhou University. The sperm had been stored in liquid nitrogen for at least 6 months and reinspected for quality. Eligible samples were transported to our institution for use.
Cryopreservation tubes were removed from liquid nitrogen, held at room temperature for 30 s (to allow evaporation of any liquid nitrogen on the outer surface of the tube), and then placed in a water bath or incubator at 37°C for 15–20 min to allow thawing to occur. Next, the semen sample was added to SpermGrad-30 (upper layer: 2 mL of 80% SpermGrad-30; lower layer: 2 mL of 40% SpermGrad-30; Vitrolife, Göteborg, Sweden) in a 15-mL conical centrifuge tube, and the mixture was centrifuged at 300 g for 15 min at room temperature. The supernatant was discarded, and the resulting pellet was resuspended in 2 mL of Semen G-IVF PLUS (Vitrolife, Göteborg, Sweden) and centrifuged at 100 g for 5 min. This resuspension/centrifugation step was repeated, and the resulting sperm pellet was resuspended in G-IVF PLUS medium to a final volume of 0.5 mL. The samples were incubated at 36.5°C in 5% CO2 until further use. Total progressive sperm count was calculated as sperm concentration × progressive sperm% × 0.5.
2.4. Clinical follow-up
Urine and blood levels of HCG were measured 16 days after AID at the First Hospital Affiliated to Lanzhou University (where all the female patients underwent AID). Females with a biochemical diagnosis of pregnancy were examined by ultrasonography 14 days later to establish whether there was clinical pregnancy. Patients with intrauterine pregnancy were followed-up at 5 time points: at the beginning of the first, second, and third trimesters, at delivery, and 1 year after delivery.
2.5. Statistical analysis
Data analysis was performed using SPSS 19.0 (SPSS Inc., Chicago, IL, USA). Measurement data, which were assessed for normality by the Shapiro–Wilk test, are expressed as mean ± standard deviation. Numeration data are expressed as rate, and inter-group comparisons were made using the chi-squared test. Statistical significance was established at P < .05. Multivariate logistic regression analysis, with calculation of odds ratios (ORs) and 95% confidence intervals (95%CIs), was carried out to identify factors associated with clinical pregnancy and live birth.
3. Results
3.1. Treatment using AID
A total of 1046 couples received 1805 AID treatment cycles. The 1046 females were aged between 20 and 44 years, with a mean age of 28.6 ± 4.0 years, and none were active smokers. Superovulation treatment was used in 591 of the 1805 AID cycles (32.7%). The 1805 cycles resulted in 447 clinical pregnancies (24.8%), including 18 dizygotic twin pregnancies. Miscarriage occurred in 57 of the 447 cases (12.8%) of clinical pregnancy (Table 1). Single intrauterine insemination was performed in 448 cycles and led to a clinical pregnancy in 90 cases (20.1%). Double intrauterine insemination was performed in 1357 cycles and resulted in a clinical pregnancy in 357 cases (26.3%). There were no significant differences in the proportion of cycles that achieved clinical pregnancy between subgroups based on the age of the female (Table 1). However, only 20 cycles were performed in women aged ≥40 years, and the proportion of cycles that resulted in clinical pregnancy was numerically much lower for these women (10.0%) than for younger women (23.4%–26.2%; Table 1). The miscarriage rate (as a proportion of clinical pregnancies) varied significantly with the age of the female patient (P < .001; Table 1) and was highest in females aged 35–39 years (40.6%). There was no clear difference in the proportion of cycles achieving clinical pregnancy between patients given superovulation treatment and those that were not (Table S1).
Table 1.
Pregnancy and parturition rates according to the women's ages.

3.2. Cumulative pregnancy after AID
For the analysis, the patients were divided into 5 groups based on the number of treatment cycles received (1, 2, 3, 4, or ≥5). The proportion of cycles that achieved clinical pregnancy and live birth varied with the number of treatment cycles received (P < .01; Table 2). Use of a single treatment cycle resulted in the lowest success (clinical pregnancy and live birth achieved in 23.0% and 19.5% of cycles, respectively), while the use of ≥5 cycles was associated with the highest success (clinical pregnancy and live birth achieved in 47.4% and 42.1% of cycles, respectively). Furthermore, the cumulative pregnancy rate (proportion of all patients achieving a clinical pregnancy) increased progressively from 23.0% after 1 cycle to 42.7% after ≥5 cycles (Table 2).
Table 2.
Cumulative pregnancy according to the number of AID cycles.

3.3. Effect of the total number of sperm with progressive motility injected per cycle on pregnancy rate
Intrauterine insemination was used for all AID cycles in this study. The total number of sperm with progressive motility injected per cycle was >12 × 106. There were no significant differences in pregnancy rates between subgroups based on the total number of sperm with progressive motility administered per cycle (Table 3).
Table 3.
Pregnancy rate according to total progressive sperm count per cycle.

3.4. Multivariate logistic regression analysis of factors associated with clinical pregnancy and parturition
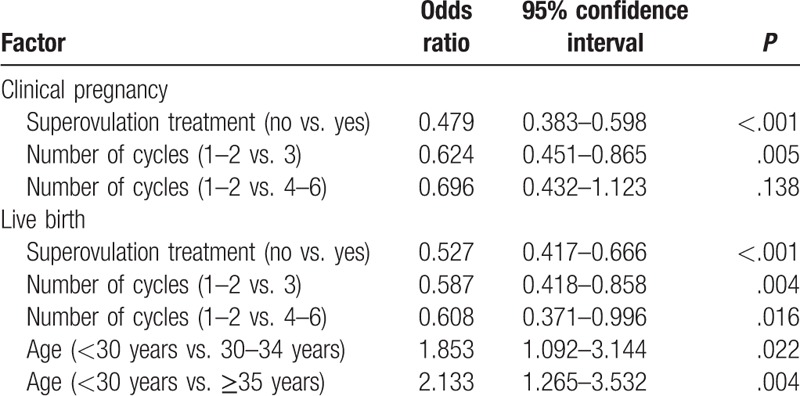
Factors significantly associated with clinical pregnancy (Table 4) were superovulation treatment (no vs. yes: OR, 0.479; 95%CI, 0.383–0.598; P < .001) and number of AID cycles (1–2 vs. 3: OR, 0.624; 95%CI, 0.451–0.865; P < .01). Factors significantly associated with live birth (Table 4) included superovulation treatment (no vs. yes: OR, 0.527; 95%CI, 0.417–0.666; P < .001), number of AID cycles (1–2 vs. 3: OR, 0.587; 95%CI, 0.418–0.858; P < .01), and age of the female patient (<30 years vs. ≥35 years: OR, 2.133; 95%CI, 1.265–3.532; P < .01).
Table 4.
Logistic regression analysis of factors associated with clinical pregnancy and live birth.
3.5. Mode of delivery and birth defects
There were 447 cases of clinical pregnancy after AID, including 57 cases of miscarriage (12.8%), 2 cases of ectopic pregnancy (0.4%), 3 cases of second trimester induction of labor, and 1 case of fetal death (stillbirth at a gestational age of 38 weeks plus 5 days). The reasons for second trimester induction of labor were one case each of trisomy-21 syndrome (male fetus), cleft lip and palate (female fetus), and pregnancy-induced hypertension. The one case of fetal death occurred in a male fetus who showed no obvious signs of deformity. There were 384 live births, with 188 babies delivered by caesarean section (49.0%) and 196 by natural birth (51.0%). Approximately 6% of all births were premature (delivery after 28 weeks of pregnancy but before 37 weeks). One of the liveborn babies had hexadactyly of the left hand and no other abnormalities; neither the female patient receiving AID nor the donor had hexadactylism.
3.6. Birth weights of the liveborn babies
The 384 live births resulted in a total of 402 babies being born (366 singleton babies and 18 pairs of dizygotic twins). There were 217 (54%) male offspring and 185 (46%) female offspring. The average birth weights of the 217 male and 185 female offspring were 3205 ± 358 g and 3173 ± 439 g, respectively.
4. Discussion
An important finding of this retrospective study was that the proportion of AID cycles resulting in a live birth decreased as the age of the female patient increased. Furthermore, clinical pregnancy and live birth were achieved in a higher proportion of cycles when at least 3 cycles of AID were used. Most patients in this study were aged < 35 years, and only 20 cycles were administered to women aged ≥40 years. The proportion of clinical pregnancies resulting in early miscarriage was notably higher in patients aged 35–39 years than in younger women. Interestingly, only 10.0% of cycles achieved a clinical pregnancy in females aged ≥40 years. Although no miscarriages were observed in patients aged ≥40 years, this should be interpreted with caution due to the low number of pregnant women in this group. Collectively, our findings suggest that female age is an important factor influencing pregnancy outcome after AID, in agreement with previous research by Ferrara et al.[10] and Botchana et al.[11] Increasing age is associated with an acceleration of follicular atresia, a reduction in ovarian reserve, a decrease in oocyte quality, and a decline in fertility.[12] Hence, female age is one of the most important factors affecting the success of artificial insemination.
In this study, 1046 females received 1805 cycles of AID, with each patient administered between 1 and 6 treatment cycles. The cumulative clinical pregnancy rate increased markedly with the number of treatment cycles up to 4 cycles, but increasing the number of cycles beyond 4 was without any obvious additional benefit. Song et al.[13] reported that the cumulative pregnancy rate increased with the number of cycles used, reaching 41.1% after 3 cycles. Guan et al.[14] also observed a progressive increase in cumulative live birth rate to a value of 24.9% after 5 cycles, with no further improvement after additional treatment cycles. Other research also supports the use of multiple AID treatment cycles. For example, Khalil et al.[15] and Vitthala et al.[16] found that the cumulative live birth rate rose gradually as the number of cycles increased from 1 to 6, reaching values of around 60% after 6 cycles. Guerif et al.[17] studied a treatment course consisting of up to 12 cycles of AID and found that the cumulative live birth rate reached 52.6% after the seventh cycle but did not notably increase after subsequent cycles. The findings of the present study are broadly consistent with those of Song et al.[13] but differ from those of Guerif et al.[17] The latter study analyzed more AID cycles than the present investigation, and the discrepancy between our results and those of Guerif et al.[17] might be due to the following factors. 1) The traditional views of Chinese people place great pressure on couples to conceive; thus, patients who had undergone a certain number of unsuccessful AID treatment cycles would likely consider other approaches thought to have higher success rates. 2) In China, medical costs are the responsibility of the individual who is receiving the treatment; therefore, multiple attempts at AID impose a substantial financial burden on families. 3) There were relatively few patients who received more than 3 cycles of AID in our study, necessitating further investigations in China with an expanded sample size.
Repeated AID failure places both psychological and financial burdens on patients. Conception is a complex physiological process, and factors that might contribute to infertility should be considered when repeated failure of AID occurs. For example, a greater number of sperm with forward motility,[14] a higher number of mature follicles during ovarian stimulation,[18] and good endometrial receptivity[19] are all thought to contribute to higher rates of pregnancy. Other factors potentially involved include abnormal fertilization mechanisms,[20] the special response of the endometrial implantation window, gamete abnormalities, zygote dysplasia, and abnormalities of the immune system.[21] In this study, assisted reproductive therapy was unsuccessful in 109 patients who received multiple cycles of AID treatment even after transfer to IVF-ET. Among these 109 patients, 4 females had chromosomal abnormalities and 2 showed follicular dysplasia.
As pointed out in Document No. 176 issued by the China National Health and Family Planning Commission in 2003, semen specimens used for intrauterine insemination should contain ≥12 × 106 sperm with forward motility. In the current study, all the semen samples provided by the sperm banks reached this standard and were eligible for intrauterine insemination. The proportion of cycles that resulted in clinical pregnancy did not vary with total number of sperm with forward motility, suggesting that beyond a certain threshold level, a further increase in sperm number does not significantly improve the odds of pregnancy after AID. This conclusion would be consistent with the findings of Pittrof et al.,[22] who suggested a cutoff value of 1.1 × 106 for the number of sperm needed for artificial insemination. However, some controversy remains. Guan et al.[14] and Liu et al.[23] concluded that pregnancy rate increased significantly with the total number of sperm with forward motility in the semen. Multicenter studies with large sample sizes are needed to better characterize the effects of sperm parameters on AID outcome.
China has a high rate of cesarean section. Cesarean section was used to deliver nearly half the 16 million babies born in China in 2010,[24] and this rate is much higher than that recommended as ideal by the World Health Organization (10%–15%).[25] In this study, 188 of the 384 live births (49.0%) were delivered by caesarean section, in line with other findings in China. There are several possible reasons for the high cesarean section rate in China: 1) cesarean section is a reliable method of delivery, and postoperative recovery is good; 2) economic development in China has led to cesarean section being affordable to many couples; 3) cesarean section avoids certain obstetric complications that can occur during natural birth such as those arising from malposition or cephalopelvic disproportion; 4) most Chinese couples choose to have only one child because of family planning policy, and many of these couples select cesarean section as the delivery method due to the perceived risks and uncertainties associated with natural labor; and 5) the social status of females in China has increased in recent years, empowering women to make decisions regarding the management of pain during childbirth, including the decision to give birth by cesarean section rather than by natural delivery. At present, the Chinese Ministry of Health promotes natural childbirth. Furthermore, China implemented a Two-Children Policy in 2016, so the rate of cesarean section may change in the future.
The average weights of the male and female offspring born after AID did not differ notably from median birth weights published in June 2009 by the National Health and Family Planning Commission of China (“Growth development reference value for Chinese children under 7 years of age”). This would suggest that children born after AID have comparable birth weights to children born after a natural pregnancy.
The well-being of neonates, infants, and children is a major public health issue. According to the Chinese Ministry of Health in 2012, approximately 900,000 children are born with birth defects in China each year, and the overall rate of birth defects is about 5.6%. International surveys have reported birth defect rates after AID of 0.9%–5.4%, similar to rates seen after natural pregnancies.[3,26] The regions in which birth defect rates have been investigated may have special characteristics. Taking Lanzhou as an example, the birth defect rate varied between 0.86 and 1.36 between 2010 and 2014[27]; the most common defects were cleft lip and palate, congenital heart disease, multiple malformation syndrome, and polydactyly. Among the live births in this study, there was 1 case of hexadactyly and 2 cases in which labor was induced, representing 0.7% of all fetuses from conception to the second trimester. This corroborates previous findings that AID does not increase the risk of birth defects in the offspring.
This study has some limitations. First, this study was retrospective and therefore subject to inherent shortcomings. Second, sample sizes were relatively small for some categories, which affected the multivariate analyses. Third, forward progressive sperm count was the only parameter available to us for the analysis, so the possible influence of other measures of semen quality on outcomes could not be assessed. Therefore, prospective studies with larger sample sizes are required to properly define which factors are associated with AID outcome.
In summary, semen samples provided by domestic human sperm banks yielded an acceptable clinical pregnancy rate, with pregnancy outcome after AID closely correlated with female age. AID should be repeated for at least 3 cycles in cases of nonsuccess, as this will increase the cumulative pregnancy rate. Furthermore, successful pregnancy after AID was not associated with an increased rate of birth defects. Therefore, AID is reliable and effective as an assisted reproductive technology.
Author contributions
Conceptualization: Aiping Zhang, Xuehong Zhang, Weihua Wang.
Data curation: Aiping Zhang, Xiaoling Ma, Lili Zhang.
Formal analysis: Aiping Zhang, Xiaoling Ma, Lili Zhang, Xuehong Zhang, Weihua Wang.
Project administration: Xuehong Zhang, Weihua Wang.
Writing – original draft: Aiping Zhang.
Writing – review & editing: Xiaoling Ma, Lili Zhang, Xuehong Zhang, Weihua Wang.
Supplementary Material
Footnotes
Abbreviations: AID = artificial insemination with donor sperm, E2 = estradiol, HMG = human menopausal gonadotropins, ICSI = intracytoplasmic sperm injection, IVF-ET = in vitro fertilization-embryo transfer, LH = luteinizing hormone, LUF = luteinized un-ruptured follicle, PCOS = polycystic ovary syndrome.
Conflict of interests: The authors declare that they have no conflicts of interest.
Supplemental Digital Content is available for this article.
References
- [1].Kumar N, Singh AK. Trends of male factor infertility, an important cause of infertility: a review of literature. J Hum Reprod Sci 2015;8:191–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Ombelet W, Van Robays J. Artificial insemination history: hurdles and milestones. Facts Views Vis Obgyn 2015;7:137–43. [PMC free article] [PubMed] [Google Scholar]
- [3].Lansac J, Royere D. Follow-up studies of children born after frozen sperm donation. Hum Reprod Update 2001;7:33–7. [DOI] [PubMed] [Google Scholar]
- [4].Sun Y, Gregersen H, Yuan W. Chinese health care system and clinical epidemiology. Clin Epidemiol 2017;9:167–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Gregoire AT, Mayer RC. The Impregnators. (William Pancoast), (Addison Davis Hard). Fertil Steril 1965;16:130–4. [PubMed] [Google Scholar]
- [6].Thijssen A, Creemers A, Van der Elst W, et al. Predictive factors influencing pregnancy rates after intrauterine insemination with frozen donor semen: a prospective cohort study. Reprod Biomed Online 2017;34:590–7. [DOI] [PubMed] [Google Scholar]
- [7].Zuzuarregui JL, Meseguer M, Garrido N, et al. Parameters affecting the results in a program of artificial insemination with donor sperm. A 12-year retrospective review of more than 1800 cycles. J Assist Reprod Genet 2004;21:109–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Isley L, Callum P. Genetic evaluation procedures at sperm banks in the United States. Fertil Steril 2013;99:1587–91. [DOI] [PubMed] [Google Scholar]
- [9].Adams DH, Clark RA, Davies MJ, et al. A meta-analysis of sperm donation offspring health outcomes. J Dev Orig Health Dis 2017;8:44–55. [DOI] [PubMed] [Google Scholar]
- [10].Ferrara I, Balet R, Grudzinskas JG. Intrauterine insemination with frozen donor sperm. Pregnancy outcome in relation to age and ovarian stimulation regime. Hum Reprod 2002;17:2320–4. [DOI] [PubMed] [Google Scholar]
- [11].Botchan A, Hauser R, Gamzu R, et al. Results of 6139 artificial insemination cycles with donor spermatozoa. Hum Reprod 2001;16:2298–304. [DOI] [PubMed] [Google Scholar]
- [12].Nelson SM, Telfer EE, Anderson RA. The ageing ovary and uterus: new biological insights. Hum Reprod Update 2013;19:67–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Song G, Zheng WW, Zhong XY, et al. Analysis of the parameters affecting the pregnancy rate and clinical value of artificial insemination by donor. Reprod Contracep 2014;34:410–09. [Google Scholar]
- [14].Guan HT, Zheng Y, Wang JJ, et al. Relationship between donor sperm parameters and pregnancy outcome after intrauterine insemination: analysis of 2821 cycles in 1355 couples. Andrologia 2016;48:29–36. [DOI] [PubMed] [Google Scholar]
- [15].Khalil MR, Rasmussen PE, Erb K, et al. Intrauterine insemination with donor semen. An evaluation of prognostic factors based on a review of 1131 cycles. Acta Obstet Gynecol Scand 2001;80:342–8. [DOI] [PubMed] [Google Scholar]
- [16].Vitthala S, Gelbaya TA, Hunter H, et al. Stimulated intrauterine insemination (SIUI) and donor insemination (DI) as first line management for a selected subfertile population: the Manchester experience. J Assist Reprod Genet 2008;25:431–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Guerif F, Fourquet F, Marret H, et al. Cohort follow-up of couples with primary infertility in an ART programme using frozen donor semen. Hum Reprod 2002;17:1525–31. [DOI] [PubMed] [Google Scholar]
- [18].van Rumste MM, Custers IM, van der Veen F, et al. The influence of the number of follicles on pregnancy rates in intrauterine insemination with ovarian stimulation: a meta-analysis. Hum Reprod Update 2008;14:563–70. [DOI] [PubMed] [Google Scholar]
- [19].Heger A, Sator M, Pietrowski D. Endometrial receptivity and its predictive value for IVF/ICSI-outcome. Geburtshilfe Frauenheilkd 2012;72:710–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kuczynski W, Dhont M, Grygoruk C, et al. Rescue ICSI of unfertilized oocytes after IVF. Hum Reprod 2002;17:2423–7. [DOI] [PubMed] [Google Scholar]
- [21].Swain JE, Pool TB. ART failure: oocyte contributions to unsuccessful fertilization. Hum Reprod Update 2008;14:431–46. [DOI] [PubMed] [Google Scholar]
- [22].Pittrof RU, Shaker A, Dean N, et al. Success of intrauterine insemination using cryopreserved donor sperm is related to the age of the woman and the number of preovulatory follicles. J Assist Reprod Genet 1996;13:310–4. [DOI] [PubMed] [Google Scholar]
- [23].Liu J, Wen J, Wang B, et al. Effects of sperm-treated methods and total motile sperm count on pregnancy outcome. Chin J Birth Health & Heredity 2006;33:83. [Google Scholar]
- [24].Hellerstein S, Feldman S, Duan T. China's 50% caesarean delivery rate: is it too high? BJOG 2015;122:160–4. [DOI] [PubMed] [Google Scholar]
- [25].World Health Organization. WHO Statement on Caesarean Section Rates. WHO reference number: WHO/RHR/15.02, 2015. Available at: https://www.who.int/reproductivehealth/publications/maternal_perinatal_health/cs-statement/en/. [Google Scholar]
- [26].Parazzini F, Cipriani S, Bulfoni G, et al. The risk of birth defects after assisted reproduction. J Assist Reprod Genet 2015;32:379–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Pan L, Liang F, Chang RX, et al. Analysis of the Perinatal Birth Defects in Lanzhou from 2010 to 2014. Chinese Primary Health Care 2016;30:36–8. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


