Abstract
Background
Intracellular tenofovir diphosphate concentrations are markedly increased in HIV/HCV coinfected individuals receiving tenofovir disoproxil fumarate (TDF) with sofosbuvir-containing treatment. Sofosbuvir may inhibit the hydrolysis of TDF to tenofovir, resulting in increased concentrations of the disoproxil or monoester forms, which may augment cell loading. We sought to quantify tenofovir disoproxil and monoester concentrations in individuals receiving TDF with and without ledipasvir/sofosbuvir.
Methods
HIV/HCV coinfected participants receiving TDF-based therapy were sampled pre-dose and 1 and 4 h post-dose prior to and 4 weeks after initiating ledipasvir/sofosbuvir. Tenofovir disoproxil was not detectable. Tenofovir monoester in plasma and tenofovir diphosphate in PBMC and dried blood spots (DBS) were quantified using LC-MS/MS. Geometric mean ratios (week 4 versus baseline) and 95% CIs were generated for the pharmacokinetic parameters. P values reflect paired t-tests.
Results
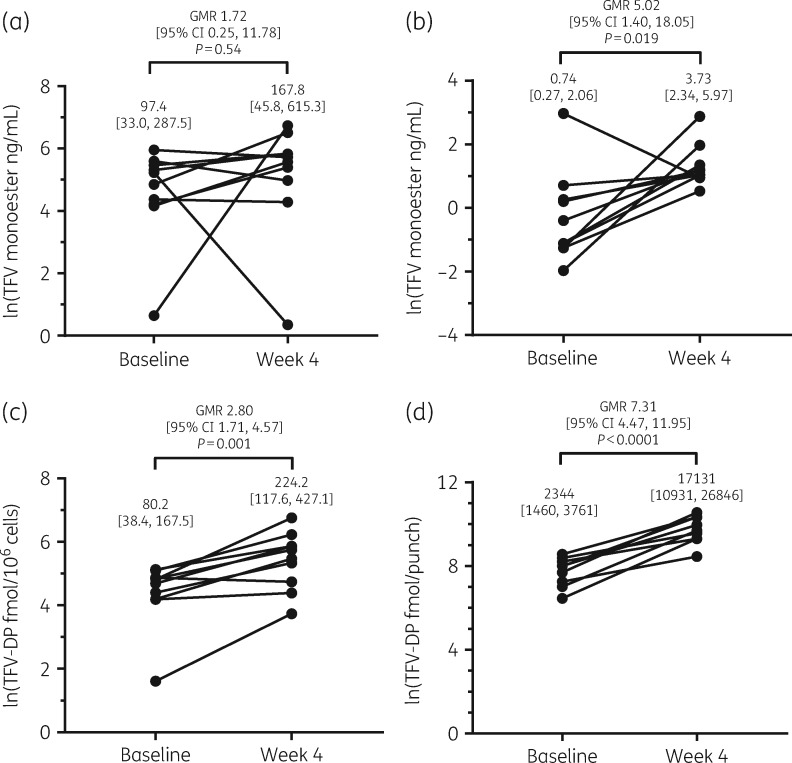
Ten participants had complete data. At baseline, geometric mean (95% CI) tenofovir monoester plasma concentrations at 1 and 4 h post-dose were 97.4 ng/mL (33.0–287.5) and 0.74 ng/mL (0.27–2.06), respectively. With ledipasvir/sofosbuvir, tenofovir monoester concentrations at 4 h post-dose were 5.02-fold higher (95% CI 1.40–18.05; P = 0.019), but did not significantly differ at 1 h post-dose (1.72-fold higher, 95% CI 0.25–11.78; P = 0.54), possibly due to absorption variability. Tenofovir diphosphate in PBMC and DBS were increased 2.80-fold (95% CI 1.71–4.57; P = 0.001) and 7.31-fold (95% CI 4.47–11.95; P < 0.0001), respectively, after 4 weeks of ledipasvir/sofosbuvir.
Conclusions
Tenofovir monoester concentrations were increased in individuals receiving TDF with ledipasvir/sofosbuvir, consistent with inhibition of TDF hydrolysis. Additional studies are needed to determine the clinical relevance of this interaction.
Introduction
There are an estimated 2.3 million individuals living with HIV and HCV worldwide.1 Persons living with HIV/HCV coinfection are at an increased risk for multiple liver-related morbidities in comparison with HCV monoinfection.2 Thus, HCV treatment is critical to improve health outcomes in this population. Several direct-acting antiviral (DAA) combinations are approved for HCV treatment, many of which only require once-daily dosing for as little as 8 weeks of therapy. Despite the short treatment duration for HCV, HIV requires lifelong therapy and should not be interrupted during the course of HCV treatment.3 Thus, drug–drug interactions between DAAs and antiretroviral medications are of concern.
Both sofosbuvir and tenofovir are key components of multiple recommended HCV and HIV treatment regimens, respectively, and have a low potential for causing or being susceptible to significant drug–drug interactions. However, we recently found that intracellular concentrations of tenofovir diphosphate were significantly increased in individuals with HIV/HCV coinfection taking tenofovir disoproxil fumarate (TDF) in combination with sofosbuvir-containing HCV treatment.4 Tenofovir diphosphate concentrations in dried blood spot (DBS) samples were increased 4.3-fold after 12 weeks of sofosbuvir/ribavirin and 17.8-fold after 8 weeks of ledipasvir/sofosbuvir treatment, whereas tenofovir concentrations in plasma appeared unchanged with sofosbuvir/ribavirin, and were only 2.1-fold higher with ledipasvir/sofosbuvir. The mechanisms behind this drug–drug interaction are unclear.
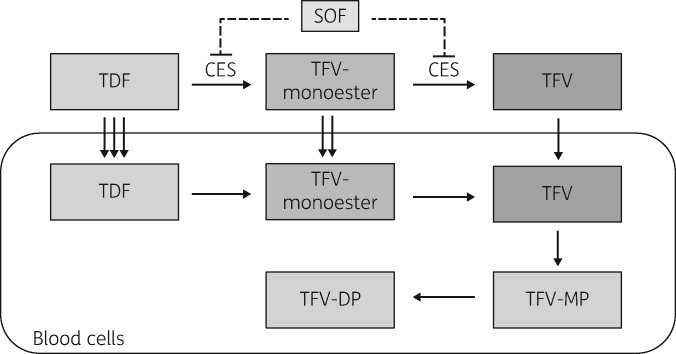
Tenofovir disoproxil is a prodrug initially hydrolysed to tenofovir monoester and subsequently to tenofovir (Figure 1). Tenofovir is then phosphorylated by host enzymes to the active moiety, tenofovir diphosphate. Early in vitro studies indicated that carboxylesterases were responsible for the initial hydrolysis of tenofovir disoproxil to tenofovir monoester, and the monoester was then converted into tenofovir by phosphodiesterases.5 Recent work by Shen et al.6,7 suggests that sofosbuvir reduces TDF hydrolysis via inhibition of carboxylesterase 2 (CES2). However, clinical/pharmacokinetic data to support this proposed drug–drug interaction are not available, and there are variable data on the clinical significance of drug–drug interactions via carboxylesterases.8 Prior studies suggested that TDF conversion through tenofovir monoester was rapid, resulting in tenofovir as the only detectable circulating moiety in the plasma.9 However, Nye et al.10 recently found a metabolite presumed to be tenofovir monoester while developing an LC-MS/MS method for tenofovir, emtricitabine and efavirenz, which was detected in plasma in ‘considerable quantities’ up to 4 h post-dose.
Figure 1.
Overview of TDF hydrolysis and proposed drug–drug interaction mechanism between TDF and sofosbuvir. Dashed lines indicate hypothetical mechanism of inhibition of TDF hydrolysis by sofosbuvir. CES, carboxylesterase; SOF, sofosbuvir; TDF, tenofovir disoproxil fumarate; TFV-monoester, tenofovir monoester; TFV, tenofovir; TFV-MP, tenofovir monophosphate; TFV-DP, tenofovir diphosphate.
Tenofovir monoester is more lipophilic than tenofovir,10 and thus may contribute to blood cell loading in individuals similarly to tenofovir disoproxil. Higher tenofovir disoproxil and/or tenofovir monoester concentrations may result from inhibition of TDF hydrolysis with the concomitant use of sofosbuvir-containing therapy, which could lead to enhanced blood cell loading. This may explain the several-fold increased levels of intracellular tenofovir diphosphate in persons receiving this combination. Thus, we sought to determine whether tenofovir disoproxil or tenofovir monoester were quantifiable in individuals with HIV/HCV coinfection receiving TDF, and whether concentrations of these moieties were increased following the initiation of ledipasvir/sofosbuvir treatment.
Patients and methods
This was a single-centre study conducted at the University of Colorado Anschutz Medical Campus (NCT02588287). This study was approved by the Colorado Multiple Institutional Review Board. All participants provided written informed consent. Eligible participants included persons living with HIV/HCV between 18 and 60 years of age, and on TDF in combination with a ritonavir-boosted HIV PI as part of standard care for at least 30 days prior to initiating ledipasvir/sofosbuvir treatment. Key exclusion criteria included women currently or planning to become pregnant, an estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2 and any medical, social or mental health issue(s) that could interfere with study participation or study outcomes. Enrolment began in December 2015, with the last follow-up visit in September 2016.
Pharmacokinetic assessments were performed prior to (baseline) and 4 weeks after initiating ledipasvir/sofosbuvir treatment. All participants received a standardized meal (macrocontrolled 600 calorie meal consisting of 15% protein, 35% fat and 50% carbohydrates) and study medications were administered under directly observed therapy at both pharmacokinetic assessments. Blood samples for pharmacokinetic analysis were obtained pre-dose (time 0) and 1 and 4 h post-dose. DBS and PBMC were processed from the time 0 sample, and plasma was collected at all aforementioned timepoints. Tenofovir disoproxil was not detectable in plasma and thus could not be quantified. Tenofovir monoester in plasma was quantified using a novel UPLC-MS method.11 This method was linear over a range of 0.1 to 500 ng/mL using a 1/concentration2 weighted calibration curve. Samples exceeding the upper limit of quantification were diluted and reanalysed. Tenofovir in plasma and intracellular tenofovir diphosphate in PBMC and DBS were quantified using validated LC-MS/MS methods as previously described.12,13 Statistical analyses were performed in Stata/IC (v15.1). Pharmacokinetic data were log-transformed to generate geometric mean ratios (GMR) with 95% CIs. P values reflect two-sided paired t-tests.
Results
Study population
Twelve participants were enrolled, of whom 10 had data available at baseline and week 4 for comparison. Of the two participants who did not complete the week 4 visit, one did not start ledipasvir/sofosbuvir therapy and the other had poor adherence to ART and was removed from the study. Of the 10 participants who completed baseline and week 4 visits, 8 were male and 2 were female and 30% were white, 40% were black and 30% were Hispanic/Latino. Mean (SD) age, weight and eGFR were 48.2 (10.4) years, 72.1 (14.3) kg and 98.7 (29.9) mL/min/1.73 m2, respectively. Three participants received ritonavir-boosted atazanavir and seven received ritonavir-boosted darunavir. The majority of participants were infected with HCV genotype 1a (70%), two with genotype 1 b and one with mixed genotype 1a + 1b.
Pharmacokinetic results
At baseline, geometric mean (95% CI) tenofovir monoester plasma concentrations at 1 and 4 h post-dose were 97.4 ng/mL (33.0–287.5) and 0.74 ng/mL (0.27–2.06), respectively (see Figure 2). Following the initiation of ledipasvir/sofosbuvir, these increased to 167.8 ng/mL (45.8–615.3) and 3.73 ng/mL (2.34–5.97), respectively. Tenofovir monoester concentrations at 1 h post-dose were higher but not significantly different between study visits, likely due to sampling variability during the absorption phase (GMR 1.72, 95% CI 0.25–11.78; P = 0.54). However, tenofovir monoester concentrations at 4 h post-dose were 5.02-fold higher (95% CI 1.40–18.05; P = 0.019) with ledipasvir/sofosbuvir. Tenofovir monoester was not detectable pre-dose at baseline or week 4. Plasma concentrations of tenofovir at time 0 (pre-dose), 1 and 4 h post-dose were increased 1.34-fold (95% CI 1.09–1.65; P = 0.011), 1.83-fold (95% CI 0.51–6.54; P = 0.31) and 1.43-fold (95% CI 1.29–1.58, P < 0.0001), respectively, at week 4 versus baseline. Tenofovir diphosphate concentrations in PBMC and DBS with ledipasvir/sofosbuvir increased 2.80-fold (95% CI 1.71–4.57; P = 0.001) and 7.31-fold (95% CI 4.47–11.95; P < 0.0001), respectively, after 4 weeks.
Figure 2.
Geometric mean (95% CI) plasma tenofovir monoester concentrations at baseline and after 4 weeks of ledipasvir/sofosbuvir (week 4) at (a) 1 h post-dose and (b) 4 h post-dose. Intracellular tenofovir diphosphate concentrations in (c) PBMC and (d) DBS were also significantly increased at week 4 in comparison with baseline.
Discussion
This study demonstrated that tenofovir monoester was quantifiable in plasma among persons with HIV/HCV coinfection receiving TDF-based therapy. Furthermore, concentrations of this moiety were increased following the initiation of ledipasvir/sofosbuvir treatment, potentially explaining the drug–drug interaction that was previously discovered in individuals on sofosbuvir-based therapy in combination with TDF. These results contradict prior literature suggesting tenofovir monoester was not detectable in the plasma of humans taking TDF14,15 and extends the work of Nye et al.,10 which identified a compound with a mass-to-charge ratio corresponding in elemental composition to tenofovir monoester.
Initial studies on the pharmacology of TDF reported its conversion to tenofovir as rapid and spontaneous, resulting in no detectable tenofovir monoester in plasma.9 However, in conjunction with the analyses published in this article, Brooks et al.11 demonstrated tenofovir monoester exhibits quantifiable pharmacokinetics in vivo following a single TDF/emtricitabine dose. Baseline tenofovir monoester concentrations in this study were higher than those quantified in the healthy volunteer study at 1 and 4 h post-dose, where geometric mean concentrations at the same timepoints were ∼39 ng/mL and 0.17 ng/mL, respectively. These differences may be driven in part by differences in fasted versus fed state15 and the concomitant use of PIs.16,17 The ∼5-fold increase in tenofovir monoester at 4 h post-dose was higher than the concomitant elevation of tenofovir in plasma (∼1.5-fold) but comparable to the 7.3-fold and 2.8-fold higher tenofovir diphosphate concentrations in DBS and PBMC, respectively.
Sofosbuvir was previously shown to increase TDF recovery through an unidentified mechanism in Caco-2 cells.18 Shen et al.6,7 demonstrated that sofosbuvir inhibited TDF hydrolysis via inhibition of CES2 in liver and kidney microsomes, and in intestinal and liver homogenates of mice treated with sofosbuvir.7 CES2 is highly expressed in the intestinal tract8 and thus CES2 inhibition could result in enhanced delivery of the disoproxil and monoester forms in portal blood. Unfortunately, tenofovir disoproxil is unstable in human plasma and was not quantifiable in this study (data not shown). However, tenofovir monoester was quantifiable. Tenofovir monoester is more lipophilic than tenofovir and has been shown to transfer across Caco-2 cell layers, whereas tenofovir transfer was not detected.19 Therefore, elevated tenofovir monoester levels may enhance cellular delivery and subsequently increase intracellular concentrations of the active tenofovir diphosphate form. Our findings are consistent with the hypothesis that sofosbuvir-mediated inhibition of TDF hydrolysis contributes to the mechanism for higher intracellular tenofovir diphosphate concentrations observed in persons taking these therapies concomitantly.
In addition to our hypothesis above, other drug–drug interaction mechanisms may also contribute to increased concentrations of tenofovir monoester and tenofovir diphosphate during sofosbuvir-based therapy. CES2 may not exclusively convert TDF to the monoester form, and other carboxylesterase subtypes, esterases and lipases have been implicated in TDF hydrolysis.20 Boosted PIs also inhibit various drug transporters, including P-glycoprotein, and carboxylesterases.16,17 Concomitant PIs in our study were consistent between baseline and week 4 in all participants. While sofosbuvir does not inhibit common efflux transporters, such as P-glycoprotein, breast cancer resistance protein (BCRP) or MDR-associated proteins,21 ledipasvir does inhibit P-glycoprotein and BCRP, for which both sofosbuvir and tenofovir disoproxil are substrates. Ledipasvir increases the AUC of sofosbuvir by 130%18 and tenofovir by ∼40%–98%,21 depending on the concomitant antiretroviral administered. These AUC increases were concurrent with increases in peak concentrations, suggesting inhibition of first-pass metabolism. The magnitude of increase in intracellular tenofovir diphosphate concentrations in DBS was markedly higher with the combination of ledipasvir/sofosbuvir versus sofosbuvir with ribavirin in previous studies [17.8-fold (95% CI 12.77–24.86) versus 4.3-fold (95% CI 2.46–7.67), respectively].4 Collectively, a combination of drug–drug interactions from PIs, inhibition of CES2 by sofosbuvir and drug efflux transporters by ledipasvir may result in elevated tenofovir disoproxil and tenofovir monoester, driving blood cell loading of tenofovir diphosphate in this and the prior study.4
Our findings raise new questions about the importance of interactions occurring at the level of nucleotide prodrug conversion to the cellular pharmacology of this class of drugs. Enhanced delivery of the disoproxil or monoester forms could lead to enhanced antiviral activity, particularly within the liver. However, renal proximal tubule damage has been associated with higher tenofovir exposures,22 which brings into question the role that tenofovir monoester may play in these toxicities if increased delivery of the monoester form is contributing to circulating tenofovir levels in the blood. The magnitude of increase in tenofovir diphosphate PBMC concentrations in our study is similar to the ∼2.4–7-fold increases observed with tenofovir alafenamide fumarate (TAF)-containing therapy.23,24 More research is needed to examine potential relationships between intracellular tenofovir diphosphate concentrations and treatment-related toxicities. Tenofovir diphosphate concentrations in DBS are used to determine cumulative medication adherence to tenofovir-based therapies owing to the long t½ of tenofovir diphosphate in this cell type,25,26 which can be altered by concomitant antiretroviral medications in persons living with HIV.27 The magnitude of tenofovir diphosphate increase that occurs with sofosbuvir-containing regimens precludes the ability to use this measure for adherence interpretations. However, the active anabolite of sofosbuvir, 007 triphosphate, is quantifiable in DBS and could alternatively serve as a direct measure of cumulative DAA adherence in persons on sofosbuvir-containing therapy.28
There are limitations to this study. First, a limited sampling strategy was used as the original focus of the study was to measure changes in plasma tenofovir concentrations, but tenofovir monoester peaks at 0.5 h post-dose and has a t½ of ∼0.44 h.11 Thus, true peak concentrations of tenofovir monoester were likely missed and AUC calculations were not possible. Additionally, this was a small sample size and two different participants had low tenofovir monoester concentrations at 1 h post-dose compared with the rest of the study participants, one at baseline and the other 4 weeks after initiating ledipasvir/sofosbuvir. All participants received a standardized breakfast and took an observed dose of study medication during the pharmacokinetic assessments. The mechanisms behind these observations are unclear, but could be attributed in part to delays in absorption. Tenofovir diphosphate in DBS is also influenced by cumulative adherence to TDF. Two participants had DBS levels that were below the threshold of 1250 fmol/punch, which has been identified as a cut-off for levels associated with seven doses per week in persons on pre-exposure prophylaxis (PrEP).25,28 Overall, DBS concentrations measured at baseline in our study were comparable to those measured in virologically suppressed persons living with HIV on boosted PI therapy [2344 (95% CI 1460–3761) versus 1890 (95% CI 1704–2095) fmol/punch, respectively].27
In summary, this study revealed increased concentrations of tenofovir monoester following the initiation of ledipasvir/sofosbuvir in persons living with HIV and HCV. These findings are consistent with elevated tenofovir monoester resulting from inhibition of TDF hydrolysis by sofosbuvir, resulting in enhanced cell loading by the disoproxil or monoester forms, and thus higher intracellular tenofovir diphosphate concentrations with concomitant use. Further research is needed to characterize the pharmacokinetic profile of tenofovir monoester and its potential relevance to cell loading in humans, and establish the magnitude and clinical relevance of drug–drug interactions occurring at the level of nucleotide prodrug conversion.
Acknowledgements
We thank the study participants, clinical staff at the University of Colorado Clinical Translational Research Center (CTRC) and members of the Colorado Antiviral Pharmacology Laboratory. These data were previously presented at the 18th International Workshop on Clinical Pharmacology in Baltimore, MD, USA (22–24 May 2018).
Funding
This study was supported in part through the National Institute on Drug Abuse at the National Institutes of Health (R01DA040499) and the National Institutes of Health/National Center for Advancing Translational Sciences Colorado Clinical and Translational Science Award Grant Number UL1 TR002535.
Transparency declarations
P. L. A. receives research funding (paid to his institution) and donated study medication from Gilead Sciences. J. J. K. receives research funding (paid to her institution) from ViiV Healthcare and Gilead Sciences, and donated study medication from Gilead Sciences for an NIH-sponsored study. The remaining authors have none to declare.
Disclaimer
The contents of this article are the authors’ sole responsibility and do not necessarily represent official NIH views.
References
- 1. Platt L, Easterbrook P, Gower E. et al. Prevalence and burden of HCV co-infection in people living with HIV: a global systematic review and meta-analysis. Lancet Infect Dis 2016; 16: 797–808. [DOI] [PubMed] [Google Scholar]
- 2. Chen TY, Ding EL, Seage GR III. et al. Meta-analysis: increased mortality associated with hepatitis C in HIV-infected persons is unrelated to HIV disease progression. Clin Infect Dis 2009; 49: 1605–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.AASLD-IDSA. Unique Populations. Patients With HIV/HCV Coinfection http://www.hcvguidelines.org/full-report/hcv-testing-and-linkage-care.
- 4. MacBrayne CE, Marks KM, Fierer DS. et al. Effects of sofosbuvir-based hepatitis C treatment on the pharmacokinetics of tenofovir in HIV/HCV-coinfected individuals receiving tenofovir disoproxil fumarate. J Antimicrob Chemother 2018; 73: 2112–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Naesens L, Bischofberger N, Augustijns P. et al. Antiretroviral efficacy and pharmacokinetics of oral bis(isopropyloxycarbonyloxymethyl)-9-(2-phosphonylmethoxypropyl)adenine in mice. Antimicrob Agents Chemother 1998; 42: 1568–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shen Y, Yan B.. Covalent inhibition of carboxylesterase-2 by sofosbuvir and its effect on the hydrolytic activation of tenofovir disoproxil. J Hepatol 2017; 66: 660–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shen Y, Yan B.. Inhibition of carboxylesterase 2 (CES2) by sofosbuvir: metabolism-reduced potency, in vivo inhibition and reduced activation of the anti-HIV drug tenofovir disoproxil. Drug Metab Pharmacokinet 2018; 33: S53. [Google Scholar]
- 8. Laizure SC, Herring V, Hu Z. et al. The role of human carboxylesterases in drug metabolism: have we overlooked their importance? Pharmacotherapy 2013; 33: 210–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Murphy RA, Valentovic MA.. Factors contributing to the antiviral effectiveness of tenofovir. J Pharmacol Exp Ther 2017; 363: 156–63. [DOI] [PubMed] [Google Scholar]
- 10. Nye LC, Gray N, McClure M. et al. Identification of a novel human circulating metabolite of tenofovir disoproxil fumarate with LC-MS/MS. Bioanalysis 2015; 7: 643–52. [DOI] [PubMed] [Google Scholar]
- 11. Brooks KM, Ibrahim ME, Castillo-Mancilla JR. et al. Pharmacokinetics of tenofovir-monoester and association with intracellular tenofovir-diphosphate following single-dose tenofovir disoproxil fumarate. J Antimicrob Chemother 2019; 74: 2352–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zheng JH, Rower C, McAllister K. et al. Application of an intracellular assay for determination of tenofovir-diphosphate and emtricitabine-triphosphate from erythrocytes using dried blood spots. J Pharm Biomed Anal 2016; 122: 16–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Delahunty T, Bushman L, Fletcher CV.. Sensitive assay for determining plasma tenofovir concentrations by LC/MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci 2006; 830: 6–12. [DOI] [PubMed] [Google Scholar]
- 14. Kearney BP, Flaherty JF, Shah J.. Tenofovir disoproxil fumarate: clinical pharmacology and pharmacokinetics. Clin Pharmacokinet 2004; 43: 595–612. [DOI] [PubMed] [Google Scholar]
- 15. Geboers S, Haenen S, Mols R. et al. Intestinal behavior of the ester prodrug tenofovir DF in humans. Int J Pharm 2015; 485: 131–7. [DOI] [PubMed] [Google Scholar]
- 16. Tong L, Phan TK, Robinson KL. et al. Effects of human immunodeficiency virus protease inhibitors on the intestinal absorption of tenofovir disoproxil fumarate in vitro. Antimicrob Agents Chemother 2007; 51: 3498–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rhoades JA, Peterson YK, Zhu HJ. et al. Prediction and in vitro evaluation of selected protease inhibitor antiviral drugs as inhibitors of carboxylesterase 1: a potential source of drug-drug interactions. Pharm Res 2012; 29: 972–82. [DOI] [PubMed] [Google Scholar]
- 18.Center for Drug Evaluation and Research. Clinical Pharmacology and Biopharmaceutics Review(s) Ledipasvir/Sofosbuvir 2014. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2014/205834Orig1s000ClinPharmR.pdf.
- 19.Center for Drug Evaluation and Research. Clinical Pharmacology and Biopharmaceutics Review(s) Tenofovir Disoproxil Fumarate 2001. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2001/21-356_Viread_biopharmr.pdf.
- 20. Moss DM, Domanico P, Watkins M. et al. Simulating intestinal transporter and enzyme activity in a physiologically based pharmacokinetic model for tenofovir disoproxil fumarate. Antimicrob Agents Chemother 2017; 61: e00105-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Harvoni [Package Insert]. Foster City, CA, USA: Gilead Sciences, Inc, 2017. [Google Scholar]
- 22. Moss DM, Neary M, Owen A.. The role of drug transporters in the kidney: lessons from tenofovir. Front Pharmacol 2014; 5: 248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Podany AT, Bares SH, Havens J. et al. Plasma and intracellular pharmacokinetics of tenofovir in patients switched from tenofovir disoproxil fumarate to tenofovir alafenamide. AIDS 2018; 32: 761–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ruane PJ, DeJesus E, Berger D. et al. Antiviral activity, safety, and pharmacokinetics/pharmacodynamics of tenofovir alafenamide as 10-day monotherapy in HIV-1-positive adults. J Acquir Immune Defic Syndr 2013; 63: 449–55. [DOI] [PubMed] [Google Scholar]
- 25. Castillo-Mancilla JR, Zheng JH, Rower JE. et al. Tenofovir, emtricitabine, and tenofovir diphosphate in dried blood spots for determining recent and cumulative drug exposure. AIDS Res Hum Retroviruses 2013; 29: 384–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Anderson PL, Liu AY, Castillo-Mancilla JR. et al. Intracellular tenofovir-diphosphate and emtricitabine-triphosphate in dried blood spots following directly observed therapy. Antimicrob Agents Chemother 2018; 62: e01710-01717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Castillo-Mancilla JR, Morrow M, Coyle RP. et al. Tenofovir diphosphate in dried blood spots is strongly associated with viral suppression in individuals with HIV infection. Clin Infect Dis 2018; doi:10.1093/cid/ciy708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jimmerson L, Morrow M, MaWhinney. et al. Intracellular 007-TP concentrations are associated with gradients of adherence to ledipasvir/sofosbuvir. In: Reviews in Antiviral Therapy & Infectious Diseases, Baltimore, MD,2018. Abstract O_02. 19th International Workshop on Clinical Pharmacology of Antiviral Therapy.