Abstract
Diffuse white matter (WM) response to traumatic brain injury (TBI) and transplantation of human bone marrow stromal cells (hMSCs) after the injury were non-invasively and dynamically investigated. Male Wistar rats (300-350g) subjected to TBI were intravenously injected with 1 ml of saline (n=10) or with hMSCs in suspension (~3×106 hMSCs, n=10) 1-week post-TBI. MRI measurements of T2-weighted imaging and diffusional kurtosis imaging (DKI) were acquired on all animals at multiple time points up to 3-months post-injury. Functional outcome was assessed using the Morris water maze test. DKI-derived metrics of fractional anisotropy (FA), axonal water fraction (AWF) and radial kurtosis (RK) longitudinally reveal an evolving pattern of structural alteration post-TBI occurring in the brain region remote from primary impact site. The progressive structural change is characterized by gradual disruption of WM integrity at an early stage (weeks post-TBI), followed by spontaneous recovery at a later stage (months post-TBI). Transplantation of hMSCs post-TBI promotes this structural plasticity as indicated by significantly increased FA and AWF in conjunction with substantially elevated RK at the later stage. Our long-term imaging data demonstrate that hMSC therapy leads to modified temporal profiles of these metrics, inducing an earlier presence of enhanced structural remodeling, which may contribute to improved functional recovery.
Keywords: Traumatic brain injury, Bone marrow stromal cells, structural change, DKI, MRI
1. Introduction
As a leading cause of mortality and disability in the young population worldwide, traumatic brain injury (TBI) has been extensively studied in pre-clinical (Aertker et al., 2016; Schouten et al., 2004; Xiong et al., 2018) and clinical (Aertker et al., 2016; Cox et al., 2017; Harting et al., 2008) settings. Although the search for treatments that can effectively improve outcomes in patients with TBI is underway (Xiong et al., 2015), remarkable advancement has been attained in understanding of biological and pathogenic mechanisms underlying the disease (Aertker et al., 2016; Xiong et al., 2018). Robust data demonstrate that the main pathological hallmarks of TBI include neuronal cell death and white matter (WM) damage that, following primary insult, progressively affect the injured brain and evoke neurological deficits (Gold et al., 2013; Schouten et al., 2004). Unfortunately, none of previous pharmacological approaches has achieved their goals to impede TBI-triggered neurological deficits, known as secondary brain injury, and to improve functional recovery (Parr et al., 2007; Sharma et al., 2015; Xiong et al., 2015).
Cell therapy, with the potential to induce brain plasticity and WM repair (Gold et al., 2013; Maegele and Schaefer, 2008; Schouten et al., 2004; Xiong et al., 2010; Xiong et al., 2018), offers an alternative to traditional pharmacological strategies. Cell engraftment, such as transplantation of human bone marrow stromal cells (hMSCs), exerts therapeutic effects in treatment of several central nervous system (CNS) disorders, including TBI (Chopp and Li, 2002; Darkazalli et al., 2016; Jiang et al., 2011; Li et al., 2012). It has been increasingly recognized that the protective and regenerative action provided by cell administration after TBI, which attenuates WM degeneration, plays an important role in neurological functional improvement (Pati et al., 2016; Rolfe and Sun, 2015). However, how this promising therapy dynamically and diffusely impacts the integrity of WM in the injured brain, thereby contributing to the reduction of post-TBI behavioral dysfunction, remains yet to be largely explored.
The changes of WM integrity, arising from brain pathology (e.g. TBI) and in response to therapeutic intervention (e.g. cell engraftment), have been investigated using diffusion tensor imaging (DTI) (Bosnell et al., 2008; Farbota et al., 2012; Jiang et al., 2011; Li et al., 2017b). As an extension of DTI, diffusional kurtosis imaging (DKI) offers additional information to quantify the non-Gaussian diffusion effects that occur in the brain as a result of its microstructural complexity (Benitez et al., 2014; Jensen et al., 2005; Jensen and Helpern, 2010). By offering both DTI-compatible metrics and diffusional kurtosis estimates with increased sensitivity and specificity (Cheung et al., 2009; Douglas et al., 2015; Hui et al., 2008; Spampinato et al., 2017a; Stokum et al., 2015; Umesh Rudrapatna et al., 2014; Veraart et al., 2011), DKI permits characterization of WM damage such as axonal loss (Benitez et al., 2014; Falangola et al., 2014; Fieremans et al., 2011) and myelin breakdown (Falangola et al., 2014; Guglielmetti et al., 2016; Spampinato et al., 2017b) related to neurodegenerative processes. With more precise in vivo detection of tissue microstructural changes by acquiring information not available to conventional DTI, DKI can provide deeper insight into the mechanisms underlying WM alterations associated with the brain injury as well as the impact of treatment strategies.
Growing preclinical evidence obtained from functional assessments indicates that a long-term observation, i.e. a minimum survival period of 2-months post-TBI, is needed to reveal the course of injury and for examining the effects of cell-based therapy (Gold et al., 2013). However, very few prior rodent TBI studies meet this time frame requirement (< 10%) (Gold et al., 2013; Mahmood et al., 2006), particularly for in vivo imaging studies on cell therapy (Li et al., 2017a; Skardelly et al., 2011). Regarding the diffuse structural alteration that directly results from a focal insult, there is a strong need to have a prolonged observation period which provides information on the temporal profiles of cerebral microstructural changes after TBI and in response to cell therapy.
With DKI-derived multiple metrics that reflect the specific aspects of tissue structural status, the purpose of this study was to longitudinally probe WM alteration after TBI during an extended period of in vivo observation time (3-months post-TBI), and to demonstrate the effects of hMSC administration following TBI on WM integrity. Our investigation focused on the non directly injured cerebral region in the brain, which may experience diffuse axonal injury (DAI) (Grassi et al., 2018; Meythaler et al., 2001; Perez et al., 2014) and on the therapeutic effects of hMSC therapy on this DAI (Al Jumah and Abumaree, 2012; Li et al., 2011; Li et al., 2017a).
2. Results
2.1. Temporal profiles of FA in CC+EC and cortex regions
Fig. 2 shows the dynamic changes of FA with time after TBI in CC+EC (Fig. 2A) and cortical (Fig. 2B) regions. With or without cell treatment, TBI resulted in a significant reduction of FA from the pre-injury status in CC+EC region, followed by a reversal of FA with different temporal profiles for the cell- and saline-treated groups (Fig. 2A). The reduction of FA persisted throughout the experimental period in the saline-treated group. In contrast, the significantly decreased FA was absent in the cell-treated group at 2-months and 3-months after TBI. Compared to saline administration, cell treatment led to an earlier and more rapid recovery of FA (starting from 3-weeks for the cell-treated group vs. 2-months for the saline-treated group). Statistical group differences were found at 5-weeks and 3-months post-TBI with significantly higher FA present in the cell-treated group than in the saline-treated group. Regardless of cell or saline intervention, however, similar temporal profiles of FA were shown in the cortex region with no significant difference between two groups at every observation time point and no evident changes with time after TBI compared to the uninjured status for each group (Fig. 2B).
Fig. 2. Temporal profiles of FA in CC+EC (A) and cortex (B) regions.
Compared to the preinjury, a significant reduction of FA after TBI in CC+EC region (A) is observed in both groups. This situation persists in the saline-treated group throughout the experimental period, whereas the significantly decreased FA is absent in the cell-treated group at later stage (2-months and 3-months) due to an earlier and greater recovery of FA (starting from 3-weeks for the cell-treated group vs. 2-months for the saline-treated group). Statistical group differences are found at 5-weeks and 3-months post-TBI. Regardless of cell or saline intervention, however, similar temporal profiles of FA are shown in cortex region (B) with no significant differences between two groups at all time points and no significant changes with time after TBI compared to the preinjury status for each group.
2.2. Temporal profiles of AWF in CC+EC region
As demonstrated in Fig. 3, TBI resulted in dynamic changes of AWF. In the CC+EC regions of saline- and cell-treated groups, the temporal profiles of AWF exhibited an initial decline and then recovery to some extent at later stage after TBI with a significant decreased AWF from the pre-injury status being observed between 2-weeks to 3-weeks post-TBI. Compared to saline administration, cell treatment led to an earlier and greater reversal of AWF (starting from 3-weeks for the cell-treated group vs. 5-weeks for the saline-treated group). Statistical group differences were observed at 3-weeks, 2-months and 3-months post-TBI with significantly higher AWF being detected in the cell-treated group than in the saline-treated group.
Fig. 3. Temporal profiles of AWF in CC+EC region.
After TBI, the significant decrease of AWF at an earlier stage (2-weeks to 3-weeks) and reversal of AWF at a later stage in CC+EC region are present in both groups. Cell administration results in an earlier and stronger recovery of AWF (starting from 3-weeks for the cell-treated group vs. 5-weeks for the saline-treated group). Statistical group differences are found at 3-weeks, 2-months and 3-months post-TBI.
As a WM tract integrity (WMTI) metric, AWF evaluation was not performed in the cortical region.
2.3. Temporal profiles of Kurtosis assessments in CC+EC and cortex regions
Changes of Kurtosis metrics with and without cell transplantation after TBI are shown in Fig. 4. In the CC+EC region (Fig. 4A-4C), higher MK values (Fig. 4A) in the cell-treated group than in the saline-treated group were observed at most observation time points with significant group differences being found at 3-weeks post-TBI. Although MK tended toward increase for both groups, particularly at later stage after TBI, significantly elevated MK compared to the pre-injury was only present in the cell-treated group (Fig. 4A, 2-months post-TBI). TBI resulted in an overall increased AK (Fig. 4B) in the CC+EC region that persisted for the entire observation period regardless of cell or saline intervention. Compared to saline administration after TBI, cell transplantation led to a different temporal profile pattern of RK (Fig. 4C) by substantially reversing the decline of RK in the CC+EC region at later stage of TBI despite the dramatically decreased RK present in both groups observed at the earlier stage of TBI (1-weeks to 3-weeks post-TBI). In the cortex (Fig. 4D-F), however, similar temporal profiles for the cell- and saline-treated groups in these separate Kurtosis metrics (i.e., MK (Fig. 4D), AK (Fig. 4E) and RK (Fig. 4F)) were found with no significant difference between two groups at each observation time point and no significant changes with time after TBI compared to the pre-injury status for each group.
Fig. 4. Changes of Kurtosis in CC+EC (A-C) and cortex (D-F) regions.
In CC+EC region (A-C), higher MK (A) values in the cell-treated group than in the saline-treated group at most observation time points exhibit with significant group difference present at 3-weeks post-TBI. Compared to the pre-injury, significantly increased MK at a later stage of TBI is found in the cell-treated group (A, 2-momths post-TBI). TBI leads to increased AK (B) during the experimental period regardless of cell or saline intervention afterwards, while cell engraftment after TBI substantially reverses the reduction of RK (C) at the later stage of TBI despite the dramatically decreased RK present in both groups at the earlier stage of TBI (1-weeks to 3-weeks). In the cortex region (D-F), similar temporal profiles for the cell- and saline-treated groups in MK (D), AK (E) and RK (F) are found with no significant differences between two groups at all observation time points and no significant changes with time after TBI compared to the pre-injury status for each group.
2.4. Functional outcome
The mMWM allows for assessment of both spatial learning and searching strategy of animals studied (Choi et al., 2006; Darwish et al., 2012). An increased amount of time spent in the target quadrant indicates an increased memory for the location and the subsequent “searching” in that quadrant in the hope of finding the platform (Tucker et al., 2018).
Fig. 5 illustrates the results of mMWM. Improved neurological performance, as evidenced by spending significantly longer time in the correct quadrant, was detected in the cell-treated group compared to the saline-treated group.
Fig. 5. Functional outcome (mMWM).
Improved neurological performance, as evidenced by spending significantly longer time in the correct quadrant, was detected in the cell-treated group compared to the saline-treated group.
3. Discussion
Using an advanced imaging technique of DKI and a well-established animal model of TBI, diffuse WM damage resulting from a focal insult and therapeutic effect of hMSC administration after injury on preservation of microstructural integrity were non-invasively and dynamically investigated. By monitoring in vivo over an extended period of observation time (up to 3-months post-TBI), DKI-derived metrics of FA, AWF and RK longitudinally reveal an evolving pattern of structural alteration post-TBI occurring in the brain region remote from primary impact site. The progressive structural change after TBI is characterized by gradual disruption of WM integrity at an earlier stage (weeks post-TBI), followed by spontaneous recovery to a certain extent at a later stage (months post-TBI). Transplantation of hMSCs after TBI promotes such adaptive structural plasticity and induces an earlier presence of augmented recovery phase. Our data further demonstrate that the increased structural integrity likely benefits from the cell therapy-enhanced neuronal repair (Ding et al., 2013; Li et al., 2005; Xin et al., 2013), such as axonal and myelin remodeling, which contributes to the reduction of functional deficits.
As a powerful imaging method capable of probing microstructural abnormalities in the living biological tissues, DTI has been widely applied to study brain structural pathology, including TBI (Bosnell et al., 2008; Douglas et al., 2015; Farbota et al., 2012; Jiang et al., 2011; Li et al., 2017b). However, DTI has a well-recognized limitation, arising from its assumption of Gaussian water diffusion, which makes DTI yield only a fraction of the information potentially accessible with diffusion weighted imaging (Fieremans et al., 2011). Among the proposed techniques aimed to assess the non-Gaussian aspect of water diffusion, DKI, a minimal extension of DTI, enables the quantification of non-Gaussian water diffusion through the estimation of diffusional kurtosis (Douglas et al., 2015; Jensen et al., 2005; Jensen and Helpern, 2010). This imaging technique provides both conventional diffusion metrics and additional derivative indices related to the diffusional kurtosis, such as FA, AWF, MK and RK that have been demonstrated by experimental (Falangola et al., 2014; Nie et al., 2015; Weber et al., 2015) and clinical (Benitez et al., 2014; Falangola et al., 2008; Gong et al., 2013; Zhu et al., 2015) studies as markers of WM integrity. Both FA and AWF are most applicable to well-defined WM tracts (Benitez et al., 2014). While FA characterizes the degree of directional coherence of WM fiber bundles, AWF, defined by a two-compartment WM model as water volume in the intra-axonal space relative to water volume in the extra-axonal space (Fieremans et al., 2011), is highly sensitive to the variation of axonal density (Benitez et al., 2014; Falangola et al., 2014; Fieremans et al., 2011). As a measure of the heterogeneity of the diffusion environment (Jensen and Helpern, 2010), kurtosis describes the state of restricted diffusion with a higher value reflecting a more severe condition in diffusional heterogeneity. Accordingly, MK corresponds to the average of the kurtosis over all possible diffusion directions, more faithfully representing the complexity of tissue microstructure. In directionally ordered WM, the direction of diffusion tensor eigenvector with the largest diffusion eigenvalue (the principal or axial direction) is typically aligned with the axon bundles. AK and RK, the axial and radial components of MK, therefore correspond to the kurtosis along the axons and the kurtosis averaged over all directions perpendicular to the axons, respectively. Among these kurtosis indices, RK is most affected by myelin integrity with a decreased value being indicative of a lessened diffusion restriction in the direction perpendicular to the axons or myelin breakdown (Falangola et al., 2014; Guglielmetti et al., 2016; Spampinato et al., 2017b).
TBI imposes DAI (Grassi et al., 2018; Meythaler et al., 2001; Perez et al., 2014) and treatment of TBI with MSCs leads to improved functional recovery, which is partially attributed to the cell-evoked neuroregenerative potential (Pati et al., 2016; Schouten et al., 2004). But how the DAI evolves in the injured rodent brain over a period of 3-months post-TBI, and more importantly, how cell engraftment attanuates this diffuse dynamic event, thereby promoting structural integrity, have thus far not been well investigated. To address these issues in the current study, DKI-derived metrics sensitive to the changes of tissue microstructures were employed. To reveal the subtle abnormalities of well-defined WM tracts where these metrics are most appropriate and powerful (Benitez et al., 2014; Fieremans et al., 2011), our investigation focused on the CC+EC, the principal and largest WM structure with strongly oriented axon bundles. To assess the diffuse structural response to TBI and cell intervention post-TBI, CC+EC in non directly injured hemisphere (contralateral side) of the brain (Fig. 1E, CC+EC) was longitudinally monitored. This ROI selection is based on the fact that the structural integrity of normal appearing WM in the contralateral hemisphere represents the state of globally affected neural tissue and plays a significant role in determining the overall post-injury functional performance (Etherton et al., 2017; Vernooij et al., 2009). For comperison, the gray matter (GM) region encompassing the entire motor and somatosensory cortex (Fig. 1E, cortex) in the contralateral side of the brain was also evaluated. Since the objective of the present study is to investigate the structural response to TBI and cell therapy post-TBI, sham animals without induced traumatic injury were therefore not included. Instead, two directly relevant types of controls were designed into our study, preinjury animals as non-injury controls (naive controls) and saline-treated animals as non-cell-treated controls (vehicle controls).
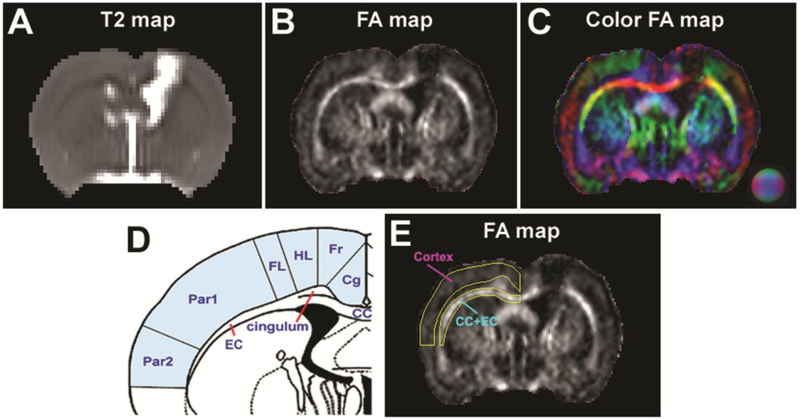
Fig. 1. A representative image slice of T2 and FA maps (cell-treated animal, 3-weeks post-TBI) illustrating the creation of ROIs.
A: T2 map showing the contusional lesion (hyperintense area in the cortical region) which extends to the subcortical region underneath, causing the damage to corpus callosum (CC) and external capsule (EC) as well as an enlarged lateral ventricle in the ipsilaleral side of the brain. B: FA map correspondingly reflecting the impact-induced FA reduction in the cortex and underlying CC+EC region while depicting this principal WM structure in the brain (as bright). C: Direction-encoded color FA map further distinguishing CC+EC (with distinct edge) from surrounding tissue regions (such as cingulum and striatum) by revealing additional structural details, which therefore serves as reference to accurately create the regions of interest (ROIs). D: Schematic anatomical diagram (bregma −0.8) illustrating the cortex (light blue shading) and CC+EC regions in the contralateral side of the brain that are chosen for MRI assessment (cg: cingulate cortex. Fr: frontal cortex. HL: hindlimb area of cortex. FL: forelimb area of cortex. Par1: parietal cortex area 1. Par2: parietal cortex area 2). E: FA map with ROIs separately encompassing the selected cortex and CC+EC regions. Referring to the anatomical diagram (D), ROIs are manually delineated on color FA map (C) and copied onto the different parametric maps (including FA, AWF, MK, AK and RK) for quantitative evaluation.
In agreement with prior works (Dinkel et al., 2014; Haber et al., 2017; Perez et al., 2014; Wu et al., 2010), dramatic decrease of FA in WM following TBI, denoting structural disruption, was observed (Fig. 2A). Without cell transplantation, this significantly reduced FA persisted throughout the experimental period although spontaneous recovery of FA to a certain extent was evident at later stage of TBI. Such recovery pattern, however, was remarkably modified by cell intervention, leading to an earlier and larger reversal of FA (Fig. 2A). This observation indicates that cell treatment retards the loss of directionality and intensity of WM bundles that results from both axonal compromise and myelin injury (Benitez et al., 2014; Douglas et al., 2015; Harris et al., 2016). The dynamic evaluation of AWF in the same WM structure (Fig. 3) confirmed that this region underwent axonal damage, as presented by a reduced AWF (Benitez et al., 2014; Buyukturkoglu et al., 2018) at the early stage after TBI for both saline- and cell-treated groups. AK, that increased with time post-TBI (Fig. 4B), reflects a more constrained diffusion along the axons compared to the non-injured status which indicates axonal injury (Haber et al., 2017; Sullivan et al., 2013; Sun et al., 2006) and the impact of reactive gliosis (Kou and VandeVord, 2014; Zhuo et al., 2012) although myelin impairment may also contribute to this reduction of diffusion (Perez et al., 2014). RK, however, decreased at the early stage following TBI regardless of treatment strategies (cell or saline, Fig. 4C), suggesting a less restricted diffusion in the direction perpendicular to axons or reduced myelin integrity (Falangola et al., 2014; Harris et al., 2016; Sun et al., 2006). This progressive neural tissue damage in the contralateral hemisphere of the brain distant from the local contusion is indicative of diaschisis (Carrera and Tononi, 2014). Axon and myelin abnormalities in the WM regions remote from but neuroanatomically connected to the site of initial insult have been well documented (Garbuzova-Davis et al., 2016; Weishaupt et al., 2016). In line with this distal but connecting phenomenon, our data show that CCI-produced TBI leads to injury of the CC+EC in the ipsilateral side of the brain (Fig. 1B), which provokes destructive alteration of the CC+EC in the contralateral side of the brain as evidenced by the impairment of axons (Fig. 3) and myelin (Fig. 4C). While limited recovery from both axonal and myelin damage were reflected by the reversal of AWF and RK at later stage of TBI in the saline-treated animals, administration of hMSCs dramatically enhanced structural remodeling, inducing a more pronounced recovery phase present at an earlier time point (Fig. 3, Fig. 4C). Consistent with previous studies (Armstrong et al., 2016; Garbuzova-Davis et al., 2016; Mierzwa et al., 2015; Weishaupt et al., 2016), our data show that WM damage after TBI is a dynamic process involving axon and myelin pathology that could start at very early stage after injury (within 1-week post-TBI,) and persist to the chronic stage (Fig. 3, Fig. 4C). Our measurements also demonstrate that cell intervention attenuates the WM compromise by reducing axonal loss (Fig. 3) and myelin breakdown (Fig. 4C), which diffusely modify the WM status in the trauma-injured brain and may correspondingly promote the recovery of neurological function (Fig. 5).
Previous studies have shown the efficacy of engrafted MSCs in facilitating neuronal repair in the injured brain (Al Jumah and Abumaree, 2012; Ding et al., 2013; Shu et al., 2018). These graft functions are associated with microstructural changes, leading to increased axonal regeneration (Ding et al., 2013; Morita et al., 2016), reduced demyelination (Gordon et al., 2010; Shu et al., 2018) and enhanced remyelination (Akiyama et al., 2002; Al Jumah and Abumaree, 2012; Shu et al., 2018). The mechanisms underlying these cell-induced structural benefits likely include down-regulating inflammatory cytokines (Aertker et al., 2016; Cox et al., 2017; Shu et al., 2018), up-regulating neurotrophic factors (Aertker et al., 2016; Mahmood et al., 2004; Shu et al., 2018), secreting soluble factors (Al Jumah and Abumaree, 2012; Menge et al., 2012; Pati et al., 2011) and exosomes (Xin et al., 2014; Xiong et al., 2017), enhancing blood-brain barrier reconstitution (Li et al., 2017a; Menge et al., 2012; Pati et al., 2011) and attenuating hypoperfusion (Li et al., 2011; Liu et al., 2016; Ukai et al., 2007) that act globally to modulate the inflammatory and inhibitory microenvironment after TBI for widespread neuronal remodeling. Supporting these findings, our in vivo data demonstrate the therapeutic effects of MSCs on diffuse preservation of structural integrity in the trauma-injured brain and show that cell administration after TBI reduces the course of injury, possibly, by rescuing both axons and myelin from the initial injury. In respect of the modified structural evolution after hMSC intervention (Fig. 2A, Fig. 3 and Fig. 4C), the earlier improvement of disrupted structure (compared to saline treatment) appears to result from cell-mediated protective effects (Al Jumah and Abumaree, 2012; Xiong et al., 2010) that hinder the progress of myelin breakdown and axonal degeneration, while the greater magnitude in recovery of compromised structural integrity may be attributed to the cell-evoked regenerative potential (Aertker et al., 2016; Schouten et al., 2004) that stimulates axon and myelin repair.
It is important to identify the temporal profile of progressive development of brain trauma, e.g. structural alteration, particularly for a severe TBI as produced by our experimental model (Li et al., 2011; Li et al., 2017b), since increasing injury severity is associated with a more prolonged recovery (Markgraf et al., 2001). Our data reveal a temporal course of TBI-induced structural change that occurs soon after injury and advances to a worse level, followed by a limited recovery (Fig. 2A, 3A, 4C). This time dependent pattern of structural change in the rodent brain is consistent with the essential feature of human TBI characterized by injury-initiated functional loss and subsequent behavioral improvement (Farbota et al., 2012; Perez et al., 2014; Stokum et al., 2015). More importantly, the rescuing effects of cell transplantation on WM integrity, predominantly detectable at the chronic stage (Gordon et al., 2010; Morita et al., 2016; Shu et al., 2018), can also be identified within our selected experimental time frame (Fig. 2A, 3A, 4C). To probe the temporal profile of WM response in animals to both TBI and cell intervention after TBI, an observation period of 3-months post-TBI appears to be a proper survival time.
In summary, our long-term dynamic data demonstrate the therapeutic effects of hMSCs on diffuse WM integrity in the trauma-injured brain. As a result of cell intervention, increased neural tissue remodeling is observed, as indicated by significantly increased FA and AWF in conjunction with substantially elevated RK in WM region at the later stage of TBI. Cell administration leads to modified temporal profiles of these metrics, represented by an earlier presence of increased recovery phase, compared to those present in animals without cell engraftment. These observations suggest that cell-enhanced neuroprotective and neuroregenerative effects, that not only attenuate demyelination and axonal degeneration, but also facilitate remyelination and regrowth of axons, are involved in the dynamically increased structural integrity, which yields improved functional outcome. Our data indicate that DKI can detect microstructural alteration, arising from both brain trauma injury and cell therapy.
4. Experimental procedures
All experimental procedures were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee (IACUC#1316) of Henry Ford Health System.
4.1. Animal model and experimental groups
A well-established pre-clinical rodent model of TBI (Dixon et al., 1991; Xiong et al., 2013), the controlled cortical impact (CCI) model, was employed in the present study. The detailed operating procedures of CCI have been previously described (Li et al., 2011; Li et al., 2012). Briefly, the rectal temperature of anesthetized rats (male Wistar rats, 300 to 350g, n = 20) was maintained at ~37°C with a feedback-regulated water-heating pad throughout the operation. The head of each animal was mounted in a stereotactic frame and two 10-mm-diameter craniotomies, one in each hemisphere, were performed adjacent to the central suture, midway between the lambda and the bregma, with special attention being paid to leave the dura mater over the cortex intact. The left craniotomy confined the location of experimental impact, while the right one allowed for the lateral movement of cortical tissue. With a pneumatic piston containing a 6-mm-diameter tip, a unilateral brain injury was induced by delivering a single impact (at a velocity of 4 m/sec) to the left exposed and intact dura mater (reaching a depth of 2.5 mm below the dura mater layer). The injury produced by this CCI setting results in a severe TBI, affecting both the cortical (e.g., primary motor and somatosensory cortex) and subcortical (e.g., hippocampus) structures. Following the operation, the bone flap was replaced and sealed with bone wax, and the skin was sutured. For analgesia, Buprenex (0.05 mg/kg, s.q.) was administered to each animal after the injury.
The hMSCs were purchased from Theradigm (Baltimore, MD). The cells were suspended in phosphate-buffered saline (PBS) prior to injection into rats, which was performed at 1 week post-TBI. Rats subjected to brain injury were randomized to two treatment groups, cell-treated (n = 10) and saline-treated (n = 10) group. A bolus of the cell suspension (~ 3×106 hMSCs in 1 ml PBS) was slowly infused over a 5-minute period into the tail vein of each rat in the cell-treated group using a Hamilton syringe. The needle was left in place for 1 minute before withdrawal to minimize cell leakage, and the injection site was compressed for a short time to reduce bleeding. Replacing the cell suspension with the same amount of saline, each animal in the saline-treated group underwent the identical procedures as those in the cell-treated group.
4.2. MR imaging and data processing
MR imaging was performed using ClinScan 7T system (Siemens, Erlanger, Germany). A birdcage type coil was used as the transmitter and a quardrature half-volume coil as the receiver. The animal was securely fixed on a MR-compatible holder equipped with an adjustable nose cone for administration of anesthetic gases and stereotaxic ear bars to immobilize the head. For reproducible positioning of the animal in the magnet, a fast gradient echo imaging sequence was used at the beginning of each MRI session. During image acquisition, anesthesia was maintained by a gas mixture of 1.0% - 1.5% isoflurane in medical air (1.0L/min), and rectal temperature was kept at 37±0.5°C using a feedback-controlled water bath (YSI Inc, Yellow Springs, OH) underneath the animal. T2-weighted imaging (T2WI) and diffusional kurtosis imaging (DKI) were repeatedly acquired for all animals at multiple time points up to 3 months following TBI (1 day pre-TBI, weekly afterwards for 3 weeks, then 5 weeks, 2 months and 3 months). All rats were killed after the final in vivo MRI scans.
T2WI was acquired using a multi-slice (13 slices, 1 mm thick), multi-echo (6 echoes) sequence with echo times (TE) of 15, 30, 45, 60, 75 and 90 ms, and a repetition time (TR) of 4.5 s. Images were produced with a 32 × 32 mm2 field of view (FOV) and a 128 × 64 image matrix. T2 maps were calculated on a voxel-wise basis by linear least-squares fit of the logarithm of the signal intensity versus TE (Eigentool image analysis software, Henry Ford Health System, MI, USA).
A single shot spin-echo echo planar imaging diffusion sequence with 20 diffusion-encoding directions and three b values (0, 900 and 1800 s/mm2) was used for DKI acquisition. Other imaging parameters were TR/TE = 10000/50 ms, δ/Δ = 10/18 ms, field of view =32 × 32 mm2, matrix = 128×128, in-plane resolution = 0.25×0.25 mm2, number of averages = 2. Thirteen axial slices were collected with 0.2mm gap and slice thickness of 0.8 mm. Both direction-encoded and conventional fractional anisotropy (FA) maps were generated by DTIstudio (Johns Hopkins University, Baltimore, MD. USA). DKI-derived metrics, including axonal water fraction (AWF), mean kurtosis (MK), axial kurtosis (AK) and radial kurtosis (RK), were calculated using an in-house software programmed in Matlab.
With a representative coronal image slice (cell-treated animal, 3-weeks post-TBI), typical injury in the brain produced by the CCI model and regions of interest (ROIs) for the current study are exhibited in Fig. 1. The contusional lesion can be easily identified on T2 map (Fig. 1A, hyperintense area in the cortical region). The injury primarily imposed onto the cortex extends to subcortical region underneath, causing the damage to corpus callosum (CC) and external capsule (EC) as well as an enlarged lateral ventricle in the ipsilateral side of the brain (Fig. 1A). The structural alterations induced by the contusion are correspondingly reflected on the FA map (Fig. 1B), with apparently reduced FA present in both the injured cortex and underlying CC+EC region. Although specific WM area, e.g. CC+EC region, is well depicted on the FA map as bright (Fig. 1B), there is uncertainty in identification of a ROI to specifically extract the anatomical WM structure from the nearby tissue areas. By revealing additional structural details, the direction-encoded color FA map (Fig. 1C), however, further distinguishes CC+EC (with distinct edge) from surrounding tissue regions (such as cingulum and striatum), particularly in the contralateral side of the brain where our longitudinal assessments were conducted to detect the diffuse effects of TBI and engraftment of hMSCs post-TBI. Referring to the anatomical diagram (Fig. 1D), ROIs that separately encompass CC+EC and cortex regions are, therefore, reliably delineated on the color FA map (Fig. 1C) and copied onto the different parametric maps (including FA, AWF, MK, AK and RK), as presented by FA map here (Fig. 1E), for quantitative evaluation. Using ImageJ (Schneider et al., 2012), these ROIs were created on the fixed coronal slice (Fig. 1E) at every observation time point for each animal studied.
4.3. Modified Morris water maze test (mMWM)
To evaluate the long-term functional outcome of spatial learning acquisition and memory retention, the mMWM (Mahmood et al., 2011) was used. The testing system consisted of a circular tank (180cm in diameter and 45cm high) filled with 30°C water and a hidden-platform (15cm in diameter and 35cm high) set inside the tank 1.5cm below the surface of the water. The pool was located in a large test room decorated with visual spatial clues (such as pictures, lamps and so forth) that remained constant during the study and enabled the rats to orientate themselves spatially. For descriptive data collection, the pool was subdivided into four equal quadrants (designated as northeast, southeast, northwest and southwest) and an automated tracking system (HVS Image, San Diego, CA, USA) was employed for recording the latency to reach the escape platform and the path length taken to the platform.
The rats were tested for five consecutive days, four trials per day with an inter-trial interval of 30min, at the last week of study period (from day 85 to 89 after TBI). Each trial was initiated by placing the animal randomly at one of four start locations (North, South, East and West) and allowing 90s for the animal to find the submerged platform. The platform was put in a randomly changing position within the northeast (NE) quadrant throughout the test period (e.g., sometimes equidistant from the center and edge of the pool, against the wall, near the center of the pool, and at the edges of the NE quadrant). After locating the platform, the animal was allowed to remain on the platform for 15s before being returned to its cage. If the animal failed to find the platform within 90s, the experiment was terminated and a maximum score of 90s was assigned. In this case, the animal was guided to the platform and allowed for 15s of exploration before being returned to its cage. The percentage of time traveled within the NE (correct) quadrant was calculated relative to the total amount of time spent swimming before reaching the platform. The advantage of this modified version of the water maze is that with the platform being relocated randomly within the target quadrant, each trial effectively acts as a probe trial.
4.4. Statistical analysis
Analysis of variance and covariance (ANCOVA) was employed to compare the group difference in MRI measurements (FA, AWF, MK, AK and RK) and functional assessments (mMWM) with the independent factor of treatment and dependent factor of time. Analysis began with testing the treatment group and time interaction, followed by testing the group difference at each time point and the time effect for each treatment group if the interaction or the overall group/time effect was detected at the 0.05 level. A subgroup analysis would be considered as exploratory analysis if the interaction or main effect of group/time was not detected at the 0.05 level. Results are presented as mean ± standard error (SE). Statistical significance was inferred for p ≤ 0.05.
Highlights.
TBI results in a diffuse WM damage, as revealed by DKI-derived metrics.
Changes of FA, AWF and RK in normal-appearing WM region are monitored up to 3-months post-TBI.
Transplantation of hMSCs after TBI promotes neural tissue remodeling.
Transplantation of hMSCs after TBI induces an earlier presence of augmented recovery phase.
DKI can detect microstructural alteration, arising from both TBI and cell therapy.
Acknowledgments
We thank Dr. Changsheng Qu for animal experiment assistance. This work was supported by National Institutes of Health RO1 NS064134.
Footnotes
Conflicts of interests
The authors have declared that no conflict of interest exists.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aertker BM, Bedi S, Cox CS Jr., 2016. Strategies for CNS repair following TBI. Exp Neurol. 275 Pt 3, 411–426. [DOI] [PubMed] [Google Scholar]
- Akiyama Y, Radtke C, Honmou O, Kocsis JD, 2002. Remyelination of the spinal cord following intravenous delivery of bone marrow cells. Glia. 39, 229–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Jumah MA, Abumaree MH, 2012. The immunomodulatory and neuroprotective effects of mesenchymal stem cells (MSCs) in experimental autoimmune encephalomyelitis (EAE): a model of multiple sclerosis (MS). Int J Mol Sci. 13, 9298–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong RC, Mierzwa AJ, Marion CM, Sullivan GM, 2016. White matter involvement after TBI: Clues to axon and myelin repair capacity. Exp Neurol. 275 Pt 3, 328–333. [DOI] [PubMed] [Google Scholar]
- Benitez A, Fieremans E, Jensen JH, Falangola MF, Tabesh A, Ferris SH, Helpern JA, 2014. White matter tract integrity metrics reflect the vulnerability of late-myelinating tracts in Alzheimer's disease. Neuroimage Clin. 4, 64–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosnell R, Giorgio A, Johansen-Berg H, 2008. Imaging white matter diffusion changes with development and recovery from brain injury. Dev Neurorehabil. 11, 174–86. [DOI] [PubMed] [Google Scholar]
- Buyukturkoglu K, Fleyser L, Byrd D, Morgello S, Inglese M, 2018. Diffusion Kurtosis Imaging Shows Similar Cerebral Axonal Damage in Patients with HIV Infection and Multiple Sclerosis. J Neuroimaging. 28, 320–327. [DOI] [PubMed] [Google Scholar]
- Carrera E, Tononi G, 2014. Diaschisis: past, present, future. Brain. 137, 2408–22. [DOI] [PubMed] [Google Scholar]
- Cheung MM, Hui ES, Chan KC, Helpern JA, Qi L, Wu EX, 2009. Does diffusion kurtosis imaging lead to better neural tissue characterization? A rodent brain maturation study. Neuroimage. 45, 386–92. [DOI] [PubMed] [Google Scholar]
- Choi SH, Woodlee MT, Hong JJ, Schallert T, 2006. A simple modification of the water maze test to enhance daily detection of spatial memory in rats and mice. J Neurosci Methods. 156, 182–93. [DOI] [PubMed] [Google Scholar]
- Chopp M, Li Y, 2002. Treatment of neural injury with marrow stromal cells. Lancet Neurol. 1, 92–100. [DOI] [PubMed] [Google Scholar]
- Cox CS Jr., Hetz RA, Liao GP, Aertker BM, Ewing-Cobbs L, Juranek J, Savitz SI, Jackson ML, Romanowska-Pawliczek AM, Triolo F, Dash PK, Pedroza C, Lee DA, Worth L, Aisiku IP, Choi HA, Holcomb JB, Kitagawa RS, 2017. Treatment of Severe Adult Traumatic Brain Injury Using Bone Marrow Mononuclear Cells. Stem Cells. 35, 1065–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darkazalli A, Ismail AA, Abad N, Grant SC, Levenson CW, 2016. Use of human mesenchymal stem cell treatment to prevent anhedonia in a rat model of traumatic brain injury. Restor Neurol Neurosci. 34, 433–41. [DOI] [PubMed] [Google Scholar]
- Darwish H, Mahmood A, Schallert T, Chopp M, Therrien B, 2012. Mild traumatic brain injury (MTBI) leads to spatial learning deficits. Brain Inj. 26, 151–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding X, Li Y, Liu Z, Zhang J, Cui Y, Chen X, Chopp M, 2013. The sonic hedgehog pathway mediates brain plasticity and subsequent functional recovery after bone marrow stromal cell treatment of stroke in mice. J Cereb Blood Flow Metab. 33, 1015–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinkel J, Drier A, Khalilzadeh O, Perlbarg V, Czernecki V, Gupta R, Gomas F, Sanchez P, Dormont D, Galanaud D, Stevens RD, Puybasset L, 2014. Long-term white matter changes after severe traumatic brain injury: a 5-year prospective cohort. AJNR Am J Neuroradiol. 35, 23–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon CE, Clifton GL, Lighthall JW, Yaghmai AA, Hayes RL, 1991. A controlled cortical impact model of traumatic brain injury in the rat. J Neurosci Methods. 39, 253–62. [DOI] [PubMed] [Google Scholar]
- Douglas DB, Iv M, Douglas PK, Anderson A, Vos SB, Bammer R, Zeineh M, Wintermark M, 2015. Diffusion Tensor Imaging of TBI: Potentials and Challenges. Top Magn Reson Imaging. 24, 241–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etherton MR, Wu O, Cougo P, Giese AK, Cloonan L, Fitzpatrick KM, Kanakis AS, Boulouis G, Karadeli HH, Lauer A, Rosand J, Furie KL, Rost NS, 2017. Integrity of normal-appearing white matter and functional outcomes after acute ischemic stroke. Neurology. 88, 1701–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falangola MF, Jensen JH, Babb JS, Hu C, Castellanos FX, Di Martino A, Ferris SH, Helpern JA, 2008. Age-related non-Gaussian diffusion patterns in the prefrontal brain. J Magn Reson Imaging. 28, 1345–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falangola MF, Guilfoyle DN, Tabesh A, Hui ES, Nie X, Jensen JH, Gerum SV, Hu C, LaFrancois J, Collins HR, Helpern JA, 2014. Histological correlation of diffusional kurtosis and white matter modeling metrics in cuprizone-induced corpus callosum demyelination. NMR Biomed. 27, 948–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farbota KD, Bendlin BB, Alexander AL, Rowley HA, Dempsey RJ, Johnson SC, 2012. Longitudinal diffusion tensor imaging and neuropsychological correlates in traumatic brain injury patients. Front Hum Neurosci. 6, 160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fieremans E, Jensen JH, Helpern JA, 2011. White matter characterization with diffusional kurtosis imaging. Neuroimage. 58, 177–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbuzova-Davis S, Haller E, Tajiri N, Thomson A, Barretta J, Williams SN, Haim ED, Qin H, Frisina-Deyo A, Abraham JV, Sanberg PR, Van Loveren H, Borlongan CV, 2016. Blood-Spinal Cord Barrier Alterations in Subacute and Chronic Stages of a Rat Model of Focal Cerebral Ischemia. J Neuropathol Exp Neurol. 75, 673–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold EM, Su D, Lopez-Velazquez L, Haus DL, Perez H, Lacuesta GA, Anderson AJ, Cummings BJ, 2013. Functional assessment of long-term deficits in rodent models of traumatic brain injury. Regen Med. 8, 483–516. [DOI] [PubMed] [Google Scholar]
- Gong NJ, Wong CS, Chan CC, Leung LM, Chu YC, 2013. Correlations between microstructural alterations and severity of cognitive deficiency in Alzheimer's disease and mild cognitive impairment: a diffusional kurtosis imaging study. Magn Reson Imaging. 31, 688–94. [DOI] [PubMed] [Google Scholar]
- Gordon D, Pavlovska G, Uney JB, Wraith DC, Scolding NJ, 2010. Human mesenchymal stem cells infiltrate the spinal cord, reduce demyelination, and localize to white matter lesions in experimental autoimmune encephalomyelitis. J Neuropathol Exp Neurol. 69, 1087–95. [DOI] [PubMed] [Google Scholar]
- Grassi DC, Conceicao DMD, Leite CDC, Andrade CS, 2018. Current contribution of diffusion tensor imaging in the evaluation of diffuse axonal injury. Arq Neuropsiquiatr. 76, 189–199. [DOI] [PubMed] [Google Scholar]
- Guglielmetti C, Veraart J, Roelant E, Mai Z, Daans J, Van Audekerke J, Naeyaert M, Vanhoutte G, Delgado YPR, Praet J, Fieremans E, Ponsaerts P, Sijbers J, Van der Linden A, Verhoye M, 2016. Diffusion kurtosis imaging probes cortical alterations and white matter pathology following cuprizone induced demyelination and spontaneous remyelination. Neuroimage. 125, 363–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber M, Hutchinson EB, Sadeghi N, Cheng WH, Namjoshi D, Cripton P, Irfanoglu MO, Wellington C, Diaz-Arrastia R, Pierpaoli C, 2017. Defining an Analytic Framework to Evaluate Quantitative MRI Markers of Traumatic Axonal Injury: Preliminary Results in a Mouse Closed Head Injury Model. eNeuro. 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris NG, Verley DR, Gutman BA, Sutton RL, 2016. Bi-directional changes in fractional anisotropy after experiment TBI: Disorganization and reorganization? Neuroimage. 133, 129–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harting MT, Baumgartner JE, Worth LL, Ewing-Cobbs L, Gee AP, Day MC, Cox CS Jr., 2008. Cell therapies for traumatic brain injury. Neurosurg Focus. 24, E18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui ES, Cheung MM, Qi L, Wu EX, 2008. Towards better MR characterization of neural tissues using directional diffusion kurtosis analysis. Neuroimage. 42, 122–34. [DOI] [PubMed] [Google Scholar]
- Jensen JH, Helpern JA, Ramani A, Lu H, Kaczynski K, 2005. Diffusional kurtosis imaging: the quantification of non-gaussian water diffusion by means of magnetic resonance imaging. Magn Reson Med. 53, 1432–40. [DOI] [PubMed] [Google Scholar]
- Jensen JH, Helpern JA, 2010. MRI quantification of non-Gaussian water diffusion by kurtosis analysis. NMR Biomed. 23, 698–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Q, Qu C, Chopp M, Ding GL, Davarani SP, Helpern JA, Jensen JH, Zhang ZG, Li L, Lu M, Kaplan D, Hu J, Shen Y, Kou Z, Li Q, Wang S, Mahmood A, 2011. MRI evaluation of axonal reorganization after bone marrow stromal cell treatment of traumatic brain injury. NMR Biomed. 24, 1119–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kou Z, VandeVord PJ, 2014. Traumatic white matter injury and glial activation: from basic science to clinics. Glia. 62, 1831–55. [DOI] [PubMed] [Google Scholar]
- Li L, Jiang Q, Qu CS, Ding GL, Li QJ, Wang SY, Lee JH, Lu M, Mahmood A, Chopp M, 2011. Transplantation of marrow stromal cells restores cerebral blood flow and reduces cerebral atrophy in rats with traumatic brain injury: in vivo MRI study. J Neurotrauma. 28, 535–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Chopp M, Ding GL, Qu CS, Li QJ, Lu M, Wang S, Nejad-Davarani SP, Mahmood A, Jiang Q, 2012. MRI measurement of angiogenesis and the therapeutic effect of acute marrow stromal cell administration on traumatic brain injury. J Cereb Blood Flow Metab. 32, 2023–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Chopp M, Ding G, Li Q, Mahmood A, Jiang Q, 2017a. Chronic global analysis of vascular permeability and cerebral blood flow after bone marrow stromal cell treatment of traumatic brain injury in the rat: A long-term MRI study. Brain Res. 1675, 61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Chopp M, Ding G, Qu C, Nejad-Davarani SP, Davoodi-Bojd E, Li Q, Mahmood A, Jiang Q, 2017b. Diffusion-Derived Magnetic Resonance Imaging Measures of Longitudinal Microstructural Remodeling Induced by Marrow Stromal Cell Therapy after Traumatic Brain Injury. J Neurotrauma. 34, 182–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Chen J, Zhang CL, Wang L, Lu D, Katakowski M, Gao Q, Shen LH, Zhang J, Lu M, Chopp M, 2005. Gliosis and brain remodeling after treatment of stroke in rats with marrow stromal cells. Glia. 49, 407–17. [DOI] [PubMed] [Google Scholar]
- Liu CB, Huang H, Sun P, Ma SZ, Liu AH, Xue J, Fu JH, Liang YQ, Liu B, Wu DY, Lu SH, Zhang XZ, 2016. Human Umbilical Cord-Derived Mesenchymal Stromal Cells Improve Left Ventricular Function, Perfusion, and Remodeling in a Porcine Model of Chronic Myocardial Ischemia. Stem Cells Transl Med. 5, 1004–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maegele M, Schaefer U, 2008. Stem cell-based cellular replacement strategies following traumatic brain injury (TBI). Minim Invasive Ther Allied Technol. 17, 119–31. [DOI] [PubMed] [Google Scholar]
- Mahmood A, Lu D, Chopp M, 2004. Intravenous administration of marrow stromal cells (MSCs) increases the expression of growth factors in rat brain after traumatic brain injury. J Neurotrauma. 21, 33–9. [DOI] [PubMed] [Google Scholar]
- Mahmood A, Lu D, Qu C, Goussev A, Chopp M, 2006. Long-term recovery after bone marrow stromal cell treatment of traumatic brain injury in rats. J Neurosurg. 104, 272–7. [DOI] [PubMed] [Google Scholar]
- Mahmood A, Qu C, Ning R, Wu H, Goussev A, Xiong Y, Irtenkauf S, Li Y, Chopp M, 2011. Treatment of TBI with collagen scaffolds and human marrow stromal cells increases the expression of tissue plasminogen activator. J Neurotrauma. 28, 1199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markgraf CG, Clifton GL, Aguirre M, Chaney SF, Knox-Du Bois C, Kennon K, Verma N, 2001. Injury severity and sensitivity to treatment after controlled cortical impact in rats. J Neurotrauma. 18, 175–86. [DOI] [PubMed] [Google Scholar]
- Menge T, Zhao Y, Zhao J, Wataha K, Gerber M, Zhang J, Letourneau P, Redell J, Shen L, Wang J, Peng Z, Xue H, Kozar R, Cox CS Jr., Khakoo AY, Holcomb JB, Dash PK, Pati S, 2012. Mesenchymal stem cells regulate blood-brain barrier integrity through TIMP3 release after traumatic brain injury. Sci Transl Med. 4, 161ra150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meythaler JM, Peduzzi JD, Eleftheriou E, Novack TA, 2001. Current concepts: diffuse axonal injury-associated traumatic brain injury. Arch Phys Med Rehabil. 82, 1461–71. [DOI] [PubMed] [Google Scholar]
- Mierzwa AJ, Marion CM, Sullivan GM, McDaniel DP, Armstrong RC, 2015. Components of myelin damage and repair in the progression of white matter pathology after mild traumatic brain injury. J Neuropathol Exp Neurol. 74, 218–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita T, Sasaki M, Kataoka-Sasaki Y, Nakazaki M, Nagahama H, Oka S, Oshigiri T, Takebayashi T, Yamashita T, Kocsis JD, Honmou O, 2016. Intravenous infusion of mesenchymal stem cells promotes functional recovery in a model of chronic spinal cord injury. Neuroscience. 335, 221–31. [DOI] [PubMed] [Google Scholar]
- Nie X, Hamlett ED, Granholm AC, Hui ES, Helpern JA, Jensen JH, Boger HA, Collins HR, Falangola MF, 2015. Evidence of altered age-related brain cytoarchitecture in mouse models of down syndrome: a diffusional kurtosis imaging study. Magn Reson Imaging. 33, 437–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parr AM, Tator CH, Keating A, 2007. Bone marrow-derived mesenchymal stromal cells for the repair of central nervous system injury. Bone Marrow Transplant. 40, 609–19. [DOI] [PubMed] [Google Scholar]
- Pati S, Khakoo AY, Zhao J, Jimenez F, Gerber MH, Harting M, Redell JB, Grill R, Matsuo Y, Guha S, Cox CS, Reitz MS, Holcomb JB, Dash PK, 2011. Human mesenchymal stem cells inhibit vascular permeability by modulating vascular endothelial cadherin/beta-catenin signaling. Stem Cells Dev. 20, 89–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pati S, Muthuraju S, Hadi RA, Huat TJ, Singh S, Maletic-Savatic M, Abdullah JM, Jaafar H, 2016. Neurogenic plasticity of mesenchymal stem cell, an alluring cellular replacement for traumatic brain injury. Curr Stem Cell Res Ther. 11, 149–57. [DOI] [PubMed] [Google Scholar]
- Perez AM, Adler J, Kulkarni N, Strain JF, Womack KB, Diaz-Arrastia R, Marquez de la Plata CD, 2014. Longitudinal white matter changes after traumatic axonal injury. J Neurotrauma. 31, 1478–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolfe A, Sun D, 2015. Stem Cell Therapy in Brain Trauma: Implications for Repair and Regeneration of Injured Brain in Experimental TBI Models Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects. Vol., 2015 by Taylor & Francis Group, LLC, Boca Raton FL. [PubMed] [Google Scholar]
- Schneider CA, Rasband WS, Eliceiri KW, 2012. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 9, 671–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schouten JW, Fulp CT, Royo NC, Saatman KE, Watson DJ, Snyder EY, Trojanowski JQ, Prockop DJ, Maas AI, McIntosh TK, 2004. A review and rationale for the use of cellular transplantation as a therapeutic strategy for traumatic brain injury. J Neurotrauma. 21, 1501–38. [DOI] [PubMed] [Google Scholar]
- Sharma A, Sane H, Kulkarni P, Yadav J, Gokulchandran N, Biju H, Badhe P, 2015. Cell therapy attempted as a novel approach for chronic traumatic brain injury - a pilot study. Springerplus. 4, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu J, He X, Li H, Liu X, Qiu X, Zhou T, Wang P, Huang X, 2018. The Beneficial Effect of Human Amnion Mesenchymal Cells in Inhibition of Inflammation and Induction of Neuronal Repair in EAE Mice. J Immunol Res. 2018, 5083797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skardelly M, Gaber K, Burdack S, Scheidt F, Hilbig H, Boltze J, Forschler A, Schwarz S, Schwarz J, Meixensberger J, Schuhmann MU, 2011. Long-term benefit of human fetal neuronal progenitor cell transplantation in a clinically adapted model after traumatic brain injury. J Neurotrauma. 28, 401–14. [DOI] [PubMed] [Google Scholar]
- Spampinato MV, Chan C, Jensen JH, Helpern JA, Bonilha L, Kautz SA, Nietert PJ, Feng W, 2017a. Diffusional Kurtosis Imaging and Motor Outcome in Acute Ischemic Stroke. AJNR Am J Neuroradiol. 38, 1328–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spampinato MV, Kocher MR, Jensen JH, Helpern JA, Collins HR, Hatch NU, 2017b. Diffusional Kurtosis Imaging of the Corticospinal Tract in Multiple Sclerosis: Association with Neurologic Disability. AJNR Am J Neuroradiol. 38, 1494–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokum JA, Sours C, Zhuo J, Kane R, Shanmuganathan K, Gullapalli RP, 2015. A longitudinal evaluation of diffusion kurtosis imaging in patients with mild traumatic brain injury. Brain Inj. 29, 47–57. [DOI] [PubMed] [Google Scholar]
- Sullivan GM, Mierzwa AJ, Kijpaisalratana N, Tang H, Wang Y, Song SK, Selwyn R, Armstrong RC, 2013. Oligodendrocyte lineage and subventricular zone response to traumatic axonal injury in the corpus callosum. J Neuropathol Exp Neurol. 72, 1106–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun SW, Liang HF, Trinkaus K, Cross AH, Armstrong RC, Song SK, 2006. Noninvasive detection of cuprizone induced axonal damage and demyelination in the mouse corpus callosum. Magn Reson Med. 55, 302–8. [DOI] [PubMed] [Google Scholar]
- Tucker LB, Velosky AG, McCabe JT, 2018. Applications of the Morris water maze in translational traumatic brain injury research. Neurosci Biobehav Rev. 88, 187–200. [DOI] [PubMed] [Google Scholar]
- Ukai R, Honmou O, Harada K, Houkin K, Hamada H, Kocsis JD, 2007. Mesenchymal stem cells derived from peripheral blood protects against ischemia. J Neurotrauma. 24, 508–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umesh Rudrapatna S, Wieloch T, Beirup K, Ruscher K, Mol W, Yanev P, Leemans A, van der Toorn A, Dijkhuizen RM, 2014. Can diffusion kurtosis imaging improve the sensitivity and specificity of detecting microstructural alterations in brain tissue chronically after experimental stroke? Comparisons with diffusion tensor imaging and histology. Neuroimage. 97, 363–73. [DOI] [PubMed] [Google Scholar]
- Veraart J, Poot DH, Van Hecke W, Blockx I, Van der Linden A, Verhoye M, Sijbers J, 2011. More accurate estimation of diffusion tensor parameters using diffusion Kurtosis imaging. Magn Reson Med. 65, 138–45. [DOI] [PubMed] [Google Scholar]
- Vernooij MW, Ikram MA, Vrooman HA, Wielopolski PA, Krestin GP, Hofman A, Niessen WJ, Van der Lugt A, Breteler MM, 2009. White matter microstructural integrity and cognitive function in a general elderly population. Arch Gen Psychiatry. 66, 545–53. [DOI] [PubMed] [Google Scholar]
- Weber RA, Hui ES, Jensen JH, Nie X, Falangola MF, Helpern JA, Adkins DL, 2015. Diffusional kurtosis and diffusion tensor imaging reveal different time-sensitive stroke-induced microstructural changes. Stroke. 46, 545–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weishaupt N, Zhang A, Deziel RA, Tasker RA, Whitehead SN, 2016. Prefrontal Ischemia in the Rat Leads to Secondary Damage and Inflammation in Remote Gray and White Matter Regions. Front Neurosci. 10, 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu TC, Wilde EA, Bigler ED, Li X, Merkley TL, Yallampalli R, McCauley SR, Schnelle KP, Vasquez AC, Chu Z, Hanten G, Hunter JV, Levin HS, 2010. Longitudinal changes in the corpus callosum following pediatric traumatic brain injury. Dev Neurosci. 32, 361–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin H, Li Y, Liu Z, Wang X, Shang X, Cui Y, Zhang ZG, Chopp M, 2013. MiR-133b promotes neural plasticity and functional recovery after treatment of stroke with multipotent mesenchymal stromal cells in rats via transfer of exosome-enriched extracellular particles. Stem Cells. 31, 2737–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin H, Li Y, Chopp M, 2014. Exosomes/miRNAs as mediating cell-based therapy of stroke. Front Cell Neurosci. 8, 377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y, Mahmood A, Chopp M, 2010. Neurorestorative treatments for traumatic brain injury. Discov Med. 10, 434–42. [PMC free article] [PubMed] [Google Scholar]
- Xiong Y, Mahmood A, Chopp M, 2013. Animal models of traumatic brain injury. Nat Rev Neurosci. 14, 128–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y, Zhang Y, Mahmood A, Chopp M, 2015. Investigational agents for treatment of traumatic brain injury. Expert Opin Investig Drugs. 24, 743–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y, Mahmood A, Chopp M, 2017. Emerging potential of exosomes for treatment of traumatic brain injury. Neural Regeneration Research. 12, 19–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y, Mahmood A, Chopp M, 2018. Current understanding of neuroinflammation after traumatic brain injury and cell-based therapeutic opportunities. Chin J Traumatol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Zhuo C, Qin W, Wang D, Ma X, Zhou Y, Yu C, 2015. Performances of diffusion kurtosis imaging and diffusion tensor imaging in detecting white matter abnormality in schizophrenia. Neuroimage Clin. 7, 170–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuo J, Xu S, Proctor JL, Mullins RJ, Simon JZ, Fiskum G, Gullapalli RP, 2012. Diffusion kurtosis as an in vivo imaging marker for reactive astrogliosis in traumatic brain injury. Neuroimage. 59, 467–77. [DOI] [PMC free article] [PubMed] [Google Scholar]