Abstract
Background:
Immune checkpoint inhibitors (ICI) are being increasingly utilized in the front-line (1L) setting of metastatic clear-cell renal cell carcinoma (mccRCC). Limited data exist on responses and survival on second-line (2L) VEGFR-TKI therapy after 1L ICI therapy.
Patients and methods:
This is a retrospective study of mccRCC patients treated with 2L VEGFR-TKI after progressive disease (PD) with 1L ICI. Patients were treated at MD Anderson Cancer Center or Memorial Sloan Kettering Cancer Center between December 2015 and February 2018. Objective response was assessed by blinded radiologists’ review using RECIST v1.1. Descriptive statistics and Kaplan-Meier method were utilized.
Results:
Seventy patients were included in the analysis. Median age at mccRCC diagnosis was 59 years; 8 patients (11%) had IMDC favorable-risk, 48 (69%) had intermediate-risk, and 14 (20%) had poor-risk disease. As 1L therapy, 12 patients (17%) received anti-PD-(L)1 monotherapy with nivolumab or atezolizumab, 33 (47%) received nivolumab plus ipilimumab, and 25 (36%) received combination anti-PD-(L)1 plus bevacizumab. 2L TKI therapies included pazopanib, sunitinib, axitinib, and cabozantinib. On 2L TKI therapy, one patient (1.5%) achieved a complete remission (CR), 27 patients (39.7%) a partial response (PR), and 36 patients (52.9%) stable disease (SD). Median progression-free survival (mPFS) was 13.2 months (95% CI: 10.1, NA). Forty-five percent of subjects required a dose reduction, and twenty-seven percent of patients discontinued treatment due to toxicity.
Conclusions:
In this retrospective study of patients with mccRCC receiving 2L TKI monotherapy following 1L ICI, we observed 2L antitumor activity and tolerance comparable to historical data for 1L TKI.
Keywords: metastatic kidney cancer, renal cell carcinoma, RCC, clear cell renal cell carcinoma, ccRCC, immunotherapy refractory, VEGFR-TKI, therapy sequence, immune checkpoint inhibitor, immunotherapy
Introduction:
The incidence of kidney cancer worldwide in 2018 was greater than 400,000 cases; in the metastatic setting, this disease is typically incurable [1, 2]. Clear cell renal cell carcinoma (ccRCC) is primarily associated with mutations in the VHL gene, which has led to the development of VEGF-receptor tyrosine kinase inhibitors (VEGFR-TKI) as anti-cancer therapies in ccRCC [3–6]. From 2006 to 2017, the standard of care in metastatic ccRCC (mccRCC) shifted in the front-line setting to VEGF targeted therapies [7–9]. In second and subsequent lines of therapy, VEGFR-TKIs, mTOR inhibitors, and immune checkpoint inhibitors (ICI) have been frequently utilized [10–12].
Nivolumab, a monoclonal antibody targeting programmed death-1 (PD-1) was the first ICI to be approved in advanced RCC, showing OS benefit over everolimus [13]. More recently, in CheckMate 214, a pivotal randomized phase 3 trial, nivolumab and ipilimumab demonstrated statistically superior median OS and higher objective response rate (ORR), in patients with IMDC intermediate- and poor-risk disease compared with sunitinib [14, 15]. These results led to the FDA approval of combination nivolumab and ipilimumab in the front-line setting for treatment of mccRCC.
The mccRCC treatment landscape is further rapidly changing with the exploration of combinations of ICI and anti-VEGF therapies. The results of the IMmotion-151 were recently reported and the combination of atezolizumab, an anti-PD-L1 antibody, with bevacizumab, an anti-VEGF therapy, was superior to sunitinib in terms of PFS (11.2 vs. 7.7 months, HR 0.74, p = 0.02) and ORR (43% vs. 35%) in PD-L1 positive patients, per investigator assessment [16], opening the possibility of another non-TKI containing ICI-based regimen in the frontline. The JAVELIN Renal 101 Phase 3 trial has now been reported as a TKI/ICI registration trial meeting (one of) its primary endpoints, demonstrating superior PFS for the combination of axitinib and avelumab over sunitinib in PD-L1 positive patients (13.8 vs. 7.2 months, HR 0.61) [17]. In addition, the KEYNOTE-426 trial has demonstrated both PFS and OS advantage of axitinib plus pembrolizumab over sunitinib (mPFS 15.1 months vs. 11.1 months, HR 0.69), which may lead to further approvals in the first-line landscape [18].
With front-line approval of the combination of nivolumab and ipilimumab and upcoming data on anti-VEGF/ICI combination therapy, understanding responses of subsequent therapies is needed. This retrospective study of patients with mccRCC reports on ORR, progression-free survival (PFS), safety of second line (2L) VEGFR-TKI, and OS after progressive disease with front-line (1L) ICI-based, non-TKI containing therapy.
Patients and Methods:
We conducted this retrospective, multicenter study after IRB approval was obtained at the two participating centers. A combined de-identified secure database was constructed of 70 patients with mccRCC treated from December 2015 to February 2018 at MD Anderson Cancer Center and Memorial Sloan Kettering Cancer Center with a 2L VEGFR-TKI after progressive disease with 1L ICI. All patients had previously received 1L ICI in the setting of clinical trials (nivolumab vs. nivolumab-ipilimumab vs. nivolumab-bevacizumab in NCT02210117, nivolumab-ipilimumab in NCT02231749, atezolizumab-bevacizumab in NCT02420821, atezolizumab in NCT01984242, and nivolumab-ipilimumab in NCT01472081).
Baseline demographic and clinical data were collected by individual chart review and included gender, age, IMDC risk score at time of 2L therapy start, nephrectomy status, presence of sarcomatoid dedifferentiation, metastatic sites, previously received ICI regimen, and choice of 2L TKI. Histologic diagnosis of ccRCC was made or confirmed in each case via review of tumor specimens by dedicated genitourinary pathologists at either of the two participating sites. During 2L TKI therapy, patients were managed per best practice established at the participating centers sites. Charts were reviewed for individual treatment courses with dedicated attention to treatment dose adjustments and reasons for treatment discontinuations. Radiographic response assessment was provided by two blinded radiologists, who assessed all cross-sectional scans obtained to evaluate extent of disease per RECIST v1.1 [19].
Continuous variables were summarized using descriptive statistics, and categorical data were tabulated with frequency and percentage. The Kaplan-Meier method was applied to estimate time-to-event outcomes. OS and PFS times were calculated from the start of 2L TKI.
Results:
Patient Characteristics:
Among the 70 patients, 50 (71%) were male. The median age at diagnosis with metastatic involvement of RCC was 59 years (range 44 – 75). Forty-three patients (61%) initially presented with Stage IV disease. At the time of 2L TKI therapy initiation, 8 patients (11%) had IMDC favorable-risk disease, 48 patients (69%) had intermediate-risk disease, and 14 patients (20%) had poor–risk disease by IMDC criteria. Sixty patients (86%) had previously undergone nephrectomy. All patients included in this analysis had clear-cell histologic subtype and 14 patients (20%) had evidence of sarcomatoid dedifferentiation. At the time of 2L treatment with VEGFR-TKI, the most common site of metastatic disease was lung in 61 patients (87%), followed by lymph nodes in 48 patients (69%). Additional details on demographics and presentation are summarized in Table 1.
Table 1:
Patient Characteristics
| Variable | N (%) |
|---|---|
| Gender | |
| Male | 50 (71) |
| Female | 20 (29) |
| Median age at mRCC diagnosis, years (range) | 59 (43.6–74.8) |
| Stage at initial diagnosis of RCC | |
| Stage I–III | 27 (39) |
| Stage IV | 43 (61) |
| IMDC risk score at time of 2L TKI start | |
| Favorable | 8 (11) |
| Intermediate | 48 (69) |
| Poor | 14 (20) |
| Nephrectomy status | |
| Status post nephrectomy | 60 (86) |
| Primary in-situ | 10 (14) |
| Histology | |
| Clear cell | 70 (100) |
| Sarcomatoid dedifferentiation | 14 (20) |
| Sites of metastatic disease at TKI start | |
| Lung | 61 (87) |
| Bone | 35 (50) |
| Liver | 12 (17) |
| Lymph node | 48 (69) |
| Adrenal | 22 (31) |
| First-line ICI | |
| Anti-PD-(L)1 single agent | 12 (17) |
| PD-1 + CTLA-4 blockade (followed by maintenance anti-PD-1) | 33 (47) |
| PD-(L)1 + anti-VEGF therapy | 25 (36) |
| Reason for discontinuation of 1L ICI | |
| Progressive disease | 58 (83) |
| Toxicity | 12 (17) |
| Median duration on ICI, months (range) | 5.9 (0.4–25.2) |
| Choice of second-line TKI and breakdown of IMDC risk score [fav/int/poor] | |
| pazopanib | 19 (27) [3/14/2] |
| sunitinib | 6 (9) [0/2/4] |
| axitinib | 25 (36) [4/16/5] |
| cabozantinib | 20 (28) [1/16/3] |
mRCC: metastatic RCC; 2L: second line; TKI: tyrosine kinase inhibitor; ICI: immune checkpoint inhibitor; PD-1: programmed death-1; CTLA-4: cytotoxic T-lymphocyte-associated protein 4; PD-(L)1: Programmed death ligand-1; VEGF: vascular endothelial growth factor
First and Second Line Treatment:
All patients in this study had previously received 1L ICI therapy in the context of a clinical trial. Twelve patients (17%) received an anti-PD-1/PD-(L)1 inhibition antibody monotherapy (nivolumab or atezolizumab) as 1L treatment. Thirty-three patients (47%) received dual immune checkpoint blockade with nivolumab and ipilimumab as 1L treatment, where patients received induction therapy with nivolumab plus ipilimumab followed by single-agent nivolumab maintenance therapy. Twenty-five patients (36%) received 1L treatment with the combination of an anti-PD-(L)1 antibody plus anti-VEGF therapy, with either the combination of nivolumab plus bevacizumab or the combination of atezolizumab plus bevacizumab. Fifty-eight patients (83%) discontinued 1L ICI therapy due to progressive disease, while 12 patients (17%) discontinued therapy due to toxicity.
All 70 patients had resolution of prior Grade 3/4 adverse events and evidence of progressive disease at the time of initiation of 2L TKI therapy. The median time from discontinuation of ICI therapy to initiation of TKI therapy was 4.2 weeks (range 0.0 – 84.9). The selection of 2L TKI therapy was at the discretion of the treating physician. Nineteen patients (27%) received pazopanib, 6 patients (9%) received sunitinib, 25 patients (36%) received axitinib, and 20 (28%) received cabozantinib.
Best Response to 2L TKI therapy:
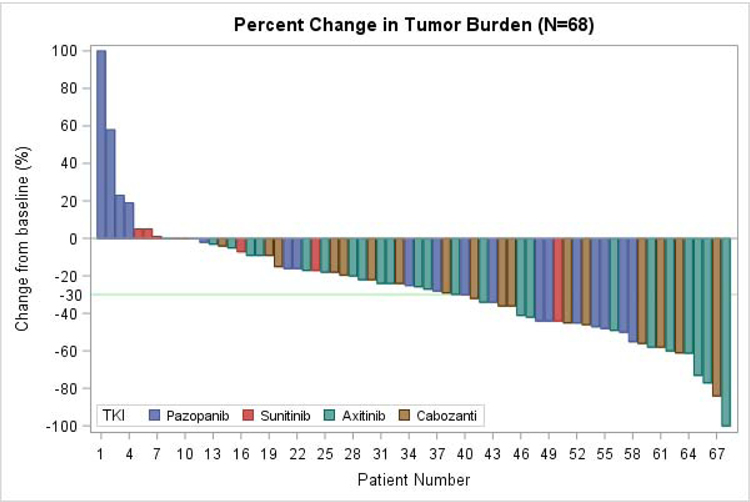
Sixty-eight patients had evaluable disease to assess response to 2L TKI. As best overall response (BOR) per RECIST v1.1, 1 patient (1.5%) achieved a complete response (CR), and 27 patients (39.7%) had a partial response (PR), adding to an objective response rate (ORR) of 41%. Thirty-six patients (52.9%) had stable disease (SD), for a disease control rate (DCR) of 94%. Only four patients (6%) had progressive disease (PD) as best response to 2L TKI. A waterfall plot of BOR is shown in Figure 1, broken down by 2L TKI received.
Figure 1:
Waterfall Plot of Best Overall Response
68 patients with evaluable disease included, with overall best responses broken down by choice of 2L TKI. 1 patient achieved CR, 27 with PR, 36 with SD, 4 with PD. TKI: tyrosine kinase inhibitor
As demonstrated in Table 2, responses to 2L TKI were noted in patients within all groups categorized. The median time to best response was 3.9 months. Nine patients (12.9%) were treated beyond RECIST v1.1 PD in the setting of ongoing clinical benefit according to the treating physician; in three of these patients, dose escalation of the TKI was offered, and in another local ablative therapy to a painful bone lesion was offered. The patient who achieved a CR was treated with axitinib and maintains this response for over one year despite prolonged treatment breaks due to side effects.
Table 2:
Responses
| Variable | n Total = 68 | CR/PR (n) | SD (n) | PD (n) |
|---|---|---|---|---|
| Male | 49 | 18 | 27 | 4 |
| Female | 19 | 10 | 9 | 0 |
| Stages I-III at presentation | 26 | 14 | 12 | 0 |
| Stage IV at presentation | 42 | 14 | 24 | 4 |
| IMDC favorable-risk | 7 | 3 | 4 | 0 |
| IMDC intermediate-risk | 47 | 22 | 22 | 3 |
| IMDC poor-risk | 14 | 3 | 10 | 1 |
| Nephrectomy | 58 | 26 | 29 | 3 |
| Primary in-situ | 10 | 2 | 7 | 1 |
| 1L anti-PD-1 single agent | 12 | 6 | 5 | 1 |
| 1L anti-PD-1 + anti-CTLA-4 | 32 | 14 | 16 | 2 |
| 1L anti-PD-(L)1 + anti-VEGF | 24 | 8 | 15 | 1 |
| 2L pazopanib | 19 | 8 | 8 | 3 |
| 2L sunitinib | 6 | 1 | 4 | 1 |
| 2L axitinib | 24 | 10 | 14 | 0 |
| 2L cabozantinib | 19 | 9 | 10 | 0 |
CR: complete remission; PR: partial response; SD: stable disease; PD: progression of disease; IMDC: international metastatic database consortium; PD-1: programmed death-1; CTLA-4: cytotoxic T-lymphocyte-associated protein 4; PD-(L)1: Programmed death ligand-1; VEGF: vascular endothelial growth factor
Survival:
As noted in Figure 2, median PFS (mPFS) on 2L TKI was 13.2 months (95% CI 10.1– NA). The 1-year PFS probability was 52.5%, and the 2-year PFS rate was 34.2%. Twenty-two patients had died of progressive disease by the time of data analysis. The median time for follow-up for surviving patients was 14.9 months. Median overall survival was not reached. As noted in Figure 3, the 1-year actuarial survival probability was 79.6% (95% CI 70.2 – 90.3). The 2-year actuarial survival probability was 57.6%.
Figure 2:
Progression-Free Survival (PFS)
Median progression free survival by Kaplan-Meier method was 13.2 months (95% CI 10.1 – NA).
Figure 3:
Overall Survival (OS)
Median overall survival by Kaplan-Meier was not reached. The 1 year survival probability was 79.6% (95% CI 70.2% – 90.3%).
Duration of Therapy:
At time of data analysis, 25 patients (35.7%) were still on active 2L TKI therapy, while 45 patients (64.3%) had discontinued 2L TKI therapy. Of those off therapy, 33 patients (73%) had discontinued due to PD, while 12 patients (27%) had discontinued due to toxicity. The estimated median duration of 2L TKI therapy was 10.1 months (95% CI 6.9 – 15.2). Figure 4 shows a swimmer’s plot of duration of therapy by 2L TKI received.
Figure 4:
Duration of 2L TKI Therapy
All 70 patients were included in this swimmer’s plot for duration of therapy, broken down by choice of 2L TKI. TKI end denotes that 2L TKI was discontinued either for progressive disease or for toxicity.
Patient Subgroups:
Table 3 shows a breakdown of mPFS, OS probability at 1 year, and median 2L TKI duration for various patient subgroups defined by baseline clinical characteristics and treatment received. The table provides summary statistics but treatment-subgroup interactions were not tested due to the small sample sizes and the retrospective nature of the study. Patients previously treated with 1L anti-PD-(L)1 monotherapy (n=12) had mPFS of 7.5 months on 2L TKI, those previously treated with 1L nivolumab-ipilimumab (n=33) had mPFS of 11.9 months on 2L TKI, and those treated with 1L anti-PD-(L)1 + bevacizumab (n=25) had mPFS of 20.8 months on 2L TKI. Interestingly, while the IMDC good risk patients had the highest OS rate at 1 year, the longest mPFS and median 2L TKI duration was in the IMDC intermediate risk group population. There were very few patients treated with 2L sunitinib, but numerically most of these patients had poor risk disease and had the shortest mPFS and OS rate at 1 year (see Table 3). In the patients with sarcomatoid de-differentiation, the ORR was 54%.
Table 3:
Subgroup Analysis of mPFS, OS Rate at 1 Year, and Median Duration of TKI
| Variable | n | mPFS (mo) (95% CI) |
OS probability 1 yr (95% CI) |
Median 2L TKI Duration (mo) (95% CI) |
|---|---|---|---|---|
| Stages I–III at presentation | 27 | 15.2 (13.2, NA) |
0.84 (0.70, 1.0) |
13.2 (7.5, NA) |
| Stage IV at presentation | 43 | 11.0 (6.9, NA) |
0.77 (0.65, 0.92) |
7.9 (5.1, 16.6) |
| IMDC favorable-risk | 8 | 8.4 (6.1, NA) |
1.0 (1.0, 1.0) |
6.2 (6.1, NA) |
| IMDC intermediate-risk | 48 | 16.6 (13.2, NA) |
0.91 (0.83, 1.0) |
15.2 (10.1, NA) |
| IMDC poor-risk | 14 | 4.0 (3.7, NA) |
0.31 (0.14, 0.70) |
3.8 (3.5, NA) |
| Nephrectomy | 60 | 15.2 (11.0, NA) |
0.83 (0.73, 0.94) |
11.0 (6.9, NA) |
| Primary in-situ | 10 | 7.4 (4.2, NA) |
0.60 (0.34, 1.0) |
6.9 (4.2, NA) |
| 1L anti-PD-(L)1 single agent | 12 | 7.5 (5.9, NA) |
0.74 (0.53, 1.0) |
6.9 (3.6, NA) |
| 1L anti-PD-1 + anti-CTLA-4 | 33 | 11.9 (8.6, NA) |
0.81 (0.69, 0.96) |
10.3 (6.9, 16.6) |
| 1L anti-PD-(L)1 + anti-VEGF | 25 | 20.8 (10.1, NA) |
0.80 (0.63, 1.0) |
20.8 (6.2, NA) |
| 2L pazopanib | 19 | 24.4 (6.1, NA) |
0.89 (0.75, 1.0) |
5.1 (3.8, NA) |
| 2L sunitinib | 6 | 3.6 (0.9, NA) |
0.33 (0.11, 1.0) |
3.2 (0.9, NA) |
| 2L axitinib | 25 | 13.2 (8.6, NA) |
0.87 (0.74, 1.0) |
10.3 (8.0, NA) |
| 2L cabozantinib | 20 | 15.2 (7.9, NA) |
0.74 (0.54, 1.0) |
15.2 (7.9, NA) |
mPFS: median progression-free survival; OS: overall survival; TKI: tyrosine kinase inhibitor; IMDC: international metastatic database consortium; PD-1: programmed death-1; CTLA-4: cytotoxic T-lymphocyte-associated protein 4; PD-(L)1: Programmed death ligand-1; VEGF: vascular endothelial growth factor
Safety:
As noted above, 12 patients (27%) discontinued therapy due to adverse events (AEs). Three of the 12 patients had previously discontinued ICI therapy due to toxicity. Five of these 12 patients discontinued 2L TKI due to transaminitis (all on pazopanib), four due to fatigue or anorexia/weight loss, two due to safety concerns of continuing TKI with radiographic evidence of aortic dissections, and one due to gastrointestinal bleeding. Thirty-two patients (45.7%) required at least one dose reduction of 2L TKI during their course due to AEs. Time from 1L ICI therapy to 2L TKI therapy did not correlate to severity of toxicity; dichotomizing the whole population per time between IL IO and 2L TKI as <1 month and >1 month, 17% of patients in each arm had to discontinue 2L TKI due to toxicity. There were no deaths related to TKI therapy. Further details on the 12 patients requiring 2L TKI discontinuation are provided in Table 4 (supplementary data).
Table 4.
Supplementary Data: Discontinuation of 2L TKI Due to Toxicity
| Pt requiring 2L TKI discontinuation due to toxicity | 1L regimen | 1L ICI discontinued for PD or toxicity? | Time from 1L ICI to 2L TKI (weeks) | 2L TKI | Toxicity leading to 2L TKI discontinuation | Time to initial toxicity onset (weeks) | Dose modification utilized (Y/N) |
|---|---|---|---|---|---|---|---|
| 1 | PD-1 + CTLA-4 blockade | PD | 3.7 | Pazopanib | Transaminitis | 2.0 | N |
| 2 | PD-1 + CTLA-4 blockade | PD | 5.1 | Pazopanib | Fatigue, anorexia | 0.3 | Y |
| 3 | PD-1 + CTLA-4 blockade | PD | 2.9 | Axitinib | Fatigue, anorexia | 0.3 | Y |
| 4 | PD-1 blockade | PD | 2.1 | Axitinib | Aortic Dissection | 31.9 | Y |
| 5 | PD-1 blockade | PD | 12.1 | Pazopanib | Transaminitis | 7.6 | N |
| 6 | PD-1 blockade + bevacizumab | Toxicity | 0.0 | Pazopanib | Transaminitis | 14.3 | Y |
| 7 | PD-1 blockade + bevacizumab | Toxicity | 3.0 | Axitinib | Fatigue, rash | 2.2 | Y |
| 8 | PD-1 blockade | PD | 11.6 | Pazopanib | Anorexia, weight loss | 1.2 | Y |
| 9 | PD-L1 blockade + bevacizumab | PD | 3.0 | Pazopanib | Transaminitis | 4.7 | N |
| 10 | PD-L1 blockade | PD | 4.6 | Sunitinib | GI bleed | 10.0 | N |
| 11 | PD-1 + CTLA-4 blockade | PD | 8.3 | Pazopanib | Transaminitis | 12.9 | Y |
| 12 | PD-1 + CTLA-4 blockade | PD | 4.7 | Pazopanib | Aortic Dissection | 8.3 | N |
Discussion:
The landscape of mccRCC treatment has shifted dramatically in the last year with the introduction of ICI therapy in 1L treatment, particularly in intermediate- and poor-risk disease by IMDC [15]. Furthermore, evolving data with ICI-VEGF-directed combination therapies in the 1L setting may well lead to further approvals in this area in the near future [16, 20]. With ICI therapy moving up in the treatment paradigm of mccRCC, TKI previously developed in and approved for the first and second line space will now be considered as standard agents following progression on ICI therapy.
Prospective data on efficacy for previously approved TKI in the post-ICI space is extremely sparse. Choueiri and colleagues noted that the PFS superiority of cabozantinib over everolimus on the phase III METEOR trial extended to the subgroup of patients previously treated with PD-1 or PD-(L)1 therapies with PFS HR 0.22 (95% CI 0.07–0.65), but their findings were limited by small sample size (n=34), and the authors provided no details on rate of radiographic response, extent of prior therapies or TKI safety in this particular subgroup of patients [12]. Further prospective data will be forthcoming from Ornstein et al. on responses with 2L axitinib dose titration after 1L ICI [21].
Prior publications of multicenter retrospective reports have suggested that TKIs have safety and efficacy following checkpoint inhibitor therapy. Albiges et al. previously reported on 44 patients from 4 academic centers in the US and Europe who received VEGF directed therapy subsequent to prior PD-1/PD-(L)1 directed treatments [22]. Overall extent of prior therapies varied with <20% of patients having received ICI therapy in the 1st line, and a significant proportion of subjects with 3 or more lines prior to post-ICI TKI. Median time to treatment failure on post-ICI TKI was reported at 6.9 months in this mixed population. Auvray et al. reported recently on 33 patients who received 2L TKI after 1L nivolumab plus ipilimumab. Median PFS was reported at 8 months with first-generation TKI and 7 months with second-generation TKI; interestingly, patients with duration of response greater than 6 months on 1L ICI appeared to have longer durations of response to 2L TKI [23].
Nadal and colleagues reported on a heterogeneous cohort of 70 patients receiving TKI treatment subsequent to ICI monotherapy (n=49) or ICI-based combinations (n=29) [24]. Again, >70% of patients were previously exposed to antiangiogenic therapy, some having received prior mTOR or cytokine therapy as well. Some had received a prior TKI in combination with ICI therapy; these patients had numerically lower response to TKI monotherapy than those treated with single-agent checkpoint inhibitors (ORR 10% vs. 36%). The study further suggested TKI therapy could be safely applied with AE profile similar to historic comparisons.
Here, we retrospectively report on a cohort of 70 patients with a well-defined clinical scenario: all had received only one prior ICI-containing regimen that was delivered in the first-line space and all patients were VEGFR-TKI naïve. There is some heterogeneity in our population given that 45 patients (64%) were exposed to ICI only in 1L, while 25 patients (36%) where exposed to ICI + bevacizumab in 1L; however, both of these front-line approaches are relevant in the current treatment landscape. In the US, the systemic therapy options for mccRCC have changed over the past year, particularly with the introduction of nivolumab plus ipilimumab therapy as 1L therapy for patients with IMDC intermediate- and poor-risk disease [15]. Furthermore, evolving data with ICI-anti-VEGF combination therapies in the 1L setting will likely lead to future approvals in this setting [16]. With ICI therapy moving upfront in the treatment paradigm of mccRCC, the data from this study suggests that patients have a robust response to 2L TKI. The ORR of 41%, a median PFS of 13.2 months, and a 1-year OS probability of 80% nearly parallels the historical comparison for 1L TKI treatment [8].
Appreciating notable differences in efficacy with single-agent vs. combination ICI therapy in the first-line setting [15, 25] as well as the fact that TKI have varying efficacy when directly compared [26, 27] one should not attempt to draw conclusions regarding associations between 2L TKI outcomes and prior ICI regimen in this dataset. Acknowledging such limitations to retrospective data review and the small sample size of subgroup analyses, we noted some interesting trends. We noted responses to 2L TKI regardless of frontline ICI choice. Interestingly, the IMDC intermediate group population had the longest mPFS and longest median TKI duration. This could reflect the fact that these patients have more disease modification and benefit from upfront ICI therapy than the favorable risk group, or this could be related to the small number of favorable risk group patients in this cohort. The pazopanib cohort demonstrated the longest mPFS and OS rate at 1 year, while the cabozantinib cohort had the longest median duration of TKI therapy. Whether this is a reflection of differences in mechanism of action / kinome profile and lingering effects of prior ICI therapy on the tumor microenvironment or simply artefactual, (i.e. reflecting small numbers in each subgroup) is not clear and could only be addressed in larger cohorts.
Another limitation of retrospective studies is the limited ability to accurately capture all side effects, leading to an underestimation of adverse events, particularly Gr 1/2 events. Powles et al. have previously demonstrated that there can be more VEGFR-TKI related side effects after prior PD-(L)1 exposure [28], and it is likely that low-grade toxicities such a fatigue and other quality-of-life measures were not fully reflected in this retrospective cohort.
Our data provide estimates for efficacy outcomes when designing trials with 2L TKI after 1L ICI therapy such as nivolumab + ipilimumab which is now the standard of care for patients with advanced or metastatic clear-cell RCC with intermediate- or poor-risk disease. These data may inform the design of trials testing novel agents in combination with VEGFR-TKI in the 2L or 3L setting after 1L ICI + ICI or 1L ICI monotherapy or 1L ICI + bevacizumab therapy.
We are witnessing the introduction of unprecedented numbers of treatment options for the treatment of patients with mRCC, but much remains to be discovered about patient selection, optimal combinations, and treatment sequencing. While it is encouraging that responses to 2L TKI after 1L ICI are robust, ideally, further such questions should be addressed on prospective trials.
Conclusion:
In this retrospective study, we observed a high ORR of 41%, median PFS of 13.2 months, and 1-yr survival probability of 79.6% in patients with mccRCC treated with 2L TKI after 1L ICI. Very few patients had outright PD on 2L TKI after 1L ICI. Tolerance was comparable to prior experience with these approved agents. Further studies are needed to evaluate optimal combination strategies and sequencing of therapies in mccRCC.
Immunotherapy (ICI) is moving to front-line treatment in metastatic RCC for intermediate- and poor-risk patients
Little data exists on responses to second-line (2L) TKI after front-line (1L) ICI
Our data showed that 2L VEGFR-TKI have ORR 41% and DCR 94% after 1L ICI–containing regimen
Median PFS on 2L VEGFR-TKI after 1L ICI in metastatic clear-cell RCC was 13.2 mo.
Safety and tolerability of 2L VEGFR-TKI after 1L ICI is typical of class effects
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Research support: none
Data Presented: Oral presentation at KCA Conference Nov 2018 (Miami, FL) and poster presentation at GU ASCO February 2019 (San Francisco, CA)
Disclaimers: none
References:
- 1.Data WC. Global cancer statistics for the most common cancers. In. 2018.
- 2.SEER. Cancer Stat Facts: Kidney and Renal Pelvis Cancer. In. Bethesda, MD: 2017. [Google Scholar]
- 3.Choueiri TK, Motzer RJ. Systemic Therapy for Metastatic Renal-Cell Carcinoma. New England Journal of Medicine 2017; 376: 354–366. [DOI] [PubMed] [Google Scholar]
- 4.Nickerson ML, Jaeger E, Shi Y et al. Improved identification of von Hippel-Lindau gene alterations in clear cell renal tumors. Clin Cancer Res 2008; 14: 4726–4734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cancer Genome Atlas Research Network. Comprehensive molecular characterization of clear cell renal cell carcinoma. Nature 2013; 499: 43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaelin WG Jr. The von Hippel-Lindau tumour suppressor protein: O2 sensing and cancer. Nat Rev Cancer 2008; 8: 865–873. [DOI] [PubMed] [Google Scholar]
- 7.Motzer RJ, Hutson TE, Tomczak P et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med 2007; 356: 115–124. [DOI] [PubMed] [Google Scholar]
- 8.Motzer RJ, Hutson TE, Cella D et al. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med 2013; 369: 722–731. [DOI] [PubMed] [Google Scholar]
- 9.Barata PC, Rini BI. Treatment of renal cell carcinoma: Current status and future directions. CA Cancer J Clin 2017. [DOI] [PubMed]
- 10.NCCN. Kidney Cancer. In Version 3.2018 Edition. 2018.
- 11.Hutson TE, Lesovoy V, Al-Shukri S et al. Axitinib versus sorafenib as first-line therapy in patients with metastatic renal-cell carcinoma: a randomised open-label phase 3 trial. Lancet Oncol 2013; 14: 1287–1294. [DOI] [PubMed] [Google Scholar]
- 12.Choueiri TK, Escudier B, Powles T et al. Cabozantinib versus everolimus in advanced renal cell carcinoma (METEOR): final results from a randomised, open-label, phase 3 trial. Lancet Oncol 2016; 17: 917–927. [DOI] [PubMed] [Google Scholar]
- 13.Motzer RJ, Escudier B, McDermott DF et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med 2015; 373: 1803–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heng DY, Xie W, Regan MM et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter study. J Clin Oncol 2009; 27: 5794–5799. [DOI] [PubMed] [Google Scholar]
- 15.Motzer RJ, Tannir NM, McDermott DF et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. New England Journal of Medicine 2018; 378: 1277–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Motzer RJ, Powles T, Atkins MB et al. IMmotion151: A Randomized Phase III Study of Atezolizumab Plus Bevacizumab vs Sunitinib in Untreated Metastatic Renal Cell Carcinoma (mRCC). Journal of Clinical Oncology 2018; 36: 578–578. [Google Scholar]
- 17.Motzer RJ, Penkov K, Haanen J et al. Avelumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med 2019; 380: 1103–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rini BI, Plimack ER, Stus V et al. Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med 2019; 380: 1116–1127. [DOI] [PubMed] [Google Scholar]
- 19.Eisenhauer EA, Therasse P, Bogaerts J et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45: 228–247. [DOI] [PubMed] [Google Scholar]
- 20.Carlo MI, Voss MH, Motzer RJ. Checkpoint inhibitors and other novel immunotherapies for advanced renal cell carcinoma. Nat Rev Urol 2016; 13: 420–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ornstein MC, Pal SK, Wood LS et al. Prospective phase II multi-center study of individualized axitinib (Axi) titration for metastatic renal cell carcinoma (mRCC) after treatment with PD-1 / PD-L1 inhibitors. Journal of Clinical Oncology 2018; 36: 4517–4517. [Google Scholar]
- 22.Albiges L, Fay AP, Xie W et al. Efficacy of targeted therapies after PD-1/PD-L1 blockade in metastatic renal cell carcinoma. Eur J Cancer 2015; 51: 2580–2586. [DOI] [PubMed] [Google Scholar]
- 23.Auvray M, Auclin E, Barthelemy P et al. Second-line targeted therapies after nivolumab-ipilimumab failure in metastatic renal cell carcinoma. Eur J Cancer 2019; 108: 33–40. [DOI] [PubMed] [Google Scholar]
- 24.Nadal R, Amin A, Geynisman DM et al. Safety and clinical activity of vascular endothelial growth factor receptor (VEGFR)-tyrosine kinase inhibitors after programmed cell death 1 inhibitor treatment in patients with metastatic clear cell renal cell carcinoma. Ann Oncol 2016; 27: 1304–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choueiri TK, Fishman MN, Escudier B et al. Immunomodulatory Activity of Nivolumab in Metastatic Renal Cell Carcinoma. Clin Cancer Res 2016; 22: 5461–5471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Choueiri TK, Halabi S, Sanford BL et al. Cabozantinib Versus Sunitinib As Initial Targeted Therapy for Patients With Metastatic Renal Cell Carcinoma of Poor or Intermediate Risk: The Alliance A031203 CABOSUN Trial. J Clin Oncol 2017; 35: 591–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rini BI, Escudier B, Tomczak P et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet 2011; 378: 1931–1939. [DOI] [PubMed] [Google Scholar]
- 28.Powles T, Motzer RJ, Escudier B et al. Outcomes based on prior therapy in the phase 3 METEOR trial of cabozantinib versus everolimus in advanced renal cell carcinoma. Br J Cancer 2018; 119: 663–669. [DOI] [PMC free article] [PubMed] [Google Scholar]