Abstract
Lacunar strokes are appropriately named for their ability to cavitate and form ponds or “little lakes” (Latin: lacune -ae meaning pond or pit is a diminutive form of lacus meaning lake). They account for a substantial proportion of both symptomatic and asymptomatic ischemic strokes. In recent years, there have been several advances in the management of large vessel occlusions. New therapies such as non-vitamin K antagonist oral anticoagulants and left atrial appendage closure have recently been developed to improve stroke prevention in atrial fibrillation; however, the treatment of small vessel disease-related strokes lags frustratingly behind. Since Fisher characterized the lacunar syndromes and associated infarcts in the late 1960s, there have been no therapies specifically targeting lacunar stroke. Unfortunately, many therapeutic agents used for the treatment of ischemic stroke in general offer only a modest benefit in reducing recurrent stroke while adding to the risk of intracerebral hemorrhage and systemic bleeding. Escalation of antithrombotic treatments beyond standard single antiplatelet agents has not been effective in long-term lacunar stroke prevention efforts, unequivocally increasing intracerebral hemorrhage risk without providing a significant benefit. In this review, we critically review the available treatments for lacunar stroke based on evidence from clinical trials. For several of the major drugs, we summarize the adverse effects in the context of this unique patient population. We also discuss the role of neuroprotective therapies and neural repair strategies as they may relate to recovery from lacunar stroke.
Keywords: cerebrovascular diseases, stroke, lacunar stroke, cerebral small vessel disease
Introduction and Basic Definitions
Lacunar strokes (LS), which account for approximately 20–30% of all ischemic strokes,(1,2) are largely due to the pathologic consequences of underlying cerebral small vessel disease (cSVD). While LS is one sequela of cSVD, cSVD is a major contributor to several neurological comorbidities, including vascular cognitive impairment, gait disorders, and intracerebral hemorrhage (ICH).(3–8) In addition to lacunar cavitations, cSVD has many neuroimaging manifestations including white matter hyperintensities (WMH), cerebral microbleeds, dilated perivascular spaces, superficial cortical siderosis, and brain atrophy. By the historical classification schema outlined in the Trial of Org 10172 in Acute Stroke Treatment (TOAST), small artery occlusions (LS) were defined as meeting the following criteria: 1) a traditional lacunar syndrome without cortical signs, 2) supporting features such as hypertension and diabetes mellitus, 3) the lack of an infarct explaining deficits on computed tomography (CT)/magnetic resonance imaging (MRI) examination or a subcortical lesion less than 15 mm in diameter, and 4) the absence of features that suggest a high likelihood of cardioembolism or embolism from upstream arterial stenosis greater than 50%.(9)
With advances in CT and MRI technology leading to the detection of asymptomatic infarcts, more recent terminology developed by the STandards for ReportIng Vascular changes on nEuroimaging (STRIVE) consortium separates the lesion attributed to small vessel disease into several subtypes: recent small subcortical infarct and lacune of presumed vascular origin.(10) This terminology reflects a more complete understanding of the dynamic nature of cSVD such that not all subcortical infarcts form lacunar cavities but instead may form acute and chronic WMH.(11,12) Furthermore, while subcortical infarctions are often accompanied by the clinical syndromes originally described by Fisher and others(13), subcortical infarctions can be identified incidentally by diffusion-weighted imaging (DWI) sequences on MRI scans.(14–17)
By consensus definitions set forth by the STRIVE consortium, recent small subcortical infarcts can be up to 20 mm in axial diameter on MRI and are attributed to infarcts in the territory of the arteriole perforator.(10) Lesions larger than 20 mm in the basal ganglia are excluded from this definition, because they may be caused by an infarct affecting several arteriolar penetrators. Lacunes of presumed vascular origin are the end products of small subcortical infarcts (see Figure 1), although they can sometimes be difficult to distinguish from old hemorrhages or large perivascular spaces. These lacunes are frequently observed on neuroimaging scans of neurologically “healthy” individuals, although they confer an increased risk of symptomatic stroke and vascular cognitive impairment.(5,18–23) Lacunes of presumed vascular origin are subcortical, round, fluid-filled spaces between 3 mm and 15 mm (smaller than subcortical infarcts due to chronic involution of tissue). Compared to dilated perivascular spaces, lacunes have a surrounding T2 hyperintense rim and are usually larger than the 3 mm size cutoff for these lesions.(10,24,25) Lacunes are classically found in deep locations, although lobar lacunes have been recently characterized,(26) and are likely associated with cerebral amyloid angiopathy (CAA) (see Figure 2). For purposes of this review, small subcortical infarcts and their accompanying clinical features will be referred to as LS.
Figure 1. Lacunar Stroke.
Recent small subcortical infarct (yellow arrow) appears bright on the DWI sequence and dark on the ADC sequence. In the early subacute phase, there may be a corresponding WMH seen on T2 FLAIR. A small subcortical infarct may involute over time and form a fluid-filled cavity or lacune seen on the contralateral hemisphere of this patient (red arrow). Note the presence of a surrounding T2 hyperintense rim which is a useful finding to help distinguish a lacune from a dilated perivascular space. DWI: diffusion-weighted imaging; ADC: apparent diffusion coefficient; WMH: white matter hyperintensity; FLAIR: fluid-attenuated inversion recovery.
Figure 2. Lobar Lacune.
Lobar lacunes are a recently described entity which can be observed on T1 and T2 FLAIR sequences (blue arrows). This brain scan was taken from a patient with probable CAA. Consistent with this example, lobar lacunes are more associated with CAA than other cerebral small vessel diseases. Notably, on the SWI sequence, multiple posterior cortical microbleeds (red arrows) are seen; however, there is no microbleed corresponding to the lobar lacune. FLAIR: fluid-attenuated inversion recovery; CAA: cerebral amyloid angiopathy; SWI: susceptibility-weighted imaging. Images were graciously provided by Elif Gökçal.
In recent years, a type of infarct termed cerebral microinfarcts have gained much attention (see Figure 3).(27) These microscopic lesions (0.2 mm to 3 mm) are observed during pathological studies(28) and occasionally by high field strength MRIs.(27,29–31) They occur commonly in patients with memory impairment and are independent predictors of vascular dementia.(30–32) The burden of these infarcts can be daunting, with some estimates suggesting hundreds to thousands of infarcts in a single brain.(33–35) These cerebral microinfarcts may be responsible for some of the devastating sequalae of small vessel disease. Understanding their role may provide further insight into LS pathogenesis allowing for the development of highly-specific molecular targets. Because their significance is unclear, at the current time, cerebral microinfarcts should be diagnosed and treated in a similar fashion as LS, as they likely represent smaller versions of LS. With the exception of comborbid ICH,(14) when cerebral microinfarcts are encountered, a thorough evaluation for embolic sources should be performed.
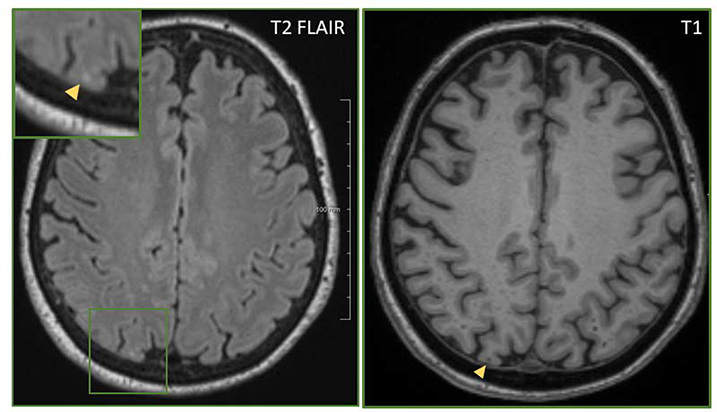
Figure 3. Cerebral Microinfarct.
Cerebral microinfarcts are less than 3 mm in diameter. They are hyperintense on T2 FLAIR (left, inset) and hypointense on T1 (right).
While there have been several recent advances in the treatment of large vessel occlusions that are impacting systems of care,(36,37) the treatment of LS has lagged in comparison. In this review, we will discuss the current therapies available for cSVD-related infarcts and discuss their role in the acute and chronic settings. Our focus will be on LS due to sporadic, non-amyloid cSVD, although it is important to note that LS may also be caused by embolism, branch artery atheroma, or CAA.(26,38,39) Throughout this review, non-amyloid cSVD will be referred to simply as cSVD(4). As determined from population-based studies, hypertension is most influential risk factor involved in cSVD formation and progression, although several additional risk factors may be involved.(40,41)
Methodology
Articles from January 1960 to December 2018 were located using the PubMed (National Center for Biotechnology Information, National Library of Medicine) database. The following search terms were employed: “lacunar stroke AND treatment” without any exclusionary terms. The search was limited to full-text articles available in English. Several additional references were selected by reviewing the reference lists of relevant publications. In our search, we excluded literature pertaining to other stroke subtypes including large artery atherosclerosis and cardioembolism.
Employing the above search strategy yielded a total of 1072 articles. Articles were independently screened by 2 authors (A.S.D. and R.W.R.) for their relevance to lacunar stroke therapy. After the screening process, 263 articles were ultimately selected for incorporation in this paper, generating a semi-systematic review. This review is organized by the various major categories of therapies trialed for LS: thrombolysis, anticoagulation, antiplatelet agents, and selective serotonin reuptake inhibitors (SSRIs). We then focus on risk factor modulation: blood pressure manipulation, glycemic control, and hyperlipidemia control. In these sections, we make the distinction between acute and secondary prevention efforts when they are both mentioned. Following these sections, we discuss neuroprotective and neural repair strategies. Because of the numerous trials involving thrombolytic and antiplatelet therapy, we have summarized this information in two tables.
General Therapeutic Considerations
In reviewing approaches for therapy and prevention of LS, there are several important considerations. A distinction should be made between prevention, acute reperfusion, neuroprotection, and chronic repair augmentation therapies. Each phase has different mechanisms and different potential therapeutic targets, and many factors influence outcomes after stroke.(42)
Whether therapies are applied in the acute or chronic setting, the benefits of treatment must be carefully weighed against the risks of systemic bleeding and ICH. Both CAA-related and non-CAA-related cSVD pose an increased risk of ICH, which has a high rate of recurrence(43,44) and carries significant morbidity and mortality.(45,46) Neuroimaging findings, such as cerebral microbleeds (either the presence or absence of these lesions),(47,48) WMH,(49,50) dilated perivascular spaces,(51) and superficial cortical siderosis,(47) influence this risk and should be taken into consideration when deciding on LS therapy.(46) Since antithrombotic agents elevate ICH risk in healthy populations,(52) applying these therapies to patients with cSVD may have an even greater effect on ICH risk. Not only is there an increased risk of ICH within the stroke territory in the early phase, but there is also an increased risk of ICH in remote territories, especially with the use of thrombolytic agents or anticoagulants.(53–57) Therefore, a thorough understanding of these agents and their usage in LS is critical even when there is a reasonable concurrent indication for their use.
Thrombolysis
In 1995, the National Institute of Neurological Disorders and Stroke (NINDS) recombinant tissue plasminogen activator (tPA) trial revolutionized the approach to acute stroke management.(53) The European Cooperative Acute Stroke Study (ECASS)(58) was published just before the NINDS trial, and five additional trials followed, including ECASS II,(59) ECASS III,(60) Alteplase ThromboLysis for Acute Noninterventional Therapy in Ischemic Stroke (ATLANTIS) A/B,(61,62) EchoPlanar Imaging Thrombolytic Evaluation Trial (EPITHET),(63,64) and the third International Stroke Trial (IST-3).(65,66) All of these trials evaluated the efficacy of tPA in various time windows ranging from 0 to 6 hours from symptom onset. Based on the results of these trials, the standard of care is intravenous (IV) tPA for patients with acute ischemic stroke of adequate disability presenting within 4.5 hours of symptom onset. This indication includes patients with acute LS.(67)
Although there is some controversy regarding its efficacy in the LS compared to other stroke subtypes,(68) the majority of trials support the use of IV tPA in this population. In the original NINDS trial, of the total LS patients that received tPA, 63% had a favorable outcome (mRS ≤ 1) at 3 months compared to 40% in the control group.(53) This was the greatest risk reduction among stroke subtypes. Yet, in IST-3, which evaluated tPA administered up to 6 hours, while the overall analysis demonstrated a functional improvement at 18 months in the treatment arm, the subgroup analysis of LS patients revealed a nonsignificant trend toward worse outcomes at 3 months in the treatment arm.(66) It should be noted that both the NINDS and IST-3 trials determined stroke subtype by purely clinical examination without modern neuroimaging. In a post-hoc analysis of ECASS, it was demonstrated that fewer than 20% of patients with a clinical lacunar syndrome had a corresponding lacunar infarct identified on a CT scan performed a week later.(69) This observation, which has been replicated by other groups,(70) highlights the practical problem that stroke type cannot be reliably determined rapidly enough to direct tPA treatment decisions.
In addition to the randomized trials of tPA, several other studies have addressed three major questions: 1) Is there a benefit of administering tPA in LS patients? 2) Is the benefit of tPA in LS similar to the benefit in other stroke subtypes? 3) Do LS have unique ICH risks compared to other stroke subtypes when given thrombolytic therapy? Regarding the first question, several studies have demonstrated that LS patients who receive tPA have improved functional outcomes and shorter hospital stays.(71,72) However, these positive results have not been replicated by all studies.(73,74) In nearly all the studies assessing the benefit of tPA in LS compared to other subtypes, the efficacy of thrombolysis was similar regardless of stroke type. As shown in Table 1, even the randomized trials did not always include a uniform approach to diagnosing LS. Results from observational data are highly variable with some suggesting higher ICH risk and others lower ICH risk, but an overall benefit is suggested by most studies. Observational data are also prone to confounding by indication, but overall the results are positive, consistent with randomized controlled trials. Unfortunately, several of these studies do not include control groups (i.e. LS patients who did not receive tPA), so it is difficult to ascertain whether these patients had favorable outcomes as a result of receiving tPA (or whether it is because LS have a favorable outcome in general(75)). Although these studies used different methodologies and yielded somewhat mixed results, it is likely that tPA is beneficial and safe for LS. Acknowledging the limitations of the data, the benefit of tPA for LS appears to outweigh the risk of symptomatic ICH and should be given in the acute setting if criteria are met.(67) Indeed this recommendation conforms to current guidelines.(67) The mechanism by which tPA is effective in cSVD-related infarcts is poorly understood. As mentioned above, embolism(76,77,86,78–85) or, more commonly, in situ formation of microthrombi in small, distal vessels is the final step that leads to ischemia in small vessel disease.(71,87) tPA may promote recanalization of these vessels thereby restoring perfusion.
Table 1. Thrombolysis trials in lacunar stroke.
Note in parenthetical numbers in the study size column represent the size of lacunar stroke treatment group and lacunar stroke control group, respectively. ICH: intracerebral hemorrhage; LS: lacunar stroke; MC: multicenter; SC: single-center; PS: prospective; RS: retrospective; RT: randomized; LAA: large artery atherosclerosis, SVD: small vessel disease; CE: cardioembolic; O: other; UD: undetermined; TACI: total anterior cerebral infarct; PACI: partial anterior circulation infarct; LACI: lacunar infarction; POCI: posterior circulation infarct; PE: physical exam; TOAST: trial of ORG 10172 in acute stroke treatment classification; OCSP: Oxfordshire Community Stroke Project classification; ICD: International Classification of Diseases; MRI: magnetic resonance imaging; CT: computed tomography; NR: not reported; BI: Barthel index; mRS: modified Rankin scale; NIHSS: National Institutes of Health Stroke Scale (NIHSS); GOS: Glasgow Outcome Scale (GOS); OHS: Oxford Handicap Scale; sICH: symptomatic intracerebral hemorrhage; aICH: asymptomatic intracerebral hemorrhage
| Trial | Study Design | Groups | Study Size | Subtyping Method | Assessment of Stroke | Outcome Measurement | Results | ICH Events |
|---|---|---|---|---|---|---|---|---|
| NINDS (1995)(53) | RT, MC | LAA, SVD, CE; control groups | 624 (51/30) | PE | PE/CT | BI, mRS, NIHSS, GOS (3 m) | favorable outcome (mRS < 2) in lacunar treatment group compared to placebo | no subgroup analysis, overall ICH rate was 6.4% in treatment group compared to 0.6% (p = 0.001) |
| Hsia et al. (2003)(254) | RS, two-center | LAA, SVD, CE, O, UD | 90 (7/0) | TOAST | PE/CT or MRI | mRS and BI (1 m and 3 m) | non-significant trend toward better outcome in lacunar group | 0 ICH in SVD group (not significantly different from other stroke subtypes) |
| Cocho et al. (2006)(68) | RS, SC | LS, non-LS | 44 (11/33) | OCSP/ TOAST | PE/CT or MRI | mRS (3 m) | proportion patients with good outcome (mRS 0 – 1) was similar (27% LS vs. 60% non-LS, p = 0.083) | 0 sICH; aICH in 1 LS group, 3 in non-LS group (p = 0.99) |
| Hwang et al. (2008)(74) | RS, SC | LS; control group | 76 (29/47) [12/26] in SVD subgroup | OCSP/ TOAST | PE/MRI | mRS (3 m) | similar outcome between treatment and control group (31% vs. 23% with favorable response (p = 0.463) | 2 with aICH |
| Fluri et al. (2010)(255) | PS, MC | LS, non-LS | 1048 (65/0) | TOAST | PE/CT or MRI | mRS (3 m) | trend toward better outcome in lacunar group | ICH 12.3% (4.6% sICH) in LS group and 13.4% (5.3% sICH) in non-LS group (p > 0.8) |
| Mustanoja et al. (2011)(256) | RS, SC | LAA, SVD, CE, O, UD, mixed | 957 (101/0) | TOAST | PE/CT or MRI | mRS (3 m) | SVD group with better outcomes and lower mortality | 0 sICH in SVD group |
| Fuentes et al. (2012)(257) | PS, MC | LAA, SVD, CE, O, mixed | 1479 (60/0) | ICD-10 | PE/CT | mRS (3 m) | no difference among stroke subtype at 24 h, nonsignificant trend toward worse outcome in SVD group at 3 m | NR |
| IST-3 (2012)(66) | MC, RT | TACI, PACI, LACI, POCI, O | 3035 (168/164) | PE | PE/CT or MRI | OHS (6 m) | nonsignificant trend toward worse outcome in LACI treatment group vs. control (59.5% vs. 62.8%) (p = 0.91) | no subgroup analysis, overall ICH rate was 7% in treatment group compared to 1% in control (p < .0001) |
| Shobha et al. (2013)(258) | RS, MC, case-cohort | TACI, PACI, LACI, POCI; control groups | 11503 (195/2001) | OCSP | PE/CT or MRI | 90-d mortality, mRS (discharge) | no difference in thrombolysis benefit between LACI, PACI, or TACI | 2.1% ICH rate (1.5% sICH) in LACI subtype, least among other stroke subtypes |
| Griebe et al. (2014)(73) | PS, SC | LS; control groups | 537 (69/468) | ASCO | PE/MRI | mRS, NIHSS (3 m) | more favorable clinical improvement within 5 days, median mRS was similar in treatment vs. control group (3 m) | 0 sICH, aICH 11.6% in treatment group compared to 1.9% in control group (p = 0.001) |
| Lahoti et al. (2014)(71) | RS, MC | LS, non-LS; control gorups | 256 (102/54) | PE/MRI | PE/MRI | mRS (3 m) | higher excellent outcomes (mRS 0 – 1) in non-LS treatment group (p < 0.01), higher perfect (mRS 0) outcomes in LS treatment group (p < 0.01) | 2 sICH in treatment group compared to 0 sICH in control group |
| Pan et al. (2016)(259) | RS, SC | LAA, SVD, CE, UD | 471 (82/0) | TOAST | PE | mRS (discharge) | highest proportion of favorable outcomes in SVD group compared to stroke subtypes (p < 0.01) | 1 patient with sICH and 2 with any ICH (lower than any other subtype) |
| Chen et al. (2016)(260) | PS, MC | LAA, SVD, CE, O, UD; control groups (NIHSS ≤ 5) | 383 (30/56) | TOAST | PE/CT or MRI | mRS (3 m) | no difference in proportion of favorable outcomes in SVD treatment and SVD control groups (73% vs. 80%, p = 0.42) | no subgroup analysis, 1 ICH overall |
| Zivanovic et al. (2017)(72) | RS, SC | LS; control group | 81 (36/45) | OCSP | NR | mRS (discharge) | shorter hospital stays in LS treatment group (9.5 d vs. 14.3 d, p = 0.004), higher proportion of excellent functional outcomes (mRS 0 – 1) in LS treatment group (41.7% vs. 15.6%, p = 0.01) | NR |
| Eggers et al. (2017)(261) | MC, non-RT | LS, non-LS, control group | 10632 (496/3492) | TOAST | PE/CT or MRI | mRS (discharge, 3 m) | better functional outcome in LS treatment group compared to placebo (p < 0.001), similar degree of improvement between LS and non-LS groups | 1% in LS treatment group compared to 0.2% in control LS group, 2.7% in non-LS treatment group compared to 1.0% in non-LS control group (p = 0.02) |
Anticoagulation
Fisher postulated that anticoagulation was inappropriate for lipohyalinosis-related strokes because of the red blood cell extravasation and microhemorrhages that are associated with these lesions.(13) Several lines of evidence in the modern era support this judgment. Anticoagulation-related ICH studies indicate that leukoaraiosis, a marker of cSVD, is a risk factor for hemorrhage.(88–90) The Stroke Prevention in Reversible Ischemia Trial (SPIRIT) demonstrated that leukoaraiosis is an independent risk factor for ICH in patients on anticoagulation (in the setting of atrial fibrillation).(91) So, there is evidence that anticoagulation should be avoided in such patients, unless there is a separate strong indication for anticoagulation without any alternative.(92)
In the acute phase of LS, few studies have examined the role of heparin and heparin-based therapies. Subcutaneous heparin was most notably studied in the first IST which enrolled nearly 20,000 patients within 48 hours of stroke symptoms.(93) In this multicenter, randomized, placebo-controlled trial (n = 19,435), patients were administered either unfractionated heparin (5000 IU or 12,500 IU twice daily) or aspirin 300 mg daily during the 14 days after ischemic stroke. Within this 14-day time frame, the heparin group had fewer recurrent ischemic strokes than the control group (2.9% vs. 3.8%), however this benefit was counterbalanced by a commensurate increase in ICH (1.2% vs. 0.4%). In both heparin and aspirin treatment groups, there was a trend toward fewer deaths in the first 14 days. At 6 months, heparin conferred no benefit over aspirin for the outcomes of death or functional status, and, at higher doses, it increased the risk of intracranial and extracranial hemorrhage. In the IST, 24% of patients that were enrolled had LS, but there was no subgroup analysis of outcome based on stroke subtype.
Similar negative findings were reported in TOAST, a randomized, double-blind, placebo-controlled trial that administered a 7-day course of danaparoid sodium (a low-molecular weight heparinoid) to subjects (n = 1281) within 24 hours of stroke symptom onset.(94) Although there was a trend toward a favorable response in the treatment group at 7 days, this benefit was not observed at 90 days. Similar to IST, increased intracranial bleeding was observed in the treatment group within 10 days. In this study, 24.5% of subjects in the treatment arm and 23.3% in the control arm had LS. At 3 months, this group had highest frequency of favorable outcomes compared to other stroke types, although there was no difference in outcome between the control and treatment groups. In light of these findings, heparin is not indicated in acute LS management.
For secondary prevention of LS, data to support the avoidance of anticoagulation comes from the Warfarin-Aspirin Recurrent Stroke Study (WARSS).(95) In that study, warfarin, at INR range of 1.4 to 2.8, or aspirin 325 mg was administered to “non-cardioembolic” ischemic stroke patients within 30 days of stroke. The largest stroke subtype in both the warfarin and aspirin groups was LS (> 55% in each group). Assessing the probability of an event at two-years, recurrent ischemic stroke was observed in 17.8% of patients taking warfarin compared to 16% of patients taking aspirin (HR 1.13, CI 0.92 to 1.38). The rate of major hemorrhage was higher in the warfarin arm [2.22 vs. 1.49 per 100 patient-years, rate ratio: 1.48 (0.93–2.44)], and minor hemorrhages occurred at a higher rate in warfarin arm [1.61 (1.38–1.89)]. Warfarin showed no benefit over aspirin in patients with cSVD (2-year probably of event: 17.1% vs. 15.2%, p = 0.31). Similar findings were reported in a two-year, prospective study (n = 386) by Evans et al. examining stroke subtype in patients with atrial fibrillation.(96) Warfarin (INR goal 2.0 to 3.0) was superior to aspirin (70 mg to 300 mg) in patients who initially presented with cardioembolic stroke but not in patients that presented with LS in the setting of atrial fibrillation (rate of recurrent stroke was 8.8% versus 8.9%). Moreover, there was an increased risk of hemorrhage in the warfarin compared to the aspirin group (2.5% vs 0.6%, p < 0.05).
For secondary prevention strategies in patients with CAA, anticoagulation is contraindicated, perhaps even for those with atrial fibrillation. The presence of CAA-related neuroimaging markers such as lobar cerebral microhemorrhages and cortical superficial siderosis suggests CAA and increases the risk of anticoagulant-associated hemorrhage.(46,92,97–99) Non-vitamin K antagonist oral anticoagulants have been shown to confer similar risks of poor functional outomes and morbidities(100,101) when compared to warfarin, except with perhaps decreased in-hospital mortalites.(52) This increased hemorrhagic risk raises the question of safety in patients with indications for anticoagulation, such as atrial fibrillation. The risk of hemorrhage appears to rise with increasing burden of cerebral microbleeds with relative risks up to 5 to 14 fold with 5 or more microbleeds.(102–104) In such cases, the decision to anticoagulate must be based on the absolute risk of hemorrhage and the competing absolute risk of ischemic stroke. In the setting of atrial fibrillation, this ischemic stroke risk rises with increasing CHA2DS2-VASc score.(105) Consideration of nonpharmacological methods such as left atrial appendage closure (LAAC) in nonvalvular atrial fibrillation patients with cSVD can be reasonable. Further studies are needed to fully elucidate the role of non-vitamin K antagonist oral anticoagulants vs. LAAC in patients with atrial fibrillation and concurrent cSVD. Because of this uncertainty, some recent NOAC studies have been designed to exclude patients with LS given this elevated ICH risk, including a trial testing the prevention of vascular events in patients with coronary or peripheral vascular disease.(106) At the current time, NOACs and warfarin should be avoided for the sole treatment of LS.
Antiplatelet Agents
Antiplatelet agents are recommended for acute ischemic stroke when patients are not candidates for tPA or intra-arterial therapy. Among the options for antiplatelet therapy, the most robust data and safety information exist for aspirin. Aspirin inhibits cyclooxygenase, reducing the production of thromboxane A2 and inhibiting platelet aggregation irreversibly. In the general population, low-dose aspirin does not increase the risk of ICH or subdural hematoma (SDH).(107) Furthermore, in survivors of deep hemorrhages, aspirin does not seem to increase ICH recurrence risk suggesting that is safe to use in cSVD.(108,109) In addition to aspirin, dipyridamole, an adenosine deaminase and phosphodiesterase inhibitor, and clopidogrel, a thienopyridine that inhibits platelet aggregation, have also been evaluated extensively and are commonly used for secondary stroke prevention. These agents have also been studied in combination with aspirin as dual antiplatelet therapy (DAPT).
Two major trials evaluated aspirin in the acute phase of ischemic stroke: The aforementioned IST(93) and the Chinese Acute Stroke Trial (CAST).(110) In IST (discussed previously given its data on heparin), the patients treated with 300 mg aspirin experienced a reduction in recurrent stroke at 14 days (2.8% vs. 3.9%; 2p < 0.001), and non-fatal stroke or death (11.3% vs. 12.4%; 2p = 0.02). Importantly, the risk of hemorrhagic strokes was similar between aspirin and controls (0.9% vs. 0.8%), although aspirin increased the risk of extracranial bleeding. The CAST randomized 21,000 patients within 48 hours of stroke to a 4-week course of 160 mg of aspirin or placebo. Results of this study revealed a 14% reduction in mortality at 30 days with aspirin usage (3.3% vs. 3.9%; 2p = 0.04). In the same time frame, there were fewer ischemic strokes in the aspirin group compared to placebo (1.6% vs. 2.1%; p = 0.01) although there was a slight increase in the number of hemorrhagic strokes with aspirin (1.1 vs. 0.9, 2p > 0.1). In the subset of patients with LS (n = 6120, ~30% in each group), there was a 10% reduction in the relative risk of stroke recurrence or mortality at 30 days. It should be noted that in both IST and CAST, only a CT scan (and no MRI) was done in the majority of cases prior to randomization, limiting the accuracy of assigned stroke mechanisms. In light of more recent data on the risk and timing of recurrent stroke after transient ischemic attacks (TIAs) or minor ischemic strokes (discussed below), the positive findings in IST and CAST likely represent prevention of early recurrent stroke rather than effective treatment of the index stroke. Based on these data, aspirin is recommended for early treatment after acute ischemic stroke.
In acute ischemic stroke of mild severity, dual antiplatelet therapy (DAPT) may be more effective than single-agent therapy when administered early, without significantly increasing ICH.(111) However, the efficacy and risks of DAPT have not been evaluated for LS specifically. The Clopidogrel in High-Risk Patients with Acute Nondisabling Cerebrovascular Events (CHANCE) trial, which administered DAPT (versus 75 mg aspirin monotherapy) within 24 hours of presentation in Chinese patients with minor stroke (NIHSS < 4) or TIA for 3 weeks after the index event, demonstrated a reduction in stroke recurrence at 90 days (8.2% vs. 11.7%, p < 0.001) without a corresponding increase in hemorrhagic stroke.(112) However, subgroup analyses of CHANCE suggest that the greatest benefit of DAPT likely occurs in patients with embolic strokes.(113,114) The Platelet-Oriented Inhibition in New TIA and minor ischemic stroke (POINT) trial tested a similar regimen of DAPT against aspirin (50 to 325 mg) monotherapy, but treated for 3 months rather than 3 weeks.(115) The early benefit of DAPT was again demonstrated; however, there were higher rates of major hemorrhage (p = 0.02) but not symptomatic intracranial hemorrhage in the treatment group at 3 months. The confirmation of the POINT trial suggests that the beneficial effect seen in CHANCE was not specific to the Chinese population. The benefits of DAPT are realized within the first few weeks, but additional DAPT confers added risk without further benefit.
Aspirin is also the mainstay of antiplatelet therapy in the chronic setting for secondary prevention of ischemic stroke in those without indications for anticoagulation. The greatest body of evidence for aspirin’s benefit for secondary prevention is summarized in a meta-analysis, the 2002 Antithrombotic Trialists Collaboration (ATC).(116) The study analyzed 195 trials comparing aspirin to placebo for the prevention of stroke, myocardial infarction, and vascular death in patients enrolled after a prior vascular event. A 22% risk reduction for ischemic stroke was observed in patients treated with antiplatelet agents (aspirin being by far the most common agent used). The 2009 ATC yielded similar results demonstrating that aspirin reduced the risk of any serious vascular event by 19% and ischemic stroke by 22%.(117) A smaller study showed that stopping antiplatelet therapy in high risk patients may increase the risk of stroke.(118)
Ticlopidine is a thienopyridine agent that irreversibly binds to the P2Y12 ADP receptor subtype to inhibit platelet aggregation. As seen in Table 2, ticlopidine (administered 1 week to 4 months after stroke) was shown to reduce the risk of LS, and may be more effective than aspirin in selected populations.(119–124) However, neutropenia was a prominent side effect, which has limited its use as a first-line agent.
Table 2. Antiplatelet therapy in lacunar stroke.
Note in parenthetical numbers in the study size column represent the percentage of LS. DB: double-blind; R: randomized; C: controlled; MC: multicenter; ASA: aspirin; DP: dipyridamole; TP: ticlopidine; CS: cilostazol; CD: clopidogrel; IS: ischemic stroke; TIA: transient ischemic attack; JCS: Joint Committee for Stroke; NE: neurological examination; CT: computed tomography; OCSP: Oxfordshire Community Stroke Project; MRI: magnetic resonance imaging; TOAST: trial of ORG 10172 in acute stroke treatment classification; NINDS: National Institute of Neurological Disorders and Stroke; RRR: relative risk reduction; LS: lacunar stroke; MI: myocardial infarction; DAPT: dual antiplatelet therapy; GI: gastrointestinal; ICH: intracerebral hemorrhage
| Study | Design | Treatment Groups | Enrollment; Duration | LS Size (136) | Stroke Classification | Outcome | Adverse Events |
|---|---|---|---|---|---|---|---|
| AICLA (1983)(131) | DB, MC, R | 330 mg ASA/330 mg ASA + 75 mg DP/placebo | IS within 1 y; 3 y | 98 (16%) | JCS (NE and CT) | fatal/nonfatal stroke lower in ASA vs. placebo (p < 0.05) and ASA + DP group (p < 0.06) | 2 deaths from ICH overall; similar systemic bleeding in DP + ASA and ASA |
| CATS (1989)(120) | DB, MC, R, C | 500 mg TP/placebo | 1 w – 4 m after IS; 2 y | 274 (26%) | NE | 30% RRR for combined IS, MI, or death in TP vs. placebo (p = 0.006) | increased neutropenia in TP |
| ESPS-2 (1996)(132) | DB, MC, R, C | 50 mg ASA/400 mg DP/50 mg ASA + 400 mg DP/placebo | IS within 3 m; 2 y | 2,600 (59%) | NE | IS risk reduced by 18% in ASA (p = 0.013), 16% in DP (p = 0.039), 37% in ASA + DP group (p < 0.001) | highest all-site and GI bleeding in ASA group |
| IST (1997)(93) | open R, MC | 300 mg ASA/control | within 48 h after IS; 6 m | 4,616 (24%) | OCSP (CT scan) | IS recurrence and death within 14 days reduced (2.8% vs. 3.9%; 2p < 0.001) | no increase in hemorrhagic stroke |
| CAST (1997)(110) | MC, R, C | 160 mg ASA/placebo | within 48 h after IS; 4 w | 6,263 (30%) | OCSP | reduction in mortality and recurrent IS (1.6% vs. 2.1%; 2p = 0.01) | increase in hemorrhagic stroke (1.1% vs. 0.9%; 2p > 0.1) |
| CSPS (2000)(138) | DB, R, C | 100 mg CS BID/placebo | IS within 1–6 m; 2 y | 794 (74%) | NE and CT/MRI | stroke reduction by 43.3% with CS in LS group (p = 0.0373) | similar ICH in CS vs. placebo, no increase in GI bleeding |
| AAASPS (2003)(123) | DB, MC, R | 500 mg TP/650 mg ASA | 7–90 d after IS; 2 y | 1,221 (68%) | TOAST and CT/MRI | stroke, MI, or vascular death similar between TP and ASA | similar rate of GI bleeding |
| MATCH (2004)(129) | DB, MC, R, C | 75 mg CD + 75 mg ASA/75 mg CD | IS/TIA within 3 m; 18 m | 3,148 (53%) | TOAST | no difference among IS, MI, or vascular death between groups | increased ICH and GI bleeding with CD + ASA (p < 0.0001) |
| ESPRIT (2006)(133) | MC, R, C | 30–325 mg ASA + 200 mg DP BID/30–325 mg ASA | IS within 6 m; 3.5 y | 1,377 (50%) | NE and CT/MRI | death from all causes, non-fatal IS/MI lower with combination therapy | similar fatal ICH and major bleeding |
| FASTER (2007)(262)* | MC, R, C | 75 mg CD + 325 mg ASA/325 mg ASA | IS within 24 h; 90 d | 113 (29%) | TOAST (CT or MRI) | similar IS rate in CD and ASA (7.1% vs. 10.8%; p = 0.19) | 2 ICH in CD vs. 0 in placebo (p = 0.5) |
| PRoFESS (2008)(134) | DB, MC, R, C | 25 mg ASA + 200 mg DP BID/75 mg CD | IS within 90 d; 2.5 y | 10,578 (52%) | NE and CT/MRI | no difference in recurrent IS between groups | increased intracranial bleeding in ASA + DP vs. CD |
| Uchiyama et al. (2009)(263) | DB, MC, R | 75 mg CD/200 mg TP | IS within 8 d; 26 or 52 w | 1,341 (73%) | NE and CT/MRI | no difference in IS, MI, or vascular death between CD and TP | similar rate of major hemorrhage |
| CSPS2 (2010)(139) | DB, MC, R | 100 mg CS BID/81 mg ASA | IS within 26 w; 2.4 y | 1,743 (65%) | NINDS-III and CT/MRI | IS recurrence lower in CS vs. ASA (2.76% vs. 3.71%; p = 0.0357) | in LS group, fewer hemorrhagic strokes in CS vs. ASA (0.36% vs. 1.20%, p = 0.003) |
| SPS3 (2012)(126) | DB, MC, R | 325 mg ASA + 75 mg CD/325 mg ASA | IS within 180 d; 3.4 y | 3,020 (100%) | NE and MRI | similar IS recurrence in DAPT and ASA; increased mortality in DAPT (0.04% vs. 0.03%; p = 0.004) | higher major hemorrhage in DAPT vs. ASA (2.1%/yr vs. 1.1%, p < 0.001); similar ICH rate |
| SOCRATES (2016)(153) | DB, MC, R | 90 mg ticagrelor BID/100 mg ASA | IS within 24 h; 90 d | 3,839 (29%) | NE and CT/MRI | similar IS, MI, or death between ticagrelor and ASA (7.3% vs. 8.0%, p = 0.40) | similar ICH between ticagrelor and ASA (0.2% vs. 0.3%, p = 0.30) |
trial terminated early
Clopidogrel has a mechanism of action similar to ticlopidine and does not cause neutropenia, making is an attractive candidate for secondary stroke prevention. The Clopidogrel versus AsPirin in patients at Risk of Ischaemic Events (CAPRIE) trial compared 75 mg clopidogrel with 325 mg aspirin in recent (≥ 1 week, but ≤ 6 months) stroke, myocardial infarction, or symptomatic peripheral arterial disease.(125) In this multicenter, randomized, blinded trial, there was no reduction in recurrent stroke with clopidogrel over 1.9 years (7.15% vs. 7.71% p = 0.26), although there was a reduction in the composite endpoint of ischemic stroke, myocardial infarction, or vascular death (driven mostly by benefit seen in patients with pre-existing peripheral vascular disease). In addition, patients on clopidogrel and aspirin had similar rates of both extracranial and intracranial hemorrhage. No subgroup analysis of LS was performed in this study however.
The Secondary Prevention of Small Subcortical Strokes 3 (SPS3) trial specifically examined the role of DAPT in secondary stroke prevention after LS.(126) This multicenter, double-blinded study enrolled patients (n = 3020) who had MRI-confirmed LS within 180 days and randomized them to aspirin 325 mg or aspirin 325 mg plus clopidogrel 75 mg (median time to randomization was over two months from LS). Over a 3.4-year period, the risk of stroke recurrence was similar in each group (2.5% with DAPT and 2.7% with aspirin monotherapy; p = 0.48) although there was a trend toward increased ICH with DAPT. In this study, the extracranial hemorrhage rate, mainly gastrointestinal, was almost doubled with DAPT (2.1% vs. 1.1%, p < 0.001). As seen in other studies such as the Atrial Fibrillation Clopidogrel Trial with Irbesartan for Prevention of Vascular Events (ACTIVE) trial,(127) ICH rates increased with DAPT, although this finding has not been replicated in all trials.(128) In SPS3, mortality was increased in the DAPT group (113 vs. 77 deaths; p = 0.004).
Several other trials have included LS patients in comparisons of single- versus dual-agent antiplatelet therapy. The Management of AtheroThrombosis with Clopidogrel in High-risk patients (MATCH) trial randomized patients with recent (within 90 days) stroke or TIA (n = 3,148) to either clopidogrel 75 mg or aspirin 75 mg plus clopidogrel 75 mg, and included over 50% LS in both treatment arms.(129) The median time to enrollment after stroke was 15 days.(130) At 18 months, there was no difference in the combined endpoint of ischemic stroke, myocardial infarction, or vascular death. Furthermore, major bleeding (mostly gastrointestinal) and symptomatic ICH were increased in the DAPT arm. DAPT’s efficacy has also been evaluated using dipyridamole and aspirin. In the 1983 French study Accidents Ischemiques Cerebraux Lies a l’Atherosclerose (AICLA) which examined placebo vs. aspirin vs. aspirin/dipyridamole, in a subset of LS patients, a significant reduction in recurrent stroke risk was observed in the aspirin monotherapy and combination therapy groups compared to placebo.(131) Dipyridamole was also studied in the 1996 European Stroke Prevention Study-2 (ESPS-2),(132) and again in the 2006 European/Australasian Stroke Prevention in Reversible Ischaemia Trial (ESPRIT) (Table 2), which both demonstrated a reduction in recurrent stroke without increasing ICH risk.(133) However, when the combination of twice daily dipyridamole 200 mg and aspirin 25 mg was compared to clopidogrel 75 mg in the Prevention Regimen for Effectively Avoiding Second Strokes (PRoFESS) trial, there was no difference in stroke recurrence rates between groups.(134) Moreover, the DAPT group experienced more intracranial bleeding than clopidogrel alone. Collectively, these trials suggest that DAPT should be avoided in the chronic treatment of LS.(130,135,136) Not surprisingly, using more aggressive antiplatelet therapy regimens with triple antiplatelet therapy has not been shown to reduce stroke risk, but only add to the risk of intracranial hemorrhage.(137)
In addition to aspirin, dipyridamole, and clopidogrel, several other antiplatelet agents have been developed as monotherapies for the secondary prevention of LS. Cilostazol, used most often for peripheral vascular disease, is a phosphodiesterase III inhibitor that promotes vasodilation. Evidence for its efficacy in LS comes from the Cilostazol Stroke Prevention Study (CSPS) conducted in Japan, which reported a 2.3% absolute risk reduction in the annual stroke rate compared to placebo (3.0% vs. 5.2%, p = 0.04) in LS patients (mean time to randomization 83 days after stroke).(138) When compared with aspirin in CSPS II, there was a 0.95% absolute risk reduction in the annual incidence of stroke in the cilostazol group (2.8% vs. 3.7%, p = 0.04).(139,140) Among patients with LS (randomized within 26 weeks after stroke), there was a trend toward decreased recurrent stroke with cilostazol use (6.8% vs. 9.7%, p = 0.09) over a 2.4 year period. Furthermore, in LS patients, the hemorrhagic stroke risk was less in patients taking cilostazol vs. aspirin,(141) a finding that was replicated in the Cilostazol versus Aspirin for Secondary Ischemic Stroke Prevention (CASISP) study.(142) The authors postulated that since LS may be related to endothelial dysfunction and failed autoregulation, treatment with cilostazol may be an appropriate therapy given its vasodilatory properties. Furthermore, cilostazol has been shown to decrease the pulsatility index (PI), a marker of cSVD, in transcranial Doppler studies at 90 days in acute LS.(143) In small studies, other combinations of cilostazol such as cilostazol-probucol or cilostazol-edaravone have also demonstrated improved functional outcomes in patients with silent LS.(144,145) Interestingly, cilostazol is being studied as a long-term DAPT option in conjunction with aspirin or clopidrogrel in the Cilostazol Stroke Prevention Study for Antiplatelet Combination (CSPS.com) study (ClinicalTrials.gov identifier: NCT01995370).(146) Given that chronic DAPT with aspirin and clopidogrel have failed because of their increased hemorrhage risks (see above), cilostazol in combination with other agents may be safe due to cilostazol’s reduced risk of hemorrhage compared to aspirin (based on CSPS II and CASISP mentioned above).(139,140,142) A subgroup analysis of LS was performed in CSPS.com, although the findings of this trial remain unpublished.
While the studies of cilostazol seem promising, it is not used as a first-line agent in many countries for two major reasons: 1) Headache is a common side effect with cilostazol use that affects 10% of cilostazol users.(147,148) 2) The majority of studies using cilostazol (including CSPS.com) were performed in Asian populations; therefore, its efficacy in non-Asian populations remains unclear.(149) However, the current study, LACunar Intervention (LACI-2) Trial-2 (ClinicalTrials.gov identifier: NCT03451591) will address whether cilostazol will be beneficial in preventing LS and slowing the progression of cSVD in non-Asian populations. At the current time, it can be concluded that cilostazol is likely an acceptable alternative as a secondary prevention strategy in LS. Moreover, it may be useful in patients with aspirin-resistance who are able to tolerate its side effects.(150)
Another antiplatelet agent used for secondary prevention in LS is ticagrelor, which has been adopted as a staple for use in acute coronary syndrome (ACS).(151) Ticagrelor is a direct-acting agent which reversibly binds to the P2Y12 receptor on platelets.(152) In the Platelet Inhibition and Patient Outcomes (PLATO) trial for ACS,(151), there was an increase in hemorrhagic stroke rates with ticagrelor compared to clopidogrel (23 [0.2%] vs. 13 [0.1%], p = 0.1) while ischemic stroke rates were similar. Recently, the Acute Stroke or Transient Ischemic Attack Treated with Aspirin or Ticagrelor and Patient Outcomes (SOCRATES) trial (n = 13,199) compared twice daily ticagrelor 90 mg with aspirin 100 mg for 90 days in patients with small strokes (NIHSS ≤ 5) within 24 hours of symptom onset.(153) In a subgroup analysis of LS, there was no significant difference among stroke, myocardial infarction, or death within 90 days.(154) ICH was rare in this study, but did not differ significantly between ticagrelor and aspirin.
Serotonin Reuptake Inhibition
There is some literature that suggests that the use of SSRIs may be efficacious post-stroke.(155,156) Much of this evidence derives from the Fluoxetine for Motor Recovery after Acute Ischemic Stroke (FLAME) trial(157) and other smaller randomized controlled trials.(158–161) FLAME was a double-blind, placebo-controlled trial conducted at nine centers in France that randomized patients (n = 118) with recent (within 5 to 10 days) ischemic stroke and hemiplegia/hemiparesis to receive fluoxetine 20 mg or placebo for 3 months. At 90 days, patients in the treatment arm had a significant improvement in motor symptoms, as measured by the Fugl-Meyer motor scale (FMS), and disability, as measured by the modified Rankin Scale (mRS). In the FLAME trial, only 3% of patients in the fluoxetine group and 10% of patients in the control group had LS. By contrast, in the recent double-blind, placebo-controlled Fluoxetine on Functional Outcomes after Acute Stroke (FOCUS) trial, fluoxetine 20 mg or placebo was administered to patients (n = 3,127) for 6 months. This study, which included 15% LS, showed that fluoxetine administration did not improve disability as measured by mRS at 90 days; however, it did not assess FMS as a surrogate for motor function.(162)
Although SSRIs may be beneficial after stroke, there are also conflicting data suggesting that SSRIs cause an increased risk of ICH.(163–168) In one population-based cohort, it was shown that this risk was the greatest in the first 30 days of SSRI use and when used in combination with oral anticoagulants.(168) In a meta-analysis of several SSRI trials, there was a slight increase in ICH risk with SSRI use, but the absolute rate of ICH with SSRI use was low.(169) Although the use of fluoxetine in LS is probably safe, given that patients with cSVD are already at an increased risk of ICH, it should be judiciously used in high-risk LS patients, such as those with previous ICH, extensive WMH, cerebral microbleeds, and concurrent anticoagulation use. Further studies specifically evaluating ICH risk in LS patients taking SSRIs are needed.
Blood Pressure Manipulation and Flow Augmentation
The current guidelines for acute ischemic stroke regardless of stroke subtype are to allow patients to autoregulate blood pressure to maintain perfusion for 24 hours, up to a systolic blood pressure of 220 mmHg for patients that did not receive tPA and 180 mmHg for those that received tPA.(67,170) However, the role for permissive hypertension in LS has not been fully examined, and the role for therapeutic hypertension induced with vasopressors is controversial.(171) While therapeutic hypertension is thought to primarily benefit patients with large vessel occlusion,(172) there may be some role for permissive or induced hypertension in the treatment of stuttering lacunes.(173) Alternative therapies to augment cerebral blood flow such as expanding the circulation with albumin infusions have been used with varying efficacy.(174–177) However, in a randomized, double-blinded trial examining high-dose albumin treatment for ischemic stroke (ALIAS) (of which about one-fifth were LS), administration of albumin within 5 hours of stroke did not improve mRS or NIHSS at 90 days and was associated with more pulmonary edema.(178) After the acute phase of autoregulation (generally 24 hours post-symptom onset), blood pressure should be gradually reduced to normotension as rapid overcorrection may worsen stroke symptoms.
For primary and secondary prevention of LS, there is a large body of evidence for aggressive blood pressure control. At a population level, it has been suggested that the incidence of LS has been declining due to improved management of hypertension in the modern era.(40,179) Specifically for LS, hypertension doubles the risk of recurrent stroke while diabetes increases it by 1.5-fold.(180) Furthermore, while blood pressure control is accepted as essential in all strokes,(181) there is a paucity of evidence regarding specific blood pressure targets for each stroke subtype. In one small study, however, hypertension was more commonly seen with acute LS, independent of pre-stroke hypertension.(182) Although the PRoFESS study did not perform a subgroup analysis of stroke subtypes, 52% of the study population (n = 20,332) included LS.(183) In that study, patients treated with telmisartan approximately 15 days after stroke achieved an average blood pressure reduction of 3.8/2.0 mmHg. However, after a 2.5-year follow-up, there was no difference in recurrent stroke or ICH with telmisartan therapy. What remained unclear however, was whether greater reductions of above 3.8/2.0 mmHg would result in improved outcomes. The SPS3 study provided additional insight into this question.
SPS3 investigated more aggressive blood pressure targets specifically in LS.(184) 3020 patients with recent (180 days) MRI-confirmed LS were randomized to a systolic blood pressure goal of 130 mm Hg to 149 mmHg vs. a goal of < 130 mmHg. Although there was no significant reduction in stroke rate (2.77% vs. 2.25%, p = 0.08), the risk of ICH was reduced with lower blood pressure targets (0.11% vs. 0.29%, p = 0.03). The mean systolic blood pressure achieved in the higher blood pressure target arm was 138 mmHg compared to 127 mmHg in the lower target arm. Given that cSVD itself poses a greater risk of ICH, a systolic blood pressure goal of <130 mmHg for recent LS is reasonable and is concordant with recent recommendations set forth by the American College of Cardiology.(185) Importantly, in the SPS3 study, there were few adverse events reported in patients randomized to a lower blood pressure target suggesting that aggressive blood pressure control is safe and may be efficacious.(186)
Glycemic Control and Diabetes Management
In acute ischemic stroke, current guidelines recommend treatment of hyperglycemia.(67,170) Hyperglycemia is due in part to cortisol and norepinephrine release during acute ischemic stroke and may have deleterious effects due to free radical production.(187) Although there is ample evidence to suggest that hyperglycemia at presentation is associated with worse outcomes,(188) there are limited data suggesting that treating hyperglycemia improves outcomes.(189,190) Not only is diabetes a risk factor for all stroke subtypes(191), but it is an independent risk factor for first-time LS(192–194) and portends worse outcomes.(195) For primary prevention, the United Kingdom Prospective Diabetes Study (UKPDS) compared Type 2 diabetic patients who were given intensive treatment (average HbA1c 7.0%) to traditional (average HbA1c 7.9%) and showed no significant reduction in stroke incidence (p = 0.52). However, this study may not have been sufficiently powered to detect a stroke-specific relationship and/or the intensive control may not have been “intensive enough” to substantially impact stroke incidence.(196) Regarding secondary prevention, in the SPS3 study, about 37% of all patients were noted to have diabetes mellitus.(197) These patients were found to have increased intracranial atherosclerosis, a predilection to develop posterior circulation strokes, and more white matter disease burden.(198) Mortality, recurrent stroke, and myocardial infarction risks were doubled in this patient cohort.(197)
Pioglitazone, a thiazolidinedione, may be helpful for reducing vascular events after ischemic stroke. In the Insulin Resistance Intervention after Stroke Trial (IRIS), insulin-resistant individuals (n= 3,876) with recent stroke were randomized to 45 mg pioglitazone daily vs. placebo.(199) At 4.8 years, patients randomized to the treatment arm had decreased rates of stroke and myocardial infarction compared to placebo (9.0% vs. 11.8%, p = 0.007). Furthermore, the incidence of diabetes within that time frame was reduced in patients that received pioglitazone (3.8% vs. 7.7%, p < 0.001), although there was an increased incidence of fractures in this group. Roughly 30% of patients in both the treatment and placebo groups were composed of patients with LS.(200) Recently, pioglitazone’s benefit has been extended to patients with prediabetes, suggesting that it may be more widely adopted as a secondary prevention strategy in the future.(201)
Controlling Hyperlipidemia and Statins
While it is well-established that hypercholesterolemia is a risk factor for large vessel atherosclerosis, there is conflicting data pertaining to hyperlipidemia as a risk factor for cSVD and LS.(202–207) Regarding treatment of hypercholesteremia, one meta-analysis revealed that statins reduced the incidence of all strokes through a reduction in LDL.(208) In one early study of patients with cerebrovascular or other occlusive artery disease, there was a 28% reduction in ischemic stroke when patients were given simvastatin 40 mg daily.(209) The Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial proved that high-intensity statin treatment results in a reduction in subsequent fatal stroke and cardiovascular events in patients without coronary artery disease.(210) A subsequent subgroup analysis showed that there was no difference in treatment outcome regardless of stroke subtype, suggesting that atorvastatin may be beneficial for LS. However, this study was limited in that it was not powered to assess such differences.(211) In a post-hoc analysis of SPARCL, there was an increased risk of hemorrhage among patients with baseline cSVD-related LS who received statins (2.8% vs. 0.57% in placebo arm, HR 4.99) although this was counterbalanced by a reduction in ischemic stroke recurrence (11.2% vs. 14.6%, HR 0.76).(212,213) Therefore, in most cases, statins should be used for the treatment of LS unless the preexisting risk of hemorrhage is unacceptably high (recurrent ICH, multiple microbleeds, etc.).(214) The StATins Use in IntRacerebral Hemorrhage PatieNts (SATURN) study (National Institutes of Health/National Institute of Neurological Disorders and Stroke) examining the safety of statin continuation after spontaneous lobar ICH will provide additional insight as to their safety in lacunar stroke.
Experimental Agents for Neuroprotection and Neural Repair
A final category of potential therapeutics consists of agents that may offer neuroprotection or augment neural repair. Neuroprotective agents are intended to act acutely to protect the ischemic brain before it is irreversibly infarcted, while agents designed to augment repair aim to replace lost elements and promote plasticity. The former is most likely to be effective in the first few hours following stroke, while the latter is most likely to be effective within the first few weeks to months after stroke.(215)
When developing agents that target brain parenchyma, the elements of the neurovascular unit should be considered. Although it has been applied more readily to large-vessel occlusions,(216–218) the neurovascular unit as a concept relates to the interactions between neurons, astrocytes, microglia, endothelial cells, and smooth muscle cells. Collectively, the unit is involved in maintaining synapses, regulating neurotransmitters, energy metabolism, the blood-brain barrier, and blood flow. Several agents targeting the health of the neurovascular unit have been tested in cell culture and animal models of stroke; however, none have translated into effective therapies for human patients.(219,220) Major pathways that have been targeted include neuronal death mechanisms, excitotoxicity, and inflammation.(216,217,221) NMDA receptor antagonists are among the most commonly evaluated neuroprotective therapies given their propensity for reducing excitotoxicity in acute stroke. Agents such as NA-1, thought to reduce NMDA-mediated injury, as well as magnesium, which has other pleiotropic effects,(222,223) have shown some promise in preliminary trials.(223–226) In addition, modulation of the ACE2-Ang-(1–7)-Mas axis in stroke patients has gained attention in recent years(227) given its demonstrated efficacy in models of small and large vessel stroke.(228–231)
Among the possible reasons for the failure of neuroprotective agents to translate clinically is the underestimation of white matter injury in human stroke. While a human brain is composed of nearly 50% white matter, most rodent brains are composed of only 15%(232) illustrating that many standard rodent models fail to mimic human white matter disease. This highlights the need for an improved understanding of both white matter ischemia and the need to develop preclinical models that specifically target white matter. An important distinction between white matter and gray matter is that white matter blood flow is lower than that of gray matter and contains fewer collateral networks.(219) In addition, unique modulation of intracellular calcium between gray and white matter leads to differences in cell death mechanisms such that white matter may be more susceptible to injury during stroke.(233–237) Because of these distinctions, the concept of the oligovascular unit has been applied to strokes that preferentially affect the white matter.(238)
In addition to neuroprotective strategies, several targets for augmenting neural repair have been proposed including axonal sprouting, neurogenesis, gliogenesis, and neuronal excitability.(215) Neural repair specifically affecting the white matter (which is more relevant to LS) has only been studied in recent years. These agents are intended to work by stabilizing the blood-brain barrier, repairing the oligovascular unit, modulating glial scarring, augmenting remyelination, and preventing axonal degeneration.(238–240) When white matter undergoes ischemia, endogenous repair mechanisms are initiated involving oligodendrocyte precursor cells,(241) which proliferate, migrate, and differentiate into myelinating oligodendrocytes.(242,243) In the process of glial scar formation, several cell surface and extracellular matrix proteins have been shown to inhibit axonal growth and impair the ability of oligodendrocytes to myelinate new axons. These detrimental molecules include the neurite outgrowth inhibitor (NOGO), ephrin ligands, and chondroitin proteoglycans.(244) In mice, NOGO receptor 1 blockade was shown to overcome remyelination failure after LS and stimulate functional recovery.(245)
In recent years, agents that augment neural repair, such as cerebrolysin, have shown promise in human studies.(246) With these neural repair augmentation strategies, it is important to note that the timing of administration is crucial. Many agents that augment repair can have deleterious effects in the acute setting.(215) For example, drugs that inhibit tonic inbitory GABA signaling or augment excitatory AMPA glutamatergic signaling increase infarct size when given 3 to 5 days after stroke. However, when given after this period, they instead enhance motor recovery.(247,248)
“Stuttering” Lacunes
LS are occasionally preceded by repetitive TIAs, described as “capsular warning syndrome” given the propensity for the resultant stroke to be found in the internal capsule.(249,250) This acute “stuttering lacune” is of particular interest as viable tissue at risk might be eventually lost. While the exact pathophysiology of the stuttering lacune is unclear, it may be a result of hemodynamic compromise of a small penetrating vessel.(249) Various therapies for this type of stroke have been trialed, although none have proven efficacy in a large, randomized cohort. In one case series of 7 patients, administration of 300 mg of clopidogrel resulted in a resolution of symptoms in 4 patients and stabilization of symptoms in the other 3.(251) Intracranial hemorrhage was not observed in this small series, although the mean age of the participants was less than 65. Others have speculated that anticoagulation may have a role for the acutely stuttering lacune, especially the non-vitamin K antagonist oral anticoagulants which appear to have an improved safety profile.(252) In one early series of 4 patients with progressive weakness, heparin did not prevent worsening of neurological symptoms.(253) Nonpharmacological strategies for the stuttering lacune have included therapeutic hypertension as well as volume expansion without any clear benefit.(173,249) Given the small nature of these studies, no clear recommendation can be given for the treatment of this unique subtype of LS. However, as with LS in general, anticoagulation should be avoided.
Conclusion
Currently, there are limited therapies available for the treatment of LS (see Figure 4). In the acute setting, thrombolysis with IV tPA in LS is the standard of care and its efficacy is well-supported by several trials. In the chronic setting, antiplatelet agents are the mainstay of therapy, although their effect on reducing stroke recurrence is modest. Oral anticoagulants and combination antithrombotics are not indicated for prevention of LS recurrence, and they are known to increase ICH risk in this setting. Modulating risk factors such as diabetes, hypercholesterolemia, and hypertension continue to play key roles in reducing stroke recurrence. Neuroprotection and neural repair augmentation are promising, as the development of agents that specifically promote the health of the oligovascular unit could aid in reducing stroke morbidity and mortality. Further research into these areas may allow for the generation of targeted therapeutics capable of minimizing parenchymal damage acutely, while aiding in repair subacutely.
Figure 4. Safety of Therapies for Lacunar Stroke.
This schematic highlights the major therapies that have been trialed in lacunar stroke. The harmful therapies (left side) should be avoided in most lacunar stroke patients. The safe therapies are listed on the right side.
Key Points.
Lacunes are subcortical, round, fluid-filled spaces between 3 mm and 15 mm.
Cerebral microinfarcts are microscopic lesions 0.2 mm to 3 mm that are observed by high resolution T1, FLAIR, and double inversion recovery MRI sequences.
Cerebral microinfarcts should be managed similarly to lacunar strokes.
Cerebral small vessel disease confers an increased risk of intracranial hemorrhage even without the use of antithrombotics.
Tissue plasminogen activator should be administered to lacunar stroke patients.
Anticoagulation including non-vitamin K antagonist oral anticoagulants should be avoided in lacunar stroke patients unless there is a separate indication for anticoagulation without any alternative.
Left atrial appendage closure should be considered in patients with lacunar stroke and atrial fibrillation.
Aspirin is the mainstay of therapy for lacunar stroke in both acute and chronic settings.
Dual antiplatelet therapy as a long-term secondary prevention strategy should be avoided.
Clopidogrel and cilostazol monotherapy are acceptable alternatives for secondary lacunar stroke prevention.
Selective serotonin reuptake inhibitors appear to be safe in lacunar stroke, although their efficacy is unclear.
A systolic blood pressure goal of less than 130 mmHg is reasonable in the outpatient setting.
Pioglitazone may be reasonable in lacunar stroke patients with insulin resistance.
Statin therapy should be used in most cases of lacunar stroke.
In the unique case of the “stuttering lacune”, no therapy beyond antiplatelet regimens can be recommended.
Neuroprotection and neural repair strategies are promising, and may play major roles in the future of lacunar stroke therapy.
Acknowledgements
The authors would like to thank Eng H. Lo for his critical revision of this manuscript and Elif Gökçal for her contribution to the figures.
Sources of Funding
Funding for this study was provided by grants from the National Institutes of Health (M. Edip Gurol, NS083711; Robert W. Regenhardt, NS065743).
ACRONYM
- ACS
acute coronary syndrome
- ACTIVE
Atrial Fibrillation Clopidogrel Trial with Irbesartan for Prevention of Vascular Events
- ADC
apparent diffusion coefficient
- aICH
asymptomatic intracerebral hemorrhage
- AICLA
Accidents Ischemiques Cerebraux Lies a l’Atherosclerose
- ALIAS
Albumin Treatment for Ischemic Stroke
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- ASA
aspirin
- ATC
Antithrombotic Trialists Collaboration
- ATLANTIS
Alteplase ThromboLysis for Acute Noninterventional Therapy in Ischemic Stroke
- BI
Barthel index
- C
controlled
- CAA
cerebral amyloid angiopathy
- CAPRIE
Clopidogrel versus AsPirin in patients at Risk of Ischaemic Events
- CASISP
Cilostazol versus Aspirin for Secondary Ischemic Stroke Prevention
- CAST
Chinese Acute Stroke Trial
- CD
clopidogrel
- CE
cardioembolic
- CHANCE
Clopidogrel in High-Risk Patients with Acute Nondisabling Cerebrovascular Events
- CI
Confidence interval
- CS
cilostazol
- CSPS
Cilostazol Stroke Prevention Study
- CSPS.com
Cilostazol Stroke Prevention Study for Antiplatelet Combination (CSPS.com)
- cSVD
cerebral small vessel disease
- CT
computed tomography
- DAPT
dual antiplatelet therapy
- DB
double-blind
- DP
dipyridamole
- DWI
diffusion-weighted imaging
- ECASS
European Cooperative Acute Stroke Study
- EPITHET
EchoPlanar Imaging Thrombolytic Evaluation Trial
- ESPRIT
European/Australasian Stroke Prevention in Reversible Ischaemia Trial
- ESPS-2
European Stroke Prevention Study-2
- FLAIR
fluid-attenuated inversion recovery
- FLAME
Fluoxetine for Motor Recovery after Acute Ischemic Stroke
- FMS
Fugl-Meyer motor scale
- FOCUS
Functional Outcomes after Acute Stroke
- GABA
γ-aminobutyric acid
- GI
gastrointestinal
- GOS
Glasgow Outcome Scale
- HbA1c
hemoglobinA1c
- HR
Hazard ratio
- ICD
International Classification of Diseases
- ICH
intracerebral hemorrhage
- INR
international normalized ratio
- IRIS
Insulin Resistance Intervention after Stroke
- IS
ischemic stroke
- IST-3
Third International Stroke Trial
- IU
International Unit
- IV
intravenous
- JCS
Joint Committee for Stroke
- LAA
large artery atherosclerosis
- LAAC
left atrial appendage closure
- LACI
lacunar infarction
- LACI-2
LACunar Intervention-2
- LS
lacunar stroke
- MATCH
Management of AtheroThrombosis with Clopidogrel in High-risk patients
- MC
multicenter
- mg
milligram
- MI
myocardial infarction
- mm
millimeter
- mmHg
millimeter of mercury
- MRI
magnetic resonance imaging
- mRS
modified Rankin scale
- NE
neurological examination
- NIHSS
National Institutes of Health Stroke Scale
- NINDS
National Institute of Neurological Disorders and Stroke
- NMDA
N-methyl-D-aspartate receptor
- NOAC
non–vitamin K antagonist oral anticoagulants
- NOGO
neurite outgrowth inhibitor
- NR
not reported
- O
other
- OCSP
Oxfordshire Community Stroke Project
- OHS
Oxford Handicap Scale
- PACI
partial anterior circulation infarct
- PE
physical exam
- PI
pulsatility index
- PLATO
Platelet Inhibition and Patient Outcomes
- POCI
posterior circulation infarct
- POINT
Platelet-Oriented Inhibition in New TIA and minor ischemic stroke
- PRoFESS
Prevention Regimen for Effectively Avoiding Second Strokes
- PS
prospective
- R
randomized
- RRR
relative risk reduction
- RS
retrospective
- RT
randomized
- SATURN
StATins Use in IntRacerebral Hemorrhage PatieNts
- SC
single-center
- SDH
subdural hematoma
- sICH
asymptomatic intracerebral hemorrhage
- SOCRATES
Acute Stroke or Transient Ischemic Attack Treated with Aspirin or Ticagrelor and Patient Outcomes
- SPARCL
Stroke Prevention by Aggressive Reduction in Cholesterol Levels
- SPIRIT
Stroke Prevention in Reversible Ischemia Trial
- SPS3
Secondary Prevention of Small Subcortical Strokes 3
- SSRI
selective serotonin reuptake inhibitors
- STRIVE
STandards for ReportIng Vascular changes on nEuroimaging
- SWI
susceptibility-weighted imaging
- TACI
total anterior cerebral infarct
- TIA
transient ischemic attack
- TOAST
Trial of ORG 10172 in Acute Stroke Treatment
- TP
ticlopidine
- tPA
tissue plasminogen activator
- UD
undetermined
- UKPDS
United Kingdom Prospective Diabetes Study
- WARSS
Warfarin-Aspirin Recurrent Stroke Study
- WMH
white matter hyperintensities
Footnotes
Conflicts of Interest/Disclosures
The authors report no conflicts of interest or disclosures.
References
- 1.Moran C, Phan TG, Srikanth VK. Cerebral small vessel disease: a review of clinical, radiological, and histopathological phenotypes. Int J Stroke. 2012. January 24;7(1):36–46. [DOI] [PubMed] [Google Scholar]
- 2.Arboix A, Marti-Vilalta JL. New concepts in lacunar stroke etiology: the constellation of small-vessel arterial disease. Cerebrovasc Dis. 2004;17 Suppl 1:58–62. [DOI] [PubMed] [Google Scholar]
- 3.de Groot JC, de Leeuw FE, Oudkerk M, van Gijn J, Hofman A, Jolles J, et al. Cerebral white matter lesions and cognitive function: the Rotterdam Scan Study. Ann Neurol. 2000. February;47(2):145–51. [DOI] [PubMed] [Google Scholar]
- 4.Pantoni L Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010. July;9(7):689–701. [DOI] [PubMed] [Google Scholar]
- 5.Vermeer SE, Prins ND, den Heijer T, Hofman A, Koudstaal PJ, Breteler MMB. Silent Brain Infarcts and the Risk of Dementia and Cognitive Decline. N Engl J Med. 2003. March 27;348(13):1215–22. [DOI] [PubMed] [Google Scholar]
- 6.Gurol ME. Molecular Neuroimaging in Vascular Cognitive Impairment. Stroke. 2016. April;47(4):1146–52. [DOI] [PubMed] [Google Scholar]
- 7.Whitman GT, Tang Y, Lin A, Baloh RW, Tang T. A prospective study of cerebral white matter abnormalities in older people with gait dysfunction. Neurology. 2001. September 25;57(6):990–4. [DOI] [PubMed] [Google Scholar]
- 8.Regenhardt RW, Das AS, Lo EH, Caplan LR. Advances in Understanding the Pathophysiology of Lacunar Stroke. JAMA Neurol. 2018. October 1;75(10):12733–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adams H, Adams H, Bendixen B, Bendixen B, Kappelle L, Kappelle L, et al. Classification of Subtype of Acute Ischemic Stroke. Stroke. 1993;23(1):35–41. [DOI] [PubMed] [Google Scholar]
- 10.Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013. August;12(8):822–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moreau F, Patel S, Lauzon ML, McCreary CR, Goyal M, Frayne R, et al. Cavitation After Acute Symptomatic Lacunar Stroke Depends on Time, Location, and MRI Sequence. Stroke. 2012. July 1;43(7):1837–42. [DOI] [PubMed] [Google Scholar]
- 12.Shi Y, Wardlaw JM. Update on cerebral small vessel disease: a dynamic whole-brain disease. Stroke Vasc Neurol. 2016. September 30; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fisher CM. Lacunar strokes and infarcts: a review. Neurology. 1982;32(8):871–6. [DOI] [PubMed] [Google Scholar]
- 14.Kimberly WT, Gilson A, Rost NS, Rosand J, Viswanathan A, Smith EE, et al. Silent ischemic infarcts are associated with hemorrhage burden in cerebral amyloid angiopathy. Neurology. 2009. April 7;72(14):1230–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kang D-W, Han M-K, Kim H-J, Yun S-C, Jeon S-B, Bae H-J, et al. New ischemic lesions coexisting with acute intracerebral hemorrhage. Neurology. 2012. August 28;79(9):848–55. [DOI] [PubMed] [Google Scholar]
- 16.Auriel E, Gurol ME, Ayres A, Dumas AP, Schwab KM, Vashkevich A, et al. Characteristic distributions of intracerebral hemorrhage-associated diffusion-weighted lesions. Neurology. 2012. December 11;79(24):2335–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gioia LC, Kate M, Choi V, Sivakumar L, Jeerakathil T, Kosior J, et al. Ischemia in Intracerebral Hemorrhage Is Associated With Leukoaraiosis and Hematoma Volume, Not Blood Pressure Reduction. Stroke. 2015. June;46(6):1541–7. [DOI] [PubMed] [Google Scholar]
- 18.Vermeer SE, Koudstaal PJ, Oudkerk M, Hofman A, Breteler MMB. Prevalence and risk factors of silent brain infarcts in the population-based Rotterdam Scan Study. Stroke. 2002. January;33(1):21–5. [DOI] [PubMed] [Google Scholar]
- 19.Vermeer SE, Hollander M, van Dijk EJ, Hofman A, Koudstaal PJ, Breteler MM, et al. Silent brain infarcts and white matter lesions increase stroke risk in the general population: the Rotterdam Scan Study. Stroke. 2003. May;34(5):1126–9. [DOI] [PubMed] [Google Scholar]
- 20.Vermeer SE, Den Heijer T, Koudstaal PJ, Oudkerk M, Hofman A, Breteler MMB, et al. Incidence and risk factors of silent brain infarcts in the population-based Rotterdam Scan Study. Stroke. 2003. January;34(2):392–6. [DOI] [PubMed] [Google Scholar]
- 21.Vermeer SE, Longstreth WT, Koudstaal PJ. Silent brain infarcts: a systematic review. Lancet Neurol. 2007;6(7):611–9. [DOI] [PubMed] [Google Scholar]
- 22.Bernick C, Kuller L, Dulberg C, Longstreth WT, Manolio T, Beauchamp N, et al. Silent MRI infarcts and the risk of future stroke: the cardiovascular health study. Neurology. 2001. October 9;57(7):1222–9. [DOI] [PubMed] [Google Scholar]
- 23.Longstreth WT, Bernick C, Manolio TA, Bryan N, Jungreis CA, Price TR. Lacunar infarcts defined by magnetic resonance imaging of 3660 elderly people: the Cardiovascular Health Study. Arch Neurol. 1998. September;55(9):1217–25. [DOI] [PubMed] [Google Scholar]
- 24.Bokura H, Kobayashi S, Yamaguchi S. Distinguishing silent lacunar infarction from enlarged Virchow-Robin spaces: a magnetic resonance imaging and pathological study. J Neurol. 1998. February;245(2):116–22. [DOI] [PubMed] [Google Scholar]
- 25.Ding J, Sigurðsson S, Jónsson PV., Eiriksdottir G, Charidimou A, Lopez OL, et al. Large Perivascular Spaces Visible on Magnetic Resonance Imaging, Cerebral Small Vessel Disease Progression, and Risk of Dementia. JAMA Neurol. 2017. September 1;74(9):1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pasi M, Boulouis G, Fotiadis P, Auriel E, Charidimou A, Haley K, et al. Distribution of lacunes in cerebral amyloid angiopathy and hypertensive small vessel disease. Neurology. 2017. June 6;88(23):2162–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith EE, Schneider JA, Wardlaw JM, Greenberg SM. Cerebral microinfarcts: The invisible lesions. Lancet Neurol. 2012;11(3):272–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brundel M, de Bresser J, van Dillen JJ, Kappelle LJ, Biessels GJ. Cerebral microinfarcts: a systematic review of neuropathological studies. J Cereb Blood Flow Metab. 2012;32(3):425–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wityk RJ. Cerebral Cortical Microinfarcts on 3-T Magnetic Resonance Imaging. JAMA Neurol. 2017. April 1;74(4):385. [DOI] [PubMed] [Google Scholar]
- 30.van Veluw SJ, Hilal S, Kuijf HJ, Ikram MK, Xin X, Yeow TB, et al. Cortical microinfarcts on 3T MRI: Clinical correlates in memory-clinic patients. Alzheimer’s Dement. 2015. Dec;11(12):1500–9. [DOI] [PubMed] [Google Scholar]
- 31.Hilal S, Sikking E, Shaik MA, Chan QL, van Veluw SJ, Vrooman H, et al. Cortical cerebral microinfarcts on 3T MRI: A novel marker of cerebrovascular disease. Neurology. 2016. October 11;87(15):1583–90. [DOI] [PubMed] [Google Scholar]
- 32.Longstreth WT, Sonnen JA, Koepsell TD, Kukull WA, Larson EB, Montine TJ, et al. Associations between microinfarcts and other macroscopic vascular findings on neuropathologic examination in 2 databases. Alzheimer Dis Assoc Disord. 2009;23(3):291–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Westover MB, Bianchi MT, Yang C, Schneider JA, Greenberg SM. Estimating cerebral microinfarct burden from autopsy samples. Neurology. 2013. April 9;80(15):1365–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Auriel E, Westover MB, Bianchi MT, Reijmer Y, Martinez-Ramirez S, Ni J, et al. Estimating Total Cerebral Microinfarct Burden From Diffusion-Weighted Imaging. Stroke. 2015. August;46(8):2129–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Veluw SJ, Shih AY, Smith EE, Chen C, Schneider JA, Wardlaw JM, et al. Detection, risk factors, and functional consequences of cerebral microinfarcts. Lancet Neurol. 2017. September;16(9):730–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goyal M, Menon BK, Zwam WH Van, Dippel DWJ, Mitchell PJ, Demchuk AM, et al. Endovascular thrombectomy after large-vessel ischaemic stroke : a meta-analysis of individual patient data from fi ve randomised trials. 2016;1723–31. [DOI] [PubMed]
- 37.Regenhardt RW, Mecca AP, Flavin SA, Boulouis G, Lauer A, Zachrison KS, et al. Delays in the Air or Ground Transfer of Patients for Endovascular Thrombectomy. Stroke. 2018. June;49(6):1419–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chowdhury D, Wardlaw JM, Dennis MS. Are multiple acute small subcortical infarctions caused by embolic mechanisms? J Neurol Neurosurg Psychiatry. 2004. October 1;75(10):1416–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Caplan LR. Lacunar infarction and small vessel disease: pathology and pathophysiology. J stroke. 2015;17(1):2–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lammie GA. Hypertensive cerebral small vessel disease and stroke. Brain Pathol. 2002. July;12(3):358–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Dijk EJ, Prins ND, Vrooman HA, Hofman A, Koudstaal PJ, Breteler MMB. Progression of Cerebral Small Vessel Disease in Relation to Risk Factors and Cognitive Consequences: Rotterdam Scan Study. Stroke. 2008. October 1;39(10):2712–9. [DOI] [PubMed] [Google Scholar]
- 42.Regenhardt RW, Biseko MR, Shayo AF, Mmbando TN, Grundy SJ, Xu A, et al. Opportunities for intervention: stroke treatments, disability and mortality in urban Tanzania. Int J Qual Heal Care. 2018;1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hill MD, Silver FL, Austin PC, Tu JV. Rate of stroke recurrence in patients with primary intracerebral hemorrhage. Stroke. 2000. January;31(1):123–7. [DOI] [PubMed] [Google Scholar]
- 44.Biffi A, Greenberg MS. Aspirin and recurrent intracerebral hemorrhage in cerebral amyloid angiopathy. 2010;04:693–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van Asch CJ, Luitse MJ, Rinkel GJ, van der Tweel I, Algra A, Klijn CJ. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol. 2010. February;9(2):167–76. [DOI] [PubMed] [Google Scholar]
- 46.Haley KE, Greenberg SM, Gurol ME. Cerebral Microbleeds and Macrobleeds: Should They Influence Our Recommendations for Antithrombotic Therapies? Curr Cardiol Rep. 2013. December 13;15(12):425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boulouis G, Van Etten ES, Charidimou A, Auriel E, Morotti A, Pasi M, et al. Association of key magnetic resonance imaging markers of cerebral small vessel disease with hematoma volume and expansion in patients with lobar and deep intracerebral hemorrhage. JAMA Neurol. 2016;73(12):1440–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pasi M, Marini S, Morotti A, Boulouis G, Xiong L, Charidimou A, et al. Cerebellar Hematoma Location: Implications for the Underlying Microangiopathy. Stroke. 2017. January;49(1):STROKEAHA.117.019286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Charidimou A, Boulouis G, Haley K, Auriel E, van Etten ES, Fotiadis P, et al. White matter hyperintensity patterns in cerebral amyloid angiopathy and hypertensive arteriopathy. Neurology. 2016. February 9;86(6):505–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lou M, Al-Hazzani A, Goddeau RP, Novak V, Selim M. Relationship Between White-Matter Hyperintensities and Hematoma Volume and Growth in Patients With Intracerebral Hemorrhage. Stroke. 2010. January 1;41(1):34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Charidimou A, Boulouis G, Pasi M, Auriel E, van Etten ES, Haley K, et al. MRI-visible perivascular spaces in cerebral amyloid angiopathy and hypertensive arteriopathy. Neurology. 2017. March 21;88(12):1157–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Inohara T, Xian Y, Liang L, Matsouaka RA, Saver JL, Smith EE, et al. Association of Intracerebral Hemorrhage Among Patients Taking Non–Vitamin K Antagonist vs Vitamin K Antagonist Oral Anticoagulants With In-Hospital Mortality. JAMA. 2018. February 6;319(5):463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med. 1995. December 14;333(24):1581–7. [DOI] [PubMed] [Google Scholar]
- 54.Pantoni L, Fierini F, Poggesi A. Thrombolysis in Acute Stroke Patients with Cerebral Small Vessel Disease. Cerebrovasc Dis. 2014;37(1):5–13. [DOI] [PubMed] [Google Scholar]
- 55.Neumann-Haefelin T, Hoelig S, Berkefeld J, Fiehler J, Gass A, Humpich M, et al. Leukoaraiosis Is a Risk Factor for Symptomatic Intracerebral Hemorrhage After Thrombolysis for Acute Stroke. Stroke. 2006. October 1;37(10):2463–6. [DOI] [PubMed] [Google Scholar]
- 56.Kongbunkiat K, Wilson D, Palumbo V, Hill MD, Jung S, Mattle HP, et al. and functional outcome after acute stroke thrombolysis. 2017;0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shi Z-S, Loh Y, Liebeskind DS, Saver JL, Gonzalez NR, Tateshima S, et al. Leukoaraiosis Predicts Parenchymal Hematoma After Mechanical Thrombectomy in Acute Ischemic Stroke. Stroke. 2012. July 1;43(7):1806–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hacke W, Kaste M, Fieschi C, Toni D, Lesaffre E, von Kummer R, et al. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. The European Cooperative Acute Stroke Study (ECASS). Jama. 1995. October 4;274(13):1017–25. [PubMed] [Google Scholar]
- 59.Hacke W, Kaste M, Fieschi C, von Kummer R, Davalos A, Meier D, et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second European-Australasian Acute Stroke Study Investigators. Lancet (London, England). 1998. October 17;352(9136):1245–51. [DOI] [PubMed] [Google Scholar]
- 60.Hacke W, Kaste M, Bluhmki E, Brozman M, Davalos A, Guidetti D, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008. September 25;359(13):1317–29. [DOI] [PubMed] [Google Scholar]
- 61.Albers GW, Clark WM, Madden KP, Hamilton SA. ATLANTIS trial: results for patients treated within 3 hours of stroke onset. Alteplase Thrombolysis for Acute Noninterventional Therapy in Ischemic Stroke. Stroke. 2002. February;33(2):493–5. [DOI] [PubMed] [Google Scholar]
- 62.Clark WM, Wissman S, Albers GW, Jhamandas JH, Madden KP, Hamilton S. Recombinant tissue-type plasminogen activator (Alteplase) for ischemic stroke 3 to 5 hours after symptom onset. The ATLANTIS Study: a randomized controlled trial. Alteplase Thrombolysis for Acute Noninterventional Therapy in Ischemic Stroke. Jama. 1999. December 1;282(21):2019–26. [DOI] [PubMed] [Google Scholar]
- 63.Davis SM, Donnan GA, Parsons MW, Levi C, Butcher KS, Peeters A, et al. Effects of alteplase beyond 3 h after stroke in the Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET): a placebo-controlled randomised trial. The LancetNeurology. 2008. April;7(4):299–309. [DOI] [PubMed] [Google Scholar]
- 64.Nagakane Y, Christensen S, Brekenfeld C, Ma H, Churilov L, Parsons MW, et al. EPITHET: Positive Result After Reanalysis Using Baseline Diffusion-Weighted Imaging/Perfusion-Weighted Imaging Co-Registration. Stroke. 2011. January;42(1):59–64. [DOI] [PubMed] [Google Scholar]
- 65.group I-3 collaborative, Sandercock P, Wardlaw JM, Lindley RI, Dennis M, Cohen G, et al. The benefits and harms of intravenous thrombolysis with recombinant tissue plasminogen activator within 6 h of acute ischaemic stroke (the third international stroke trial [IST-3]): a randomised controlled trial. Lancet (London, England). 2012. June 23;379(9834):2352–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.group I-3 collaborative. Effect of thrombolysis with alteplase within 6 h of acute ischaemic stroke on long-term outcomes (the third International Stroke Trial [IST-3]): 18-month follow-up of a randomised controlled trial. The LancetNeurology. 2013. August;12(8):768–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. 2018. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2018 Jan 24;STR.0000000000000158. [Google Scholar]
- 68.Cocho D, Belvís R, Martí-Fàbregas J, Bravo Y, Aleu A, Pagonabarraga J, et al. Does thrombolysis benefit patients with lacunar syndrome? Eur Neurol. 2006;55(2):70–3. [DOI] [PubMed] [Google Scholar]
- 69.Toni D, Iweins F, von Kummer R, Busse O, Bogousslavsky J, Falcou A, et al. Identification of lacunar infarcts before thrombolysis in the ECASS I study. Neurology. 2000. February 8;54(3):684–8. [DOI] [PubMed] [Google Scholar]
- 70.Potter G, Doubal F, Jackson C, Sudlow C, Dennis M, Wardlaw J. Associations of Clinical Stroke Misclassification (‘Clinical-Imaging Dissociation’) in Acute Ischemic Stroke. Cerebrovasc Dis. 2010;29(4):395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lahoti S, Gokhale S, Caplan L, Michel P, Samson Y, Rosso C, et al. Thrombolysis in Ischemic Stroke Without Arterial Occlusion at Presentation. Stroke. 2014. September;45(9):2722–7. [DOI] [PubMed] [Google Scholar]
- 72.Zivanovic Z, Slankamenac P, Vlahovic D, Radovanovic B, Ruzicka-Kaloci S, Rabi-Zikic T, et al. Intravenous thrombolysis in lacunar stroke. J Neurol Sci. 2017. October 15;381:1128. [Google Scholar]
- 73.Griebe M, Fischer E, Kablau M, Eisele P, Wolf ME, Chatzikonstantinou A, et al. Thrombolysis in patients with lacunar stroke is safe: an observational study. J Neurol. 2014. February 24;261(2):405–11. [DOI] [PubMed] [Google Scholar]
- 74.Hwang Y-H, Seo J-G, Lee H-W, Park S-P, Suh C-K. Early Neurological Deterioration following Intravenous Recombinant Tissue Plasminogen Activator Therapy in Patients with Acute Lacunar Stroke. Cerebrovasc Dis. 2008;26(4):355–9. [DOI] [PubMed] [Google Scholar]
- 75.Norrving B Long-term prognosis after lacunar infarction. Lancet Neurol. 2003. April;2(4):238–45. [DOI] [PubMed] [Google Scholar]
- 76.Lodder J, Bamford JM, Sandercock PA, Jones LN, Warlow CP. Are hypertension or cardiac embolism likely causes of lacunar infarction? Stroke. 1990. March;21(3):375–81. [DOI] [PubMed] [Google Scholar]
- 77.Arboix A, Marti-Vilalta JL. Presumed cardioembolic lacunar infarcts. Stroke. 1992. December;23(12):1841–2. [DOI] [PubMed] [Google Scholar]
- 78.Arboix A, Rexach M, Subira M, Pujadas R. Complex aortic atheroma plaques: study of 71 patients with lacunar infarcts. Med Clin (Barc). 2012. February 25;138(4):160–4. [DOI] [PubMed] [Google Scholar]
- 79.Laloux P, Brucher JM. Lacunar infarctions due to cholesterol emboli. Stroke. 1991. November;22(11):1440–4. [DOI] [PubMed] [Google Scholar]
- 80.Gan R, Sacco RL, Kargman DE, Roberts JK, Boden-Albala B, Gu Q. Testing the validity of the lacunar hypothesis: the Northern Manhattan Stroke Study experience. Neurology. 1997. May;48(5):1204–11. [DOI] [PubMed] [Google Scholar]
- 81.Del Bene A, Makin SDJ, Doubal FN, Inzitari D, Wardlaw JM. Variation in risk factors for recent small subcortical infarcts with infarct size, shape, and location. Stroke. 2013;44(11):3000–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Norrving B Lacunar infarction: embolism is the key: against. Stroke. 2004. July;35(7):1779–80. [DOI] [PubMed] [Google Scholar]
- 83.Jackson C, Sudlow C. Are lacunar strokes really different? A systematic review of differences in risk factor profiles between lacunar and nonlacunar infarcts. Stroke. 2005;36(4):891–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sacco SE, Whisnant JP, Broderick JP, Phillips SJ, O’Fallon WM. Epidemiological characteristics of lacunar infarcts in a population. Stroke. 1991. October;22(10):1236–41. [DOI] [PubMed] [Google Scholar]
- 85.Ay H, Oliveira-Filho J, Buonanno FS, Ezzeddine M, Schaefer PW, Rordorf G, et al. Diffusion-weighted imaging identifies a subset of lacunar infarction associated with embolic source. Stroke. 1999;30(12):2644–50. [DOI] [PubMed] [Google Scholar]
- 86.Macdonald RL, Kowalczuk A, Johns L. Emboli enter penetrating arteries of monkey brain in relation to their size. Stroke. 1995. July;26(7):1241–7. [DOI] [PubMed] [Google Scholar]
- 87.Huang Y-C, Tsai Y-H, Lee J-D, Weng H-H, Lin L-C, Lin Y-H, et al. Hemodynamic Factors May Play a Critical Role in Neurological Deterioration Occurring within 72 hrs after Lacunar Stroke. Wiart M, editor. PLoS One. 2014. October 23;9(10):e108395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pantoni L, Inzitari D. New clinical relevance of leukoaraiosis. European Task Force on Age-Related White Matter-Changes. Stroke. 1998. February;29(2):543. [DOI] [PubMed] [Google Scholar]
- 89.Smith EE, Rosand J, Knudsen KA, Hylek EM, Greenberg SM. Leukoaraiosis is associated with warfarin-related hemorrhage following ischemic stroke. Neurology. 2002. July 23;59(2):193–7. [DOI] [PubMed] [Google Scholar]
- 90.Inzitari D Leukoaraiosis: An Independent Risk Factor for Stroke? Stroke. 2003. August 1;34(8):2067–71. [DOI] [PubMed] [Google Scholar]
- 91.Gorter JW. Major bleeding during anticoagulation after cerebral ischemia: patterns and risk factors. Stroke Prevention In Reversible Ischemia Trial (SPIRIT). European Atrial Fibrillation Trial (EAFT) study groups. Neurology. 1999. October 12;53(6):1319–27. [DOI] [PubMed] [Google Scholar]
- 92.van Etten ES, Auriel E, Haley KE, Ayres AM, Vashkevich A, Schwab KM, et al. Incidence of Symptomatic Hemorrhage in Patients With Lobar Microbleeds. Stroke. 2014. August 1;45(8):2280–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.The International Stroke Trial (IST): a randomised trial of aspirin, subcutaneous heparin, both, or neither among 19435 patients with acute ischaemic stroke. International Stroke Trial Collaborative Group. Lancet (London, England). 1997. May 31;349(9065):1569–81. [PubMed] [Google Scholar]
- 94.The Publications Committee for the Trial of ORG 10172 in Acute Stroke Treatment Investigators. Low Molecular Weight Heparinoid, ORG 10172 (Danaparoid), and Outcome After Acute Ischemic Stroke: A Randomized Controlled Trial. JAMA J Am Med Assoc. 1998;279(16):1265–72. [PubMed] [Google Scholar]
- 95.Mohr JP, Thompson JL, Lazar RM, Levin B, Sacco RL, Furie KL, et al. A comparison of warfarin and aspirin for the prevention of recurrent ischemic stroke. N Engl J Med. 2001. November 15;345(20):1444–51. [DOI] [PubMed] [Google Scholar]
- 96.Evans a, Perez I, Yu G, Kalra L. Should stroke subtype influence anticoagulation decisions to prevent recurrence in stroke patients with atrial fibrillation? Stroke. 2001;32(12):2828–32. [DOI] [PubMed] [Google Scholar]
- 97.Lee SH, Bae HJ, Kwon SJ, Kim H, Kim YH, Yoon BW, et al. Cerebral microbleeds are regionally associated with intracerebral hemorrhage. Neurology. 2004. January 13;62(1):72–6. [DOI] [PubMed] [Google Scholar]
- 98.Charidimou A, Boulouis G, Xiong L, Jessel MJ, Roongpiboonsopit D, Ayres A, et al. Cortical superficial siderosis and first-ever cerebral hemorrhage in cerebral amyloid angiopathy. Neurology. 2017. April 25;88(17):1607–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Charidimou A, Peeters AP, Jager R, Fox Z, Vandermeeren Y, Laloux P, et al. Cortical superficial siderosis and intracerebral hemorrhage risk in cerebral amyloid angiopathy. Neurology. 2013. November 5;81(19):1666–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hankey GJ. Intracranial Hemorrhage and Novel Anticoagulants for Atrial Fibrillation: What Have We Learned? Curr Cardiol Rep. 2014. May 20;16(5):480. [DOI] [PubMed] [Google Scholar]
- 101.Wilson D, Seiffge DJ, Traenka C, Basir G, Purrucker JC, Rizos T, et al. Outcome of intracerebral hemorrhage associated with different oral anticoagulants. Neurology. 2017. May 2;88(18):1693–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wilson D, Charidimou A, Ambler G, Fox ZV., Gregoire S, Rayson P, et al. Recurrent stroke risk and cerebral microbleed burden in ischemic stroke and TIA. Neurology. 2016. October 4;87(14):1501–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Charidimou A, Karayiannis C, Song T-J, Orken DN, Thijs V, Lemmens R, et al. Brain microbleeds, anticoagulation, and hemorrhage risk. Neurology. 2017. December 5;89(23):2317–26. [DOI] [PubMed] [Google Scholar]
- 104.Wilson D, Ambler G, Shakeshaft C, Brown MM, Charidimou A, Al-Shahi Salman R, et al. Cerebral microbleeds and intracranial haemorrhage risk in patients anticoagulated for atrial fibrillation after acute ischaemic stroke or transient ischaemic attack (CROMIS-2): a multicentre observational cohort study. Lancet Neurol. 2018. June;17(6):539–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lip GYH, Nieuwlaat R, Pisters R, Lane DA, Crijns HJGM. Refining Clinical Risk Stratification for Predicting Stroke and Thromboembolism in Atrial Fibrillation Using a Novel Risk Factor-Based Approach. Chest. 2010. February;137(2):263–72. [DOI] [PubMed] [Google Scholar]
- 106.Eikelboom JW, Connolly SJ, Bosch J, Dagenais GR, Hart RG, Shestakovska O, et al. Rivaroxaban with or without Aspirin in Stable Cardiovascular Disease. N Engl J Med. 2017. October 5;377(14):1319–30. [DOI] [PubMed] [Google Scholar]
- 107.Cea Soriano L, Gaist D, Soriano-Gabarró M, Bromley S, García Rodríguez LA. Low-dose aspirin and risk of intracranial bleeds. Neurology. 2017. November 28;89(22):2280–7. [DOI] [PubMed] [Google Scholar]
- 108.Viswanathan A, Chabriat H. Cerebral Microhemorrhage. Stroke. 2006. February 1;37(2):550–5. [DOI] [PubMed] [Google Scholar]
- 109.Chong B-H, Chan K-H, Pong V, Lau K-K, Chan Y-H, Zuo M-L, et al. Use of aspirin in Chinese after recovery from primary intracranial haemorrhage. Thromb Haemost. 2012. December 21;107(2):241–7. [DOI] [PubMed] [Google Scholar]
- 110.CAST: randomised placebo-controlled trial of early aspirin use in 20,000 patients with acute ischaemic stroke. CAST (Chinese Acute Stroke Trial) Collaborative Group. Lancet (London, England). 1997. June 7;349(9066):1641–9. [PubMed] [Google Scholar]
- 111.Wong KSL, Wang Y, Leng X, Mao C, Tang J, Bath PMW, et al. Early Dual Versus Mono Antiplatelet Therapy for Acute Non-Cardioembolic Ischemic Stroke or Transient Ischemic Attack: An Updated Systematic Review and Meta-Analysis. Circulation. 2013. October 8;128(15):1656–66. [DOI] [PubMed] [Google Scholar]
- 112.Wang Y, Pan Y, Zhao X, Li H, Wang D, Johnston SC, et al. Clopidogrel With Aspirin in Acute Minor Stroke or Transient Ischemic Attack (CHANCE) TrialCLINICAL PERSPECTIVE. Circulation. 2015. July 7;132(1):40–6. [DOI] [PubMed] [Google Scholar]
- 113.Liu L, Wong KSL, Leng X, Pu Y, Wang Y, Jing J, et al. Dual antiplatelet therapy in stroke and ICAS. Neurology. 2015. September 29;85(13):1154–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jing J, Meng X, Zhao X, Liu L, Wang A, Pan Y, et al. Dual Antiplatelet Therapy in Transient Ischemic Attack and Minor Stroke With Different Infarction Patterns. JAMA Neurol. 2018. June 1;75(6):711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Johnston SC, Easton JD, Farrant M, Barsan W, Conwit RA, Elm JJ, et al. Clopidogrel and Aspirin in Acute Ischemic Stroke and High-Risk TIA. N Engl J Med. 2018. July 19;379(3):215–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Collaboration AT. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002. January 12;324(7329):71–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Collaboration AT (ATT), Baigent C, Blackwell L, Collins R, Emberson J, Godwin J, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet (London, England). 2009. May 30;373(9678):1849–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sibon I, Orgogozo JM. Antiplatelet drug discontinuation is a risk factor for ischemic stroke. Neurology. 2004. April 13;62(7):1187–9. [DOI] [PubMed] [Google Scholar]
- 119.Gent M, Blakely JA, Easton JD, Ellis DJ, Hachinski VC, Harbison JW, et al. The Canadian American Ticlopidine Study (CATS) in thromboembolic stroke. Design, organization, and baseline results. Stroke. 1988. October;19(10):1203–10. [DOI] [PubMed] [Google Scholar]
- 120.Gent M, Blakely JA, Easton JD, Ellis DJ, Hachinski VC, Harbison JW, et al. The Canadian American Ticlopidine Study (CATS) in thromboembolic stroke. Lancet (London, England). 1989. June 3;1(8649):1215–20. [DOI] [PubMed] [Google Scholar]
- 121.Harbison JW. Ticlopidine versus aspirin for the prevention of recurrent stroke. Analysis of patients with minor stroke from the Ticlopidine Aspirin Stroke Study. Stroke. 1992. December;23(12):1723–7. [DOI] [PubMed] [Google Scholar]
- 122.Weisberg LA. The efficacy and safety of ticlopidine and aspirin in non-whites: analysis of a patient subgroup from the Ticlopidine Aspirin Stroke Study. Neurology. 1993. January;43(1):27–31. [DOI] [PubMed] [Google Scholar]
- 123.Gorelick PB, Richardson D, Kelly M, Ruland S, Hung E, Harris Y, et al. Aspirin and ticlopidine for prevention of recurrent stroke in black patients: a randomized trial. Jama. 2003. June 11;289(22):2947–57. [DOI] [PubMed] [Google Scholar]
- 124.Weisberg LA. Retrospective analysis of aspirin and ticlopidine in preventing recurrent stroke following an initial lacunar infarct. J Stroke Cerebrovasc Dis. 1995;5(1):44–8. [DOI] [PubMed] [Google Scholar]
- 125.Committee CS. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). CAPRIE Steering Committee. Lancet (London, England). 1996. November 16;348(9038):1329–39. [DOI] [PubMed] [Google Scholar]
- 126.Investigators S, Benavente OR, Hart RG, McClure LA, Szychowski JM, Coffey CS, et al. Effects of clopidogrel added to aspirin in patients with recent lacunar stroke. N Engl J Med. 2012. August;367(9):817–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.ACTIVE Investigators, Connolly SJ, Pogue J, Hart RG, Hohnloser SH, Pfeffer M, et al. Effect of Clopidogrel Added to Aspirin in Patients with Atrial Fibrillation. N Engl J Med. 2009. May 14;360(20):2066–78. [DOI] [PubMed] [Google Scholar]
- 128.Bhatt DL, Fox KA, Hacke W, Berger PB, Black HR, Boden WE, et al. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med. 2006. April 20;354(16):1706–17. [DOI] [PubMed] [Google Scholar]
- 129.Diener HC, Bogousslavsky J, Brass LM, Cimminiello C, Csiba L, Kaste M, et al. Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): randomised, double-blind, placebo-controlled trial. Lancet (London, England). 2004;364(9431):331–7. [DOI] [PubMed] [Google Scholar]
- 130.Field TS, Nakajima M, Benavente OR. Combination Aspirin and Clopidogrel for Secondary Prevention of Ischemic Stroke. Curr Treat Options Cardiovasc Med. 2013. June 29;15(3):348–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bousser MG, Eschwege E, Haguenau M, Lefaucconnier JM, Thibult N, Touboul D, et al. “AICLA” controlled trial of aspirin and dipyridamole in the secondary prevention of athero-thrombotic cerebral ischemia. Stroke. 1983;14(1):5–14. [DOI] [PubMed] [Google Scholar]
- 132.Diener HC, Cunha L, Forbes C, Sivenius J, Smets P, Lowenthal A. European Stroke Prevention Study. 2. Dipyridamole and acetylsalicylic acid in the secondary prevention of stroke. J Neurol Sci. 1996. November;143(1–2):1–13. [DOI] [PubMed] [Google Scholar]
- 133.Group ES, Halkes PH, van Gijn J, Kappelle LJ, Koudstaal PJ, Algra A. Aspirin plus dipyridamole versus aspirin alone after cerebral ischaemia of arterial origin (ESPRIT): randomised controlled trial. Lancet (London, England). 2006. May 20;367(9523):1665–73. [DOI] [PubMed] [Google Scholar]
- 134.Sacco RL, Diener H-C, Yusuf S, Cotton D, Ôunpuu S, Lawton WA, et al. Aspirin and Extended-Release Dipyridamole versus Clopidogrel for Recurrent Stroke. N Engl J Med. 2008. September 18;359(12):1238–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Diener H-C, Weber R. Clopidogrel Added to Aspirin Adds No Benefit but Bleeding Risk in Patients With Recent Lacunar Stroke. Stroke. 2013. March 1;44(3):861–3. [DOI] [PubMed] [Google Scholar]
- 136.Kwok CS, Shoamanesh A, Copley HC, Myint PK, Loke YK, Benavente OR. Efficacy of Antiplatelet Therapy in Secondary Prevention Following Lacunar Stroke: Pooled Analysis of Randomized Trials. Stroke. 2015. April 1;46(4):1014–23. [DOI] [PubMed] [Google Scholar]
- 137.Bath PM, Woodhouse LJ, Appleton JP, Beridze M, Christensen H, Dineen RA, et al. Antiplatelet therapy with aspirin, clopidogrel, and dipyridamole versus clopidogrel alone or aspirin and dipyridamole in patients with acute cerebral ischaemia (TARDIS): a randomised, open-label, phase 3 superiority trial. Lancet. 2017. December 20; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gotoh F, Tohgi H, Hirai S, Terashi A, Fukuuchi Y, Otomo E, et al. Cilostazol stroke prevention study: A placebo-controlled double-blind trial for secondary prevention of cerebral infarction. J Stroke Cerebrovasc Dis. 2000;9(4):147–57. [DOI] [PubMed] [Google Scholar]
- 139.Shinohara Y, Katayama Y, Uchiyama S, Yamaguchi T, Handa S, Matsuoka K, et al. Cilostazol for prevention of secondary stroke (CSPS 2): an aspirin-controlled, double-blind, randomised non-inferiority trial. The LancetNeurology. 2010. October;9(10):959–68. [DOI] [PubMed] [Google Scholar]
- 140.Uchiyama S Results of the Cilostazol Stroke Prevention Study II (CSPS II): a randomized controlled trial for the comparison of cilostazol and aspirin in stroke patients. Rinsho Shinkeigaku. 2010. November;50(11):832–4. [DOI] [PubMed] [Google Scholar]
- 141.Uchiyama S, Shinohara Y, Katayama Y, Yamaguchi T, Handa S, Matsuoka K, et al. Benefit of cilostazol in patients with high risk of bleeding: subanalysis of cilostazol stroke prevention study 2. Cerebrovasc Dis. 2014;37(4):296–303. [DOI] [PubMed] [Google Scholar]
- 142.Huang Y, Cheng Y, Wu J, Li Y, Xu E, Hong Z, et al. Cilostazol as an alternative to aspirin after ischaemic stroke: a randomised, double-blind, pilot study. The LancetNeurology. 2008. June;7(6):494–9. [DOI] [PubMed] [Google Scholar]
- 143.Han SW, Lee SSIS, Kim SH, Lee JYHYH, Kim GS, Kim OJ, et al. Effect of cilostazol in acute lacunar infarction based on pulsatility index of transcranial Doppler (ECLIPse): a multicenter, randomized, double-blind, placebo-controlled trial. Eur Neurol. 2013;69(1):33–40. [DOI] [PubMed] [Google Scholar]
- 144.Yamashita S, Matsuzawa Y. Where are we with probucol: a new life for an old drug? Atherosclerosis. 2009. November;207(1):16–23. [DOI] [PubMed] [Google Scholar]
- 145.Yamamoto Y, Ohara T, Ishii R, Tanaka E, Murai T, Morii F, et al. A combined treatment for acute larger lacunar-type infarction. J Stroke Cerebrovasc Dis. 2011;20(5):387–94. [DOI] [PubMed] [Google Scholar]
- 146.Toyoda K, Uchiyama S, Hoshino H, Kimura K, Origasa H, Naritomi H, et al. Protocol for Cilostazol Stroke Prevention Study for Antiplatelet Combination (CSPS.com): A Randomized, Open-Label, Parallel-Group Trial. Int J Stroke. 2015. February 8;10(2):253–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Guo S, Olesen J, Ashina M. Phosphodiesterase 3 inhibitor cilostazol induces migraine-like attacks via cyclic AMP increase. Brain. 2014. November;137(11):2951–9. [DOI] [PubMed] [Google Scholar]
- 148.Hiatt WR, Money SR, Brass EP. Long-term safety of cilostazol in patients with peripheral artery disease: The CASTLE study (Cilostazol: A Study in Long-term Effects). J Vasc Surg. 2008. February;47(2):330–336.e2. [DOI] [PubMed] [Google Scholar]
- 149.Galyfos G, Sianou A. Cilostazol for Secondary Prevention of Stroke: Should the Guidelines Perhaps Be Extended? Vasc Spec Int. 2017. September 30;33(3):89–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lee J-H, Cha J-K, Lee SJ, Ha S-W, Kwon SU. Addition of cilostazol reduces biological aspirin resistance in aspirin users with ischaemic stroke: a double-blind randomized clinical trial. Eur J Neurol. 2010. March;17(3):434–42. [DOI] [PubMed] [Google Scholar]
- 151.Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009. September 10;361(11):1045–57. [DOI] [PubMed] [Google Scholar]
- 152.Storey RF, Husted S, Harrington RA, Heptinstall S, Wilcox RG, Peters G, et al. Inhibition of platelet aggregation by AZD6140, a reversible oral P2Y12 receptor antagonist, compared with clopidogrel in patients with acute coronary syndromes. J Am Coll Cardiol. 2007. November 6;50(19):1852–6. [DOI] [PubMed] [Google Scholar]
- 153.Johnston SC, Amarenco P, Albers GW, Denison H, Easton JD, Evans SR, et al. Ticagrelor versus Aspirin in Acute Stroke or Transient Ischemic Attack. N Engl J Med. 2016. May 10; [DOI] [PubMed] [Google Scholar]
- 154.Amarenco P, Albers GW, Denison H, Easton JD, Evans SR, Held P, et al. Efficacy and safety of ticagrelor versus aspirin in acute stroke or transient ischaemic attack of atherosclerotic origin: a subgroup analysis of SOCRATES, a randomised, double-blind, controlled trial. Lancet Neurol. 2017. April;16(4):301–10. [DOI] [PubMed] [Google Scholar]
- 155.Mead GE, Hsieh CF, Lee R, Kutlubaev MA, Claxton A, Hankey GJ, et al. Selective serotonin reuptake inhibitors (SSRIs) for stroke recovery. Cochrane database Syst Rev. 2012. November 14;11:CD009286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Mead GE, Hsieh CF, Lee R, Kutlubaev M, Claxton A, Hankey GJ, et al. Selective serotonin reuptake inhibitors for stroke recovery: a systematic review and meta-analysis. Stroke. 2013. March;44(3):844–50. [DOI] [PubMed] [Google Scholar]
- 157.Chollet F, Tardy J, Albucher JF, Thalamas C, Berard E, Lamy C, et al. Fluoxetine for motor recovery after acute ischaemic stroke (FLAME): a randomised placebo-controlled trial. The LancetNeurology. 2011. February;10(2):123–30. [DOI] [PubMed] [Google Scholar]
- 158.Acler M, Robol E, Fiaschi A, Manganotti P. A double blind placebo RCT to investigate the effects of serotonergic modulation on brain excitability and motor recovery in stroke patients. J Neurol. 2009. July;256(7):1152–8. [DOI] [PubMed] [Google Scholar]
- 159.Zittel S, Weiller C, Liepert J. Citalopram improves dexterity in chronic stroke patients. Neurorehabil Neural Repair. 2008;22(3):311–4. [DOI] [PubMed] [Google Scholar]
- 160.Pariente J, Loubinoux I, Carel C, Albucher JF, Leger A, Manelfe C, et al. Fluoxetine modulates motor performance and cerebral activation of patients recovering from stroke. Ann Neurol. 2001. December;50(6):718–29. [DOI] [PubMed] [Google Scholar]
- 161.Gerdelat-Mas A, Loubinoux I, Tombari D, Rascol O, Chollet F, Simonetta-Moreau M. Chronic administration of selective serotonin reuptake inhibitor (SSRI) paroxetine modulates human motor cortex excitability in healthy subjects. Neuroimage. 2005. August 15;27(2):314–22. [DOI] [PubMed] [Google Scholar]
- 162.Dennis M, Mead G, Forbes J, Graham C, Hackett M, Hankey GJ, et al. Effects of fluoxetine on functional outcomes after acute stroke (FOCUS): a pragmatic, double-blind, randomised, controlled trial. Lancet. 2018. December 4; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Bak S, Tsiropoulos I, Kjaersgaard JO, Andersen M, Mellerup E, Hallas J, et al. Selective serotonin reuptake inhibitors and the risk of stroke: a population-based case-control study. Stroke. 2002. June;33(6):1465–73. [DOI] [PubMed] [Google Scholar]
- 164.Kharofa J, Sekar P, Haverbusch M, Moomaw C, Flaherty M, Kissela B, et al. Selective Serotonin Reuptake Inhibitors and Risk of Hemorrhagic Stroke. Stroke. 2007. November 1;38(11):3049–51. [DOI] [PubMed] [Google Scholar]
- 165.Douglas I, Smeeth L, Irvine D. The use of antidepressants and the risk of haemorrhagic stroke: a nested case control study. Br J Clin Pharmacol. 2011. January;71(1):116–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.de Abajo FJ, Jick H, Derby L, Jick S, Schmitz S. Intracranial haemorrhage and use of selective serotonin reuptake inhibitors. Br J Clin Pharmacol. 2000. July;50(1):43–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Chen V, Guo JJ, Li H, Wulsin L, Patel NC. Risk of Cerebrovascular Events Associated with Antidepressant Use in Patients with Depression: A Population-Based, Nested Case-Control Study . Ann Pharmacother. 2008. February 15;42(2):177–84. [DOI] [PubMed] [Google Scholar]
- 168.Renoux C, Vahey S, Dell’Aniello S, Boivin J-F. Association of Selective Serotonin Reuptake Inhibitors With the Risk for Spontaneous Intracranial Hemorrhage. JAMA Neurol. 2017. February 1;74(2):173. [DOI] [PubMed] [Google Scholar]
- 169.Hackam DG, Mrkobrada M. Selective serotonin reuptake inhibitors and brain hemorrhage: A meta-analysis. Neurology. 2012. October 30;79(18):1862–5. [DOI] [PubMed] [Google Scholar]
- 170.Jauch EC, Saver JL, Adams HP, Bruno A, Connors JJ, Demaerschalk BM, et al. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2013. March 1;44(3):870–947. [DOI] [PubMed] [Google Scholar]
- 171.Rordorf G, Cramer SC, Efird JT, Schwamm LH, Buonanno F, Koroshetz WJ. Pharmacological elevation of blood pressure in acute stroke. Clinical effects and safety. Stroke. 1997. November;28(11):2133–8. [DOI] [PubMed] [Google Scholar]
- 172.Regenhardt RW, Das AS, Stapleton CJ, Chandra RV. Blood Pressure and Penumbral Sustenance in Stroke from Large vessel Occlusion. Front Neurol. 2017;8(July). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lalive PH, Mayor I, Sztajzel R. The Role of Blood Pressure in Lacunar Strokes Preceded by TIAs . Cerebrovasc Dis. 2003;16(1):88–90. [DOI] [PubMed] [Google Scholar]
- 174.Belayev L, Liu Y, Zhao W, Busto R, Ginsberg MD. Human albumin therapy of acute ischemic stroke: marked neuroprotective efficacy at moderate doses and with a broad therapeutic window. Stroke. 2001. February;32(2):553–60. [DOI] [PubMed] [Google Scholar]
- 175.Liu Y, Belayev L, Zhao W, Busto R, Belayev A, Ginsberg MD. Neuroprotective effect of treatment with human albumin in permanent focal cerebral ischemia: histopathology and cortical perfusion studies. Eur J Pharmacol. 2001. October 5;428(2):193–201. [DOI] [PubMed] [Google Scholar]
- 176.Pascual-Leone A, Anderson DC, Larson DA. Volume therapy in orthostatic transient ischemic attacks. Stroke. 1989. September;20(9):1267–70. [DOI] [PubMed] [Google Scholar]
- 177.Frey JL. Hemodilution therapy for lacunar stroke: Treatment results in 10 consecutive cases. J Stroke Cerebrovasc Dis. 1992;2(3):136–45. [DOI] [PubMed] [Google Scholar]
- 178.Ginsberg MD, Palesch YY, Hill MD, Martin RH, Moy CS, Barsan WG, et al. High-dose albumin treatment for acute ischaemic stroke (ALIAS) Part 2: a randomised, double-blind, phase 3, placebo-controlled trial. The LancetNeurology. 2013. November;12(11):1049–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Lammie GA, Brannan F, Slattery J, Warlow C. Nonhypertensive cerebral small-vessel disease. An autopsy study. Stroke. 1997. November;28(11):2222–9. [DOI] [PubMed] [Google Scholar]
- 180.Arboix A, Blanco-Rojas L, Martí-Vilalta JL. Advancements in understanding the mechanisms of symptomatic lacunar ischemic stroke: translation of knowledge to prevention strategies. Expert Rev Neurother. 2014;14(3):261–76. [DOI] [PubMed] [Google Scholar]
- 181.Rashid P, Leonardi-Bee J, Bath P. Blood pressure reduction and secondary prevention of stroke and other vascular events: a systematic review. Stroke. 2003. November;34(11):2741–8. [DOI] [PubMed] [Google Scholar]
- 182.Altmann M, Thommessen B, Rønning OM, Reichenbach AS, Fure B. Blood pressure differences between patients with lacunar and nonlacunar infarcts. Brain Behav. 2015. August;5(8):e00353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Yusuf S, Diener H-C, Sacco RL, Cotton D, Ôunpuu S, Lawton WA, et al. Telmisartan to Prevent Recurrent Stroke and Cardiovascular Events. N Engl J Med. 2008. September 18;359(12):1225–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Group SS, Benavente OR, Coffey CS, Conwit R, Hart RG, McClure LA, et al. Blood-pressure targets in patients with recent lacunar stroke: the SPS3 randomised trial. Lancet (London, England). 2013. August 10;382(9891):507–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Dennison Himmelfarb C, et al. 2017. ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary. Hypertension. 2017 Nov 13;HYP.0000000000000066. [Google Scholar]
- 186.Hankey GJ, Lacey B. Optimum Blood Pressure Target After Lacunar Stroke: Pro Side of the Argument. Hypertension. 2014. May 1;63(5):918–22. [DOI] [PubMed] [Google Scholar]
- 187.Anderson RE, Tan WK, Martin HS, Meyer FB. Effects of glucose and PaO2 modulation on cortical intracellular acidosis, NADH redox state, and infarction in the ischemic penumbra. Stroke. 1999. January;30(1):160–70. [DOI] [PubMed] [Google Scholar]
- 188.Capes SE, Hunt D, Malmberg K, Pathak P, Gerstein HC. Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview. Stroke. 2001. October;32(10):2426–32. [DOI] [PubMed] [Google Scholar]
- 189.Lindsberg PJ, Roine RO. Hyperglycemia in acute stroke. Stroke. 2004. February;35(2):363–4. [DOI] [PubMed] [Google Scholar]
- 190.Gray CS, Hildreth AJ, Sandercock PA, O’Connell JE, Johnston DE, Cartlidge NE, et al. Glucose-potassium-insulin infusions in the management of post-stroke hyperglycaemia: the UK Glucose Insulin in Stroke Trial (GIST-UK). Lancet Neurol. 2007. May;6(5):397–406. [DOI] [PubMed] [Google Scholar]
- 191.Jorgensen H, Nakayama H, Raaschou HO, Olsen TS. Stroke in patients with diabetes. The Copenhagen Stroke Study. Stroke. 1994. October;25(10):1977–84. [DOI] [PubMed] [Google Scholar]
- 192.You R, McNeil JJ, O’Malley HM, Davis SM, Donnan GA. Risk factors for lacunar infarction syndromes. Neurology. 1995. August;45(8):1483–7. [DOI] [PubMed] [Google Scholar]
- 193.Tuttolomondo A, Pecoraro R, Di Raimondo D, Arnao V, Clemente G, Della Corte V, et al. Stroke subtypes and their possible implication in stroke prevention drug strategies. Curr Vasc Pharmacol. 2013. November;11(6):824–37. [DOI] [PubMed] [Google Scholar]
- 194.Karapanayiotides T, Piechowski-Jozwiak B, van Melle G, Bogousslavsky J, Devuyst G. Stroke patterns, etiology, and prognosis in patients with diabetes mellitus. Neurology. 2004. May 11;62(9):1558–62. [DOI] [PubMed] [Google Scholar]
- 195.Megherbi SE, Milan C, Minier D, Couvreur G, Osseby GV, Tilling K, et al. Association between diabetes and stroke subtype on survival and functional outcome 3 months after stroke: data from the European BIOMED Stroke Project. Stroke. 2003. March;34(3):688–94. [DOI] [PubMed] [Google Scholar]
- 196.Air EL, Kissela BM. Diabetes, the metabolic syndrome, and ischemic stroke: epidemiology and possible mechanisms. Diabetes Care. 2007. December;30(12):3131–40. [DOI] [PubMed] [Google Scholar]
- 197.Palacio S, McClure LA, Benavente OR, Bazan C 3rd, Pergola P, Hart RG. Lacunar strokes in patients with diabetes mellitus: risk factors, infarct location, and prognosis: the secondary prevention of small subcortical strokes study. Stroke. 2014. September;45(9):2689–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Ichikawa H, Kuriki A, Kinno R, Katoh H, Mukai M, Kawamura M. Occurrence and clinicotopographical correlates of brainstem infarction in patients with diabetes mellitus. J Stroke Cerebrovasc Dis. 2012. November;21(8):890–7. [DOI] [PubMed] [Google Scholar]
- 199.Kernan WN, Viscoli CM, Furie KL, Young LH, Inzucchi SE, Gorman M, et al. Pioglitazone after Ischemic Stroke or Transient Ischemic Attack. N Engl J Med. 2016. April 7;374(14):1321–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Kernan WN, Viscoli CM, Dearborn JL, Kent DM, Conwit R, Fayad P, et al. Targeting Pioglitazone Hydrochloride Therapy After Stroke or Transient Ischemic Attack According to Pretreatment Risk for Stroke or Myocardial Infarction. JAMA Neurol. 2017. November 1;74(11):1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Spence JD, Viscoli CM, Inzucchi SE, Dearborn-Tomazos J, Ford GA, Gorman M, et al. Pioglitazone Therapy in Patients With Stroke and Prediabetes. JAMA Neurol. 2019. February 7; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Martinez-Sanchez P, Rivera-Ordonez C, Fuentes B, Ortega-Casarrubios MA, Idrovo L, Diez-Tejedor E. The beneficial effect of statins treatment by stroke subtype. Eur J Neurol. 2009. January;16(1):127–33. [DOI] [PubMed] [Google Scholar]
- 203.Olsen TS, Christensen RH, Kammersgaard LP, Andersen KK. Higher total serum cholesterol levels are associated with less severe strokes and lower all-cause mortality: ten-year follow-up of ischemic strokes in the Copenhagen Stroke Study. Stroke. 2007. October;38(10):2646–51. [DOI] [PubMed] [Google Scholar]
- 204.Tirschwell DL, Smith NL, Heckbert SR, Lemaitre RN, Longstreth WT Jr, Psaty BM. Association of cholesterol with stroke risk varies in stroke subtypes and patient subgroups. Neurology. 2004. November 23;63(10):1868–75. [DOI] [PubMed] [Google Scholar]
- 205.Adams RJ, Carroll RM, Nichols FT, McNair N, Feldman DS, Feldman EB, et al. Plasma lipoproteins in cortical versus lacunar infarction. Stroke. 1989;20(4):448–52. [DOI] [PubMed] [Google Scholar]
- 206.Laloux P, Galanti L, Jamart J. Lipids in ischemic stroke subtypes. Acta Neurol Belg. 2004. March;104(1):13–9. [PubMed] [Google Scholar]
- 207.Amarenco P, Labreuche J, Elbaz A, Touboul PJ, Driss F, Jaillard A, et al. Blood lipids in brain infarction subtypes. Cerebrovasc Dis. 2006;22(2–3):101–8. [DOI] [PubMed] [Google Scholar]
- 208.Amarenco P Effect of statins in stroke prevention. Curr Opin Lipidol. 2005. December;16(6):614–8. [DOI] [PubMed] [Google Scholar]
- 209.Collins R, Armitage J, Parish S, Sleight P, Peto R, Group HPSC. Effects of cholesterol-lowering with simvastatin on stroke and other major vascular events in 20536 people with cerebrovascular disease or other high-risk conditions. Lancet (London, England). 2004. March 6;363(9411):757–67. [DOI] [PubMed] [Google Scholar]
- 210.Amarenco P, Bogousslavsky J, Callahan A 3rd, Goldstein LB, Hennerici M, Rudolph AE, et al. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006. August 10;355(6):549–59. [DOI] [PubMed] [Google Scholar]
- 211.Amarenco P, Benavente O, Goldstein LB, Callahan A 3rd, Sillesen H, Hennerici MG, et al. Results of the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial by stroke subtypes. Stroke. 2009. April;40(4):1405–9. [DOI] [PubMed] [Google Scholar]
- 212.Lauer A, Greenberg SM, Gurol ME. Statins in Intracerebral Hemorrhage. Curr Atheroscler Rep. 2015;17(8):46. [DOI] [PubMed] [Google Scholar]
- 213.Goldstein MR, Mascitelli L, Pezzetta F, Goldstein LB. Hemorrhagic stroke in the stroke prevention by aggressive reduction in cholesterol level study. Neurology. 2009. April 21;72(16):1448–9. [DOI] [PubMed] [Google Scholar]
- 214.Haussen DC, Henninger N, Kumar S, Selim M. Statin Use and Microbleeds in Patients With Spontaneous Intracerebral Hemorrhage. Stroke. 2012. October;43(10):2677–81. [DOI] [PubMed] [Google Scholar]
- 215.Carmichael ST. Emergent properties of neural repair: Elemental biology to therapeutic concepts. Ann Neurol. 2016; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Moskowitz MA, Lo EH, Iadecola C. The science of stroke: mechanisms in search of treatments. Neuron. 2010. July 29;67(2):181–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Lo EH, Rosenberg GA. The neurovascular unit in health and disease: introduction. Stroke. 2009. March;40(3 Suppl):S2–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Itoh Y, Toriumi H, Ebine T, Unekawa M, Yamada S, Konoeda F, et al. Disturbance in neurovascular unit plays a pivotal role in pathophysiology of small vessel disease in the brain. Rinsho Shinkeigaku. 2012;52(11):1365–8. [DOI] [PubMed] [Google Scholar]
- 219.Lo EH, Dalkara T, Moskowitz MA. Mechanisms, challenges and opportunities in stroke. Nat Rev. 2003. May;4(5):399–415. [DOI] [PubMed] [Google Scholar]
- 220.Cheng YD, Al-Khoury L, Zivin JA. Neuroprotection for ischemic stroke: Two decades of success and failure. NeuroRX. 2004. January;1(1):36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Lo EH. Experimental models, neurovascular mechanisms and translational issues in stroke research. Br J Pharmacol. 2008. March;153 Suppl(Suppl 1):S396–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Muir KW, Lees KR. Dose optimization of intravenous magnesium sulfate after acute stroke. Stroke. 1998. May;29(5):918–23. [DOI] [PubMed] [Google Scholar]
- 223.Muir KW, Lees KR, Ford I, Davis S, Investigators IME in S (IMAGES) S. Magnesium for acute stroke (Intravenous Magnesium Efficacy in Stroke trial): randomised controlled trial. Lancet (London, England). 2004. February 7;363(9407):439–45. [DOI] [PubMed] [Google Scholar]
- 224.Hill MD, Martin RH, Mikulis D, Wong JH, Silver FL, Terbrugge KG, et al. Safety and efficacy of NA-1 in patients with iatrogenic stroke after endovascular aneurysm repair (ENACT): a phase 2, randomised, double-blind, placebo-controlled trial. The LancetNeurology. 2012. November;11(11):942–50. [DOI] [PubMed] [Google Scholar]
- 225.Aslanyan S, Weir CJ, Muir KW, Lees KR, Investigators IS. Magnesium for treatment of acute lacunar stroke syndromes: further analysis of the IMAGES trial. Stroke. 2007. April;38(4):1269–73. [DOI] [PubMed] [Google Scholar]
- 226.Saver JL, Starkman S, Eckstein M, Stratton SJ, Pratt FD, Hamilton S, et al. Prehospital use of magnesium sulfate as neuroprotection in acute stroke. N Engl J Med. 2015. February 5;372(6):528–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Bennion DM, Rosado CA, Haltigan EA, Regenhardt RW, Sumners C, Waters MF . Serum activity of angiotensin converting enzyme 2 is decreased in patients with acute ischemic stroke. J Renin-Angiotensin-Aldosterone Syst. 2016. August 3;17(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Regenhardt RW, Desland F, Mecca AP, Pioquinto DJ, Afzal A, Mocco J, et al. Anti-inflammatory effects of angiotensin-(1–7) in ischemic stroke. Neuropharmacology. 2013. August;71:154–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Regenhardt RW, Bennion DM, Sumners C. Cerebroprotective action of angiotensin peptides in stroke. Clin Sci. 2014. February 1;126(3):195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Bennion DM, Haltigan E, Regenhardt RW, Steckelings UM, Sumners C. Neuroprotective mechanisms of the ACE2-angiotensin-(1–7)-Mas axis in stroke. Curr Hypertens Rep. 2015. February;17(2):2–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Regenhardt RW, Mecca AP, Desland F, Ritucci-Chinni PF, Ludin JA, Greenstein D, et al. Centrally administered angiotensin-(1–7) increases the survival of stroke-prone spontaneously hypertensive rats. Exp Physiol. 2014. February 1;99(2):442–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Goldberg MP, Ransom BR. New light on white matter. Stroke. 2003. February;34(2):330–2. [DOI] [PubMed] [Google Scholar]
- 233.Choi DW. Ionic dependence of glutamate neurotoxicity. J Neurosci. 1987. February;7(2):369–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Choi DW. Cerebral hypoxia: some new approaches and unanswered questions. J Neurosci. 1990. August;10(8):2493–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Stys PK, Ransom BR, Waxman SG. Tertiary and quaternary local anesthetics protect CNS white matter from anoxic injury at concentrations that do not block excitability. J Neurophysiol. 1992. January;67(1):236–40. [DOI] [PubMed] [Google Scholar]
- 236.Stys PK. White matter injury mechanisms. Curr Mol Med. 2004. March;4(2):113–30. [DOI] [PubMed] [Google Scholar]
- 237.Imaizumi T, Kocsis JD, Waxman SG. Anoxic injury in the rat spinal cord: pharmacological evidence for multiple steps in Ca(2+)-dependent injury of the dorsal columns. J Neurotrauma. 1997. May;14(5):299–311. [DOI] [PubMed] [Google Scholar]
- 238.Xiao G, Hinman J. Concepts and opportunities for repair in cerebral microvascular disease and white matter stroke. Neural Regen Res. 2016. September;11(9):0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Hinman JD. The back and forth of axonal injury and repair after stroke. Curr Opin Neurol. 2014. November;27(6):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Hinman JD, Lee MD, Tung S, Vinters HV., Carmichael ST. Molecular disorganization of axons adjacent to human lacunar infarcts. Brain. 2015. March;138(3):736–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Sozmen EG, Kolekar A, Havton LA, Carmichael ST. A white matter stroke model in the mouse: Axonal damage, progenitor responses and MRI correlates. J Neurosci Methods. 2009. June 15;180(2):261–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Franklin RJM. Remyelination of the demyelinated CNS: the case for and against transplantation of central, peripheral and olfactory glia. Brain Res Bull. 2002. April;57(6):827–32. [DOI] [PubMed] [Google Scholar]
- 243.Shindo A, Liang AC, Maki T, Miyamoto N, Tomimoto H, Lo EH, et al. Subcortical ischemic vascular disease: Roles of oligodendrocyte function in experimental models of subcortical white-matter injury. J Cereb Blood Flow Metab. 2016. January 29;36(1):187–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Giger RJ, Venkatesh K, Chivatakarn O, Raiker SJ, Robak L, Hofer T, et al. Mechanisms of CNS myelin inhibition: evidence for distinct and neuronal cell type specific receptor systems. Restor Neurol Neurosci. 2008;26(2–3):97–115. [PMC free article] [PubMed] [Google Scholar]
- 245.Sozmen EG, Rosenzweig S, Llorente IL, Ditullio DJ, Machnicki M, Vinters HV. Nogo receptor blockade overcomes remyelination failure after white matter stroke and stimulates functional recovery in aged mice. 2016; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Muresanu DF, Heiss WD, Hoemberg V, Bajenaru O, Popescu CD, Vester JC, et al. Cerebrolysin and Recovery After Stroke (CARS): A Randomized, Placebo-Controlled, Double-Blind, Multicenter Trial. Stroke. 2016. January;47(1):151–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Clarkson AN, Huang BS, Macisaac SE, Mody I, Carmichael ST. Reducing excessive GABA-mediated tonic inhibition promotes functional recovery after stroke. Nature. 2010. November 11;468(7321):305–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Clarkson AN, Overman JJ, Zhong S, Mueller R, Lynch G, Carmichael ST. AMPA receptor-induced local brain-derived neurotrophic factor signaling mediates motor recovery after stroke. J Neurosci. 2011. March 9;31(10):3766–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Donnan GA, O’Malley HM, Quang L, Hurley S, Bladin PF. The capsular warning syndrome: pathogenesis and clinical features. Neurology. 1993. May;43(5):957–62. [DOI] [PubMed] [Google Scholar]
- 250.Fisher CM, Curry HB. Pure Motor Hemiplegia of Vascular Origin. Arch Neurol. 1965. July;13:30–44. [DOI] [PubMed] [Google Scholar]
- 251.Marsh EB, Llinas RH. Stuttering Lacunes: An Acute Role for Clopidogrel? J Neurol Transl Neurosci. 2014;2(1):1035. [PMC free article] [PubMed] [Google Scholar]
- 252.Yaghi S, Kamel H, Elkind MS. Potential new uses of non-vitamin K antagonist oral anticoagulants to treat and prevent stroke. Neurology. 2015. September 22;85(12):1078–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Dobkin BH. Heparin for lacunar stroke in progression. Stroke. 1983;14(3):421–3. [DOI] [PubMed] [Google Scholar]
- 254.Hsia AW, Sachdev HS, Tomlinson J, Hamilton SA, Tong DC. Efficacy of IV tissue plasminogen activator in acute stroke: does stroke subtype really matter? Neurology. 2003. July 8;61(1):71–5. [DOI] [PubMed] [Google Scholar]
- 255.Fluri F, Hatz F, Rutgers MP, Georgiadis D, Sekoranja L, Schwegler G, et al. Intravenous thrombolysis in patients with stroke attributable to small artery occlusion. Eur J Neurol. 2010. August;17(8):1054–60. [DOI] [PubMed] [Google Scholar]
- 256.Mustanoja S, Meretoja A, Putaala J, Viitanen V, Curtze S, Atula S, et al. Outcome by stroke etiology in patients receiving thrombolytic treatment: descriptive subtype analysis. Stroke. 2011. January 1;42(1):102–6. [DOI] [PubMed] [Google Scholar]
- 257.Fuentes B, Martínez-Sánchez P, Alonso de Leciñana M, Egido J, Reig-Roselló G, Díaz-Otero F, et al. Efficacy of intravenous thrombolysis according to stroke subtypes: the Madrid Stroke Network data. Eur J Neurol. 2012. December;19(12):1568–74. [DOI] [PubMed] [Google Scholar]
- 258.Shobha N, Fang J, Hill MD. Do lacunar strokes benefit from thrombolysis? Evidence from the Registry of the Canadian Stroke Network. Int J Stroke. 2013. October 11;8 Suppl A1(SA100):45–9. [DOI] [PubMed] [Google Scholar]
- 259.Pan Y-T, Lee J-D, Lin Y-H, Huang Y-C, Weng H-H, Lee M, et al. Comparisons of outcomes in stroke subtypes after intravenous thrombolysis. Springerplus. 2016. December 20;5(1):47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Chen W, Pan Y, Zhao X, Liu L, Li H, Liao X, et al. Intravenous Thrombolysis in Chinese Patients with Different Subtype of Mild Stroke: Thrombolysis in Patients with Mild Stroke. Sci Rep. 2017. December 23;7(1):2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Eggers CCJ, Bocksrucker C, Seyfang L, Austrian Stroke Unit Registry Collaborators. The efficacy of thrombolysis in lacunar stroke - evidence from the Austrian Stroke Unit Registry. Eur J Neurol. 2017. June;24(6):780–7. [DOI] [PubMed] [Google Scholar]
- 262.Kennedy J, Hill MD, Ryckborst KJ, Eliasziw M, Demchuk AM, Buchan AM, et al. Fast assessment of stroke and transient ischaemic attack to prevent early recurrence (FASTER): a randomised controlled pilot trial. The LancetNeurology. 2007. November;6(11):961–9. [DOI] [PubMed] [Google Scholar]
- 263.Uchiyama S, Demaerschalk BM, Goto S, Shinohara Y, Gotoh F, Stone WM, et al. Stroke prevention by cilostazol in patients with atherothrombosis: meta-analysis of placebo-controlled randomized trials. J Stroke Cerebrovasc Dis. 2009;18(6):482–90. [DOI] [PubMed] [Google Scholar]