Abstract
Purpose
Acute macular neuroretinopathy has been shown to be due to ischemia of the deep capillary retinal plexus and most cases occur in young women; we hypothesized that there may be an association with antiphospholipid antibodies.
Observations
We identified three patients who were diagnosed with deep capillary retinal ischemia after presenting with sudden onset of focal paracentral scotoma who tested persistently positive for antiphospholipid antibodies. All patients had high-titer prothrombin-associated antibodies and two of the three also had low-titer anticardiolipin antibodies. In all patients, the diagnosis was missed at the initial presentation. All patients experienced involvement of both eyes over time with permanent visual deficits and all were female with an average age at symptom onset of 34 years. All patients were using exogenous estrogen and had additional but previously undiagnosed symptoms or signs that may be seen in the antiphospholipid syndrome. One patient was ANA positive with a titer of 1:320, but none had lupus-specific antibodies or clinical features of lupus.
Conclusions and Importance
The persistent presence of high-titer prothrombin-associated antiphospholipid antibodies in three women with deep capillary retinal ischemia suggests this may be an important association. Prothrombin-associated antibodies (anti-prothrombin IgG and anti-phosphatidylserine-prothrombin IgG and IgM) as well as the traditional antiphospholipid antibodies (anticardiolipin IgG, IgM and IgA; anti-beta 2 glycoprotein I IgG, IgM and IgA; and the lupus anticoagulant) should be included in the diagnostic work-up of patients diagnosed with deep capillary retinal ischemia. Because of the broader health and treatment implications of high-titer antiphospholipid antibodies, further investigation into this suspected association is warranted.
Keywords: Acute macular neuroretinopathy, Paracentral acute middle maculopathy, Antiphospholipid syndrome, Optical coherence tomography angiography, Anti-Phosphatidylserine-prothrombin antibodies, Retinal ischemia
Abbreviations: SD-OCT, Spectral domain optical coherence tomography
1. Introduction
The antiphospholipid syndrome is the most common acquired hypercoagulable disorder. It is a systemic autoimmune disease characterized by the persistent presence of antiphospholipid antibodies. It is associated with an increased risk of arterial, venous and small vessel thrombosis as well as pregnancy morbidity1 and presents most commonly in women aged 20–40 years.
Acute macular neuroretinopathy has been shown to occur due to an interruption of the blood supply of the deep capillary plexus of the retina2,3 Several risk factors for acute macular neuroretinopathy have been identified, including estrogen use, pregnancy, the postpartum state and migraine. Most cases occur in young women.2 Given the shared pathogenesis and clinical phenotype, we hypothesized that deep capillary retinal ischemia may occur secondary to antiphospholipid antibodies in a subset of patients. We have identified three patients with deep capillary retinal ischemia who tested persistently positive for high titer antiphospholipid antibodies.
2. Findings
The clinical characteristics and ophthalmologic testing for the patients is summarized in Table 1. The immunological testing and antiphospholipid syndrome manifestations for the patients are summarized in Table 2.
Table 1.
Clinical characteristics and ophthalmologic testing.
| PATIENT 1 | PATIENT 2 | PATIENT 3 | |
|---|---|---|---|
| Age at first scotoma | 32 years | 26 years | 44 years |
| Gender | female | female | female |
| Eye involved | both | both | both |
| Time from first to last scotoma | 8 months | 5 years | 7 years |
| Persistent visual deficits | yes | yes | yes |
| Other eye disease | no | no | no |
| Best corrected visual acuity | 20/20 | 20/20 | 20/20 |
| Amsler visual field testing | multiple perifoveal scotoma both eyes | multiple perifoveal scotoma both eyes | multiple perifoveal scotoma both eyes |
| Optical coherence tomography | disruption of outer nuclear and outer plexiform layers | disruption of outer plexiform layer | disruption of outer plexiform layer |
| OCT angiography | focal deep capillary loss | focal deep capillary loss | focal deep capillary loss |
| Cotton wool spot | no | yes | no |
| Fluorescein angiography | normal | normal | normal |
| Vascular risk factors | labile hypertension | none | none |
| Exogenous estrogen use | yes | yes | yes |
| Brain MRI with gadolinium | normal | normal | normal |
Table 2.
Immunological testing and APS manifestations.
| PATIENT 1 | PATIENT 2 | PATIENT 3 | |
|---|---|---|---|
| Antiphospholipid antibodies—initial* | PT IgG (>=20): 43 U | PSPT IgG (>=30): >80 U aCL IgM (>=12.5): 15 U | PSPT IgG (>=30): 64 U aCL IgM (>=12.5); 10 U |
| Antiphospholipid antibodies—repeat | PT IgG (>=20): 47 U | PSPT IgG (>=30): 40 U aCL IgM (>=12.5): 14 U | PSPT IgG (>=30): 42 U aCL IgM (>=12.5); 14 U |
| Possible APS manifestations | labile hypertension | headache, tachycardia, fatigue, mitral valve thickening | livedo reticularis, memory loss, fatigue, unexplained hearing loss |
| Pregnancy | never attempted | never attempted | never attempted |
| ANA† | negative | negative | positive 1:320; ENA negative |
| C3/C4ˆ | C4 low x 2; C3 normal | normal | normal |
| Other autoimmune disease | no | no | no |
*PT = anti-prothrombin antibody; PSPT = anti-phosphatidylserine-prothrombin antibody.
aCL = anti-cardiolipin antibody; IgG = immunoglobulin G; IgM = immunoglobulin M; U = units.
†ANA = anti-nuclear antibodies; ENA = extractable nuclear antigens, i.e. analysis of specific ANA types.
ˆC3 = complement 3; C4 = complement 4.
2.1. Case 1
The patient had no past medical or ocular history except for unexplained, intermittent hypertension (to as high as 150/100) first noted in her late 20's and she had used birth control pills for many years. At age 32, she developed atraumatic pain and swelling in her right knee. This resolved spontaneously after 10–14 days, however, two weeks after the onset of the knee pain, she awoke with a paracentral scotoma in her left eye which persisted. A few weeks later, she developed a few more scotomata in the same eye. There was no eye pain, no change in her visual acuity and no headaches. She was evaluated by ophthalmology and neurology and all testing including brain MRI was reportedly negative. We do not know if formal visual field testing was performed at this time. Seven months later, she noted a new scotoma in her left eye followed by several more scotomata in both eyes which occurred over the subsequent weeks. She was referred to the University of Colorado Lions Eye Institute. Visual acuity was 20/20 in both eyes. Slit lamp examination was normal except for trace anterior vitreous cells bilaterally and funduscopic examination was normal except for rare perivascular refractile deposits in both eyes. There were no macular abnormalities, but OCT revealed focal thinning of the outer nuclear and outer plexiform layers bilaterally consistent with prior ischemia of the deep capillary plexus. Fluorescein angiography revealed a few areas of focal late vascular leakage bilaterally and OCT angiography revealed focal deep capillary drop out temporal to the fovea, corresponding to the scotoma. Antiphospholipid antibody testing was notable for a high titer anti-prothrombin IgG on two occasions 12 weeks apart. Her C4 was also low on both testing occasions--a finding seen in a subset of patients with the antiphospholipid syndrome. All of the traditional antiphospholipid antibodies (anticardiolipin, anti-beta 2 glycoprotein I and the lupus anticoagulant) were negative. Her estrogen-containing birth control was discontinued and she was treated with aspirin 81 mg daily. Labile hypertension has been reported in the antiphospholipid syndrome since the early descriptions of the syndrome and may occur due to focal renal arterial stenosis or due to autonomic dysfunction.4,5 Renal ultrasound did not find evidence of renal artery stenosis and workup for other secondary causes of hypertension was negative. Detailed review of systems otherwise was negative, she has not had other joint symptoms and she has never attempted to conceive. Her visual defects persist, but she has not had a new scotoma in the 2.5 years since her estrogen-containing birth control was stopped and aspirin was started.
2.2. Case 2
The patient was healthy when she started using birth control pills at the age of 23. At age 25, she developed severe daily headaches. The headaches lasted 30–60 minutes, were behind both eyes and were throbbing in nature. They occurred daily for months, then spontaneously resolved. At age 26, she developed a paracentral scotoma in her right eye. She was evaluated by optometry and ophthalmology, but all testing was reportedly normal and no diagnosis was made. At age 27, she developed unexplained frequent tachycardia and mild fatigue. Cardiac monitoring confirmed frequent sinus tachycardia and an echocardiogram was normal except for mild mitral valve thickening without valvular regurgitation. She did not undergo tilt table testing, but she has been treated with a beta blocker since then with symptomatic improvement. There has not been a progression of her symptoms, but she does still experience intermittent tachycardia and has mild chronic fatigue suggestive of possible mild postural tachycardia syndrome, which may occur in association with the antiphospholipid syndrome.4 She had no new issues until age 30 when frequent headaches like those she had at age 24 returned and she developed a new scotoma in her left eye. A brain MRI scan was normal. Over the next nine months she developed multiple new scotomata in both eyes. The headaches again gradually resolved spontaneously approximately six months after they started. At this point, she was evaluated at the University of Colorado Lions Eye Institute. Snellen's visual acuity was 20/20 in the right eye and 20/30 in the left eye. Slit lamp examination was normal and funduscopic examination revealed faint depigmentation in the paramacular area bilaterally and a cotton wool spot. Spectral domain optical coherence tomography (SD-OCT) revealed disruption of the outer plexiform layer bilaterally consistent with prior deep capillary retinal ischemia. Fluorescein angiography was normal, but OCT angiography revealed focal deep capillary loss. Antiphospholipid testing was notable for high titer antiphosphatidylserine-prothrombin IgG on two occasions 12 weeks apart. ANA, C3 and C4 were all normal. Her estrogen-containing oral contraception was discontinued and she was treated with aspirin 325 mg daily. She developed a new scotoma in the left eye 3 months later and aspirin was changed to apixaban 5 mg twice daily. One year later, she developed another small scotoma in the left eye and aspirin 81 mg daily was added to apixaban. She again remained stable for approximately one year when she developed another small scotoma, this time in the right eye. Aspirin was changed to clopidogrel and apixaban was continued. There has not been a recurrence in 13 months on this regimen. Given recurrent scotoma and associated fatigue, arthralgias and neuropathic pain, all of which may occur in patients with the antiphospholipid syndrome, the possibility of short-term treatment with plaquenil for 1–3 years was discussed, but she declined this option. She has not yet attempted to conceive. OCT, OCTA and Amsler grid images from Patient 2 are shown in Fig. 1.
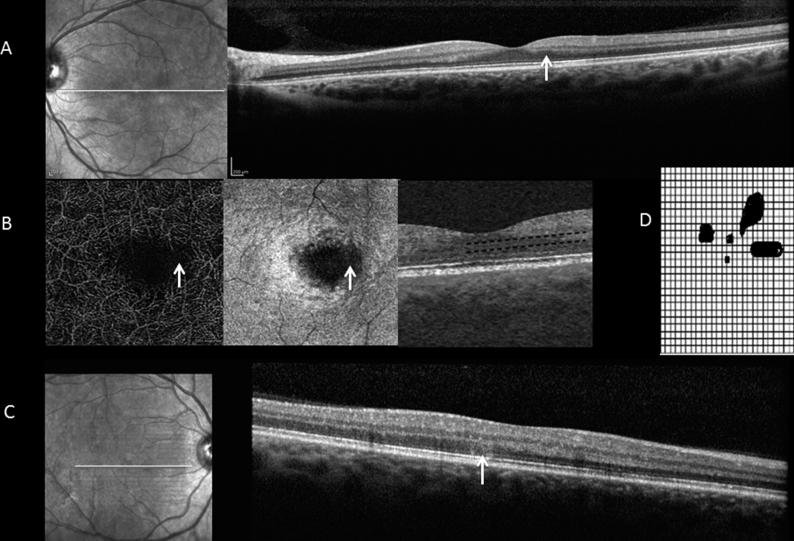
Fig. 1.
OCT, OCTA and Amsler grid from Case 2. OCT demonstrating damage to the outer plexiform layer in the left eye (arrow) (A); OCTA demonstrating deep parafoveal capillary loss in the left eye (arrow) (B); OCT demonstrating a hyper-reflective lesion in the outer retina of the right eye (arrow) (C); The corresponding Amsler grid from the right eye of this patient (D).
2.3. Case 3
There had not been significant medical or ocular history until age 45, when she developed sudden focal visual loss in her left eye while driving that affected her temporal field inferiorly. There was associated pain, but it lasted only a few minutes. There was no triggering event identified and there was no history of migraine. She was evaluated by ophthalmology within a few days. Fundoscopic examination showed bitemporal pallor and brain MRI was normal and no diagnosis was made. The deficit remained relatively stable over time, but there were periodic flares lasting 1–2 days. She initiated estrogen replacement therapy at age 51. A similar episode with sudden onset focal visual loss occurred in her right eye at age 52 and she was evaluated at the University of Colorado Lions Eye Institute. Visual field testing showed multiple perifoveal scotoma in both eyes and OCT demonstrated thinning of the outer plexiform layer bilaterally consistent with deep capillary retinal ischemia. Aspirin 325 mg daily was initiated and exogenous estrogen therapy was stopped. She was diagnosed with keratoconjuctivitis sicca and glaucoma at age 65, but there has not been recurrent scotoma in 14 years. Prior unexplained bilateral sensorineural hearing loss, which may occur in association with antiphospholipid syndrome,6 objectively improved 10 dB when aspirin was started. She also experienced fatigue, memory loss and vertigo intermittently over the last 10 years, all of which may also be seen in the antiphospholipid syndrome. Repeat brain MRI as well as serological testing for other causes of memory loss besides the antiphospholipid syndrome were negative.
3. Discussion
Antiphospholipid syndrome is an autoimmune clotting disorder estimated to affect at least 1% of the population.7 It is characterized by the persistent presence of antiphospholipid antibodies that lead to the activation of endothelial cells, platelets and monocytes and have been shown to be instrumental in the pathogenesis of the disease.8 Notably, clotting may occur in veins, arteries, and the microvasculature and there is a clear association between the antiphospholipid syndrome and several ischemic ocular conditions, including retinal ischemia.9 Trese et al.10 were the first to describe a patient with antiphospholipid antibodies and deep capillary retinal ischemia. Arf et al.11 subsequently reported a second patient with the lupus anticoagulant and prior venous and arterial thrombotic events who developed ischemia of the retinal deep capillary plexus. The present findings confirm those of the prior two case reports demonstrating the presentation with scotoma and thinning of the outer nuclear and/or outer plexiform layers in the post-acute phase as well as focal deep capillary loss on OCT angiography in all three patients. Our findings add to the prior case reports as our patients were identified retrospectively and thus inform of the natural history of undiagnosed and untreated deep capillary retinal ischemia in this context. Due to the delay in diagnosis, all patients developed bilateral recurrent lesions over 8 months to 8 years with permanent bilateral visual field deficits. Importantly, two of the three patients have stabilized for 2.5–14 years without recurrent events on aspirin and discontinuation of exogenous estrogen therapy. The other patient (patient 2) has had a more aggressive course, but she has seemed to stabilize with the combination of clopidogrel and apixaban.
All three of our patients had prothrombin-based antiphospholipid antibodies in high titer. Prothrombin-associated antiphospholipid antibodies are not currently included in the formal classification criteria for a diagnosis of definite antiphospholipid syndrome, but they have been demonstrated to be specific for the syndrome, have been associated with thrombotic complications in several publications and there is interest in adding them to the formal Sapporo criteria for the diagnosis of “definite” antiphospholipid syndrome.12 The Sapporo criteria have not been revised since 2006 and were intended to be used to create a homogeneous population of patients for research purposes in this very heterogeneous autoimmune disorder and are recognized to have significant limitations when used for diagnosis.1 Importantly, each of the present patients was using exogenous estrogen at the time of diagnosis. Estrogen use has been noted as a risk factor for deep capillary retinal ischemia with 35.6% of 101 patients reviewed in a meta-analysis having used oral contraceptive pills.2 Thus, we cannot be certain that the antiphospholipid antibodies are pathogenic in the present patients. We hypothesize they are important, however, given their high-titer, presence in all three patients, prior description of the association of antiphospholipid antibodies and deep capillary retinal ischemia in two separate cases in the medical literature, as well as the frequent additive nature of vascular risk factors. Given the very common use of oral contraception in young women and the rarity of deep capillary retinal ischemia, estrogen use alone may not be a potent enough thrombotic risk factor to cause retinal ischemia in many patients, but the combination with antiphospholipid antibodies may be more likely to result in retinal ischemia. Indeed, estrogen has been found previously to act synergistically with antiphospholipid antibodies13 and exogenous estrogen is considered a contraindication in patients with antiphospholipid antibodies for this reason.14 Of note, several “non-thrombotic” neurological manifestations may occur in patients with the antiphospholipid syndrome, including seizures, chorea, migraine, autonomic and sensory neuropathy, sensorineural hearing loss and memory loss, and some of these manifestations have been reported to rapidly improve or even resolve on anti-platelet therapy and/or anticoagulation.15 This observation led to the hypothesis by Hughes that these manifestations may occur due to vascular “sludging” and the exquisite sensitivity of the brain to adequate blood flow.16 The outer plexiform and outer nuclear layers of the retina contain photoreceptors that are rich in mitochondria and are especially vulnerable to an interruption of blood supply.17 The multiple, bilateral scotoma in all three of our antiphospholipid antibody-positive patients suggest that the retinal ischemia seen in these patients may have been the result of vascular sludging or microthrombosis. Pecen et al.18 have demonstrated capillary perfusion defects consistent with this mechanism by OCT angiography in patients with acute macular neuroretinopathy and paracentral acute middle maculopathy. The recent demonstration of macular vascular abnormalities by OCT angiography in patients with sickle cell disease19 is also supportive of a microthrombotic/sludging pathogenesis in some patients with acute macular neuroretinopathy.
The association between antiphosholipid antibodies and deep capillary retinal ischemia is important as each of our patients had bilateral involvement with permanent visual deficits and all three went undiagnosed initially. Additionally, all of the patients reported to date with antiphospholipid antibodies and deep capillary retinal ischemia (both in the present report and in the preceding two case reports) had other manifestations likely related to their antiphospholipid antibodies including suspected hip avascular necrosis,10 transient cerebral ischemia and pulmonary embolus11 and valvular thickening, severe labile hypertension, suspected postural tachycardia syndrome, memory loss, livedo reticularis and unexplained hearing loss that improved with anti-platelet therapy (present report). Additionally, the persistent presence of high-titer antiphospholipid antibodies has important implications for long-term health, including future pregnancies and risk for systemic arterial and venous thrombotic events as well as risk for the development of other autoimmune diseases. Based on the current and previous reports, it is appropriate to screen patients with deep capillary retinal ischemia for the presence of antiphospholipid antibodies, including the prothombin-associated antibodies. In addition, deep capillary retinal ischemia should be considered in patients with antiphospholipid syndrome presenting with scotoma and referral to specialty centers for advanced multimodal imaging is warranted.
4. Conclusions
The present study is limited by its retrospective and single-center nature, the concurrent use of exogenous estrogen in all three patients, as well as retrospectively-reviewed clinical-based images. We believe, however, that our report provides further evidence that antiphospholipid antibodies may represent an important association with deep capillary retinal ischemia and that this association, if confirmed by others, may have important diagnostic and therapeutic implications. Further studies into this suspected association including optimal treatment are needed given the risk of visual loss as well as of systemic thrombosis and pregnancy morbidity.
Patient consent
Approval was obtained from the University of Colorado Multiple Institutional Review Board. Patient consent was not obtained as data was collected retrospectively without the use of patient identifiers.
Conflicts of interest
The following authors have no financial disclosures: JS, AP, VP, MM.
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
Funding
No funding or grant support
Acknowledgements
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ajoc.2019.03.010.
Contributor Information
Jill R. Schofield, Email: jill.schofield@ucdenver.edu.
Alan G. Palestine, Email: Alan.palestine@ucdenver.edu.
Victoria Pelak, Email: Victoria.pelak@ucdenver.edu.
Marc T. Mathias, Email: marc.mathias@ucdenver.edu.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Garcia D., Erkan D. Diagnosis and management of the antiphospholipid syndrome. N Engl J Med. 2018 May 24;378(21):2010–2021. doi: 10.1056/NEJMra1705454. [DOI] [PubMed] [Google Scholar]
- 2.Bhavsar K.V., Lin S., Rahimy E. Acute macular neuroretinopathy: a comprehensive review of the literature. Surv Ophthalmol. 2016;61(5):538–565. doi: 10.1016/j.survophthal.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 3.Sarraf D., Rahimy E., Fawzi A.A., Sohn E. Paracentral acute middle maculopathy: a new variant of acute macular neuroretinopathy associated with retinal capillary ischemia. JAMA Ophthalmol. 2013;131(10):1275–1287. doi: 10.1001/jamaophthalmol.2013.4056. [DOI] [PubMed] [Google Scholar]
- 4.Schofield J.R., Blitshteyn S., Shoenfeld Y., Hughes G.R. Postural tachycardia syndrome (POTS) and other autonomic disorders in antiphospholipid (Hughes) syndrome (APS) Lupus. 2014;23(7):697–702. doi: 10.1177/0961203314524468. [DOI] [PubMed] [Google Scholar]
- 5.Hughes G.R.V. The Prosser-White oration 1983. Connective tissue disease and the skin. Clin Exp Dermatol. 1984;9(6):535–544. doi: 10.1111/j.1365-2230.1984.tb00856.x. [DOI] [PubMed] [Google Scholar]
- 6.Bachor E., Kremmer S., Kreuzfelder E., Jahnke K., Seidahmadi S. Antiphospholipid antibodies in patients with sensorineural hearing loss. Eur Arch Oto-Rhino-Laryngol. 2005;262(8):622–626. doi: 10.1007/s00405-004-0877-y. [DOI] [PubMed] [Google Scholar]
- 7.Chighizola C.B., Ubiali T., Meroni P.L. Treatment of thrombotic antiphospholipid syndrome: the rational of current management—an insight into future approaches. J Immunol Res. 2015:1–20. doi: 10.1155/2015/951424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salmon J.E., de Groot P.G. Pathogenic role of antiphospholipid antibodies. Lupus. 2009;17(5):405–411. doi: 10.1177/0961203308090025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Utz V.M., Tang J. Ocular manifestations of the antiphospholipid syndrome. Br J Ophthalmol. 2011;95(4):454–459. doi: 10.1136/bjo.2010.182857. [DOI] [PubMed] [Google Scholar]
- 10.Trese M.G.J., Thanos A., Yonekawa Y. Optical coherence tomography angiography of paracentral acute middle maculopathy associated with primary antiphospholipid syndrome. Ophthalmic Surg Lasers Imaging Retina. 2017;48:175–178. doi: 10.3928/23258160-20170130-13. [DOI] [PubMed] [Google Scholar]
- 11.Arf S., Sayman Muslubas I., Hocaoglu M. Retinal deep capillary plexus ischemia in a case with antiphospholipid syndrome. Retin Cases Brief Rep. 2018;12(2):106–110. doi: 10.1097/ICB.0000000000000436. [DOI] [PubMed] [Google Scholar]
- 12.Sciascia S., Sanna G., Murru V. Anti-prothrombin (aPT) and anti-phosphatidylserine/prothrombin (aPS/PT) antibodies and the risk of thrombosis in the antiphospholipid syndrome. A systematic review. Thromb Haemostasis. 2014;111(2):354–364. doi: 10.1160/TH13-06-0509. [DOI] [PubMed] [Google Scholar]
- 13.Cervera R., Asherson R.A., Font J. Chorea in the antiphospholipid syndrome. Clinical, radiologic, and immunologic characteristics of 50 patients from our clinics and the recent literature. Medicine. 1997;76(3):203–212. doi: 10.1097/00005792-199705000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Sammaritano L.R. Contraception in patients with systemic lupus erythematosus and antiphospholipid syndrome. Lupus. 2014;23(12):1242–1245. doi: 10.1177/0961203314528062. [DOI] [PubMed] [Google Scholar]
- 15.Hughes G.R.V., Cuadrado M.J., Khamastha M.A. Headache and memory loss: rapid response to heparin in the antiphospholipid syndrome. Lupus. 2001;10:778. doi: 10.1177/096120330101001103. [DOI] [PubMed] [Google Scholar]
- 16.Hughes G.R.V. Heparin, antiphospholipid antibodies and the brain. Lupus. 2012;21:1039–1040. doi: 10.1177/0961203312451336. [DOI] [PubMed] [Google Scholar]
- 17.Yu D.Y., Cringle S.J., Yu P.K. Intraretinal oxygen distribution and consumption during retinal artery occlusion and graded hyperoxic ventilation in the rat. Investig Ophthalmol Vis Sci. 2007;48(5):2290–2296. doi: 10.1167/iovs.06-1197. [DOI] [PubMed] [Google Scholar]
- 18.Pecen P.E., Smith A.G., Ehlers J.P. Optical coherence tomography angiography of acute macular neuroretinopathy and paracentral acute middle maculopathy. JAMA Ophthalmol. 2015 Dec;133(12):1478–1480. doi: 10.1001/jamaophthalmol.2015.4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Han I.C., Tadarati M., Scott A.W. Macular vascular abnormalities identified by optical coherence tomographic angiography in patients with sickle cell disease. JAMA Ophthalmol. 2015;133(11):1337–1340. doi: 10.1001/jamaophthalmol.2015.2824. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.