Abstract
Candida parapsilosis (C. parapsilosis) has become a common pathogen, especially in immunocompromised hosts. Here, we present an immunocompetent adult with greenish-black discoloration of the right first finger nail in combination with recurrent onycholysis. C. parapsilosis was isolated from the right first finger nail and was confirmed by morphological characteristics as well as by DNA molecular analysis. Patient was successfully treated with oral itraconazole in a regimen of 5 cycles of 200 mg twice daily for one week, followed by an interruption of treatment for 3 weeks. To our knowledge, this is the first report of C. parapsilosis-induced onychomycosis with recurrent onycholysis.
Keywords: Candida parapsilosis, Onychomycosis, Onycholysis, Discoloration
1. Introduction
Onychomycosis is among the most common nail disorders in adults, accounting for 15–40% of all nail diseases [1]. Candida parapsilosis (C. parapsilosis) is known to be occasionally responsible for pathological lesions of the nails [2,3]. The clinical presentations of onychomycosis caused by C. parapsilosis typically include severe dystrophy of the nail fold and plate, yellowish discoloration, and thickening and fragmentation of the plate [4].
The clinical presentation of this case is not typical of those described in the literature. The 38-year-old patient presented with onychomycosis of the right first fingernail caused by C. parapsilosis, and showed greenish-black discoloration in combination with recurrent onycholysis. The identification of the causative agent was confirmed by clinical findings, repeated fungal isolation, light microscopy, and sequencing analysis of the internal transcribed spacer (ITS) region in ribosomal RNA genes. The onychomycosis resolved completely in 5 months after treatment with oral itraconazole 200 mg twice daily for one week every month, for a total of 5 months.
2. Case
A 38-year-old female patient showed greenish-black discoloration of the proximal portion of the right first fingernail without paronychia when she was referred to our hospital on 27 January 2018 (at day 0). About twelve years ago, the right first finger nail was completely detached after an injury. It took 4 months for the finger nail to regrow (at approximately normal speed). However, the new nail exhibited a yellowish discoloration, and the discoloration gradually turned to greenish-black without pain. To our surprise, the patient reported that the finger nail would occasionally become separated from the nail bed, and would then peel off completely, followed by a full regrowth-retaining the greenish-black-discoloration-every 2–3 years. The patient did not receive any treatment during those 12 years.
The patient had no apparent underlying disease. She denied using artificial nails to protect the diseased nail. Laboratory tests at the time of presentation included a complete blood cell count, liver and renal function, venereal disease, urinalysis, stool examination, hepatitis B virus test, human immunodeficiency virus test, chest X-ray, and electrocardiogram examination. All were within normal limits or negative.
At day 0, physical examination revealed the lesion covering three-fourth of the outer edge of the nail plate with somewhat tenderness, which was schistose green changes inlaid in the buff background. The lesion, with clear edge and less lichen scale, was no significant thickening (Fig. 1A).
Fig. 1.
The lesion covered three-fourth of the outer edge of the nail plate with black-greenish discoloration (A), the skin lesion showed significant reduction over a period of 4 months of treatment (B), and the lesions fully healed 9 months later (C).
At day 0, direct microscopic examination of the shedding nails and nail plate scrapings in 20% KOH revealed fungal infection. Chain spores were observed (Fig. 2). Cell culture was negative. Subungual debris was inoculated onto Sabouraud's glucose agar (SGA) slants containing chloramphenicol (0.5 mg/ml), respectively, at 25 °C (Fig. 3A). Repeated examination of the nail sample after one week and two months, respectively, revealed the same picture on direct microscopy, and a duplicate culturing on SGA yielded growth of the same strain (Fig. 3B and C). No other fungi were isolated.
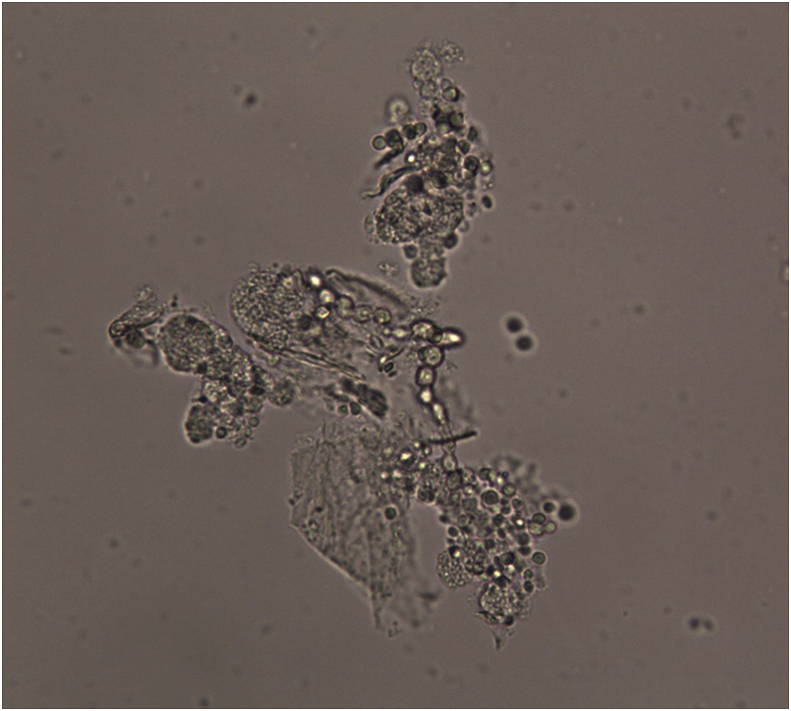
Fig. 2.
Microscopic examination revealed sub-spherical budding yeast-like cells without pseudomycelium characterizing Candida species.
Fig. 3.
On Sabourauds glucose agar at 25 °C, cream-colored yeast-like colonies firstly developed in 7 days (A), secondly grew over a period of 1 week of treatment in 7 days (B), and third time produced during 2 months of therapy in 7 days (C).
In order to identify the strain using gene analysis, we amplified the ITS, we used primers ITS1 and ITS4 [5]. In each 25μl reaction tube 5 pmol of each primer and 40 ng on template DNA were added. PCR reaction was carried out using MiniCycler ™. The pre-denaturation step at 95 °C for 5 min was followed by: denaturation at 95 °C for 2 s, annealing at 48 °C for 30 s, extension at 72 °C for 1 min. The last three steps were repeated 35 times, with a last extension 72 °C for 6 min. The PCR production were purified and sequenced. Based on a megablast search using the ITS sequence, the closest matches in NCBI's GenBank nucleotide database were Di. simplex (GenBank FJ662414; Identities 523/523 (100%), no gap (0%)). The fungus was identified using gene analysis as C. parapsilosis.
The patient received empirical treatment with oral itraconazole in 4-week cycles of 200 mg twice daily for one week, with an interruption of the drug for the following three weeks. Over a period of 16 weeks of treatment (at day+112 days), the nail condition improved satisfactorily (Fig. 1B). The onychomycosis completely resolved after another 2-cycle treatment (Fig. 1C). The patient reported no side effects from the drug.
The in vitro susceptibility of the strain to seven antifungal agents was determined using the microdilution method in accord with the guidelines of the Clinical and Laboratory Standards Institute (CLSI) M27Ed4 at day 0+10 months [6]. The minimum inhibitory concentrations (MICs) were defined as the lowest concentration at which no growth occurred which led to the results in Table 1. The treatment was never adjusted. No recurrence of cutaneous lesions was seen at a 4-month follow-up visit.
Table 1.
In vitro susceptibilities of the Candida parapsilosis to antifungal agents.
| Antifungal agent | MIC range, μg/mL |
|---|---|
| Itraconazole | <0.0313 |
| Fluconazole | <0.0625 |
| Ketoconazole | <0.0313 |
| Terbinafine | 0.0625 |
| Posaconazole | <0.0313 |
| Voriconazole | <0.0313 |
| Amphotericin B | 1 |
MIC; Minimum Inhibitor Concentration.
3. Discussion
C. parapsilosis was traditionally regarded as an accidental rather than pathogenic organism in nail isolates. However, C. parapsilosis is known to be responsible for some pathological lesions in the nails [7]. Although C. parapsilosis is found to coexist with other fungal pathogens, it has rarely been reported that onychomycosis is due to C. parapsilosis alone [4,8]. We report a clear case of onychomycosis caused by C. parapsilosis alone. C. parapsilosis was isolated three times in the lesion to the exclusion of other fungi and bacteria, especially Pseudomonas spp., supporting the view that C. parapsilosis may be responsible for this clinical manifestation.
Most cases of patients with nail infection by C. parapsilosis are associated with risk factors, such as hemodialysis, ventricular septal defect, kidney transplantation and premature birth [2,4,9]. In the present case, owing to the absence of systemic disease, the main risk factors for C. parapsilosis nail infection could be previous detachment of the nail after an injury. Although C. parapsilosis has often been isolated from skin as normal flora, topical long duration of trauma may be a risk factor for the fungal invasion.
The main clinical features of Candidal nail infection were defined as (i) hyperkeratosis of the whole nail plate with distortion of the normal curvature and distal erosion; (ii) chronic proximal paronychia with irregular transverse grooves and ridges and discoloration of the lateral margin; and (iii) isolated distal and lateral onychomycosis. However, some onychomycosis cases showed atypical Candidal clinical features. P. Gautret reported a patient with onychomycosis of twenty nails due to C. papapsilosis with blackish discoloration [8]. A 4-year-old male child with peri-membranous ventricular septal defect developed sloughed-off nails with yellowish discoloration [4]. However, the clinical presentations of greenish-black discoloration and recurrent onycholysis have not been described in the literature before. We speculate that the patient's reported recurrent onycholysis and persistent nail discoloration was due to the fungus persisting in the nail bed. Therefore, persistent infection destroyed both nail bed and nail, leading to nail separation.
The main issue from a clinical standpoint is whether removal of the organisms with systemic antifungal therapy will lead to significant improvement in nail dystrophy. C. parapsilosis can be removed by local application of amorolfine when the lesion affects only one nail [8]. When several nails are involved, an oral treatment by terbinafine or fluconazole in combination with topical application amphotericin B may be proposed, as C. parapsilosis is very sensitive to those drugs [4]. The present patient was successfully treated with oral itraconazole without any topical application. According to in vitro drug susceptibility, the isolated strain is sensitive to most antifungal drugs, and the patient had no history of antifungal drug use.
In conclusion, it is very likely that C. parapsilosis colonizes the surface of the nails easily, but will invade the nail and nail bed only in response to risk factors such as systemic disease, nail disease or trauma [10]. It is unclear what agent is responsible for the greenish-black discoloration in this and certain other cases [8], although recurrent onycholysis seems a likely result of withholding treatment for such a long time.
Conflict of interest
The authors have no conflicts of interest in connection with this study.
Acknowledgements
This work was supported in part by grants from the National Natural Science Foundation of China (NM.81773337 and 81401653) and was funded by the Shandong Traditional Chinese Medicine Science and Technology Development Plans, China (NM 2017-415), the Medical and Health Science Technology Project of Shandong Province, China (NM 2017WS345), and the Natural Science Foundation of Shandong Province, China (NM. ZR2015HL127).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.mmcr.2019.04.005.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Burns Tony, Breathnach Stephen, Cox Neil H., Griffiths Christopher., editors. Rook's Textbook of Dermatology. eighth ed. Blackwell Publishing Ltd.; 2010. chapter:36.35. [Google Scholar]
- 2.AMS F., Ventura C.G., Criado P.R. Hemodialysis and kidney transplantation as predisposing conditions to onychomycosis. Nephron. 2017;137(1):38–46. doi: 10.1159/000475674. [DOI] [PubMed] [Google Scholar]
- 3.Segal R., Kimchi A., Kritzman A., Inbar R., Segal Z. The frequency of Candida parapsilosis in onychomycosis. An epidemiological survey in Israel. Mycoses. 2000;43(9–10):349–353. doi: 10.1046/j.1439-0507.2000.00582.x. [DOI] [PubMed] [Google Scholar]
- 4.Hosuru S.S., Hamal D., Nayak N., Gokhale S. Onychomycosis due to Candida parapsilosis in a child with ventricular septal defect: an unusual predisposition. Case Rep. Pediatr. 2016;(2016):7026068. doi: 10.1155/2016/7026068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.White T.J., Bruns T., Lee S., Taylor J.M. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis M.A., Gelfand D.H., Sninsky J.J., White T.J., editors. PCR Protocols: a Guide to the Methods and Applications. Academic Press; New York: 1990. pp. 315–322. [Google Scholar]
- 6.Clinical and Laboratory Standards Institute . third ed. Clinical and Laboratory Standards Institute; Wayne, PA: 2008. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; Approved Standard. CLSI document M27-A3. [Google Scholar]
- 7.Ameen M., Lear J.T., Madan V., Mohd M.M.F., Richardson M. British Association of Dermatologists' guidelines for the management of onychomycosis 2014. Br. J. Dermatol. 2014;171(5):937–958. doi: 10.1111/bjd.13358. [DOI] [PubMed] [Google Scholar]
- 8.Gautret P., Rodier M.H., Kauffmann-Lacroix C., Jacquemin J.L. Case report and review. Onychomycosis due to Candida parapsilosis. Mycoses. 2000;43(11–12):433–435. [PubMed] [Google Scholar]
- 9.Koklu E., Gunes T., Kurtoglu S., Gokoglu S., Koklu S. Onychomycosis in a premature infant caused by Candida parapsilosis. Pediatr. Dermatol. 2007;24(2):155–156. doi: 10.1111/j.1525-1470.2007.00365.x. [DOI] [PubMed] [Google Scholar]
- 10.Tosti A., Hay R., Arenas-Guzmán R. Patients at risk of onychomycosis-risk factor identification and active prevention. J. Eur. Acad. Dermatol. Venereol. 2005;19(Suppl 1):13–16. doi: 10.1111/j.1468-3083.2005.01282.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.