Abstract
Objective
This phase I/II study sought to explore intrathecal administration of mesenchymal stem cells (MSCs) as therapeutic approach to multiple system atrophy (MSA).
Methods
Utilizing a dose-escalation design, we delivered between 10 and 200 million adipose-derived autologous MSCs intrathecally to patients with early MSA. Patients were closely followed with clinical, laboratory, and imaging surveillance. Primary endpoints were frequency and type of adverse events; key secondary endpoint was the rate of disease progression assessed by the Unified MSA Rating Scale (UMSARS).
Results
Twenty-four patients received treatment. There were no attributable serious adverse events, and injections were generally well-tolerated. At the highest dose tier, 3 of 4 patients developed low back/posterior leg pain, associated with thickening/enhancement of lumbar nerve roots. Although there were no associated neurologic deficits, we decided that dose-limiting toxicity was reached. A total of 6 of 12 patients in the medium dose tier developed similar, but milder and transient discomfort. Rate of progression (UMSARS total) was markedly lower compared to a matched historical control group (0.40 ± 0.59 vs 1.44 ± 1.42 points/month, p = 0.004) with an apparent dose-dependent effect.
Conclusions
Intrathecal MSC administration in MSA is safe and well-tolerated but can be associated with a painful implantation response at high doses. Compelling dose-dependent efficacy signals are the basis for a planned placebo-controlled trial.
Classification of evidence
This phase I/II study provides Class IV evidence that for patients with early MSA, intrathecal MSC administration is safe, may result in a painful implantation response at high doses, and is associated with dose-dependent efficacy signals.
Multiple system atrophy (MSA) is a relentlessly progressive and invariably fatal neurodegenerative disorder. It is characterized clinically by autonomic failure along with motor dysfunction that may predominantly involve parkinsonism (MSA-P) or cerebellar impairment (MSA-C).1,2 The pathologic hallmark of MSA is glial cytoplasmic α-synuclein inclusions with associated neuronal loss in selective brain regions.3–5 These inclusions are the result of accumulation and aggregation of conformationally changed α-synuclein oligomers, which are thought to be causally linked to disease progression in MSA.6,7
The precise mechanisms leading to neuronal loss are not entirely understood, but there is increasing evidence that deprivation of neurotrophic factors is playing an important role.8 Neuroinflammatory mechanisms such as microglial activation appear to also play a role, particularly in early disease stages.9,10
Considering the rapid progression and grim prognosis of this disease, it comes as no surprise that many attempts have been made to discover a disease course–modifying intervention. Recently, a large, placebo-controlled trial utilizing rifampicin, which had shown great promise in preclinical studies, failed to indicate even a trend at slowing disease progression.11 Other previous trials utilizing growth hormone, minocycline, rasagiline, riluzole, and lithium also were either inconclusive or negative.12–16 The one exception is a double-blind placebo-controlled trial of mesenchymal stem cells (MSCs), which showed significantly slower disease progression in patients treated with intracarotid and intravertebral injections of MSCs followed by IV administration.17
MSCs are known to be capable of differentiating into various cell types under appropriate conditions and to secrete neurotrophic factors exerting neuroprotective effects, including in the transgenic mouse model of MSA.18–26 This neurotrophic support along with well-documented immunomodulatory properties provide compelling scientific rationale for the positive MSC trial, with excitement dampened only by apparent safety concerns, with intra-arterial injections resulting in ischemic lesions.27
In an attempt to develop a safer route of administration along with more widespread CNS access, we sought to evaluate safety and tolerability of intrathecal autologous MSC delivery in patients with MSA, and to explore signals of potential efficacy of this approach.
Methods
Study design
A phase I/II dose-escalation trial of intrathecal injection of autologous MSCs was performed at Mayo Clinic, Rochester, Minnesota. Escalating doses of MSCs were delivered over 3 patient groups with group 1 receiving a single dose of 1 × 107 cells, group 2 receiving 2 doses of 5 × 107 cells each 1 month (±4 days) apart, and group 3 receiving 2 doses of 1 × 108 cells each 1 month apart. Advancement to the next dose cohort occurred after 8 patients had adequately tolerated a dose.
Participants
Patients with early MSA (MSA-C or MSA-P) were required to fulfill consensus criteria for probable MSA and to have at least a moderate degree of autonomic failure based on autonomic function testing. A score ≤17 (omitting the erectile dysfunction score) on part 1 of the Unified MSA Rating Scale (UMSARS) ensured enrollment of patients at a relatively early disease stage.28 Patients were excluded if they scored 24 points or less on the Mini-Mental State Examination, had a clinically significant or unstable medical or surgical condition that might preclude safe completion of the study or might affect the results of the study, had taken any investigational products within 60 days prior to baseline, or had a contraindication for MRI scanning.
Standard protocol approvals, registrations, and patient consents
The study was approved by the Mayo Clinic Institutional Review Board, and written informed consent was obtained from all participants. The trial was registered at ClinicalTrials.gov (NCT02315027).
MSC collection, preparation, and administration
A subcutaneous fat biopsy was taken from the abdomen of each participant, and up to 15 mL of subcutaneous fat was removed. The biopsy tissue was delivered to and processed in Mayo's human cellular therapy laboratory, a cGMP cell processing facility at Mayo Clinic Rochester (Immune Progenitor and Cell Therapeutics [IMPACT]). Cells were expanded ex vivo in a platelet lysate-based media (PLTMax, Mill Creek, Life Sciences, Rochester, MN). Cells were cryopreserved during release criteria analysis, which included testing for phenotype, mycoplasma, culture sterility, and cytogenetic analysis. Cells not meeting release criteria were not administered to the patient. In the event of release criteria failure or lack of adequate cell growth, one further attempt was allowed from a second biopsy. Three to five days prior to infusion, the cells were placed into culture to allow recovery and accurate dosing.
After completion of baseline assessments, all participants were admitted to Mayo's inpatient Clinical Research and Trials Unit (CRTU) the evening before, and remained hospitalized for 48 hours after MSC injection. MSCs were delivered suspended in lactated Ringer’s solution and administered within 12 hours of preparation. A lumbar spinal needle was placed in the subarachnoid space via a standard posterior, intervertebral approach between lumbar level 2 and 5; the specific level was determined individually for each patient based on anatomical considerations. After collection of CSF for baseline analysis, MSCs were infused into the CSF over 1–2 minutes via free-hand delivery by one of the study physicians, followed by a 1 mL flush with lactated Ringer’s. After cell injection, the patient was placed in a 10° Trendelenburg position and rotated side to back to side every 15 minutes for 2 hours to maximize even distribution of cells in the CSF.
Study assessments
Baseline assessments prior to MSC administration included safety laboratory studies, vital signs, general medical and neurologic examination, UMSARS (consisting of part I, which quantifies patients' symptoms and function, and part II, which quantifies findings on neurologic examination), autonomic function testing (including autonomic reflex screen and thermoregulatory sweat test), autonomic symptom assessment (using the Composite Autonomic Severity Scale [COMPASS]–select), as well as MRI of the entire neuraxis.29 In order to quantify autonomic deficits, the Composite Autonomic Severity Score (CASS), a validated instrument to quantify the overall severity and distribution of autonomic failure based on standardized autonomic testing, was derived.30 All clinical assessments were completed by the same investigator (W.S.).
During the stay in the CRTU, participants were closely observed for any adverse events (AEs) during and immediately following MSC injection. Vital signs were monitored every 15 minutes for 1 hour, then hourly for 4 hours, and then every 4 hours until dismissal. Participants were instructed to monitor temperature, blood pressure, and pulse twice daily after discharge for 4 weeks, and to chart these variables in a patient diary along with any AEs for review at subsequent study visits. Participants returned for weekly visits at Mayo's outpatient CRTU for the first 4 weeks after each MSC injection for safety laboratory studies, vital signs, general medical and neurologic examination, spinal fluid collection at 1 and 4 weeks after MSC injection, and MRI of the entire neuraxis at 3 weeks following MSC injection.
After completion of the early follow-up phase, additional late follow-up visits at the outpatient CRTU took place at 6 months and 12 months following the first MSC injection with safety blood collection, recording of AEs, vital signs, general medical and neurologic examination, UMSARS, autonomic function testing (12 months), COMPASS select, and MRI of the entire neuraxis (12 months). Additional phone visits at 3 and 9 months following the first MSC injection gathered information about AEs and UMSARS, part I. Further details of study timeline and assessments are provided in figure 1.
Figure 1. Study timeline.
Primary research question and outcome measures
The primary research question of this study was if intrathecal autologous MSC delivery in patients with early MSA is safe and adequately tolerated (Class IV evidence). The primary outcome measure of this study was therefore the type and frequency of AEs based on above described clinical and imaging data. Secondary outcome measures were designed to explore signals of potential efficacy and included assessments of (1) rate of change from baseline to 12 months (using slope analysis) in UMSARS (UMSARS total, UMSARS I, UMSARS II); (2) change from baseline to 12 months in indices reflecting autonomic symptoms (COMPASS-select) and dysfunction (CASS); (3) change in spinal fluid markers following MSC injections; and (4) survival at 12 and 24 months following the first MSC injection. Spinal fluid markers of interest included cell count, protein, cytology, as well as levels of neurotrophic factors (nerve growth factor [NGF], glial-derived neurotrophic factor [GDNF], and brain-derived neurotrophic factor [BDNF]). Neurotrophic factor levels were measured utilizing a dedicated Millipore (Burlington, MA) Luminex assay.
Statistical analysis
Descriptive statistics were used to describe demographic and parameter characteristics, including mean, SD, frequencies, and percentages as appropriate. Type, frequency, and attribution of AEs were captured using frequencies.
Rate of change in UMSARS scores was calculated for each participant by regressing scores against time (months) to estimate a participant-specific rate of increase in points per month. We used the resulting slope parameter estimate as the response feature for each participant to account for repeated measurements.31 In order to assess for an efficacy signal, we compared progression slopes with participant-specific progression slopes derived from the placebo group (n = 50) of our recently completed rifampicin treatment trial.11 This study had similar inclusion and exclusion criteria, included patients at a similar disease stage, utilized identical disease-specific instruments to assess neurologic function and deficits, and was led by the same senior investigator. We compared baseline characteristics with the 24 MSC-treated patients and found slight differences for UMSARS scores and MSA type (C vs P). We therefore proceeded to match patients on those variables using a computerized algorithm resulting in 22 matched pairs. After matching, there were no baseline differences between the groups (p values ≥0.15, using Wilcoxon rank sum and Fisher exact tests, for all baseline variables, including age, sex, baseline UMSARS scores, baseline COMPASS-select scores, MSA type, and disease duration).
Autonomic indices (COMPASS-select and CASS) were compared between baseline and 12 months. Spinal fluid markers obtained after the study intervention were compared to baseline.
Frequencies were compared using the χ2 or Fisher exact test; group differences were compared using Wilcoxon rank sum test; paired analysis utilized Wilcoxon signed rank test. All statistical tests were 2-sided, and p values < 0.05 were considered statistically significant. Analysis was performed using SAS software version 9.4 (SAS, Cary, NC).
Data availability
Anonymized data will be shared by request from any qualified investigator.
Results
Participants
A total of 27 patients with MSA were enrolled to reach the goal of 24 patients receiving MSC injections. Two patients were excluded from MSC delivery due to chromosomal abnormalities in the stem cell specimen (n = 1) and insufficient cell growth (n = 1) in 2 separate fat biopsy specimens. One patient no longer met inclusion criteria at baseline.
Of the 24 patients receiving intrathecal MSC delivery, all completed the planned 12 months of follow-up. One patient did not return for in-person evaluations at 6 and 12 months but completed follow-up visits via phone calls. There were no dropouts. Baseline characteristics of the 24 patients who received study treatment are summarized in table 1.
Table 1.
Demographic, clinical, autonomic, and autonomic characteristics on enrollment

Primary outcome
Intrathecal injection of autologous MSCs in patients with MSA was found to be safe and generally well-tolerated. There were no attributable serious AEs. A total of 50 AEs were observed related to the underlying disease (n = 7), to spinal taps (n = 9), and to the MSC product (n = 26), and nonspecific AEs attributed as probably or definitely not related to study procedures (n = 8). AEs are further detailed in table 2.
Table 2.
Adverse events, frequency, and attribution

Of particular interest were AEs attributable to the MSC product. None of those occurred among participants in group 1. Two participants (both in group 2) experienced a self-limited febrile reaction less than 10 hours in duration within 24 hours following the MSC injection. One out of 8 patients in group 2 noted fairly symmetric low back/proximal posterior lower extremity discomfort with bending over, coughing, or other maneuvers associated with straining, while all 8 were found to have changes on MRI consisting of clumping, thickening, or mild enhancement of cauda equina nerve roots near the injection site on postgadolinium sequences. Since there were no other associated symptoms or any neurologic deficits, the study proceeded to group 3. After 3 out of 4 patients in that dose group experienced similar but more severe low back/proximal posterior lower extremity discomfort, the decision was made to stop injections at that dose level and to expand group 2 by administering group 2 dosing to the remaining 4 patients who had already been enrolled (resulting in 12 patients in group 2). The additional 4 patients added to group 2 all developed transient low back/proximal posterior lower extremity discomfort, but milder than group 3 participants. The discomfort overall occurred in 8 patients, and was self-limited and resolved within 12 weeks in all but 2 participants who had more persistent low back discomfort. The above described MRI findings were seen in all participants receiving group 2 and 3 dosing and were associated with variable elevations of CSF protein and cell count. An example of the observed nerve root thickening and enhancement is shown in figure 2. CSF protein and cell count before and after MSC injection by dose group is provided in figure 3. Cytology analysis revealed no evidence of malignancy.
Figure 2. MRI illustrating cauda equina findings.
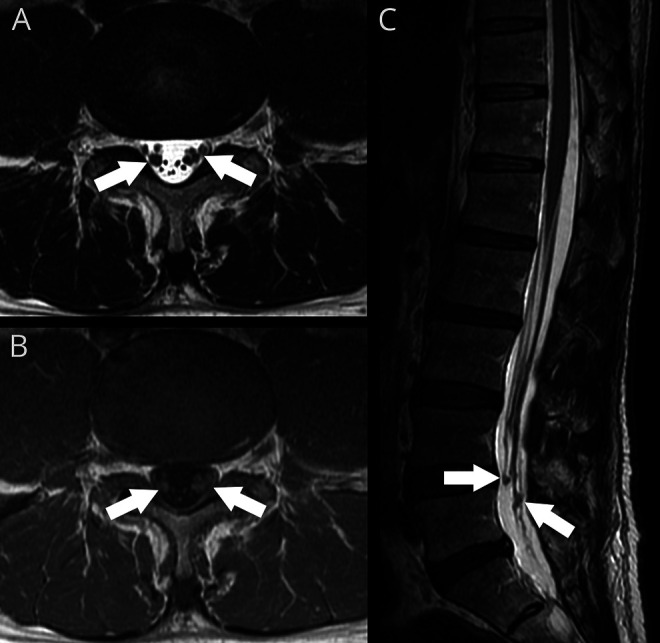
Nerve root thickening, clumping, and enhancement in a 64-year-old patient with multiple system atrophy predominantly involving cerebellar impairment 7 weeks after the first of 2 intrathecal injections of 5 × 107 autologous mesenchymal stem cells at the L4/5 interspace. Although the MRI findings were more prominent than seen in most patients, he remained asymptomatic. (A) Axial T2 image at L4/5 shows thickening of several cauda equina nerve roots. (B) Axial T1 image post gadolinium at the same level shows questionable enhancement. (C) Sagittal T2 image of the lumbar spine shows these changes centered at the site of injection with some distortion and nodular thickening of nerve roots.
Figure 3. Spinal fluid protein and cell count.
CSF protein (top) and cell count (bottom) before and after mesenchymal stem cell (MSC) injection by dose group. The low-dose group showed no significant change in CSF protein (A) and cell count (D), while medium- and high-dose groups showed a variable increase of both CSF protein (B, C) and cell count (E, F) after MSC injection. Injection time points are shown as arrows, dashed lines show the upper limit of normal for CSF protein and cell count.
Secondary outcomes
The rate of disease progression as measured using the rate of change of UMSARS total was markedly lower in MSC treated patients compared to the matched control group (0.40 ± 0.59 vs 1.44 ± 1.42 points/mo, p = 0.004, Wilcoxon rank sum test). This difference was more pronounced for the medium than the low-dose tier, suggesting a dose-dependent effect (figure 4). The small number (n = 4) of observations in the high-dose group did not lend itself to statistical comparisons, but the observed rate of change was highly variable between patients in that group (range −0.29 to 1.00). The rate of change of subcomponents of UMSARS (UMSARS I and UMSARS II) showed similar differences compared to the control group (UMSARS I 0.26 ± 0.35 vs 0.62 ± 0.53 points/month, p = 0.015; UMSARS II 0.16 ± 0.31 vs 0.80 ± 0.92, p = 0.009, figure 4).
Figure 4. Rate of disease progression (Unified MSA Rating Scale [UMSARS]).
Rate of disease progression as measured using UMSARS in mesenchymal stem cell (MSC)–treated patients vs the matched control group. UMSARS total (A–C), UMSARS I (D–F), and UMSARS II (G–I) shown as mean and SD of change per month comparing low dose and medium dose to controls (left panels), and change over time per participant in the low-dose (middle panels) and medium-dose group (right panels). Low-dose group = light blue, medium-dose group = dark blue, controls = red.
Autonomic symptoms and function assessed using COMPASS-select and CASS scores did not change between baseline and 12 months (p = 0.67 and 0.10, respectively; Wilcoxon signed rank test).
NGF was undetectable or only detected at low levels (up to 0.54 pg/mL) at baseline. While there was no significant increase following MSC administration at the lowest dose tier, NGF increased over 100-fold by 1 week after MSC administration with well-detectable levels for all patients in the medium-dose tier. NGF levels decreased but remained markedly elevated 4 weeks after administration (figure 5). NGF increased on average even more so in the few high-dose tier patients, though there was high variability. BDNF and GDNF were undetectable at baseline but became detectable in one patient (13%) in the low-dose group and in 9 (56%) for BDNF and 8 (50%) for GDNF in the medium- and high-dose groups following MSC injection.
Figure 5. Nerve growth factor (NGF) response to mesenchymal stem cell (MSC) injections.
Spinal fluid NGF levels (mean and SD) before and after intrathecal MSC injection (indicated with arrows). NGF was largely undetectable at baseline, showed a slight but nonsignificant rise after the single MSC injection in the low-dose group (light blue), but was elevated dramatically 1 week after each MSC injection in the medium-dose tier (dark blue), with still markedly elevated levels 4 weeks after each injection.
All patients survived beyond the 12 months follow-up period of the study. Extended surveillance of survival shows 21 of the 24 treated patients (88%) still alive at 2 years following the first MSC injections.
Discussion
MSA has a grim prognosis, with a median time from first symptom to death of 8–10 years, median survival from time of diagnosis of about 3 years, and median survival from enrollment in a large national prospective natural history study of only 1.8 years.32,33 There are currently no approved therapies to slow or halt disease progression. Here, we present a phase I/II dose escalation study of adipose-derived autologous MSCs delivered intrathecally to patients with MSA, based on (1) evidence to suggest that deprivation of neurotrophic factors is playing an important role in the pathophysiology of MSA; (2) evidence of the capability of exogenous MSCs to secrete neurotrophic factors exerting neuroprotective effects; and (3) a previous positive double-blind, placebo-controlled trial of intra-arterial and IV autologous MSC treatment of MSA.
Our findings support the results of the previous trial but go beyond supporting evidence that MSCs delivery in MSA is feasible and may have a role in course modification of this rapidly progressive and invariably fatal disease in a number of ways.
First, there is a safety concern about intracarotid and intravertebral artery injections, as illustrated by documentation of ischemic lesions, likely related to microembolization.17,27,34 In an earlier pilot trial, MRI lesions on diffusion-weighted imaging following intra-arterial MSC delivery were seen in as many as 64% of patients.34 The subsequent double-blind trial reported fewer (29% in the active arm) such lesions, yet one participant with multiple such infarcts developed transient neurologic deficits.17 The fact that such lesions occurred at similar frequency (35%) in the placebo group suggests that the lesions were likely related to the angiographic procedure. Patients in those studies also received IV MSC infusions. Although the blood–brain barrier has been shown to be less tight in MSA with impairment correlating with the severity of disease, the first passage through lung capillaries following venous infusion along with the remaining blood–brain barrier would be expected to comprise major hurdles in MSC access to the CNS.35
Our trial demonstrated intrathecal delivery of autologous MSCs to be safe and well-tolerated as long as the dose per administration was less than 1 × 108 cells per administration. The positional low back and posterior lower extremity discomfort observed at the medium-dose tier was generally mild and short-lived, and was in none of our participants, including those in the high-dose tier, associated with neurologic deficits. The MRI findings of thickening, clumping, or mild enhancement of cauda equina nerve roots in the area of MSC injection were seen in every patient in the medium- and high-dose tier, yet only half of the patients noted an associated low-back discomfort. With the elevated CSF protein and cell count in many of the cases and lack of neurologic deficits, the underlying mechanism is likely a reactive implantation response to MSCs, which in more severe cases may take on characteristics of reactive arachnoiditis. This phenomenon remains the subject of active research, and we anticipate being able to provide a more definitive answer as to the pathologic correlate as more autopsy material becomes available.
Second, intra-arterial delivery in carotid and vertebral arteries limits MSC delivery to the brain, while a significant burden of the disease includes the spinal cord, including intermediolateral cell column and Onuf nucleus.4,5 Intrathecal delivery of MSCs allows for more widespread delivery of MSCs across the blood–brain barrier and to the CNS. This more widespread access to the sites of α-synuclein deposition and neuronal loss is more than a theoretical advantage considering strong support for target engagement of MSCs in various disease states. For example, MSCs infused into the intrathecal space of rats were shown to migrate to the site of experimental spinal cord injury.20,25,36,37 In several experimental models, including primates, MSCs have been shown to survive and reside in the brain and spinal cord for up to 3 months after injection.19,38–40 The cells appear to intercalate between resident neural cells and maintain their typical morphology and surface marker expression.
Third, we could show that patients treated with intrathecal delivery of MSCs had a slower than expected rate of disease progression in a dose-dependent fashion. In spite of the open-label nature of this dose-escalation study, the apparent dose-dependent effect on the rate of disease progression utilizing a well-established and validated clinical instrument is further supported by (1) the comparison with patients matched on baseline disease characteristics derived from the placebo group of a recently completed double-blind controlled trial that utilized similar inclusion and exclusion criteria and studied patients at a similar disease stage; (2) a similar dose-dependent effect observed for subcomponents of that instrument; and (3) the survival data, showing that all patients in our study survived the 12 months of follow-up, which contrasts with most other MSA trials, including the rifampicin treatment trial, where 5 of the 50 patients (10%) in the placebo group died during the 48 weeks of follow-up.11 That being said, the possibility of some placebo effect related to the invasive and extensive study procedures cannot be excluded.
Finally, the observed clinical effect was associated with a marked rise in neurotrophic factors in the spinal fluid documented to be present for at least 4 weeks following MSC administration. This effect was also dose-dependent and convincingly seen only in the medium- and high-dose tiers, though concentration of most neurotrophic factors was just at or below detection threshold for the baseline samples. Even more sensitive assays are currently in development. Although documentation of a rise in neurotrophic factors following MSC administration supports one of the potential mechanisms by which MSCs may positively influence the disease course in MSA, further work is needed to delineate pathophysiologic mechanisms in MSA and the role of MSCs in affecting those mechanisms. Besides the deprivation of growth factors as a result of glial dysfunction, inflammatory mechanisms, including microglial activation and resulting oxidative stress, appear to be an important part of the pathophysiologic cascade and may be positively influenced by MSCs through their immunomodulatory properties.10,26,41–43 An effect on endogenous stem cells with resulting change of the target microenvironment is another intriguing concept.44
Although the precise mechanisms by which MSCs may positively influence the disease course in MSA remain only partially understood, there is increasingly convincing evidence based on preclinical studies and clinical trials that MSCs have an intriguing potential of modifying the disease course of MSA. Our study contributes to this evidence and adds information about the safety and tolerability of the intrathecal administration route, which we could demonstrate to be adequate, particularly for doses below 108 cells per dose. Further work is needed to define the optimal timing of repeated injections, to explore long-term efficacy, to better understand the imaging findings near the injection site, to document target engagement, and to further define the mechanism of action. Work in all these areas is currently ongoing.
In spite of this additional work needed, the available evidence combined with the poor prognosis of this disease for which no disease-modifying therapy exists postulate to simultaneously proceed with a randomized, placebo-controlled trial to provide proof of efficacy of this approach.
This study established adequate safety and tolerability of intrathecal injection of autologous, adipose-derived MSCs in MSA. In spite of its open-label nature, clinical and surrogate efficacy signals support a future phase II/III trial.
Acknowledgment
The authors thank IMPACT and Dr. Dietz for preliminary work and contribution of the cellular therapy used in this trial.
Glossary
- AE
adverse event
- BDNF
brain-derived neurotrophic factor
- CASS
Composite Autonomic Severity Score
- COMPASS
Composite Autonomic Severity Scale
- CRTU
Clinical Research and Trials Unit
- GDNF
glial-derived neurotrophic factor
- MSA
multiple system atrophy
- MSA-C
multiple system atrophy predominantly involving cerebellar impairment
- MSA-P
multiple system atrophy predominantly involving parkinsonism
- MSC
mesenchymal stem cell
- NGF
nerve growth factor
- UMSARS
Unified MSA Rating Scale
Appendix. Authors


Footnotes
Comment, page 25
Class of Evidence: NPub.org/coe
Study funding
This publication was made possible by NIH (P01NS44233, U54NS065736, K23NS075141, R01NS092625, UL1TR000135), FDA (R01FD004789), Cure MSA Foundation, and support from Mayo's Center for Regenerative Medicine. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH or FDA.
Disclosure
W. Singer reports no disclosures relevant to the manuscript. A. Dietz is an inventor of technology used as a tool in this research; the technology has been licensed to a commercial entity (Mill Creek LifeScienes). Dr. Dietz and Mayo Clinic have equity in the company and have contractual rights to receive royalties from the licensing of this technology. This research is not intended to directly enhance the value of this technology but the value of the technology may be enhanced as a consequence of the research. Dr. Dietz has governance responsibilities within this company but does not participate in his role at Mayo with any interaction with the company. These conflicts have been disclosed previously and these comments are included here as directed by the Mayo Clinic Conflict of Interest Board. A. Zeller, T. Gehrking, J. Schmelzer, A. Schmeichel, J. Gehrking, M. Suarez, D. Sletten, K. Minota Pacheco, E. Coon, P. Sandroni, E. Benarroch, R. Fealey, J. Matsumoto, J. Bower, A. Hassan, A. McKeon, A. Windebank, J. Mandrekar, and P. Low report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Gilman S, Low PA, Quinn N, et al. Consensus statement on the diagnosis of multiple system atrophy. J Auton Nerv Syst 1998;74:189–192. [PubMed] [Google Scholar]
- 2.Gilman S, Wenning GK, Low PA, et al. Second consensus conference on the diagnosis of multiple system atrophy. Neurology 2008;71:670–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benarroch EE, Schmeichel AM, Parisi JE. Depletion of mesopontine cholinergic and sparing of raphe neurons in multiple system atrophy. Neurology 2002;59:944–946. [DOI] [PubMed] [Google Scholar]
- 4.Dickson DW, Liu W, Hardy J, et al. Widespread alterations of alpha-synuclein in multiple system atrophy. Am J Pathol 1999;155:1241–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wakabayashi K, Takahashi H. Cellular pathology in multiple system atrophy. Neuropathology 2006;26:338–345. [DOI] [PubMed] [Google Scholar]
- 6.Rockenstein E, Ubhi K, Inglis C, et al. Neuronal to oligodendroglial alpha-synuclein redistribution in a double transgenic model of multiple system atrophy. Neuroreport 2012;23:259–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tong J, Wong H, Guttman M, et al. Brain alpha-synuclein accumulation in multiple system atrophy, Parkinson's disease and progressive supranuclear palsy: a comparative investigation. Brain 2010;133:172–188. [DOI] [PubMed] [Google Scholar]
- 8.Ubhi K, Rockenstein E, Mante M, et al. Neurodegeneration in a transgenic mouse model of multiple system atrophy is associated with altered expression of oligodendroglial-derived neurotrophic factors. J Neurocsci 2010;30:6236–6246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ishizawa K, Komori T, Sasaki S, Arai N, Mizutani T, Hirose T. Microglial activation parallels system degeneration in multiple system atrophy. J Neuropathol Exp Neurol 2004;63:43–52. [DOI] [PubMed] [Google Scholar]
- 10.Stefanova N, Reindl M, Neumann M, Kahle PJ, Poewe W, Wenning GK. Microglial activation mediates neurodegeneration related to oligodendroglial alpha-synucleinopathy: implications for multiple system atrophy. Mov Disord 2007;22:2196–2203. [DOI] [PubMed] [Google Scholar]
- 11.Low PA, Robertson D, Gilman S, et al. Efficacy and safety of rifampicin for multiple system atrophy: a randomised, double-blind, placebo-controlled trial. Lancet Neurol 2014;13:268–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bensimon G, Ludolph A, Agid Y, Vidailhet M, Payan C, Leigh PN. Riluzole treatment, survival and diagnostic criteria in Parkinson plus disorders: the NNIPPS study. Brain 2009;132:156–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dodel R, Spottke A, Gerhard A, et al. Minocycline 1-year therapy in multiple-system-atrophy: effect on clinical symptoms and [(11)C] (R)-PK11195 PET (MEMSA-trial). Mov Disord 2010;25:97–107. [DOI] [PubMed] [Google Scholar]
- 14.Holmberg B, Johansson JO, Poewe W, et al. Safety and tolerability of growth hormone therapy in multiple system atrophy: a double-blind, placebo-controlled study. Mov Disord 2007;22:1138–1144. [DOI] [PubMed] [Google Scholar]
- 15.Poewe W, Seppi K, Fitzer-Attas CJ, et al. Efficacy of rasagiline in patients with the parkinsonian variant of multiple system atrophy: a randomised, placebo-controlled trial. Lancet Neurol 2015;14:145–152. [DOI] [PubMed] [Google Scholar]
- 16.Saccà F, Marsili A, Quarantelli M, et al. A randomized clinical trial of lithium in multiple system atrophy. J Neurol 2013;260:458–461. [DOI] [PubMed] [Google Scholar]
- 17.Lee PH, Lee JE, Kim HS, et al. A randomized trial of mesenchymal stem cells in multiple system atrophy. Ann Neurol 2012;72:32–40. [DOI] [PubMed] [Google Scholar]
- 18.Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem 2006;98:1076–1084. [DOI] [PubMed] [Google Scholar]
- 19.Isakova IA, Baker K, Dufour J, Gaupp D, Phinney DG. Preclinical evaluation of adult stem cell engraftment and toxicity in the CNS of rhesus macaques. Mol Ther 2006;13:1173–1184. [DOI] [PubMed] [Google Scholar]
- 20.Ohta M, Suzuki Y, Noda T, et al. Bone marrow stromal cells infused into the cerebrospinal fluid promote functional recovery of the injured rat spinal cord with reduced cavity formation. Exp Neurol 2004;187:266–278. [DOI] [PubMed] [Google Scholar]
- 21.Park HJ, Bang G, Lee BR, Kim HO, LP H. Neuroprotective effect of human mesenchymal stem cells in an animal model of double toxin-induced multiple system atrophy-parkinsonism. Cell Transpl 2011;20:827–835. [DOI] [PubMed] [Google Scholar]
- 22.Park HJ, Lee PH, Bang OY, Lee G, Ahn YH. Mesenchymal stem cells therapy exerts neuroprotection in a progressive animal model of Parkinson's disease. J Neurochem 2008;107:141–151. [DOI] [PubMed] [Google Scholar]
- 23.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science 1999;284:143–147. [DOI] [PubMed] [Google Scholar]
- 24.Sanchez-Ramos J, Song S, Cardozo-Pelaez F, et al. Adult bone marrow stromal cells differentiate into neural cells in vitro. Exp Neurol 2000;164:247–256. [DOI] [PubMed] [Google Scholar]
- 25.Satake K, Lou J, Lenke LG. Migration of mesenchymal stem cells through cerebrospinal fluid into injured spinal cord tissue. Spine 2004;29:1971–1979. [DOI] [PubMed] [Google Scholar]
- 26.Stemberger S, Jamnig A, Stefanova N, Lepperdinger G, Reindl M, Wenning GK. Mesenchymal stem cells in a transgenic mouse model of multiple system atrophy: immunomodulation and neuroprotection. PLoS One 2011;6:e19808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Low PA, Gilman S. Are trials of intravascular infusions of autologous mesenchymal stem cells in patients with multiple system atrophy currently justified, and are they effective? Ann Neurol 2012;72:4–5. [DOI] [PubMed] [Google Scholar]
- 28.Wenning GK, Tison F, Seppi K, et al. Development and validation of the Unified Multiple System Atrophy Rating Scale (UMSARS). Mov Disord 2004;19:1391–1402. [DOI] [PubMed] [Google Scholar]
- 29.Lipp A, Sandroni P, Ahlskog JE, et al. Prospective differentiation of multiple system atrophy from Parkinson's disease, with and without autonomic failure. Arch Neurol 2009;66:742–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Low PA. Composite autonomic scoring scale for laboratory quantification of generalized autonomic failure. Mayo Clin Proc 1993;68:748–752. [DOI] [PubMed] [Google Scholar]
- 31.Dupont WD. Statistical Modeling for Biomedical Researchers. Cambridge: Cambridge University Press; 2002. [Google Scholar]
- 32.Low PA, Reich SG, Jankovic J, et al. Natural history of multiple system atrophy in the USA: a prospective cohort study. Lancet Neurol 2015;14:710–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coon EA, Sletten DM, Suarez MD, et al. Clinical features and autonomic testing predict survival in multiple system atrophy. Brain 2015;138:3623–3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee PH, Kim JW, Bang OY, Ahn YH, Joo IS, Huh K. Autologous mesenchymal stem cell therapy delays the progression of neurological deficits in patients with multiple system atrophy. Clin Pharmacol Ther 2008;83:723–730. [DOI] [PubMed] [Google Scholar]
- 35.Song SK, Lee SK, Lee JJ, et al. Blood-brain barrier impairment is functionally correlated with clinical severity in patients of multiple system atrophy. Neurobiol Aging 2011;32:2183–2189. [DOI] [PubMed] [Google Scholar]
- 36.Jin K, Sun Y, Xie L, et al. Comparison of ischemia-directed migration of neural precursor cells after intrastriatal, intraventricular, or intravenous transplantation in the rat. Neurobiol Dis 2005;18:366–374. [DOI] [PubMed] [Google Scholar]
- 37.Nishida K, Tanaka N, Nakanishi K, et al. Magnetic targeting of bone marrow stromal cells into spinal cord: through cerebrospinal fluid. Neuroreport 2006;17:1269–1272. [DOI] [PubMed] [Google Scholar]
- 38.Deng YB, Liu XG, Liu ZG, Liu XL, Liu Y, Zhou GQ. Implantation of BM mesenchymal stem cells into injured spinal cord elicits de novo neurogenesis and functional recovery: evidence from a study in rhesus monkeys. Cytotherapy 2006;8:210–214. [DOI] [PubMed] [Google Scholar]
- 39.Hofstetter CP, Schwarz EJ, Hess D, et al. Marrow stromal cells form guiding strands in the injured spinal cord and promote recovery. Proc Natl Acad Sci USA 2002;99:2199–2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ono K, Yoshihara K, Suzuki H, et al. Preservation of hematopoietic properties in transplanted bone marrow cells in the brain. J Neurosci Res 2003;72:503–507. [DOI] [PubMed] [Google Scholar]
- 41.Frank MH, Sayegh MH. Immunomodulatory functions of mesenchymal stem cells. Lancet 2004;363:1411–1412. [DOI] [PubMed] [Google Scholar]
- 42.Le Blanc K. Immunomodulatory effects of fetal and adult mesenchymal stem cells. Cytotherapy 2003;5:485–489. [DOI] [PubMed] [Google Scholar]
- 43.Le Blanc K, Ringdén O. Immunomodulation by mesenchymal stem cells and clinical experience. J Intern Med 2007;262:509–525. [DOI] [PubMed] [Google Scholar]
- 44.Joyce N, Annett G, Wirthlin L, Olson S, Bauer G, Nolta JA. Mesenchymal stem cells for the treatment of neurodegenerative disease. Regen Med 2010;5:933–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data will be shared by request from any qualified investigator.






