Short abstract
Background
The concept of mixed adenoneuroendocrine carcinoma (MANEC) was introduced in the 2010 World Health Organization classification of digestive neuroendocrine neoplasms. Bile duct invasion by MANEC is exceptionally rare. We herein report a case of MANEC with invasion of multiple bile ducts.
Case presentation: A 60-year-old man presented with a 7-day history of upper abdominal pain, and a mass in the cystic duct was suspected based on computed tomography findings. The patient underwent resection of the extrahepatic bile ducts with concomitant radical lymphadenectomy and Roux-en-Y cholangiojejunostomy. Large cell neuroendocrine carcinoma was detected in a component of the resected tumor. According to the pathological and immunohistochemical features of the tumor, the final histopathological diagnosis was a biliary MANEC, tumor stage T2N0M1 (Stage IIIC). The patient recovered uneventfully and was discharged from the hospital 10 days after surgery.
Conclusions
We have described a rare case of extrahepatic MANEC invading multiple bile ducts, with particular emphasis on the physician’s awareness of MANEC and its optimal treatment. MANEC arising from extrahepatic bile ducts is rare, and surgical resection is the most effective treatment method.
Keywords: Mixed adenoneuroendocrine carcinoma, neuroendocrine carcinoma, adenocarcinoma, common bile duct, common hepatic duct, surgical resection
Background
Mixed adenoneuroendocrine carcinomas (MANECs) are defined as composite neoplasms with areas of adenocarcinoma or squamous cell carcinoma intermingled with neuroendocrine carcinoma (NEC) or neuroendocrine tumor (NET), each comprising at least 30% of the neoplasm.1 Since the concept of MANEC was introduced in 2010,1 cases of MANEC have been continuously reported. MANECs usually develop in the colon or stomach2–4; those in the bile ducts are exceptionally rare. In one report, MANEC originating from the extrahepatic bile duct was poorly differentiated, malignant, and invasive.5 To our knowledge, no reports have described simultaneous invasion of MANEC into the cystic duct, common bile duct (CBD), and common hepatic duct (CHD). We herein describe a biliary MANEC with invasion of multiple bile ducts and review the current literature of MANECs.
Case presentation
A 60-year-old man presented with a 1-week history of abdominal distension. The patient had no family history of cancer. His relevant medical history included a 2-year duration of type 2 diabetes mellitus and a myocardial infarction 10 years previously. Physical examination revealed mild jaundice. Laboratory data showed an aspartate aminotransferase concentration of 262.7 U/L [reference range (RR): 15.0–40.0 U/L], alanine aminotransferase of 413.1 U/L (RR: 9.0–50.0 U/L), Y-glutamyl transpeptidase of 1105.7 U/L (RR: 10.0–60.0 U/L), alkaline phosphatase of 499.5 U/L (RR: 45.0–125.0 U/L), total bilirubin of 149.5 µmol/L (RR: 6.8–30.0 µmol/L), conjugated bilirubin of 92.1 µmol/L (RR: 0.0–8.6 µmol/L), and unconjugated bilirubin of 57.4 µmol/L (RR: 5.1–21.4 µmol/L). Tumor biomarker measurement showed a carbohydrate antigen 19-9 concentration of 109.91 U/mL (RR: <37.00 U/mL) and no abnormalities in all others, including carcinoembryonic antigen. All other laboratory data were within RRs. Abdominal triple-phase contrast computed tomography (CT) showed a well-defined mass in the cystic duct, distal CHD, and proximal CBD as well as mild dilatation of the biliary tree (Figure 1). The patient did not undergo magnetic response cholangiopancreatography to save time and avoid a further increase in the bilirubin concentration. Resection of the extrahepatic bile ducts with concomitant radical lymphadenectomy and Roux-en-Y cholangiojejunostomy was performed. Pathologic examination of the resected specimen revealed no tumor cells in the margins of the CHD or CBD. No adverse events occurred intraoperatively. The patient’s postoperative course was uneventful, and he was discharged on the 10th postoperative day.
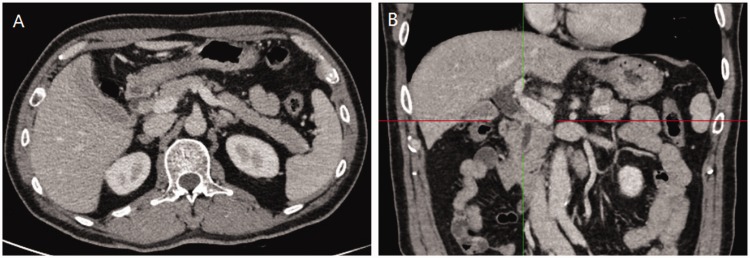
Figure 1.
Abdominal computed tomography (CT) images at the delayed phase. (a) Abdominal CT showed a low-density mass measuring 15 mm located in the common bile duct. (b) Coronal CT reconstructed image showed a striped well-defined mass in the common bile duct.
Pathologic findings
Macroscopic examination showed a cauliflower-like, solid mass measuring 1.7 × 1.5 × 0.3 cm in the distal CHD, proximal CBD, and cystic duct. No gallstones were identified in either the gallbladder or bile ducts. Histological examination revealed two cell populations. Moderately to poorly differentiated adenocarcinoma comprised the main component (approximately 65% of the whole tumor). The other component was large cell NEC (>30% of the whole tumor). The neuroendocrine component was arranged a nest pattern distributed into the adenocarcinoma component, without regularity in the arrangement (Figure 2(a)). The NET cells showed cytomorphologic features of oval cells, abundant eosinophilic cytoplasm, and partial vacuolation of the nucleus (Figure 2(b)). The mitotic rate of cancer cells was 10 to 20 cells per 10 high-power fields (Figure 2(c)). Cancer cells were detected in the lymph nodes (No. 12) adjacent to the cystic duct, while the other regional lymph nodes (Nos. 8, 9, and 13) were free of cancer cells. Immunohistochemically, the Ki-67 labeling index was 70% in the whole tumor, indicating strong proliferative activity (Figure 2(d)). The large cell NEC component was positive for chromogranin A (CgA) and synaptophysin (Syn), while the adenocarcinoma components were negative for CgA and Syn (Figure 2(e), (f)). Taken together, the CT scan and gross anatomical examination revealed a biliary tumor in the distal CHD, proximal CBD, and cystic duct. Histological examination and immunophenotyping confirmed the diagnosis of biliary MANEC. The final pathological diagnosis was T2N1M0 (Stage IIIC) according to the 2010 World Health Organization classification.
Figure 2.
Histological findings and immunophenotyping. (a) Moderately to poorly differentiated adenocarcinoma admixed with large cell neuroendocrine carcinoma [hematoxylin and eosin (HE) staining, ×40]. (b) The neuroendocrine component was arranged in a nest pattern, and the nucleus was partially vacuolated. Cytomorphologically, the neuroendocrine carcinoma cells were oval and had abundant eosinophilic cytoplasm (HE staining, ×200). (c) The neuroendocrine carcinoma cells show a high mitotic rate (HE staining, ×400). (d) Representative image of Ki-67-positive cells, indicating high proliferation in the whole tumor (immunohistochemical staining, ×100). (e) The neuroendocrine carcinoma components were strongly positive for chromogranin A (immunohistochemical staining, ×200). (f) The neuroendocrine carcinoma components were strongly positive for synaptophysin (immunohistochemical staining, ×100).
Discussion and conclusions
Biliary MANEC is extremely rare, and only a few cases have been reported to date.6 According to the latest World Health Organization classification, MANEC is graded as an NET.1 The most common histologic subtype of NET of the extrahepatic bile ducts is small cell NET; only a few cases of large cell NET of the CBD have been reported.7 The most frequent sites of extrahepatic biliary NETs are the CBD and the distal CBD (19.2%), followed by the middle CBD (17.9%), the cystic duct (16.7%), and the proximal CBD (11.5%).8 Previously reported cases of MANEC have described localization of the tumor at either the extrahepatic biliary duct or intrahepatic biliary duct.9,10 With respect to the growth pattern of the MANEC components within the tumor, the adenocarcinoma component is generally located in the superficial portion of the neoplasm, while the NEC component is mainly located in the deeper portion of the tumor.7,11
In the present case of an extrahepatic MANEC, the tumor was growing in the cystic duct, CBD, and CHD simultaneously. The NEC components showed a nest pattern and were distributed separately in the deeper portion of the tumor. The large cell NEC component accounted for >30% of the whole tumor. To the best of our knowledge, no such case has been previously reported.
Because of the absence of specific serum markers, preoperative diagnosis of MANEC is difficult.12 A preoperative diagnosis may be made by examining brush cytology specimens, but this method has a high false-negative rate and is not a conventional method.13 Contrast CT is a useful tool for diagnosis of cholangiocarcinoma, which is enhanced only in the delayed phase.14 Immunohistochemistry is the main diagnostic method for MANECs.12 Because both the small and large cell pure neuroendocrine components are diffusely positive for Syn and usually for CgA, at least two of three commonly used neuroendocrine markers (Syn, CgA, or CD56) must be abundantly expressed to establish a diagnosis of high-grade MANEC.1 Measurement of neuron-specific enolase is generally excluded because of its poor specificity. In the present case, both Syn and CgA showed strong immunohistochemical positivity in the nest growth pattern of the NEC components but negativity in the adenocarcinoma components, confirming the diagnosis of MANEC.
The prognosis of MANECs has not been thoroughly studied because of the rarity of this tumor.10 However, the prognosis of NEC of the bile duct appears to be poor. In one case report and literature review,7 57% (12/21) of affected patients died from 3 to 20 months postoperatively, and only two patients reportedly survived for >2 years. In addition, NEC in the biliary system has a high incidence of distant metastasis.7 The prognosis might be related to the proliferative fraction of the tumor. In one case, a patient with MANEC survived for 45 months after surgery because the tumor showed a low proliferative fraction (Ki-67 labeling index of 9.6%).15 In fact, the proliferation rate has been shown to provide significant prognostic information in patients with NEC.16 In terms of therapy for MANECs, surgical resection is the most effective treatment method. Multidisciplinary treatment consisting of preoperative chemotherapy, adjuvant or neoadjuvant chemotherapy, and radiation therapy may prolong the survival of patients with MANEC.7,17
In conclusion, we have herein reported a rare case of a biliary MANEC extensively infiltrating the extrahepatic bile ducts, with particular emphasis on the physician’s awareness of MANEC and its optimal treatment. MANEC arising from the extrahepatic bile duct is rare, and surgical resection is the most effective treatment method. Additional studies are required to more conclusively define the optimal management in terms of preoperative diagnosis and therapy for patients with MANECs to achieve improved outcomes.
Abbreviations
MANEC: mixed adenoneuroendocrine carcinoma; CBD: common bile duct; CHD: common hepatic duct; RR: reference range; CT: computed tomography; CgA: chromogranin A; Syn: synaptophysin; NET: neuroendocrine tumor; NEC neuroendocrine carcinoma.
Authors’ contributions
H-W Z, JQ, KK, and E-B X contributed to the data collection and analysis. MW contributed to the data analysis and interpretation and drafting of the manuscript. YL contributed to the data interpretation and critical revision of the manuscript for important intellectual content. G-Y L and G-Y W contributed to the data analysis and interpretation.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Ethics approval and consent to participate
The collection of informed consent (according to World Medical Association Declaration of Helsinki) was performed at The First Hospital of Jilin University. The study was approved by the Institutional Review Board at the First Hospital of Jilin University.
The patient provided written informed consent for publication of his clinical details and clinical images.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Rindi G, Petrone G, Inzani F. The 2010 WHO classification of digestive neuroendocrine neoplasms: a critical appraisal four years after its introduction. Endocr Pathol 2014; 25: 186–192. DOI: 10.1007/s12022-014-9313-z. [DOI] [PubMed] [Google Scholar]
- 2.Li Y, Yau A, Schaeffer D, et al. Colorectal glandular-neuroendocrine mixed tumor: pathologic spectrum and clinical implications. Am J Surg Pathol 2011; 35: 413–425. DOI: 10.1097/PAS.0b013e3182093657. [DOI] [PubMed] [Google Scholar]
- 3.Liang J, Liu S. [Clinicopathological features of the primary gastric neuroendocrine neoplasms]. Zhonghua Zhong Liu Za Zhi 2014; 36: 522–528. [PubMed] [Google Scholar]
- 4.Paspala A, Machairas N, Prodromidou A, et al. Management of MANEC of the colon and rectum: a comprehensive review of the literature. Mol Clin Oncol 2018; 9: 219–222. DOI: 10.3892/mco.2018.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang L, DeMay RM. Cytological features of mixed adenoneuroendocrine carcinoma of the ampulla: two case reports with review of literature. Diagn Cytopathol 2014; 42: 1075–1084. DOI: 10.1002/dc.23107. [DOI] [PubMed] [Google Scholar]
- 6.Acosta AM, Wiley EL. Primary biliary mixed adenoneuroendocrine carcinoma (MANEC): a short review. Arch Pathol Lab Med 2016; 140: 1157–1162. DOI: 10.5858/arpa.2015-0102-RS. [DOI] [PubMed] [Google Scholar]
- 7.Sasatomi E, Nalesnik MA, Marsh JW. Neuroendocrine carcinoma of the extrahepatic bile duct: case report and literature review. World J Gastroenterol 2013; 19: 4616–4623. DOI: 10.3748/wjg.v19.i28.4616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hosoda K, Kobayashi A, Shimizu A, et al. Neuroendocrine tumor of the common bile duct. Surgery 2016; 160: 525–526. DOI: 10.1016/j.surg.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 9.Michalopoulos N, Papavramidis TS, Karayannopoulou G, et al. Neuroendocrine tumors of extrahepatic biliary tract. Pathol Oncol Res 2014; 20: 765–775. DOI: 10.1007/s12253-014-9808-4. [DOI] [PubMed] [Google Scholar]
- 10.Zheng SL, Yip VS, Pedica F, et al. Intrahepatic bile duct mixed adenoneuroendocrine carcinoma: a case report and review of the literature. Diagn Pathol 2015; 10: 204. DOI: 10.1186/s13000-015-0439-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harada K, Sato Y, Ikeda H, et al. Clinicopathologic study of mixed adenoneuroendocrine carcinomas of hepatobiliary organs. Virchows Arch 2012; 460: 281–289. DOI: 10.1007/s00428-012-1212-4. [DOI] [PubMed] [Google Scholar]
- 12.Marsh Rde W, Alonzo M, Bajaj S, et al. Comprehensive review of the diagnosis and treatment of biliary tract cancer 2012. Part I: diagnosis-clinical staging and pathology. J Surg Oncol 2012; 106: 332–338. DOI: 10.1002/jso.23028. [DOI] [PubMed] [Google Scholar]
- 13.Noronha YS, Raza AS. Well-differentiated neuroendocrine (carcinoid) tumors of the extrahepatic biliary ducts. Arch Pathol Lab Med 2010; 134: 1075–1079. DOI: 10.1043/2008-0764-RS.1. [DOI] [PubMed] [Google Scholar]
- 14.Hennedige TP, Neo WT, Venkatesh SK. Imaging of malignancies of the biliary tract- an update. Cancer Imaging 2014; 14: 14. DOI: 10.1186/1470-7330-14-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edakuni G, Sasatomi E, Satoh T, et al. Composite glandular-endocrine cell carcinoma of the common bile duct. Pathol Int 2001; 51: 487–490. [DOI] [PubMed] [Google Scholar]
- 16.Klimstra DS, Modlin IR, Coppola D, et al. The pathologic classification of neuroendocrine tumors: a review of nomenclature, grading, and staging systems. Pancreas 2010; 39: 707–712. DOI: 10.1097/MPA.0b013e3181ec124e. [DOI] [PubMed] [Google Scholar]
- 17.Kihara Y, Yokomizo H, Urata T, et al. A case report of primary neuroendocrine carcinoma of the perihilar bile duct. BMC Surg 2015; 15: 125. DOI: 10.1186/s12893-015-0116-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.