Abstract
Background
The relationships between first-line drug concentrations and clinically important outcomes among patients with tuberculosis (TB) remain poorly understood.
Methods
We enrolled a prospective cohort of patients with new pulmonary TB receiving thrice-weekly treatment in India. The maximum plasma concentration of each drug was determined at months 1 and 5 using blood samples drawn 2 hours postdose. Subtherapeutic cutoffs were: rifampicin <8 µg/mL, isoniazid <3 µg/mL, and pyrazinamide <20 µg/mL. Factors associated with lower log-transformed drug concentrations, unfavorable outcomes (composite of treatment failure, all-cause mortality, and recurrence), and individual outcomes were examined using Poisson regression models.
Results
Among 404 participants, rifampicin, isoniazid, and pyrazinamide concentrations were subtherapeutic in 85%, 29%, and 13%, respectively, at month 1 (with similar results for rifampicin and isoniazid at month 5). Rifampicin concentrations were lower with human immunodeficiency virus coinfection (median, 1.6 vs 4.6 µg/mL; P = .015). Unfavorable outcome was observed in 19%; a 1-μg/mL decrease in rifampicin concentration was independently associated with unfavorable outcome (adjusted incidence rate ratio [aIRR], 1.21 [95% confidence interval {CI}, 1.01–1.47]) and treatment failure (aIRR, 1.16 [95% CI, 1.05–1.28]). A 1-μg/mL decrease in pyrazinamide concentration was associated with recurrence (aIRR, 1.05 [95% CI, 1.01–1.11]).
Conclusions
Rifampicin concentrations were subtherapeutic in most Indian patients taking a thrice-weekly TB regimen, and low rifampicin and pyrazinamide concentrations were associated with poor outcomes. Higher or more frequent dosing is needed to improve TB treatment outcomes in India.
Keywords: tuberculosis, pharmacokinetics, drug concentrations, subtherapeutic concentrations, unfavorable treatment outcomes
In a prospective cohort of patients with newly diagnosed pulmonary tuberculosis receiving standard thrice-weekly antituberculosis treatment in India, lower rifampicin concentration was associated with Human Immunodeficiency Virus coinfection and independently associated with unfavorable treatment outcomes.
Worldwide, tuberculosis (TB) remains the leading cause of death from a curable infectious disease. While most patients respond well to anti-TB treatment (ATT), treatment failure and recurrence remain common [1, 2], and multidrug-resistant/rifampicin-resistant (MDR/RR) TB threatens global TB control, particularly in high-TB-burden countries. India bears the greatest global burden of TB and MDR/RR-TB [3, 4]. Generally, even when treatment failure rates are low, rates of recurrence and multidrug resistance are high among previously treated patients. Optimization of drug dosing with available drugs has become a current focus in TB research [5], yet pharmacokinetic data remain limited.
Until recently, India’s Revised National TB Control Program (RNTCP) has been treating new pulmonary TB patients with thrice-weekly, directly observed treatment–short course (DOTS), a 6-month regimen using isoniazid, rifampicin, pyrazinamide, and ethambutol. Successful treatment (defined as cure or treatment completion) has been reported in 88% of patients [4, 6], yet treatment failure, recurrence, and acquired drug resistance remain unexplained in programmatic settings [2]. According to RNTCP surveillance data, nearly 12% of previously treated patients acquire MDR/RR-TB [6], and, although data on recurrence are limited, a recent study found that 158 of 1210 (13%) patients who were successfully treated under programmatic settings developed recurrent disease within 12 months [7].
Incomplete sterilization of lesions due to low TB drug concentrations may contribute to unfavorable treatment outcomes, including treatment failure and recurrence, with potential for acquired drug resistance [8–15]. Low concentrations of rifampicin and isoniazid precede acquired drug resistance, and some studies have shown that low drug exposures were predictive of clinical outcomes in TB patients, but data are limited and are conflicting [12–14, 16].
Drug concentrations in blood collected 2 hours postdosing are often used to estimate the maximum plasma concentration (Cmax) of TB drugs, and putative therapeutic targets have been proposed, yet limited data exist describing the relationships between drug dosing or dosing frequency and poor TB treatment outcomes [17]. We aimed to determine sources of variability in 2-hour postdose concentrations of rifampicin, isoniazid, and pyrazinamide and to describe the relationship between drug concentration and poor treatment outcomes among a prospective cohort of adults with newly diagnosed pulmonary TB treated with thrice-weekly DOTS under the national program in India. The results may have implications for dosing and may identify patients at particular risk for subtherapeutic drug levels and needing enhanced care, such as higher dosing, therapeutic drug monitoring, or closer clinical follow-up.
METHODS
Study Design and Setting
We conducted this study as part of an ongoing prospective study, Cohort for Tuberculosis Research by the Indo-US Partnership (CTRIUMPh), a collaboration among the National Institute for Research in Tuberculosis (NIRT) in Chennai, India; Byramjee Jeejeebhoy Government Medical College (BJGMC) in Pune, India; and Johns Hopkins University in Baltimore, Maryland. CTRIUMPh has been enrolling at NIRT and BJGMC since August 2014 as described in detail elsewhere [18].
Our subcohort was comprised of 404 adults with newly diagnosed pulmonary TB who were enrolled from August 2014 to October 2017. Inclusion criteria were age ≥18 years, body weight >30 kg, ATT for ≥2 weeks under directly observed treatment, not critically ill, willing to participate and give informed written consent, and agreeable to visiting the same DOTS center until study completion. Diagnosis and treatment was administered by RNTCP according to national guidelines [19]. All participants received category I ATT (rifampicin, isoniazid, pyrazinamide, and ethambutol for 2 months followed by rifampicin and isoniazid for 4 months). Treatment was thrice-weekly with drug dosing as follows: rifampicin 450 mg or 600 mg for body weight ≥60 kg; isoniazid 600 mg; pyrazinamide 1500 mg; and ethambutol 1200 mg. Participants were followed at 2, 4, and 8 weeks and at 5, 6, 12, 18, and 24 months following ATT initiation to determine treatment outcomes. The study was approved by the Ethics Committees of NIRT and BJGMC and the Institutional Review Board of Johns Hopkins University.
Study Procedures
A structured questionnaire was used to collect patient details, including sociodemographic and clinical characteristics. Diabetes mellitus was defined as known history of diabetes, random blood glucose ≥200 mg/dL, or glycosylated hemoglobin (HbA1C) ≥6.5%. Smoking status was dichotomized as never smoker (smoked <100 cigarettes in their lifetime and currently not smoking) or ever smoker (smoked ≥100 cigarettes in their lifetime or currently smoking). The Alcohol Use Disorders Identification Test (AUDIT) was used to classify alcoholic status as nonalcoholic (score <8) or alcoholic (score ≥8). Baseline sputum samples were collected within 7 days of ATT initiation; cultures and drug susceptibility testing were performed using standard methods. Clinical biochemistry tests (random glucose, liver, and renal function tests), routine hematologic tests, and human immunodeficiency virus (HIV) testing were performed at baseline. The blood samples for plasma pharmacokinetics were collected at least 1 visit: month 1 of intensive and/or month 5 of continuation phases of ATT; blood was collected 2 hours following an observed dose and immediately centrifuged. Plasma was then separated and stored at –20°C until analysis. Ascorbic acid was added to plasma samples prior to freezing to preserve rifampicin.
Drug Measurements
All drug estimations were conducted at the pharmacology laboratory at NIRT that undergoes external quality assurance (International Interlaboratory Quality Control Program, the Netherlands). Plasma concentrations of rifampicin, isoniazid, and pyrazinamide were determined using high-performance liquid chromatography (Shimadzu Corporation, Kyoto, Japan) according to validated methods as previously described [20, 21]. For rifampicin extraction, acetonitrile was used; C18 column at 254 nm was used for the analysis. For isoniazid and pyrazinamide extraction, para-hydrobenzaldehyde and trifluoroacetic acid were used, respectively; column C8 at 267 nm was used for the analysis. The retention time for rifampicin, pyrazinamide, and isoniazid was 1.7, 3, and 5.5 minutes, respectively. The lower limits of quantification were 0.25, 1.25, and 0.25 μg/mL, respectively. All of the drugs had <10% within and between-run variation.
TB Treatment Outcomes and Definitions
Unfavorable outcome was defined as a composite outcome of death, treatment failure, and recurrence. Death was defined as all-cause mortality. Treatment failure and recurrence were defined as Mycobacterium tuberculosis growth on liquid or solid culture ≥4 months following either ATT initiation or successful treatment, respectively; for participants with unavailable culture results, definitions of treatment failure and recurrence were based on the presence of symptoms suggestive of TB disease and acid-fast bacilli detected on smear microscopy. Successful treatment (ie, favorable outcome) was defined as treatment completion or cure (defined as the absence of symptoms suggestive of TB disease and absence of microbiological evidence of M. tuberculosis by smear or cultures at the end of treatment).
Statistical Analysis
Data analysis was performed using Stata version 15.0 software (StataCorp, College Station, Texas). This analysis included participants with drug concentrations available at either month 1 or 5 or both. Data were verified and normality checked by Shapiro-Wilk test. Categorical and continuous variables are summarized as proportion and median with interquartile range (IQR), respectively. The Mann-Whitney U test was used to compare median drug concentrations between patient groupings based on baseline characteristics. Proportions were compared using the z proportion test. Subtherapeutic drug concentrations were defined as rifampicin <8 µg/mL, isoniazid <3 µg/mL, and pyrazinamide <20 µg/mL [22]. The covariates were identified through a review of published literature and exploratory data analysis; significant variables were retained in the multivariable model. Single and multivariable linear regression analysis was performed to identify factors influencing drug concentrations, wherein the drug concentrations were transformed using log function, without adjusting for multiple testing. Because all of the participants received the exact same dose under the national program, there was no variation in the drug dosing and we excluded it from the analysis. As body mass index (BMI) is known to have an effect on drug absorption, we used the BMI that was significantly associated with Cmax of TB drugs in the univariable analysis. Univariable and multivariable Poisson regression with person-time as offset was used to identify the effect of a 1-unit decrease in drug concentration on the composite outcome, unfavorable outcome, and individual outcomes—namely, all-cause mortality, treatment failure, and recurrence. Aggregated drug concentrations from month 1 and 5 were used for isoniazid and rifampicin, and drug concentration at month 1 was used for pyrazinamide. For recurrence models, start time was the end of treatment. Statistical significance was determined at P < .05. The power achieved by the study to report the significance difference in drug concentration between the TB outcomes was >90%, as calculated using G*power, assuming an α level of 5%.
RESULTS
Of 404 patients recruited to the subcohort, 260 (64%) were male, the median age was 39.5 years (IQR, 28–50), 113 (28%) had diabetes mellitus, and 27 (7%) were HIV seropositive (Table 1). Of 404 patients with pharmacokinetic sample testing done at least at 1 visit, 390 were tested at month 1, 359 at month 5, and 315 at both the month 1 and month 5 visits. Overall, the median rifampicin concentration was 3.6 µg/mL (IQR, 1.5–6.6) at month 1 (n = 287) and 4.5 µg/mL (IQR, 1.6–8.1) at month 5 (n = 290); the median isoniazid concentration was 5.3 µg/mL (IQR, 2.4–8.4) at month 1 (n = 328) and 6.2 µg/mL (IQR, 3.3–9.0) at month 5 (n = 309); and the median pyrazinamide concentration was 37.0 µg/mL (IQR, 27.2–36.3) at month 1 (n = 292). The rifampicin concentration was subtherapeutic in the majority of patients during the intensive and continuation phases (85% at month 1 and 74% at month 5); isoniazid was subtherapeutic in approximately one-fourth of patients (29% at month 1 and 22% at month 5 for isoniazid); and pyrazinamide was subtherapeutic in 13% at month 1.
Table 1.
Baseline Characteristics Among Adults With Newly Diagnosed Pulmonary Tuberculosis in Chennai and Pune, India (N = 404)
| Characteristic | Overall, No. (%) |
|---|---|
| Median age, y (IQR) | 39.5 (28.0–50.0) |
| Male sex | 260 (64.4) |
| Smoking status | |
| Never smoker | 270 (54.7) |
| Ever smoker | 134 (33.2) |
| Median BMI, kg/m2 (IQR) | 17.8 (15.9–20.3) |
| Median TB drug dose, mg/kg (IQR) | |
| Rifampicin | 9.4 (8.2–10.9) |
| Isoniazid | 12.4 (10.8–14.5) |
| Pyrazinamide | 31.3 (27.2–36.3) |
| Cavitary lesions | |
| Absent | 184 (54.9) |
| Present | 151 (45.1) |
| Diabetes mellitus | |
| No | 291 (72.0) |
| Yes | 113 (28.0) |
| Median HbA1C, % (IQR) | 5.8 (5.1–6.5) |
| HIV status | |
| Uninfected | 377 (93.3) |
| Infected | 27 (6.7) |
| On ART | 13 (48.1) |
| Clinical laboratory studies, median (IQR) | |
| Hemoglobin, g/dL | 11.8 (10.3–13.1) |
| AST, U/L | 20.0 (15.8–26.0) |
| ALT, U/L | 15.0 (11.0–21.1) |
| ALP, IU/L | 96.5 (77.6–124) |
| Urea, mg/dL | 12.0 (7.4–20.0) |
| Creatinine, mg/dL | 0.7 (0.5–0.8) |
| TB outcome | |
| Unfavorable | 77 (19.1) |
| Failure | 38 (9.4) |
| Recurrence | 19 (4.7) |
| Death | 20 (5.0) |
| Favorable | 327 (80.9) |
Data are presented as no. (%) unless otherwise indicated.
Abbreviations: ALP, alkaline phosphatase; ALT, alanine aminotransferase; ART, antiretroviral therapy; AST, aspartate aminotransferase; BMI, body mass index; HbA1C, glycosylated hemoglobin; HIV, human immunodeficiency virus; IQR, interquartile range; TB, tuberculosis.
Factors Influencing TB Drug Concentrations
Median drug concentrations at month 1 and 5 were compared according to groupings of baseline patient characteristics (Table 2). The median rifampicin concentration was lower in patients with HIV and lower BMI; the median isoniazid concentration was lower in male patients and smokers; and the median pyrazinamide concentration was lower in male patients and among those who tested HIV positive, those who were acid-fast bacilli smear negative, and those who had a higher BMI. Focusing on specific factors (age, sex, smoking status, alcohol use, sputum smear status, diabetes mellitus, baseline isoniazid susceptibility, BMI, cavitary lesions, and HIV status), multivariable linear regression analysis indicates that HIV infection lowered the rifampicin concentration by 1.91 µg/mL, and male sex and diabetes mellitus lowered pyrazinamide concentration by 7.78 µg/mL and 4.36 µg/mL, respectively (Table 3).
Table 2.
Median Tuberculosis Drug Concentrations at Month 1 and 5 of Tuberculosis Treatment, by Exposure of Interest
| Exposure | Month 1 | Month 5 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Rifampicin | Isoniazid | Pyrazinamide | Rifampicin | Isoniazid | ||||||
| No. | µg/mL (IQR) | No. | µg/mL (IQR) | No. | µg/mL (IQR) | No. | µg/mL (IQR) | No. | µg/mL (IQR) | |
| Overall | 273 | 3.61 (1.45–6.63) | 313 | 5.32 (2.45–8.38) | 279 | 37.04 (27.66–44.94) | 282 | 4.52 (1.64–8.09) | 299 | 6.20 (3.27–8.97) |
| Age | ||||||||||
| <35 y | 101 | 3.80 (1.52–6.84) | 123 | 5.35 (2.72–8.88) | 104 | 36.77 (27.56–44.76) | 105 | 4.90 (2.27–8.47) | 111 | 6.25 (3.71–9.06) |
| ≥35 y | 172 | 3.36 (1.38–6.32) | 190 | 5.27 (2.16–8.08) | 175 | 36.31 (26.78–44.90) | 177 | 4.42 (1.51–7.77) | 188 | 6.13 (3.14–9.15) |
| P value | .691 | .292 | .916 | .338 | .500 | |||||
| Sex | ||||||||||
| Female | 93 | 3.10 (1.46–6.91) | 112 | 5.87 (3.13–8.20) | 99 | 39.24 (29.69–51.61) | 104 | 5.12 (2.30–8.84) | 107 | 6.80 (3.87–11.17) |
| Male | 180 | 3.61 (1.51–6.16) | 201 | 5.12 (2.16–8.36) | 180 | 35.39 (26.14–42.94) | 178 | 4.42 (1.51–7.57) | 192 | 5.93 (3.14–8.53) |
| P value | .687 | .181 | <.01 | .051 | <.05 | |||||
| Ever smoker | ||||||||||
| Yes | 51 | 3.68 (1.43–6.65) | 56 | 4.87 (2.19–8.45) | 51 | 37.65 (30.36–44.96) | 49 | 4.08 (1.09–7.12) | 52 | 5.29 (2.16–7.61) |
| No | 222 | 3.54 (1.48–6.47) | 257 | 5.35 (2.55–8.11) | 228 | 36.40 (26.90–44.87) | 233 | 4.90 (1.91–8.23) | 247 | 6.28 (3.71–9.55) |
| P value | .731 | .428 | .391 | .179 | <.05 | |||||
| Alcoholic | ||||||||||
| Yes | 67 | 2.50 (1.39–5.96) | 75 | 5.12 (1.78–8.65) | 67 | 36.56 (28.94–44.49) | 66 | 4.34 (1.12–7.87) | 71 | 6.17 (2.80–8.74) |
| No | 206 | 3.68 (1.51–6.64) | 238 | 5.35 (2.68–8.28) | 212 | 36.48 (26.90–45.49) | 216 | 4.63 (1.99–8.09) | 228 | 6.27 (3.46–9.43) |
| P value | .220 | .469 | .825 | .381 | .666 | |||||
| Diabetes | ||||||||||
| Yes | 94 | 3.64 (1.78–6.53) | 98 | 5.05 (2.94–7.34) | 98 | 34.95 (25.03–43.42) | 84 | 5.91 (2.13–9.51) | 90 | 5.91 (3.17–7.89) |
| No | 179 | 3.47 (1.33–6.40) | 215 | 5.35 (2.10–9.65) | 181 | 37.49 (28.26–46.17) | 198 | 4.41 (1.65–7.46) | 209 | 6.63 (3.49–10.09) |
| P value | .485 | .413 | .079 | .068 | .104 | |||||
| BMI, kg/m2 | ||||||||||
| <18.5 | 122 | 2.70 (1.15–5.74) | 144 | 5.15 (2.16–8.95) | 130 | 38.35 (28.98–47.17) | 104 | 4.51 (2.25–7.88) | 104 | 6.55 (3.10–8.80) |
| ≥18.5 | 146 | 4.27 (1.83–6.90) | 164 | 5.43 (2.75–8.09) | 145 | 35.86 (26.09–41.77) | 174 | 4.64 (1.51–8.23) | 190 | 6.15 (3.71–9.33) |
| P value | <.05 | .637 | <.05 | .784 | .947 | |||||
| AFB smear | ||||||||||
| Positive | 87 | 3.47 (1.48–6.74) | 94 | 5.74 (3.21–8.52) | 91 | 37.80 (31.64–46.26) | 15 | 4.04 (2.27–7.12) | 16 | 6.43 (3.18–7.96) |
| Negative | 175 | 3.68 (1.46–6.33) | 207 | 5.16 (2.07–8.28) | 179 | 35.89 (23.89–44.55) | 259 | 4.63 (1.65–8.26) | 273 | 6.28 (3.61–9.29) |
| P value | .763 | .252 | <.05 | .383 | .565 | |||||
| INH status | ||||||||||
| Sensitive | 138 | 3.40 (1.48–6.11) | 160 | 5.32 (2.87–8.49) | 147 | 36.70 (27.60–45.30) | 137 | 4.52 (1.93–7.58) | 146 | 6.15 (3.38–9.52) |
| Resistant | 13 | 1.83 (0.92–5.37) | 17 | 5.12 (1.76–7.55) | 15 | 44.49 (27.51–50.89) | 15 | 5.22 (2.33–6.49) | 17 | 6.91 (4.67–10.14) |
| P value | .338 | .836 | .361 | >.95 | .405 | |||||
| Cavitation | ||||||||||
| Present | 99 | 2.64 (1.33–5.47) | 114 | 4.73 (2.10–6.94) | 108 | 37.28 (27.65–46.14) | 106 | 4.59 (1.93–8.47) | 110 | 5.93 (3.27–8.13) |
| Absent | 137 | 3.79 (1.36–6.84) | 153 | 5.35 (2.52–8.65) | 139 | 36.31 (24.91–44.49) | 140 | 4.49 (1.62–8.09) | 149 | 6.17 (3.15–9.33) |
| P value | .102 | .135 | .208 | .546 | .571 | |||||
| HIV status | ||||||||||
| Infected | 16 | 2.64 (1.06–5.77) | 19 | 6.11 (0.98–11.45) | 13 | 31.88 (19.92–35.89) | 13 | 1.65 (0.95–5.36) | 18 | 4.64 (2.84–8.07) |
| Uninfected | 257 | 3.61 (1.51–6.47) | 294 | 5.27 (2.52–8.11) | 266 | 37.04 (27.60–44.96) | 269 | 4.63 (1.93–8.17) | 281 | 6.28 (3.49–9.26) |
| P value | .452 | .899 | <.05 | <.05 | .334 |
Values in bold represents statistical significance.
Abbreviations: AFB, acid-fast bacilli; BMI, body mass index; HIV, human immunodeficiency virus; INH, isoniazid; IQR, interquartile range.
Table 3.
Baseline Patient Factors Influencing Tuberculosis Drug Concentrations Using Univariable and Multivariable Linear Regression Analysis
| Factors | Rifampicin | Isoniazid | Pyrazinamide | |||
|---|---|---|---|---|---|---|
| β (95% CI) | P Value | β (95% CI) | P Value | β (95% CI) | P Value | |
| Unadjusted analysis | ||||||
| Age ≥35 y | –0.13 (–.84 to .58) | .721 | –0.25 (–1.1 to .59) | .557 | 1.97 (–1.48 to 5.42) | .262 |
| Male sex | –0.61 (–1.36 to .14) | .109 | –0.80 (–1.69 to .10) | .081 | –5.31 (–8.65 to –1.61) | .004 |
| Never smoker | –0.19 (–1.09 to .71) | .676 | –0.76 (–1.75 to .22) | .130 | 1.61 (–2.46 to 5.69) | .438 |
| Nonalcoholic | –0.60 (–1.39 to .18) | .132 | –0.18 (–1.13 to .76) | .704 | 0.14 (–3.61 to 3.88) | .943 |
| Diabetes | 0.68 (–.07 to 1.44) | .077 | –0.90 (–1.67 to –.12) | .023 | –1.21 (–4.54 to 2.11) | .475 |
| BMI <18.5 kg/m2 | –0.55 (–1.27 to .17) | .135 | –0.16 (–.96 to .65) | .705 | 4.05 (.71–7.39) | .017 |
| AFB smear positive | –0.56 (–1.41 to .28) | .192 | –0.57 (–1.34 to .20) | .144 | 5.69 (2.33–9.05) | .001 |
| INH resistant | NA | 0.80 (–1.93 to 3.53) | .566 | NA | ||
| Cavitary lesion | –0.12 (–.89 to .65) | .756 | –0.59 (–1.44 to .27) | .178 | 3.18 (–.42 to 6.79) | .084 |
| HIV positive | –1.57 (–2.83 to –.3) | .015 | –0.19 (–2.06 to 1.68) | .843 | –9.83 (–16.82 to –2.85) | .006 |
| Adjusted analysisa | ||||||
| Age ≥35 y | –0.21 (–1.16 to .73) | .657 | 0.41 (–.64 to 1.46) | .445 | 4.08 (–.08 to 8.24) | .055 |
| Male sex | –0.60 (–1.57 to .38) | .229 | –0.71 (–1.82 to .40) | .210 | –7.78 (–11.88 to –3.69) | <.001 |
| Never smoker | 0.43 (–.72 to 1.58) | .463 | –0.99 (–2.10 to .12) | .080 | 2.91 (–1.65 to 7.47) | .211 |
| Nonalcoholic | –0.55 (–1.47 to .36) | .237 | 0.42 (–.64 to 1.48) | .433 | 0.89 (–3.43 to 5.20) | .687 |
| Diabetes | 0.44 (–.44 to 1.33) | .326 | –0.77 (–1.73 to .20) | .118 | –4.36 (–8.69 to –.03) | .048 |
| BMI <18.5 kg/m2 | –0.60 (–1.41 to .21) | .147 | –0.38 (–1.28 to .51) | .405 | 1.94 (–1.89 to 5.77) | .321 |
| AFB smear positive | –0.55 (–1.56 to .45) | .277 | –0.08 (–.98 to .82) | .865 | 4.76 (.91–8.60) | .015 |
| INH resistant | NA | 2.54 (–1.60 to 6.69) | .229 | NA | ||
| Cavitary lesion | –0.08 (–.91 to .75) | .849 | –0.33 (–1.24 to .58) | .477 | 2.53 (–1.20 to 6.26) | .184 |
| HIV infected | –1.91 (–3.49 to –.32) | .018 | –0.21 (–3.10 to 2.69) | .887 | –5.49 (–15.01 to 4.04) | .259 |
Values in bold represents statistical significance.
Abbreviations: AFB, acid-fast bacilli; BMI, body mass index; CI, confidence interval; HIV, human immunodeficiency virus; INH, isoniazid; NA, not applicable.
aAdjusted for age, sex, ever smoker, alcohol use, BMI, diabetes mellitus, AFB smear positivity, and cavitary lesion on chest radiography.
Effect of TB Drug Concentrations on Treatment Outcomes
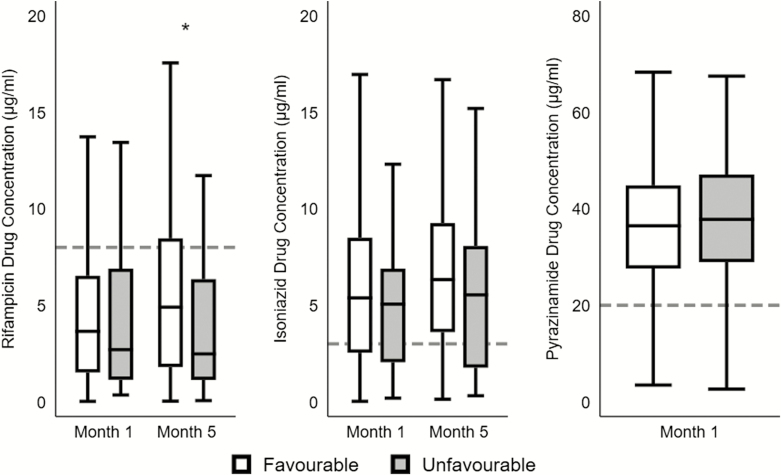
Of the 404 patients assessed, 77 (19%) had an unfavorable outcome (incidence rate [IR], 117 per 1000 person-years [PY]), including 38 (9%) with treatment failure (IR, 63.6 cases per 1000 PY), 20 (5%) deaths (IR, 43 per 1000 PY), and 19 (5%) with recurrence (IR, 41.1 per 1000 PY). Compared to favorable outcome, patients with unfavorable outcome had lower concentrations of both rifampicin (2.7 µg/mL vs 3.7 µg/mL at month 1; 2.5 µg/mL vs 4.9 µg/mL at month 5) and isoniazid (5.1 µg/mL vs 5.4 µg/mL at month 1; 5.5 µg/mL vs 6.3 µg/mL month 5) in the acute and continuation phases of ATT, but the difference only attained statistical significance for rifampicin at month 5 (P = .02) (Figure 1).
Figure 1.
Plasma concentrations of tuberculosis (TB) drugs at months 1 and 5 among adults with favorable and unfavorable TB treatment outcomes. Median 2-hour postdose plasma concentrations with interquartile range are shown (horizontal line within rectangle) for rifampicin, isoniazid, and pyrazinamide during the acute (month 1) and continuous (month 5) phases of TB treatment by treatment outcome. “Favorable” is defined as cure or treatment completion; “unfavorable” is defined as treatment failure or recurrence or all-cause mortality up to 24 months following treatment initiation. Horizontal dashed lines represent subtherapeutic cutoffs for each drug. Rifampicin levels were largely subtherapeutic throughout anti-TB treatment and significantly lower among patients with unfavorable outcome at month 5 (denoted by the asterisk).
The influence of various factors on both composite and individual unfavorable treatment outcomes was considered, such as age, sex, smoking, alcohol use, BMI, sputum smear status, diabetes mellitus, baseline isoniazid sensitivity, cavitary lesions, HIV infection, and lower concentrations of TB drugs (defined as a 1-unit [1 μg/mL]) decrease in 2-hour postdose plasma concentration). In multivariate analysis, unfavorable outcome was significantly associated with lower rifampicin concentrations (adjusted incidence rate ratio [aIRR], 1.21 [95% confidence interval {CI}, 1.01–1.47]; P = .045). Similarly, treatment failure was associated with lower rifampicin concentrations (aIRR, 1.16 [95% CI, 1.05–1.28]; P = .003). Finally, a 1-μg/mL decrease in pyrazinamide concentrations was associated with recurrence (aIRR, 1.05 [95% CI, 1.01–1.11]) (Table 4).
Table 4.
Risk of Unfavorable Outcomes by 24 Months After Tuberculosis Treatment Initiation With a 1-Unit Decrease in Drug Concentration Using Poisson Regression Analysisa
| Outcomec | Unadjusted | Adjustedb | ||
|---|---|---|---|---|
| IRR (95% CI) | P Value | aIRR (95% CI) | P Value | |
| Unfavorable outcome | ||||
| Rifampicin | 1.22 (1.02–1.46) | .026 | 1.21 (1.01–1.47) | .045 |
| Isoniazid | 1.05 (.94–1.18) | .375 | 1.03 (.92–1.16) | .585 |
| Pyrazinamide | 1.08 (1.01–1.18) | .052 | 1.27 (.88–1.83) | .207 |
| Treatment failure | ||||
| Rifampicin | 1.36 (1.04–1.26) | .005 | 1.16 (1.05–1.28) | .003 |
| Isoniazid | 1.06 (.99–1.13) | .076 | 1.06 (.99–1.14) | .100 |
| Pyrazinamide | 1.01 (.99–1.04) | .255 | 1.02 (.99–1.04) | .127 |
| All-cause mortality | ||||
| Rifampicin | 1.07 (.96–1.18) | .205 | 1.04 (.94–1.15) | .469 |
| Isoniazid | 1.05 (.97–1.14) | .212 | 1.04 (.97–1.13) | .275 |
| Pyrazinamide | 1.01 (.98–1.03) | .821 | 1.01 (.98–1.04) | .615 |
| Recurrence | ||||
| Rifampicin | 1.03 (.92–1.15) | .659 | 1.02 (.91–1.14) | .751 |
| Isoniazid | 1.04 (.94–1.15) | .483 | 1.05 (.94–1.17) | .368 |
| Pyrazinamide | 1.06 (1.01–1.11) | .015 | 1.05 (1.01–1.11) | .046 |
Values in bold represents statistical significance.
Abbreviations: aIRR, adjusted incidence rate ratio; CI, confidence interval; IRR, incidence rate ratio.
aConcentration was estimated using 2-hour postdose plasma drug concentration; aggregated data from month 1 and 5 were used for rifampicin and isoniazid; data from month 1 were used for pyrazinamide.
bModel included age, sex, ever smoker, body mass index, diabetes mellitus, acid-fast bacilli smear positivity, and cavitary lesion on chest radiography; recurrence model excluded ever smoker and used the end of treatment as start time.
cUnfavorable outcome was composite outcome of treatment failure, all-cause mortality, and recurrence.
DISCUSSION
While putative therapeutic targets have been proposed for anti-TB drugs, limited data exist describing the relationships between drug dosing or dosing frequency and unfavorable TB treatment outcomes [17]. Our prospective cohort study among adults with newly diagnosed pulmonary TB receiving first-line thrice-weekly DOTS under the national program in India found that the majority of patients had subtherapeutic concentrations of rifampicin in the intensive and continuous phases of ATT; patients with HIV coinfection were at particularly high risk for low rifampicin concentrations, and low rifampicin concentration was strongly and independently associated with composite unfavorable TB treatment outcomes as well as the individual outcome of treatment failure. Furthermore, lower pyrazinamide concentration was independently associated with risk of TB recurrence. Overall, our study adds to the body of evidence that low rifampicin concentrations are common and provides new, more robust pharmacokinetic and pharmacodynamic data to support that intermittent dosing of rifampicin is suboptimal and has an adverse impact on clinically important TB treatment outcomes.
To date, studies linking pharmacokinetic data to TB treatment outcomes have been limited and conflicting. Some studies have reported that treatment failure and longer time to culture conversion have been more frequently observed among patients with drug concentrations below the therapeutic range [23, 24], including a retrospective cohort study from Virginia (United States), where most patients who responded slowly to treatment had lower than expected 2-hour postdose concentrations of rifampicin and isoniazid [25]. In contrast, an Indonesian study observed that most patients had favorable TB treatment outcomes despite having low concentrations of rifampicin, isoniazid, and pyrazinamide under the directly observed treatment strategy [26]. Furthermore, a systematic review and meta-analysis found that only 3 of the 12 studies meeting inclusion criteria demonstrated an association between 2-hour postdose concentrations of first-line TB drugs and clinical outcomes, and no firm conclusions could be drawn due to limitations of the evidence [27]. India has been using thrice-weekly dosing of ATT instead of daily dosing. A prior study from our Chennai team, which included patients with extrapulmonary TB and pulmonary TB as well as retreatment cases, but excluded HIV patients, found that low rifampicin concentrations were associated with TB treatment failure [15]. However, the study had some notable differences from our current study. Their team had reported lower failure rates of 1% compared to 9.4% in our current study, and did not use culture to assess treatment outcomes [15]. We included HIV-infected individuals and younger patients from a second site in India (Pune), excluded retreatment cases and extrapulmonary TB cases (both of which have different odds of treatment outcomes), and used all 3 major unfavorable outcomes of treatment failure, recurrence, and death as endpoints (the prior Chennai study did not include recurrence). We also used the more sensitive HbA1C test to assess diabetes compared to random blood glucose alone. Last, we identified collinearity between BMI and drug-dose mg/kg; in contrast to the prior study, BMI was significantly associated with outcomes, and we used BMI as a variable of interest in our models. Our analysis indicates greater risk of composite treatment failure, all-cause mortality, or recurrence by 18 months following ATT with exposure to lower rifampicin concentrations. Our prior study done just in Chennai and our current study from 2 sites in India now provide compelling evidence that intermittent administration of 450–600 mg of rifampicin is inadequate, and this in turn has implications for acquired drug resistance.
The relationship between plasma drug concentrations and TB treatment outcomes is complex. While playing an important role, plasma drug concentration is only one among multiple factors that determine treatment outcome. Other factors include bacillary load, M. tuberculosis strain, minimum inhibitory concentration of the infecting strain, drug concentrations at the site of disease, genetics, extent of disease, and the patient’s immune and nutritional status [28–30]. This highlights the need for large, prospective studies in different geographic settings to capture the variability in TB patient characteristics as well as the programs and strategies for treating them. Importantly, although some studies have identified factors influencing TB treatment outcomes, most have not included drug concentrations in the analysis.
We followed a systematic and rigorous procedure to ascertain outcomes and, importantly, patients were followed up for 18 months after the end of ATT to fully capture information regarding recurrence. There are, however, some limitations. We determined 2-hour postdose plasma concentration to approximate peak drug concentrations [31], the rationale being that the bactericidal activity of all of the key first-line drugs (isoniazid, rifampicin, and pyrazinamide) is more concentration- than time-dependent and that the best single time point for these 3 drugs is 2 hours postdose, as the pharmacokinetic parameter that correlates best with the activity is Cmax rather than time above the minimum inhibitory concentration. Thus, 2-hour postdose concentrations have been consistently used in several studies, including therapeutic drug monitoring studies, to assess peak drug exposure. Furthermore, this measure requires only a single blood collection and is easy to implement in programmatic settings. However, peak concentrations could have been underestimated in patients with delayed absorption. In addition, the therapeutic ranges followed for drugs in this study represent typical values used in therapeutic drug monitoring, but have not been validated or related to treatment outcome or adverse events. The adjusted model was not performed for multiple measures and individual outcomes, but rather was used for aggregated drug concentrations at 2 time points. Finally, we did not measure ethambutol concentrations as we did not anticipate there to be a relationship between ethambutol concentrations and outcomes. Based on published literature, ethambutol at the current doses does not add meaningfully to the bactericidal activity of the regimen; it is largely included to protect against emergence of resistance of companion drugs (eg, rifampicin) if the strain turns out to be isoniazid resistant [32].
In summary, our study among adult pulmonary TB patients treated under the RNTCP in India identifies low rifampicin concentration at 2 hours postdose as an independent risk factor for unfavorable TB treatment outcomes. We now know that thrice-weekly dosing with 450–600 mg of rifampicin is suboptimal. With the change to daily ATT in India, it will be important to evaluate the pharmacokinetic–pharmacodynamic relationships to determine if further increases in dosing will be needed to provide optimal benefit in the country with the world’s largest burden of TB and MDR/RR-TB.
Notes
Acknowledgments. The authors thank the patients who took part in the study; Ms V. Sudha and Mr A. Vijayakumar for drug estimations by high-performance liquid chromatography; staff of the clinical biochemistry and hematology laboratories for blood estimations; data entry operators; field investigators engaged in patient recruitment; and staff at the Revised National Tuberculosis Control Program treatment centers in Tiruvallur and Pune. They also thank the study participants and study staff for their immense contribution. They acknowledge Katherine McIntire for reviewing and copyediting the manuscript.
Disclaimer. The contents of this publication are solely the responsibility of the authors and do not represent the official views of the Government of India Department of Biotechnology (DBT), Indian Council of Medical Research (ICMR), National Institutes of Health (NIH), or Civilian Research and Development Foundation (CRDF) Global. Any mention of trade names, commercial projects, or organizations does not imply endorsement by any of the sponsoring organizations.
Financial support. This work was primarily supported by the National Institute of Allergy and Infectious Diseases (NIAID), NIH (grant number R21AI127149 to V. M.). Research data in this manuscript were collected, in part, as part of the Regional Prospective Observational Research for Tuberculosis (RePORT) India Consortium. This work was funded in whole or in part by federal funds from the DBT; the ICMR; the Office of AIDS Research, NIAID/NIH; CRDF Global; the NIH Baltimore-Washington-India Clinical Trials Unit for the NIAID Networks (grant number UM1AI069465 to A. G.); and the NIH (grant number R01AI097494 to J. E. G.).
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. American Thoracic Society, Centers for Disease Control and Prevention, and Infectious Diseases Society of America. Treatment of tuberculosis. MMWR Recomm Rep 2003; 52(RR-11):1–77. [Google Scholar]
- 2. Chaulk CP, Moore-Rice K, Rizzo R, Chaisson RE. Eleven years of community-based directly observed therapy for tuberculosis. JAMA 1995; 274:945–51. [PubMed] [Google Scholar]
- 3. World Health Organization. Global tuberculosis report 2018. Available at: http://www.apps.who.int. Accessed 7 December 2018. [Google Scholar]
- 4. Jindani A, Nunn AJ, Enarson DA. Two 8-month regimens of chemotherapy for treatment of newly diagnosed pulmonary tuberculosis: international multicentre randomised trial. Lancet 2004; 364:1244–51. [DOI] [PubMed] [Google Scholar]
- 5. Marx FM, Floyd S, Ayles H, Godfrey-Faussett P, Beyers N, Cohen T. High burden of prevalent tuberculosis among previously treated people in southern Africa suggests potential for targeted control interventions. Eur Respir J 2016; 48:1227–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Central TB Division Directorate General of Health Services. India TB report 2018, revised national TB control programme annual status report. Available at: http://tbindia.gov.in. Accessed 7 December 2018. [Google Scholar]
- 7. Velayutham B, Chadha VK, Singla N, et al. Recurrence of tuberculosis among newly diagnosed sputum positive pulmonary tuberculosis patients treated under the Revised National Tuberculosis Control Programme, India: a multi-centric prospective study. PLoS One 2018; 13:e0200150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kimerling ME, Phillips P, Patterson P, Hall M, Robinson CA, Dunlap NE. Low serum antimycobacterial drug levels in non-HIV-infected tuberculosis patients. Chest 1998; 113:1178–83. [DOI] [PubMed] [Google Scholar]
- 9. Tappero JW, Bradford WZ, Agerton TB, et al. Serum concentrations of antimycobacterial drugs in patients with pulmonary tuberculosis in Botswana. Clin Infect Dis 2005; 41:461–9. [DOI] [PubMed] [Google Scholar]
- 10. McIlleron H, Wash P, Burger A, Norman J, Folb PI, Smith P. Determinants of rifampin, isoniazid, pyrazinamide, and ethambutol pharmacokinetics in a cohort of tuberculosis patients. Antimicrob Agents Chemother 2006; 50:1170–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Weiner M, Burman W, Vernon A, et al. Tuberculosis Trials Consortium Low isoniazid concentrations and outcome of tuberculosis treatment with once-weekly isoniazid and rifapentine. Am J Respir Crit Care Med 2003; 167:1341–7. [DOI] [PubMed] [Google Scholar]
- 12. Weiner M, Benator D, Burman W, et al. Tuberculosis Trials Consortium Association between acquired rifamycin resistance and the pharmacokinetics of rifabutin and isoniazid among patients with HIV and tuberculosis. Clin Infect Dis 2005; 40:1481–91. [DOI] [PubMed] [Google Scholar]
- 13. Um SW, Lee SW, Kwon SY, et al. Low serum concentrations of anti-tuberculosis drugs and determinants of their serum levels. Int J Tuberc Lung Dis 2007; 11:972–8. [PubMed] [Google Scholar]
- 14. Mehta JB, Shantaveerapa H, Byrd RP Jr, Morton SE, Fountain F, Roy TM. Utility of rifampin blood levels in the treatment and follow-up of active pulmonary tuberculosis in patients who were slow to respond to routine directly observed therapy. Chest 2001; 120:1520–4. [DOI] [PubMed] [Google Scholar]
- 15. Ramachandran G, Hemanth Kumar AK, Chandrasekaran V, et al. Factors influencing tuberculosis treatment outcome in adult patients treated with thrice-weekly regimens in India. Antimicrob Agents Chemother 2017; 61:e02464-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pasipanodya JG, Mcllleron H, Burger A, Wash P, Smith P, Gumbo T. Serum drug concentrations predictive of pulmonary tuberculosis. J Infect Dis 2013; 208:1464–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sekaggya-Wiltshire C, Lamorde M, Kiragga AN, et al. The utility of pharmacokinetic studies for the evaluation of exposure-response relationships for standard dose anti-tuberculosis drugs. Tuberculosis (Edinb) 2018; 108:77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gupte A, Padmapriyadarsini C, Mave V, et al. CTRIUMPH Study Team Cohort for Tuberculosis Research by the Indo-US Medical Partnership (CTRIUMPH): protocol for a multicentric prospective observational study. BMJ Open 2016; 6:e010542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Revised National TB Control Programme. Annual status report (2011). New Delhi: Central TB Division, Government of India, 2010: 98–101. [Google Scholar]
- 20. Hemanth Kumar AK, Chandra I, Geetha R, Silambu Chelvi K, Lalitha V, Prema G. A validated high performance liquid chromatography method for the determination of rifampicin and desacetyl rifampicin in plasma and urine. Indian J Pharmacol 2004; 36:231–3. [Google Scholar]
- 21. Hemanth Kumar AK, Sudha V, Geetha R. Simple and rapid liquid chromatography method for simultaneous determination of isoniazid and pyrazinamide in plasma. SAARC J Tuberc Lung Dis HIV/AIDS 2012; IX:13–8. [Google Scholar]
- 22. Alsultan A, Peloquin CA. Therapeutic drug monitoring in the treatment of tuberculosis: an update. Drugs 2014; 74:839–54. [DOI] [PubMed] [Google Scholar]
- 23. Babalik A, Babalik A, Mannix S, Francis D, Menzies D. Therapeutic drug monitoring in the treatment of active tuberculosis. Can Respir J 2011; 18:225–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Prahl JB, Johansen IS, Cohen AS, Frimodt-Moller N, Andersen AB. Clinical significance of 2 h plasma concentrations of first-line anti-tuberculosis drugs: a prospective observational study. J Antimicrob Chemother 2014; 69:2841–7. [DOI] [PubMed] [Google Scholar]
- 25. Heysell SK, Moore JL, Keller SJ, Houpt ER. Therapeutic drug monitoring for slow response to tuberculosis treatment in a state control program, Virginia, USA. Emerg Infect Dis 2010; 16:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Burhan E, Ruesen C, Ruslami R, et al. Isoniazid, rifampin, and pyrazinamide plasma concentrations in relation to treatment response in Indonesian pulmonary tuberculosis patients. Antimicrob Agents Chemother 2013; 57:3614–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mota L, Al-Efraij K, Campbell JR, Cook VJ, Marra F, Johnson J. Therapeutic drug monitoring in anti-tuberculosis treatment: a systematic review and meta-analysis. Int J Tuber Lung Dis 2016; 20:819–26. [DOI] [PubMed] [Google Scholar]
- 28. Colangeli R, Jedrey H, Kim S, et al. DMID 01-009/Tuberculosis Trials Consortium Study 22 Teams Bacterial factors that predict relapse after tuberculosis therapy. N Engl J Med 2018; 379:823–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Balabanova Y, Nikolayevskyy V, Ignatyeva O, et al. Beijing clades of Mycobacterium tuberculosis are associated with differential survival in HIV-negative Russian patients. Infect Genet Evol 2015; 36:517–23. [DOI] [PubMed] [Google Scholar]
- 30. Costa-Veiga A, Briz T, Nunes C. Unsuccessful treatment in pulmonary tuberculosis: factors and a consequent predictive model. Eur J Public Health 2018; 28:352–8. [DOI] [PubMed] [Google Scholar]
- 31. Hemanth Kumar AK, Kannan T, Chandrasekaran V, et al. Pharmacokinetics of thrice weekly rifampicin, isoniazid and pyrazinamide in adult tuberculosis patients in India. Int J Tuberc Lung Dis 2016; 20:1236–41. [DOI] [PubMed] [Google Scholar]
- 32. Law S, Benedetti A, Oxlade O, Schwartzman K, Menzies D. Comparing cost-effectiveness of standardised tuberculosis treatments given varying drug resistance. Eur Respir J 2014; 43:566–81. [DOI] [PubMed] [Google Scholar]