Abstract
Patients with chronic kidney disease (CKD) have accelerated bone loss, vascular calcification and abnormal biochemistries, together contributing to an increased risk of cardiovascular disease and fracture-associated mortality. Despite evidence of vascular pathologies and dysfunction in CKD, our group has shown that cortical bone tissue perfusion is higher in a rat model of high-turnover CKD. The goal of the present study was to test the hypothesis that parathyroid hormone (PTH) suppressive interventions would normalize cortical bone vascular perfusion in the setting of CKD. In two separate experiments, 35-week old CKD animals and their normal littermates, underwent intra-cardiac fluorescent microsphere injection to assess the effect of 10 weeks of PTH suppression (Experiment 1: calcium supplementation, Experiment 2: calcimimetic treatment) on alterations in bone tissue perfusion. In Experiment 1, CKD animals had serum blood urea nitrogen (BUN) and PTH levels significantly higher than NL (+182% and +958%; p<0.05). CKD+Ca animals had BUN levels that were similar to CKD, while PTH levels were significantly lower and comparable to NL. Both femoral cortex (+220%, p=0.003) and tibial cortex (+336, p=0.005) tissue perfusion were significantly higher in CKD animals when compared to NL; perfusion was normalized to those of NL in CKD+Ca animals. MicroCT analysis of the proximal tibia cortical porosity showed a trend toward higher values in CKD (+401%; p=0.017) but not CKD+Ca (+111%; p = 0.38) compared to NL. Experiment 2, using an alternative method of PTH suppression, showed similar results as those of Experiment 1. These data demonstrate that PTH-suppression based interventions normalize cortical bone perfusion in the setting of CKD.
Keywords: CKD-MBD, Bone blood flow, Bone vascular, Etelcalcitide
INTRODUCTION
Chronic kidney disease–mineral and bone disorder (CKD‐MBD), consisting of accelerated bone loss, vascular calcification, and abnormal biochemistries [1], contributes to an increased risk of premature death due to cardiovascular disease and fracture-associated mortality [2]. Understanding the underlying mechanisms of cardiovascular and skeletal alterations due to the sequelae of reduced kidney function, including mechanisms that overlap between cardiovascular and skeletal alterations, is crucial to developing novel ways to reduce mortality in CKD patients.
Patients with CKD have vascular dysfunction through endothelium-dependent, endothelium-independent, and/or vascular remodeling mechanisms[3], along with decreased cardiac output [4], and vascular calcification [5], suggesting that altered skeletal perfusion can be expected in CKD. Bone health and homeostasis are dependent on regulated skeletal perfusion [6] and conditions that result in altered bone perfusion have been shown to be associated with bone loss [7]. Data from our lab have shown that cortical bone perfusion is elevated in a rat model of chronically high parathyroid hormone (PTH), high remodeling CKD [8]. However, whether vascular dysfunction and the bone abnormalities are related in CKD is not known.
Sustained elevation of serum PTH in the setting of CKD has been shown, by our group and others, to contribute to compromised bone quantity and quality via elevated bone turnover and increased cortical porosity[9]. Currently, the primary goal of CKD-MBD therapy is the suppression of elevated levels of PTH [10] utilizing calcitriol (and its analogues) and calcimimetics. Our group has previously shown that the suppression of PTH levels with calcium supplementation in drinking water in rodents substantially mitigates the deleterious effects of CKD on bone parameters, essentially normalizing bone turnover and mass [11]. Calcimimetics, such as the recently FDA-approved etelcalcitide, suppress PTH without contributing to the increased vascular calcification [12] observed with calcium supplementation in rodents [11]. For this reason, the present study utilizes both calcium supplementation and a calcimimetic to evaluate the effect of PTH suppression therapies on cortical bone perfusion. We hypothesized these interventions would normalize CKD-induced elevations in cortical bone perfusion.
METHODS
Animals.
Male Cy/+ (CKD) rats, Han:SPRD rats are characterized with a spontaneous and slowly progressive kidney disease [13]. Blood urea nitrogen (BUN) and creatinine are elevated by 10 weeks of age. With progression of CKD, hyperphospatemia, hyperparathyroidism, and skeletal abnormalities develop and ultimately progress to dramatic manifestations of cardiac and vascular calcification as well as severe cortical porosity and compromised bone mechanical properties [13].
Experimental Design.
Experiment 1:
CKD animals (n=12) and their normal littermates (NL, n=6) were used to assess alterations in skeletal perfusion at 35 weeks of age (~15% normal kidney function in the Cy/+ model). The CKD treatment animals were divided into two groups (n=6/group) at 25 weeks of age: half were administered normal deionized drinking water (CKD) and the other half administered calcium gluconate (3% Ca) in the drinking water (CKD+Ca). The calcium gluconate was used as a phosphate binder with the goal of PTH suppression as shown previously [9]. Age‐matched normal (NL) littermate animals (n=6) were used as a comparator group to determine if treatment normalized skeletal perfusion. All animals were fed a casein diet (Purina AIN‐76A, Purina Animal Nutrition, Shreevport, LA, USA); 0.53% Ca and 0.56% P) during the experimental period (weeks 25–35), which has been shown to produce a more consistent kidney disease in this model [13]. At 35 weeks, all animals were assessed for serum biochemistries approximately 24 hours before undergoing in vivo microsphere injection to assess bone tissue perfusion. Tibiae, femora, and testes were collected for analyses.
Experiment 2:
In order to differentiate the effects of PTH lowering from that of increased calcium intake, CKD animals (n=6) and their normal littermates (n=4) were used to assess the effect of KP-2326 (KP), an active metabolite of the calcimimetic etalcalcitide, on CKD-induced alterations in skeletal perfusion. The CKD treatment groups (n=3/group) were administered either vehicle injections thrice weekly or KP injection thrice weekly (i.p. 1mg/kg) from age 25 to 35 weeks. At 35 weeks, all animals were assessed for serum biochemistries approximately 24 hours before undergoing in vivo microsphere injection to assess bone tissue perfusion. Tibiae were collected for analysis.
Plasma Biochemistries.
Blood plasma was analyzed for blood urea nitrogen (BUN), calcium, phosphorus, and creatinine using colorimetric assays (Point Scientific, Canton, MI, USA; or Sigma kits; Sigma, St. Louis, MO, USA). Intact PTH was determined by ELISA (Alpco, Salem, NH, USA).
Bone perfusion measurement.
Microsphere injection was performed as we previously described [14]. Briefly, under isoflurane anesthesia, polystyrene red fluorescent (580/605), 15 μm microspheres (FluoSpheres, ThermoFisher) were injected into the apex of the beating left ventricle. The animal was euthanized by cardiac dissection 60 seconds after the completion of the injection, allowing for the circulation of spheres. A total of 5.0×106 spheres/kg were injected, a number sufficient to assess perfusion in skeletal tissue [14].
Tibiae, femora, and testes were collected and weighed. Femoral diaphyses were isolated, thoroughly flushed of marrow, and weighed. Marrow was extracted from the tibial diaphysis by centrifugation; both marrow and tibial cortex were weighed. In Experiment 2, only the tibia was collected for perfusion analysis. The tibial cortex was isolated and marrow was separated by centrifugation.
Samples isolated for tissue perfusion measures were processed as previously described [14]. Briefly, bone samples were placed in a Cal-Ex Decalcifier solution for 7 days. Decalcified bone samples were degraded in 10% ethanolic potassium hydroxide (KOH) for 24 hours. Soft tissue samples (testes) were placed in KOH directly. Degraded samples were filtered through polyamide mesh filters (5 μm pore size) and placed into microcentrifuge vials along with 1mL of Cellosolve acetate (2-ethoxyethyl acetate, 98%, Sigma) to dissolve the microspheres and distribute the fluorescence into solution.
Fluorescence measurements were performed as previously described [14]. Briefly, three 100μL aliquots from each sample were placed in a 96-well V-bottom polypropylene microplate for fluorescence quantification using the SpectraMax i3x microplate reader (Molecular Devices, CA). The readings from the three aliquots were averaged to produce a single fluorescence measurement per sample. Red fluorescence was measured using an excitation of 580nm and an emission of 620nm. All data is presented as tissue fluorescence density (TFD) with units of Arbitrary Units per gram of tissue (AU/g) and scaled by 106.
μCT Imaging.
Tibiae were scanned (Skyscan 1172, 6 micron resolution) to obtain cortical morphology at a standardized location (~2.5mm distal to the proximal growth plate). Cortical parameters included total cross-sectional area, bone area, marrow area, cortical thickness, and cortical porosity. Porosity was calculated as the percent of void area within the total cross sectional area (100 – BA/TA*100). Nomenclature is reported in accordance with ASBMR guidelines [15].
Statistics.
All analyses were performed using GraphPad Prism software. Comparisons among groups were assessed by Kruskal-Wallis analysis of ranks with Dunn’s multiple comparisons post-hoc tests when appropriate. A priori α-levels were set at p ≤ 0.05 to determine statistical significance.
RESULTS
Plasma Biochemistries.
Experiment 1:
Animals with CKD had serum blood urea nitrogen (BUN) and PTH levels that were significantly higher than NL (+182% and +958%; p<0.05) (Table 1). CKD+Ca animals had BUN levels that were not different from CKD (p=0.7922), while PTH levels were significantly lower than CKD alone (p=0.004) and not different from NL (Table 1).
Table 1:
Serum biochemistries at end point (35 weeks)
| Experiment 1 | |||
|---|---|---|---|
| Normal | CKD | CKD+Ca | |
| BUN (mg/dL) | 17.8 ± 1.9* | 50.4 ± 8.0 | 49.1 ± 9.4 |
| PTH (mg/dL) | 123 ± 49 | 2105 ± 139* | 33 ± 42 |
| Experiment 2 | |||
| Normal | CKD | CKD+KP | |
| BUN (mg/dL) | 20.8 ± 1.8 | 45.7 ± 2.6 | 51.2 ± 8.7 |
| PTH (mg/dL) | 263 ± 66 | 6840 ± 6935 | 403 ± 265 |
Data presented as mean and standard deviation.
p<0.05, different from all other groups
Experiment 2:
Animals with CKD had elevated serum blood urea nitrogen (BUN) and PTH levels compared to NL (+119% and +2700%), although due to variability and low sample size, the results did not reach statistical significance (Table 1). CKD+KP animals had BUN levels that were not different from CKD, while PTH levels were lower than CKD alone and not different from NL (Table 1).
Bone geometry.
Experiment 1:
Cortical mean cross-sectional area (p=0.002) was significantly lower in CKD animals when compared to NL animals, while the calcium supplementation (CKD+Ca) group was not different from NL (Table 2). MicroCT analysis of the proximal tibia cortical porosity was higher in CKD (+401% vs. NL; p=0.017) and not different in CKD+Ca (+111% relative to NL; p = 0.38) (Table 2).
Table 2:
Proximal tibia cortical geometry
| Experiment 1 | |||
|---|---|---|---|
| Normal | CKD | CKD+Ca | |
| Cortical Area (mm2) | 7.3 ± 0.2 | 6.1 ± 0.7* | 7.1 ± 0.3 |
| Cortical Porosity (%) | 1.1 ± 2.0 | 5.7 ± 4.7 | 2.4 ± 2.3 |
| Experiment 2 | |||
| Normal | CKD | CKD+KP | |
| Cortical Area (mm2) | 6.0 ± 0.3 | 4.8 ± 1.1 | 5.5 ± 0.1 |
| Cortical Porosity (%) | 2.8 ± 0.5 | 28.3 ± 22.25* | 3.9 ± 2.2 |
Data presented as mean and standard deviation.
p<0.05, compared to Normal group
Experiment 2:
MicroCT analysis of the proximal tibia demonstrated cortical porosity was significantly higher in CKD (+925%; p=0.05) vs. NL, but not different in CKD+KP (+42%; p = 0.86) animals relative to NL. Cortical mean cross-sectional area (ANOVA, p=0.09) showed a trend toward lower values in CKD (−20%; p=0.078) compared to NL while CKD+KP (−8%; p = 0.70) animals were not different compared to NL (Table 2).
Bone perfusion measurement.
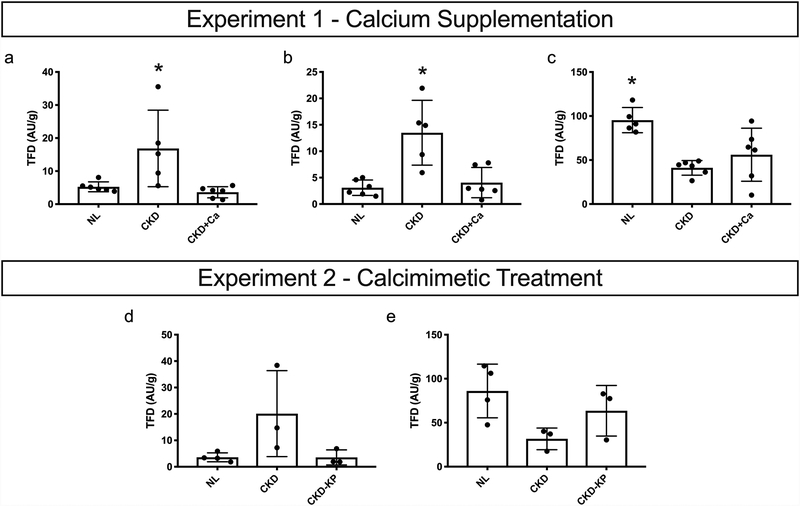
Experiment 1:
Femoral cortex (p=0.003) and tibial cortex (p=0.005) tissue perfusion were both significantly higher in CKD animals when compared to NL animals (Figure 1A&B). Calcium supplementation resulted in femoral and tibial cortical perfusion that was not different than normal. Isolated marrow perfusion (p=0.005) was significantly lower in CKD animals when compared to NL animals (Figure 1C). Calcium supplementation resulted in isolated marrow perfusion with values not different from CKD animals receiving no treatment.
Figure 1:
Bone perfusion data. Experiment 1: Tissue fluorescence density (TFD) of (A) femoral cortical bone (Kruskal-Willis, p=0.0030) (B) tibial cortical bone (Kruskal-Willis, p=0.0051) (C) tibial bone marrow (Kruskal-Willis, p=0.0047). Experiment 2: Tissue fluorescence density (TFD) of (A) tibial cortical bone (Kruskal-Willis, p=0.0505) (B) tibial bone marrow (Kruskal-Willis, p=0.0780). Dots represent data points, and error bars represent standard deviation. *p<0.05, different from all other groups
Experiment 2:
Tibial cortex (p=0.05) tissue perfusion was significantly higher in CKD animals when compared to NL animals (Figure 1D). KP treatment resulted in tibial cortical perfusion that was not different than normal. Isolated marrow perfusion showed a trend toward lower values in CKD (−63%; p=0.11) vs. NL, while CKD+KP were not different from NL (−26%; p = 0.40) (Figure 1E).
DISCUSSION
CKD causes progressive damage to bone. Bone deterioration in CKD can be characterized by changes in turnover, mineralization, and volume [1], where, most commonly, high turnover drives bone loss. Altered bone turnover/remodeling has been associated with changes in bone perfusion in a number of conditions including diabetes, disuse, aging, and estrogen withdrawal [16] }. In turn, reduced bone perfusion has also been associated with bone loss in a number of conditions [7]. Since CKD is known to be associated with both cardiac and vascular abnormalities, we investigated skeletal perfusion in the setting of CKD to provide insights into the pathophysiology of skeletal changes in CKD.
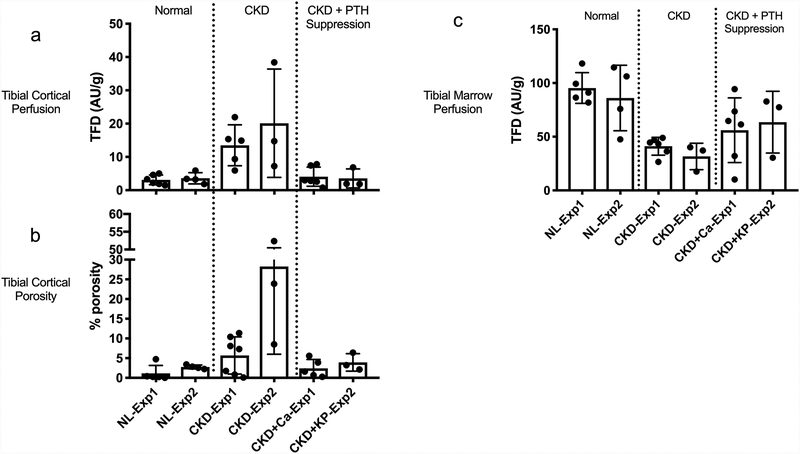
Previous studies, by our group and others, have shown that sustained elevation of serum PTH in the setting of CKD contributes to compromised bone quantity and quality [9]. In the present study, with the use of fluorescent microspheres to measure regional perfusion in bone, we show that two methods of PTH suppression normalize CKD-induced elevations in cortical bone perfusion. CKD animals with calcium supplementation and calcimimetic treatment had PTH levels that were suppressed by 98% and 94% relative to untreated CKD animals, respectively, yielding PTH levels that were not significantly different than normal animals. Our group has previously shown that the suppression of PTH levels with calcium supplementation in drinking water has substantial positive skeletal effects, essentially normalizing bone turnover and volume [11]. In the present study, calcium supplementation and calcimimetic treatment resulted in higher cortical bone area along with normalized cortical bone perfusion (Figure 2A–B). The normalization of cortical bone perfusion via two methods of PTH suppression suggests that perfusion elevations are either dependent on PTH, or dependent on a factor secondary to elevated PTH, such as increased cell metabolism. PTH suppression can reduce the direct effects of PTH on the endothelial expression of vascular endothelial growth factor [17] and consequently normalize CKD-induced elevation in cortical bone perfusion. On the other hand, PTH suppression can result in the attenuation of the increased metabolic demands [18] of high turnover CKD secondary to elevated PTH levels.
Figure 2:
Combined graphs with data from Experiments 1 & 2: (A) tibial cortical perfusion, (B) tibial cortical porosity, (C) tibial marrow perfusion
The current study demonstrates lower marrow perfusion in the setting of high PTH/high remodeling CKD in two separate experiments that is not completely normalized with either calcium supplementation or calcimimetic therapy (Figure 2C). The lack of normalization of CKD-induced lowering of marrow perfusion in the low PTH/low remodeling CKD state suggests that marrow perfusion is not dependent on the high remodeling state in the setting of CKD. Other factors including arterial perfusion pressures, myogenic, endothelial, neural and hormonal disturbances may explain alterations of marrow perfusion in the setting of CKD. The potential role of medullary hypoxia on increased bone resorption via differentiation of osteoclasts requires further investigation.
Our results should be interpreted in the context of various assumptions and limitations. One limitation of the study is that only male rats were utilized in this study. Female Cy/+ animals do not develop notable changes in cortical bone properties and thus were not studied in the current context. Injection of microspheres in the left ventricle to assess perfusion is based on a set of assumptions and has multiple limitations as discussed in our previous work [14]. While these limitations are important to consider from a technical standpoint, we have now consistently shown in several studies that in CKD in the setting of high PTH there is higher cortical perfusion. Another limitation of our current work is the low number of animals in Experiment 2 which resulted in the lack of a significant difference in NL vs.CKD PTH levels in Experiment 2. Despite this limitation, the results of calcimimetic treatment paralleled those observed with calcium-induced suppression of PTH in that both treatments normalize cortical bone perfusion, without effect on marrow perfusion (Figure 2A–C), thus suggesting that the effects may be due to PTH suppression rather than the calcium treatment. Further work will be needed to confirm the effect of PTH suppression on cortical bone and marrow perfusion.
The effect of CKD-induced perfusion elevations in cortical bone are normalized by interventions aimed at suppression of PTH. The normalization of cortical bone perfusion via two methods of PTH suppression suggests that perfusion elevations are may be PTH dependent, either directly, or indirectly mediated via another factor. Determining the mechanisms of these bone perfusion alterations and whether they are drivers, propagators, or consequences of skeletal deterioration in CKD will necessitate further work but will help untangle a key player in CKD-induced bone quality alterations.
Acknowledgements
This work was supported by a United States (U.S.) Department of Veterans Affairs grant (BX003025) to MRA. MWA was supported by F30 DK115162 and T32 AR065971 during separate portions of this work. KP-2326 was provided through a material transfer agreement with Amgen.
References
- 1.Group KUW (2017) KDIGO 2017. Clinical Practice Guideline Update for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease — Mineral and Bone Disorder (CKD-MBD). KIDNEYS 6:149–154. doi: 10.22141/2307-1257.6.3.2017.109030 [DOI] [Google Scholar]
- 2.Demer L, Tintut Y (2010) The bone–vascular axis in chronic kidney disease. Current Opinion in Nephrology and Hypertension 19:349–353. doi: 10.1097/MNH.0b013e32833a3d67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dhaun N (2006) The Endothelin System and Its Antagonism in Chronic Kidney Disease. Journal of the American Society of Nephrology 17:943–955. doi: 10.1681/ASN.2005121256 [DOI] [PubMed] [Google Scholar]
- 4.Bleeker GB, Bax JJ, Steendijk P, et al. (2006) Left ventricular dyssynchrony in patients with heart failure: pathophysiology, diagnosis and treatment. Nat Clin Pract Cardiovasc Med 3:213–219. doi: 10.1038/ncpcardio0505 [DOI] [PubMed] [Google Scholar]
- 5.Moe SM, Chen NX (2008) Mechanisms of Vascular Calcification in Chronic Kidney Disease. Journal of the American Society of Nephrology 19:213–216. doi: 10.1681/ASN.2007080854 [DOI] [PubMed] [Google Scholar]
- 6.Marenzana M, Arnett TR (2013) The Key Role of the Blood Supply to Bone. Bone Res 1:203–215. doi: 10.4248/BR201303001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arnett TR (2010) Acidosis, hypoxia and bone. Arch Biochem Biophys 503:103–109. doi: 10.1016/j.abb.2010.07.021 [DOI] [PubMed] [Google Scholar]
- 8.Aref MW, Swallow EA, Chen NX, et al. (2018) Skeletal vascular perfusion is altered in chronic kidney disease. Bone Reports 8:215–220. doi: 10.1016/j.bonr.2018.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moe SM, Chen NX, Newman CL, et al. (2014) A Comparison of Calcium to Zoledronic Acid for Improvement of Cortical Bone in an Animal Model of CKD. J Bone Miner Res 29:902–910. doi: 10.1002/jbmr.2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kandula P, Dobre M, Schold JD, et al. (2011) Vitamin D supplementation in chronic kidney disease: a systematic review and meta-analysis of observational studies and randomized controlled trials. Clin J Am Soc Nephrol 6:50–62. doi: 10.2215/CJN.03940510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Newman CL, Chen NX, Smith E, et al. (2015) Compromised vertebral structural and mechanical properties associated with progressive kidney disease and the effects of traditional pharmacological interventions. Bone 77:50–56. doi: 10.1016/j.bone.2015.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu L, Tomlinson JE, Alexander ST, et al. (2017) Etelcalcetide, A Novel Calcimimetic, Prevents Vascular Calcification in A Rat Model of Renal Insufficiency with Secondary Hyperparathyroidism. Calcif Tissue Int 101:641–653. doi: 10.1007/s00223-017-0319-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moe SM, Chen NX, Seifert MF, et al. (2009) A rat model of chronic kidney disease-mineral bone disorder. Kidney Int 75:176–184. doi: 10.1038/ki.2008.456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aref MW, Akans E, Allen MR (2017) Assessment of regional bone tissue perfusion in rats using fluorescent microspheres. Bone Reports 6:140–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bouxsein ML, Boyd SK, Christiansen BA, et al. (2010) Guidelines for assessment of bone microstructure in rodents using micro–computed tomography. J Bone Miner Res 25:1468–1486. doi: 10.1002/jbmr.141 [DOI] [PubMed] [Google Scholar]
- 16.Prisby RD, Dominguez JM, Muller-Delp J, et al. (2012) Aging and estrogen status: a possible endothelium-dependent vascular coupling mechanism in bone remodeling. PLoS ONE 7:e48564. doi: 10.1371/journal.pone.0048564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rashid G, Bernheim J, Green J, Benchetrit S (2008) Parathyroid hormone stimulates the endothelial expression of vascular endothelial growth factor. European Journal of Clinical Investigation 38:798–803. doi: 10.1111/j.1365-2362.2008.02033.x [DOI] [PubMed] [Google Scholar]
- 18.Adair TH, Gay WJ, Montani JP (1990) Growth regulation of the vascular system: evidence for a metabolic hypothesis. Am J Physiol 259:R393–404. doi: 10.1152/ajpregu.1990.259.3.R393 [DOI] [PubMed] [Google Scholar]