Abstract
Objectives:
This study gathered preliminary information on the initial feasibility of using injection Naltrexone (NTX) therapy in opioid users.
Methods:
One hundred opioid users (36% female, 8% minorities, mean age 34.5±11.4 yrs.) undergoing a health screen to determine initial eligibility for an ongoing study completed the survey.
Results:
Of the 100 respondents, 26, 16, 16, 1 and 0 reported prior treatment episodes of opioid detoxification, buprenorphine (BUP), methadone (MTD), oral NTX and injection NTX, respectively. Ninety and 71% were interested in participating in a study involving oral and/or injection NTX treatment, respectively. Reasons for not wanting to try injection NTX included fear of needles (n=13), side effects (n=7), lack of pain relief (n=12) and cost (n=3). A significantly higher percentage of those interested in injection NTX had episodes of prior opioid agonist maintenance treatment relative to those uninterested (32.4% vs 10.3%; Chi2=5.2, p<0.03). Those preferring injection NTX therapy showed a higher level of interest in this therapy (3.08±1.01 vs 1.62±1.35; Rank Sum p<0.0001) and a lower degree of interest in BUP treatment (2.96±0.93 vs 3.38±0.90; Rank Sum p< 0.03) than those not preferring injection NTX.
Conclusions:
These preliminary results suggest that those with prior, failed experience with opioid agonist maintenance treatment are more likely to consider injection NTX therapy, suggesting it may be optimal as a second-line treatment for OUD.
Keywords: Naltrexone, prescription opioids, opioid use disorder
Introduction:
Opioid dependence continues to be a serious public health problem, particularly with the dramatic rise in abuse of prescription opioids (PO) [1]. PO abuse is associated with serious negative consequences that include physiological dependence, high-risk behaviors [2, 3], overdose [4], [5], and death [6–8].
PO abusers appear to differ from heroin users in that PO user tend to be younger, have less treatment history, less severe opioid and injection use, greater social stability, and less income from illegal sources [9, 10]. PO abusers are also more likely to be female [11, 12] and may have different treatment outcomes than heroin users [9, 13, 14]. Current FDA-approved treatments for opioid dependence were developed in heroin users and efficacies of these interventions may differ in PO dependent individuals. Our project targets a critical barrier to the field in that optimal treatments for PO dependence have not been identified. Despite the scope of the PO problem, a paucity of data exists regarding those subpopulations of PO abusers most likely to benefit from a particular treatment [10, 15–17] and few studies have examined predictors, mediators, and moderators of outcome for medication treatment for opioid dependence [17–20]. Because many PO abusers have characteristics that may indicate better treatment prognosis than heroin abusers [9, 10], long-term maintenance treatment strategies with long-acting opioid agonists such as methadone or buprenorphine may not be the optimal or preferred treatment among PO abusers. At the same time, it is unclear whether maintenance treatment with the opioid antagonist naltrexone following opioid detoxification as a form of relapse prevention would be acceptable to these individuals, much less efficacious.
In 2016, Arkansas had the second highest rate of opioid prescriptions in the US [21] and more Arkansas young adults use POs than then national average (Office of Adolescent Health 2017). In 2014, 94% of Arkansans admitted to treatment for opioid use disorder used POs [22]. Thus, this study sought to gather preliminary information regarding the initial feasibility of conducting research using naltrexone as a treatment agent for this population. Specifically, this survey protocol was designed to accomplish the following: to determine treatment preferences for opioid dependence; to determine initial interest and willingness to participate in a study using a long acting form of naltrexone for maintenance treatment following opioid detoxification; and to assess whether particular subject characteristics are associated with preference for naltrexone maintenance treatment. We postulated that participants with prior episodes of treatment with opioid agonists would be less likely to consider treatment with injection NTX.
Methods
Participants:
Opioid-using male and female volunteers (≥18 years of age) were recruited to respond to this anonymous survey from those individuals attending a screening interview to determine eligibility to participate in ongoing clinical trials to treat opioid use disorder. Respondents were recruited between October 2013 and February 2016. Written consent was not obtained because the only record linking the participant to the research would have been the consent document itself and the principal risk would be the potential harm from a breach of confidentiality. Instead, a copy of the following statement was placed before the potential participant so that the individual could follow along as it was read aloud: “You are invited to participate in a survey designed to determine what people’s treatment preferences are for addressing their opioid addiction and whether you might be interested in being part of a research study that involves a long-acting form of Naltrexone (Vivitrol) for maintenance treatment after detoxification from opioids. A few questions regarding demographics and opioid addiction treatment history are included also. The survey will take approximately 5–10 minutes to complete. The information will not be linked to you in any way. Your completing this survey is completely voluntary and will not determine in any way your eligibility to participate in one of our research studies. You do not have to answer any question that makes you uncomfortable and you can end the survey at any time without penalty. Are you willing to participate?” This protocol was deemed exempt by the University of Arkansas for Medical Sciences Institutional Review Board.
Survey:
The survey interviews were conducted on the 4th floor of the Psychiatric Research Institute at University of Arkansas for Medical Sciences, Little Rock. For those who agreed to complete the survey, the interviewer proceeded to ask questions from the survey assessment, called the “Treatment Preferences for Opioid Dependence.” This 21-item questionnaire obtained basic demographic information age (yrs.), sex (male or female), race (Caucasian, African American, Native American, Asian, Other, refused), ethnicity (Latino/Hispanic, Non-Latino/Hispanic, refused), primary substance of abuse (Opioids, Cocaine, methamphetamine /Amphetamines, Alcohol, Marijuana, Other), prior treatments for opioid dependence (Detox, Buprenorphine (BUP), Methadone (MTD), Naltrexone (NTX) tablets or injection, Other), level of interest in treatment with long acting form of naltrexone and reasons for not considering it. In addition, the degree to which respondents’ drug use affected physical health, mental health, finances/work, relationships, and legal problems were measured on a scale of 0 (not at all) to extremely (4). Interviewees were not compensated for their time.
Data Analyses:
Descriptive statistics were performed to summarize the data. The sample was split into two groups based on respondents’ answers to the two questions, “Are you interested in participating in a study that involves oral NTX” or “Are you interested in participating in a study that involves injection NTX?” Single Logistic Regression models were run for continuous variables to test their effect on interest in injection NTX (Yes/No). Due to non-normality, Two-sample Wilcoxon Rank Sum tests were performed on continuous variables with level of interest in injection NTX (Yes/No) as the between-subjects factor. Dichotomous variables were analyzed using chi square tests to examine whether differences in acceptability were due to subject characteristics. Number of episodes of maintenance treatment, either with buprenorphine or methadone, was reclassified into the categories: “zero” and “one or more”. A p value < 0.05 was used to infer statistical significance. All statistical analysis was performed with SAS v9.4 (SAS Institute Inc., Cary, NC).
Results
Of the 100 respondents, 90 (90%) and 71 (71%) expressed an interest in participating in a study involving oral or injection NTX respectively. Willingness to participate in a study receiving injection NTX did not differ by gender (% Females, χ2=1.38, p=0.24), education (%> HS, χ2=0.09, p=0.77), or relationship status (% Married/In relationship, χ2=0.09, p=0.77); See Table 1. Age did not differ between those willing to participate and those not willing (Yes (mean +/− standard deviation): 35.83 +/−12.06 years, No: 31.38+/−9.08 years; odds ratio=1.04, χ2=3.04, p-value=0.0814). Similarly, years of opioid abuse (Yes: 9.28+/−7.36, No: 6.86+/−6.18; odds ratio=1.06, χ2=2.32, p-value=0.1281), legal (odds ratio=1.28, χ2=2.48, p-value=0.1151), financial (odds ratio=1.03, χ2=0.02, p-value=0.8936) or psychiatric problems (odds ratio=0.93, χ2=0.11, p-value=0.7377) also did not differ between the groups (See Table 2). All respondents identified opioids as their primary drug of abuse.
Table 1.
Subject Characteristics – Dichotomous Variables
| Measure | Interest in NTXa |
Statistical Test | P-Value | ||||
|---|---|---|---|---|---|---|---|
| Yes |
No |
||||||
| N | % | N | % | ||||
| Sex | Males |
48 | 67.6 | 16 | 55.2 | Chi-Square | 0.24 |
| Females | 23 | 32.4 | 13 | 44.8 | |||
| Education | Less than HS |
9 | 12.7 | 5 | 17.2 | Fisher | 0.84 |
| HS Graduate |
23 | 32.4 | 9 | 31.0 | |||
| Some College |
28 | 39.4 | 13 | 44.8 | |||
| Bachelor’s Degree |
7 | 9.9 | 1 | 3.4 | |||
| More Than Bachelor’s Degree | 4 | 5.6 | 1 | 3.4 | |||
| Marital Status | Single |
26 | 36.6 | 10 | 34.5 | Fisher | 0.90 |
| In Relationship |
11 | 15.5 | 3 | 10.3 | |||
| Married |
21 | 29.6 | 11 | 37.9 | |||
| Divorced |
11 | 15.5 | 5 | 17.2 | |||
| Widowed | 2 | 2.8 | 0 | 0.0 | |||
| Detox Episodes | Yes |
18 | 25.4 | 8 | 27.6 | Chi-Square | 0.82 |
| No | 53 | 74.6 | 21 | 72.4 | |||
| Buprenorphine Maintenance Episodes | Yes |
13 | 18.3 | 3 | 10.3 | Chi-Square | 0.32 |
| No | 58 | 81.7 | 26 | 89.7 | |||
| Methadone Maintenance Episodes | Yes |
14 | 19.7 | 2 | 6.9 | Chi-Square | 0.11 |
| No | 57 | 80.3 | 27 | 93.1 | |||
| BUPb/MTDc Maintenance Episodes |
1+ |
23 | 32.4 | 3 | 10.3 | Chi-Square | 0.02 |
| Zero | 48 | 67.6 | 26 | 89.7 | |||
| Recommended Buprenorphine By Others | Yes |
48 | 67.6 | 18 | 62.1 | Chi-Square | 0.65 |
| No | 23 | 32.4 | 11 | 37.9 | |||
| Recommended Methadone By Others | Yes |
28 | 39.4 | 12 | 41.4 | Chi-Square | 0.86 |
| No | 43 | 60.6 | 17 | 58.6 | |||
Long Acting Injection of Naltrexone
Buprenorphine
Methadone
Table 2.
Subject Characteristics – Continuous Variables
| Measure | Interest in Inj, NTXa | Statistical Test | P-value | |
|---|---|---|---|---|
| Yes (N=71) Mean | No (N=29) Mean | |||
| Age | 35.8 | 31.4 | Rank Sum Test | 0.11 |
| Yrs. of Opioid Abuse (Mean +/− SD) | 9.28+/−7.36 | 6.86+/−6.18 | Rank Sum Test | 0.079 |
| Detox Times | 0.70 | 0.72 | Rank Sum Test | 0.90 |
| Buprenorphine Times | 0.25 | 0.45 | Rank Sum Test | 0.39 |
| Methadone Times | 0.31 | 0.14 | Rank Sum Test | 0.13 |
| Family Support | 2.73 | 2.45 | Rank Sum Test | 0.28 |
| Legal Problems | 1.41 | 0.86 | Rank Sum Test | 0.14 |
| Financial Problems | 3.34 | 3.31 | Rank Sum Test | 0.45 |
| Psychiatric Problems | 2.51 | 2.59 | Rank Sum Test | 0.63 |
| Relationship Problems | 2.68 | 2.79 | Rank Sum Test | 0.54 |
| Physical Health Problems | 2.39 | 2.48 | Rank Sum Test | 0.70 |
| Prefer Buprenorphine Maintenance (Mean +/− SD) | 2.96+/−0.93 | 3.38+/−0.90 | Rank Sum test | 0.02 |
| Prefer Methadone Maintenance | 1.72 | 1.83 | Rank Sum Test | 0.73 |
| Prefer IM NTX (Mean +/− SD) | 2.55+/−1.12 | 0.59+/−1.02 | Rank Sum Test | <0.0001 |
| Prefer PO NTX | 2.65 | 2.07 | Rank Sum test | 0.09 |
| Prefer Outpatient Detox | 3.15 | 3.17 | Rank Sum Test | 0.75 |
| Prefer Inpatient Detox | 1.03 | 0.86 | Rank Sum Test | 0.45 |
| Interest in Feeling Clearheadedb | 3.55 | 3.55 | Rank Sum Test | 0.93 |
| Interest in Medication You Can’t Feelc | 3.34 | 3.45 | Rank Sum test | 0.38 |
| Interest in Medication Taken Daily | 3.34 | 3.45 | Rank Sum Test | 0.40 |
| Interest in Medication Taken Monthly by Inj. | 3.08+/−1.01 | 1.62+/−1.35 | Rank Sum test | <0.0001 |
Long Acting Injection Naltrexone
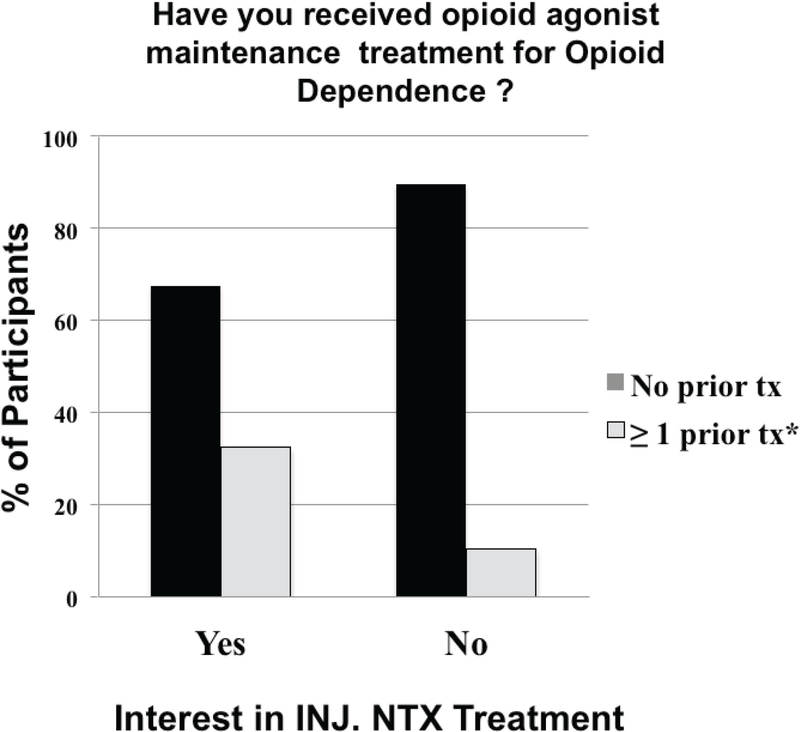
No differences were observed regarding prior episodes with specific medication-assisted treatments (p’s>0.1), with 26, 16, 16, 1 and 0 of all respondents reporting prior treatment episodes of opioid detoxification, BUP maintenance, MTD maintenance, oral NTX treatment and injection NTX treatment, respectively. However, a significantly higher percentage of those interested in injection NTX had episodes of prior opioid agonist (i.e., buprenorphine or methadone) maintenance treatment relative to those uninterested (χ2=5.3, p<0.03; Figure 1).
Figure1.
Episodes of prior opioid agonist (i.e., buprenorphine or methadone) maintenance treatment in those interested in Long Acting Injection of Naltrexone relative to those uninterested. Results of those without and with at least one prior treatment episodes are represented by the black columns and white columns, respectively.
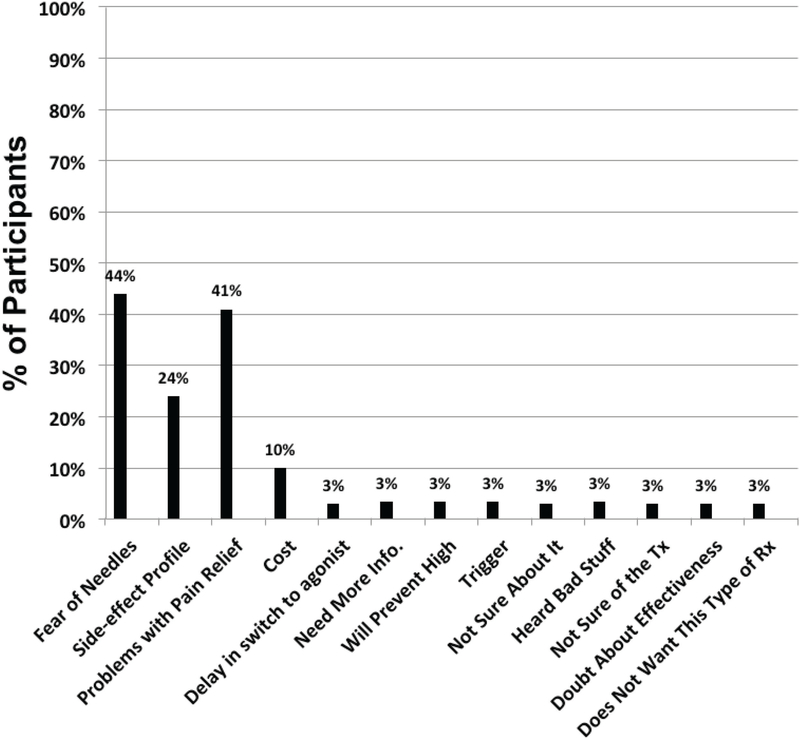
Those interested in this therapy showed a higher level of interest in injection NTX therapy (3.08+/−1.01 vs 1.62+/−1.35; Rank Sum p< 0.0001) and a lower degree of interest in BUP treatment (2.96+/−0.93 vs 3.38+/−0.90; Rank Sum p< 0.03) than those not preferring injection NTX. The two most cited reasons for not preferring injection naltrexone were fear of needles and lack of pain relief (see Figure 2).
Figure 2.
Reasons provided byparticipants for not considering Long Acting Injection of Naltrexone
Discussion
The main finding of this survey indicates that those with prior, failed experience with opioid agonist maintenance treatment are more likely to consider injection NTX therapy than those who have not had prior opioid agonist treatment experience. This result is opposite of our hypothesis. The reasons for this are unclear but stigma, poor response in a prior treatment episode and fear of withdrawal on cessation of treatment have been reported [23]. Nevertheless, this finding suggests injection NTX may be optimal as a second-line treatment for opioid use disorder in the absence of a strong campaign to educate opioid use disordered individuals about the relative benefits of this intervention.
The demographic characteristics of participants interested in injection NTX treatment generally did not differ from those who were not. In contrast, a survey of 657 injection opioid users found Caucasian race was negatively associated with willingness to consider long acting injection of naltrexone for treatment of opioid use disorder [24]. Nevertheless, in a study of acceptability of extended-release naltrexone among heroin-dependent patients in Netherlands, neither age, sex, race nor employment status predicted interest in treatment with this medication [25]. Although naltrexone has been found to be more useful in individuals with external motivation to remain abstinent from opioids (e.g., involvement in criminal justice system) [26], interest in treatment with injection NTX did not differ by degree of legal problems in our study.
Study participants identified several barriers to consideration of injection NTX therapy that are similar to those identified in surveys of acceptability among injection opioid [24] and heroin users [25]. Needle aversion, the most commonly reported issue in the current study, has also been cited as affecting acceptability of injection NTX for alcohol use disorder [27] and injectable medications for Type 2 Diabetes Mellitus [28]. Needle-free injection technology has shown promise to mitigate pain associated with injectable medications and can improve acceptance [29, 30]. The second most-cited barrier, concern about not getting pain relief, may be alleviated by educating patients about treatment options for pain management among patients on injection NTX [31]. Thus, developing a needle-free naltrexone injection along with education about pain management availability while taking injectable naltrexone may increase acceptance of this therapy among opioid-use-disordered patients.
In addition to the importance of having a broad portfolio of medication-assisted treatments available to enhance optimal matching of patients to appropriate treatment for opioid use disorder, increasing the utilization of injection NTX is important from a financial perspective. For instance, Baser et. al. [32], using retrospective claims database analysis, showed that patients who received injection NTX had lower inpatient healthcare utilization at comparable or lower total costs than those receiving oral/sublingual opioid agonist medications, including methadone and buprenorphine. This report suggests that increased utilization of injection NTX treatment may lead to lower medical costs compared with opioid agonist approaches.
To our knowledge, this is the first study to determine characteristics of individuals in an area with a high prevalence of PO abuse that are associated with willingness to consider injection NTX. A significant strength of our study is inclusion of several variables including treatments recommended by people important to participants [33], common reasons for not considering injection NTX [34, 35] and interest in taking a medication which does not make them feel like being on methadone [36] which have been associated with acceptability of injection NTX in published literature.
Our study has several limitations as well. For instance, respondents were recruited from those seeking to participate in a treatment research study. Thus, it is unclear whether these individuals may differ from the general PO abusing patient population. Moreover, respondents did not undergo structured diagnostic interviews to establish diagnosis of opioid use disorder, which limits conclusions about opioid use disordered patients. In addition, we did not obtain the specific opioids being abused by respondents. Nevertheless, given that all but three participants identified their drug of choice as opioids, mean years of regular opioid use, and varying degree of impact on various aspects of their life reported by participants suggest their data are likely generalizable to the opioid use disordered population in general. At the same time, this survey was conducted in rural Arkansas, so whether findings are generalizable to opioid users in more urban areas is unknown. Lastly, our survey has not been validated, although several questions were adopted from published surveys on similar populations.
Conclusions
Injection NTX therapy may be optimal as a second line treatment for opioid use disorder in individuals with prior treatment episodes for opioid use disorder in the absence of strong efforts to educate opioid use disordered individuals about the relative benefits of NTX therapy. Use of injection-free technology and education to mitigate fears of needles and side effects may improve overall acceptability of this approach.
Acknowledgments:
• Supported by NIDA grants 5R01DA039088–02 and 5R21DA035325–02
• Nahata, R., Mancino, M., Thostenson, J., Oliveto, A. (2017) Survey of treatment preferences for opioid use disorder. Presented at the 79th Annual Scientific Meeting of the College on Problems of Drug Dependence. June 20, 2017.
Abbreviations:
- NTX
Naltrexone
- MTD
Methadone
- BUP
Buprenorphine
- PO
Prescription Opioids
Footnotes
Conflict of Interest: The authors declare that they have no conflict of interest to report
References:
- 1.SAMHSA, Results from the 2009 National Survey on Drug Use and Health: Volume I. Summary of National Findings.. NSDUH Series H-38A, HHS Publication No. SMA 10–4586 Findings)., 2010.
- 2.DuPont RL, Prescription Drug Abuse: An Epidemic Dilemma. Journal of Psychoactive Drugs, 2010. 42(2): p. 127–132. [DOI] [PubMed] [Google Scholar]
- 3.McCabe SE, et al. , Does early onset of non-medical use of prescription drugs predict subsequent prescription drug abuse and dependence? Results from a national study. Addiction (Abingdon, England), 2007. 102(12): p. 1920–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coben JH, et al. , Hospitalizations for Poisoning by Prescription Opioids, Sedatives, and Tranquilizers. American Journal of Preventive Medicine, 2010. 38(5): p. 517–524. [DOI] [PubMed] [Google Scholar]
- 5.Cai R, et al. , Emergency Department Visits Involving Nonmedical Use of Selected Prescription Drugs in the United States, 2004–2008. Vol. 24 2010. 293–7. [DOI] [PubMed] [Google Scholar]
- 6.Paulozzi LJ and Xi Y, Recent changes in drug poisoning mortality in the United States by urban–rural status and by drug type. Pharmacoepidemiology and Drug Safety, 2008. 17(10): p. 997–1005. [DOI] [PubMed] [Google Scholar]
- 7.Hanson K, A pill problem: prescription drug abuse is the fastest growing form of substance abuse. State Legislatures, 2010. 36(3): p. 22–25. [PubMed] [Google Scholar]
- 8.K. H, A pill problem: prescription drug abuse is the fastest growing form of substance abuse. State Legislatures, 2010(March): p. 22–25. [PubMed] [Google Scholar]
- 9.Moore BA, et al. , Primary Care Office-based Buprenorphine Treatment: Comparison of Heroin and Prescription Opioid Dependent Patients. Journal of General Internal Medicine, 2007. 22(4): p. 527–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sigmon SC, Characterizing the Emerging Population of Prescription Opioid Abusers. The American Journal on Addictions, 2006. 15(3): p. 208–212. [DOI] [PubMed] [Google Scholar]
- 11.Green TC, et al. , Women who abuse prescription opioids: Findings from the Addiction Severity Index-Multimedia Version Connect prescription opioid database. Drug & Alcohol Dependence,2009. 103(1): p. 65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Back SE, et al. , Gender and Prescription Opioids: Findings from the National Survey on Drug Use and Health. Addictive behaviors, 2010. 35(11): p. 1001–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brands B, et al. , Prescription opioid abuse in patients presenting for methadone maintenance treatment. Drug & Alcohol Dependence. 73(2): p. 199–207. [DOI] [PubMed] [Google Scholar]
- 14.Weiss RD, et al. , Adjunctive counseling during brief and extended buprenorphine-naloxone treatment for prescription opioid dependence: A 2-phase randomized controlled trial. Archives of General Psychiatry, 2011. 68(12): p. 1238–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zacny J, et al. , College on Problems of Drug Dependence taskforce on prescription opioid non-medical use and abuse: position statement. Drug & Alcohol Dependence. 69(3): p. 215–232. [DOI] [PubMed] [Google Scholar]
- 16.Sigmon SC, et al. , Brief Buprenorphine Detoxification for the Treatment of Prescription Opioid Dependence: A Pilot Study. Addictive behaviors, 2009. 34(3): p. 304–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dreifuss JA, et al. , Patient characteristics associated with buprenorphine/naloxone treatment outcome for prescription opioid dependence: Results from a multisite study. Drug & Alcohol Dependence. 131(1): p. 112–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morral AR, et al. , Natural classes of treatment response. Journal of Consulting and Clinical Psychology, 1997. 65(4): p. 673–685. [DOI] [PubMed] [Google Scholar]
- 19.Ziedonis DM, et al. , Predictors of outcome for short-term medically supervised opioid withdrawal during a randomized, multicenter trial of buprenorphine–naloxone and clonidine in the NIDA clinical trials network drug and alcohol dependence. Drug & Alcohol Dependence. 99(1): p. 28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McHugh RK, et al. , Gender differences in a clinical trial for prescription opioid dependence. Journal of Substance Abuse Treatment. 45(1): p. 38–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.CDC. U.S. State Opioid Prescribing Rates. 2016 July 31, 2017. [cited 2018 May 29]; Available from: https://www.cdc.gov/drugoverdose/maps/rxstate2016.html.
- 22.SAMHSA, Treatment Episode Data Set (TEDS) 2004–2014. State Admissions to Substance Abuse Treatment Services; 2014. [Google Scholar]
- 23.Nunes EV, et al. , Treating Opioid Dependence With Injectable Extended-Release Naltrexone (XR-NTX): Who Will Respond? Journal of Addiction Medicine, 2015. 9(3): p. 238–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahamad K, et al. , Factors associated with willingness to take extended release naltrexone among injection drug users. Addict Sci Clin Pract, 2015. 10(1): p. 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zaaijer ER, et al. , Acceptability of Extended-Release Naltrexone by Heroin-Dependent Patients and Addiction Treatment Providers in the Netherlands. Substance Use & Misuse, 2016. 51(14): p. 1905–1911. [DOI] [PubMed] [Google Scholar]
- 26.O’Brien C and Cornish JW, Naltrexone for probationers and parolees. Journal of Substance Abuse Treatment, 2006. 31(2): p. 107–111. [DOI] [PubMed] [Google Scholar]
- 27.Chokron Garneau H, et al. , Barriers to initiation of extended release naltrexone among HIV-infected adults with alcohol use disorders. Journal of Substance Abuse Treatment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spain CV, et al. , Self-reported Barriers to Adherence and Persistence to Treatment With Injectable Medications for Type 2 Diabetes. Clinical Therapeutics. 38(7): p. 1653–1664.e1. [DOI] [PubMed] [Google Scholar]
- 29.Ravi A, et al. , Needle free injection technology: A complete insight. International Journal of Pharmaceutical Investigation, 2015. 5(4): p. 192–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weissmueller NT, et al. , Alternative vaccine administration by powder injection: Needle-free dermal delivery of the glycoconjugate meningococcal group Y vaccine. PLOS ONE, 2017. 12(8): p. e0183427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coluzzi F, B.F., Cuomo A, Dauri M, Leonardi C, Melotti RM, Natoli S, Romualdi P, Savoia G, Corcione A The challenge of perioperative pain management in opioid-tolerant patients. Therapeutics and Clinical Risk Management, 2017. 13: p. 1163–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baser O, et al. , Cost and Utilization Outcomes of Opioid-Dependence Treatments. The American Journal of Managed Care, 2011. Suppl 8:S235–48. [PubMed] [Google Scholar]
- 33.Fram DH, Marmo J, and Holden R, Naltrexone treatment — the problem of patient acceptance. Journal of Substance Abuse Treatment. 6(2): p. 119–122. [DOI] [PubMed] [Google Scholar]
- 34.Friedmann PD, et al. , Aversion to Injection Limits Acceptability of Extended-Release Naltrexone Among Homeless, Alcohol-Dependent Patients. Substance Abuse, 2013. 34(2): p. 94–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Swift RM, et al. , Alcoholic Patients’ Experience and Attitudes on Pharmacotherapy for Alcoholism. Journal of Addictive Diseases, 1998. 17(3): p. 35–48. [DOI] [PubMed] [Google Scholar]
- 36.Jones Hendrée E, Acceptance of Naltrexone by Pregnant Women Enrolled in Comprehensive Drug Addiction Treatment: An Initial Survey. The American Journal on Addictions, 2012. 21(3): p. 199–201. [DOI] [PubMed] [Google Scholar]