Abstract
The unique cumulative nature of human culture has often been explained by high-fidelity copying mechanisms found only in human social learning. However, transmission chain experiments in human and non-human primates suggest that cumulative cultural evolution (CCE) might not necessarily depend on high-fidelity copying after all. In this study, we test whether defining properties of CCE can emerge in a non-copying task. We performed transmission chain experiments in Guinea baboons and human children where individuals observed and produced visual patterns composed of four squares on touchscreen devices. In order to be rewarded, participants had to avoid touching squares that were touched by a previous participant. In other words, they were rewarded for innovation rather than copying. Results nevertheless exhibited fundamental properties of CCE: an increase over generations in task performance and the emergence of systematic structure. However, these properties arose from different mechanisms across species: children, unlike baboons, converged in behaviour over generations by copying specific patterns in a different location, thus introducing alternative copying mechanisms into the non-copying task. In children, prior biases towards specific shapes led to convergence in behaviour across chains, while baboon chains showed signs of lineage specificity. We conclude that CCE can result from mechanisms with varying degrees of fidelity in transmission and thus that high-fidelity copying is not necessarily the key to CCE.
Keywords: social learning, iterated learning, transmission chain, cumulative cultural evolution, primate behaviour, comparative cognition
1. Introduction
Almost every aspect of human culture evolves through time with the gradual accumulation of modifications, from stories [1] to paintings [2], social norms [3] and language [4]. In sharp contrast, it has proved extremely difficult to find evidence of cumulative culture in other animals (but see [5–9] for potential examples) or to induce cumulative culture in other species through experimental manipulations [10] (but see [11,12] for potential examples). One of the main reasons invoked to explain this sharp contrast between human and non-human animal cultures is the low copying fidelity in non-human animals' social learning [13–19]; faithful transmission can prevent the loss of cultural modifications and therefore result in cultural accumulation [14]. The ability to faithfully transmit information through high-fidelity social learning has therefore been taken as a requirement for cumulative cultural evolution (CCE).
However, there are theoretical and empirical arguments suggesting that this view might be mistaken. First, the notion of fidelity in cultural transmission is highly problematic [20]; it is unclear whether there is a critical level of fidelity required to the build-up of CCE and whether that required level of fidelity can ever be achieved [20]. Second, when fidelity can be measured, it is generally low and unlikely to sustain long-lasting cultural traditions [21], although not always (e.g. [22]). These results suggest that, even in humans, social learning is not in itself of sufficiently high fidelity to prevent the loss of cultural modifications; other mechanisms such as trial and error learning, for instance, can have a stabilizing role [23].
Furthermore, transmission chain studies in humans have shown that fundamental properties of CCE can be reproduced with social learning mechanisms that exist in non-human animals, suggesting that CCE is not dependent on special cognitive capacities unique to humans [24–26]. Claidière et al. [26], for instance, performed a transmission chain study in which baboons observed and reproduced visual patterns on touchscreen computers. The baboons were organized into chains of transmission, where each baboon was provided with the patterns produced by the previous individual in their chain. As in some human transmission chain experiments ([27] for instance), the baboons had no visual access to the behaviour of other individuals, simply the products of those behaviours. With this procedure, transmission led to the emergence of cumulative culture, as indicated by three fundamental aspects of human cultural evolution: (i) a progressive increase in performance, (ii) the emergence of systematic structure and (iii) the presence of lineage specificity [26]. Surprisingly, these results were achieved with an extremely low fidelity of pattern reproduction during the first generation of transmission (only 37% of the patterns were reproduced without errors). This initially low level of fidelity did not prevent the accumulation of modifications, and they observed a sharp increase in fidelity as patterns were passed on from generation to generation (reaching 72% in the 12th generation). Similar results have been found in transmission experiments with human participants, for example, where the transmission of miniature languages results in the emergence of languages which can be easily learned, even if the initial languages in each chain of transmission are transmitted only with very low fidelity (e.g. [28,29]). Together, these results suggest that high-fidelity transmission may not always be the cause of cumulative culture and may in fact itself be a product of CCE. Individuals may transform input variants in accordance with their prior biases, and if those biases are shared at the population level, we expect transformations in the same direction to accumulate at each transmission step. This could thus lead to the evolution of variants which are more faithfully transmitted because they match the prior biases more and more closely over generations, giving a misleading impression of high-fidelity transmission.
The vast majority of experiments on social learning and cultural transmission in humans and non-human animals focus on copying tasks (see [30–32] for reviews). In our opinion, this almost exclusive interest in copying has prevented a more neutral exploration of the mechanisms through which humans, and probably other animals, use and transmit the information gained from other individuals, and whether these other forms of social learning and transmission may result in cumulative culture (see also [33]).
Encouraged by the results of [26] showing that crucial properties of CCE can also result from initially low transmission fidelity, we decided to test whether CCE could occur in a transmission task that did not require copying. We performed an experiment with baboons and children using the same protocol as [26] but with an ‘anti-copying’ task in which the individuals were trained to avoid directly reproducing the patterns produced by a previous individual.
2. Material and methods
(a). Methods for baboons
(i). Participants and testing facility
Twelve Guinea baboons (Papio papio) belonging to a large social group of 25 from the CNRS Primate Centre in Rousset-sur-Arc (France) participated in this study. They were six males (median age 8 years, min = 5, max = 11) and six females (median age 8 years, min = 5, max = 12), all born within the primate centre.
The study was conducted in a facility developed by J.F., where baboons have free access to computerized testing booths that are installed in trailers next to their outdoor enclosure (for further information, see [34–37]).
(ii). Computer-based tasks
Each trial began with the display of a grid made of 16 squares, of which 12 were white and four green (see electronic supplementary material, video S1). Touching the display triggered the immediate abortion of the trial and the display of a green screen for 3 s (time-out). After 400 ms, all the green squares became white and, in order to obtain a food reward, the monkey had to select and touch four squares in this matrix which had not previously been highlighted in green. Touching these four squares could be done in any order and with less than 5 s between touches. Squares became black when touched to avoid being touched again and did not respond to subsequent touches. A trial was completed when four different squares had been touched. If four correct squares were touched, the trial was considered a success and the computer triggered the delivery of three to four wheat grains; otherwise, the trial was considered a failure and a green time-out screen appeared for 3 s.
The stimuli consisted of 80 × 80 pixel squares (white or green) equally spaced on a 600 × 600 pixel grid and were displayed on a black background on a 1024 × 768 pixel screen. The inter-trial interval was at least 3 s but could be much longer since the baboons chose when to initiate a trial.
(iii). Training to criterion
Training followed a progressive increase in the complexity of the task, starting with one white square and one green square, followed by a stage with an increasing number of white squares (up to 6), then by a progressively increasing number of white and green squares up to 12. Training blocks consisted of 50 non-aborted trials (aborted trials were immediately represented, and the abortion rate was very low: mean = 2.2%, min = 0.23% and max = 4.6%). Progress through training was conditioned on performing above criteria (80% success on a block of 50 random trials, excluding aborted trials).
(iv). Between-individuals transmission procedure
We followed the transmission procedure described in [26] and therefore only report the main elements here. Testing began when all 12 monkeys reached the learning criterion with 4 green squares and 12 white squares randomly placed on the grid. For each transmission chain, a first baboon was randomly selected, and this subject received the first block of 50 transmission trials consisting of randomly generated patterns. The squares touched by the first individual in responding on a given trial, whether they were correct or not, were then used as green squares on that trial for the next individual in the chain. The 50 transmission trials were randomly reordered in a new block of 50 trials that became the set of patterns shown to the next individual in that chain.
When the individuals were not involved in the transmission chain, they could perform random trials that were generated automatically by the computer and were not part of the transmission process. We ran 9 such chains with a total of 10 generations (i.e. 10 individuals in each chain), each initialized with a different set of randomly generated trials. We also made sure that each baboon did not appear more than once in each chain and performed at least 500 random trials between sets of transmission trials to avoid interference between chains (the order of the baboons in each chain was determined opportunistically). In our analyses, the last 50 responses recorded in this set of 500 random trials were compared with those obtained in the transmission chain, to infer the effects of cumulative culture. A minimum of 450 random trials therefore separated the responses to transmission trials from the responses to the random trials used in our analysis.
(b). Methods specific to children
The experimental procedure for children was as similar as possible to the experimental procedure for baboons; in this section, we detail the differences.
(i). Participants and materials
Participants were 90 English-speaking children between the ages of 5 and 7 years old (42 female, mean age = 6 years old), recruited at the hall of the Edinburgh Zoo's Budongo Trail. Four further participants were excluded from the study because they failed the pre-established criterion to achieve at least two-thirds successful trials during training.
The experiment was conducted on iPads using iOS application Pythonista 3, in a single session of approximately 3 min. All participants were rewarded with stickers at the end of the experiment.
(ii). Procedure: iPad-based tasks
The experiment was divided into two phases, a training phase and a testing phase. The training phase followed a progressive increase in the complexity of the task over three blocks, starting with a grid of two squares (one white, one red),1 then a grid of four (two red, two white) followed by the final grid of 16 (four red, 12 white). Training blocks consisted of three trials each. During testing, each trial (20 total) began with the display of a grid made of 16 squares as in the baboons' version, 12 white and four red. If four correct squares (any four of those which were not displayed in red) were touched, the trial was considered a success and the smiley face of a monkey emoji was displayed along a reward sound effect. Otherwise, the face of the monkey emoji was displayed with both hands covering the mouth along a child-friendly incorrect answer sound effect. After the monkey emoji faded away, the screen remained black for 1 s before the next trial began. At the end of the experiment, irrespective of the participant's performance, the display filled with animated stars while a reward melody was played.
(iii). Between-individuals transmission procedure
The transmission procedure was exactly as described in §2a(iv) for the baboon's version, with the only difference being the size of the testing/transmission set, which is 20 trials in this version instead of 50. We ran nine transmission chains with 10 generations. Each chain was initialized with a different set of randomly generated trials.
(c). Statistical analysis
The aim of our analysis was to evaluate the strength of the evidence for cumulative culture considering the three criteria highlighted in [26], that is, to test (i) a progressive increase in performance, (ii) the emergence of systematic structure and (iii) the presence of lineage specificity. To this aim, we first analysed the data from baboons comparing transmission versus random trials and later we analysed the data from transmission trials in children and baboons.
(i). Analysis restricted to the baboon data
We followed the procedure used in [26] to analyse the results and ran mixed-effects regression models using the lme4 package developed in R [38,39]. The type of model (linear or logistic) varied according to the dependent variable.2 All models contained a fixed effect of generation (continuous variable with the 10 generations, ranging from 0 to 9) and a fixed effect for trial type (two levels: transmission as the baseline, and random trials; 50 trials each)3 with an interaction term between them. To control for the non-independence within a given chain, models contained random intercepts for subjects and chain as well as by-subject random slopes for the effect of trial type, and by-chain slopes for the effect of generation.
(ii). Cross-species analysis between baboons and children
The models used for the cross-species analysis had a very similar structure to those described above. The only difference is that they did not contain a fixed effect for trial type, but they did contain a fixed effect for primate species (two levels: children as the baseline, and baboons) and its interaction with generation. The random-effects structure was consequently reduced to only include random intercepts for chain as well as by-chain random slopes for the effect of generation.
3. Results
(a). Is cumulative cultural evolution possible without copying in baboons?
(i). Increase in performance
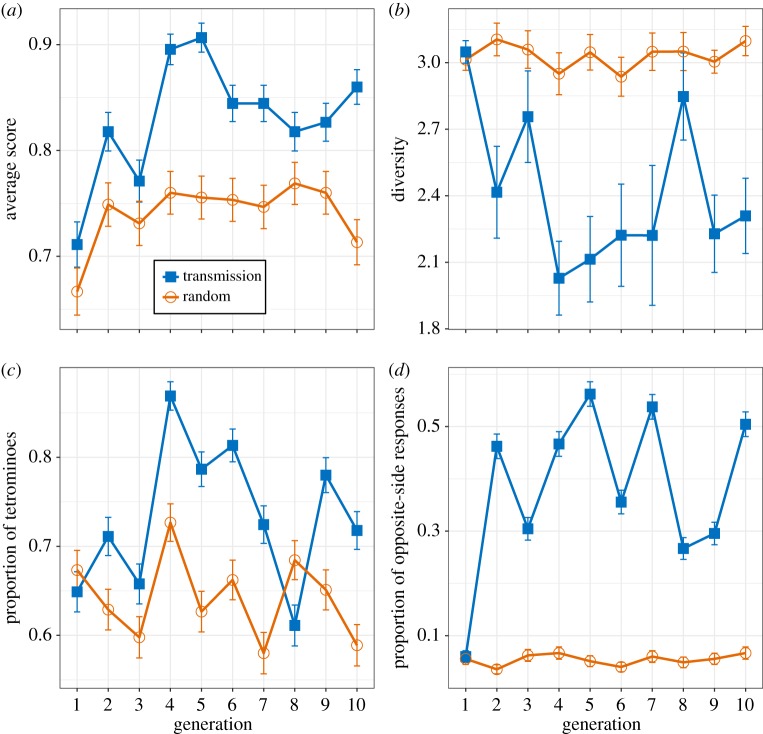
We found a progressive increase in performance over generations in transmission chains with baboons (figure 1a). Using a dependent binary variable determining the success or failure for each trial, the results of the logistic regression model suggest that the proportion of successful trials increases significantly with generation in transmission trials (β = 0.065, s.e. = 0.026, z = 2.466, p = 0.014) and that it does so significantly less in random trials (β = −0.05, s.e. = 0.019, z = −2.580, p = 0.01). This difference in the increase in performance over time between trial types reveals a clear benefit of cultural transmission.
Figure 1.
Results from transmission and random trials in baboons, depicted by blue squares and orange circles respectively. (a) Average score defined by the proportion of successful trials; (b) average Shannon's diversity index within the set of responses; (c) average proportion of tetrominoes produced; and (d) average increase in opposite-side responses. Error bars represent s.e. (Online version in colour.)
(ii). Emergence of systematic structure
One indicator of the emergence of structure is a progressive decrease in response diversity due to a focus on a subset of responses. We observed a reduction of diversity among sets of grids during transmission trials compared to random trials (figure 1b). A linear mixed-effects model with the Shannon diversity index (equal to Shannon entropy [40]) as the dependent variable suggests marginally significant reduction in diversity over generations in transmission trials (β = −0.036, s.e. = 0.018, t = −2.030, p = 0.047) and no strong evidence for a different trajectory in random trials (β = 0.038, s.e. = 0.023, t = 1.679, p = 0.095). This linear model fails to capture the sharp decrease in diversity between generations 1 and 2 and predicts a much lower diversity value for generation 1 in transmission trials (β = 2.64) than the one observed in figure 1b (greater than 3). Consequently, the difference in the overall diversity observed in figure 1b from generation 2 onwards is captured by the effect of trial type (β = 0.394, s.e. = 0.137, t = 2.888, p = 0.006), suggesting that diversity is significantly higher in random trials than in transmission trials.
To explore the type of structures that emerged during transmission and which might guide the observed decrease in diversity, we looked at the main structures found in [26], that is, tetrominoes (grids where all four squares are connected—lines, squares, L-shapes, T-shapes, S-shapes; tetrominoes will be familiar to anyone who has played Tetris). Figure 1c shows the proportion of tetrominoes produced over generations. The results from a logistic mixed regression model with a binary dependent variable representing the presence or absence of a tetromino suggest that baboons have a significant tendency to produce tetrominoes, similar across random and transmission trials (intercept, β = 1.01, s.e. = 0.217, z = 4.666, p < 0.001; trial type, β = −0.308, s.e. = 0.194, z = −1.589, p = 0.112). However, we found that the proportion of tetrominoes did not change over generations in either random (β = 0.015, s.e. = 0.018, z = 0.817, p = 0.414) or transmission trials (β = −0.027, s.e. = 0.017, z = −1.586, p = 0.113).
Further inspection of the response strategies suggested a spatial alternation of the responses (from one side of the response grid to the opposite side) between subsequent generations in transmission chains (figure 2). To quantify this, we created a binary variable that indicated if the position of the response was in a part of the screen that was opposite to that of the stimulus. We divided the screen into four quadrants: right half, left half, top half and bottom half. If the stimulus and the response were in different quadrants (left versus right or top versus bottom), we coded them as opposite-side responses (only responses that were entirely in one quadrant were considered). Figure 1d shows that the number of opposite-side responses increases sharply during the first generation and remains high compared to random trials. Results from the logistic regression model suggest that the percentage of opposite-side responses marginally increases over generations in transmission trials (β = 0.068, s.e. = 0.037, z = 1.826, p = 0.068) and not in random trials (β = −0.071, s.e. = 0.027, z = −2.648, p = 0.008). Thus although the linear model fails to capture the sharp increase in the first generation and provides weak evidence of an increase in the proportion of opposite-side responses over generations in transmission trials, it provides stronger evidence against such increase in random trials. Moreover, the model captures a significantly lower proportion of opposite-side responses in random trials than in transmission trials (β = −2.034, s.e. = 0.22, z = −9.232, p < 0.001), further confirming the difference observed in figure 1d from generation 2 onwards.
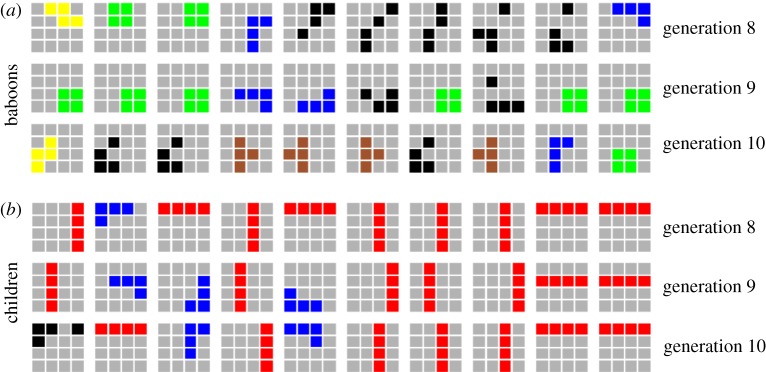
Figure 2.
Example of (a) baboons' and (b) children's example responses (extracted from their corresponding chain 5). Rows correspond to generations 8 to 10 and each row contains 10 example grids. Colouring of each grid reflects the tetromino class each pattern comes from (red for lines, green for squares, blue for L-shapes, brown for T-shapes, yellow for S-shapes, black for non-tetrominoes). (Online version in colour.)
(iii). Presence of lineage specificity
If responses are indeed dependent on those of previous generations within a given chain and independent between chains, we expect different transmission chains, or lineages, to develop different responses. For instance, one chain might converge on alternating between top and bottom responses when another might use left versus right, or one chain might contain more S-shapes and another more T-shapes. In order to assess the presence of lineage-specific systems and its potential effect on the baboons' performance, we conducted a follow-up study in which we tested the baboons’ performance on trials from the 10th generations of the nine chains (this additional experiment is presented in detail in the electronic supplementary material, §B). In one condition, the test sets were unmodified (all the trials within a set belonged to the same chain); in another condition, they were randomly pooled from different chains. If there is lineage specificity, we expect the baboons to perform better on the unmodified sets than the randomly pooled sets.
As expected, baboons were more successful in the unmodified set condition (β = 0.172, s.e. = 0.079, z = 2.161, p = 0.031; details provided in the electronic supplementary material). Importantly, the divergence between lineages is not solely due to differences in response position but also to differences in shape distributions (see electronic supplementary material).
To summarize the baboons' results, we found the three distinctive properties of CCE outlined above: an increase in score, the emergence of systematic structure in the response set and the presence of lineage specificity. These results are also in line with the core criteria for CCE outlined by Mesoudi & Thornton [32]; in this non-copying task, we observe a repeated cycle of changes in behaviour that improve performance as they are transmitted to other individuals.
(b). Are the trends in CCE without copying similar across children and baboons?
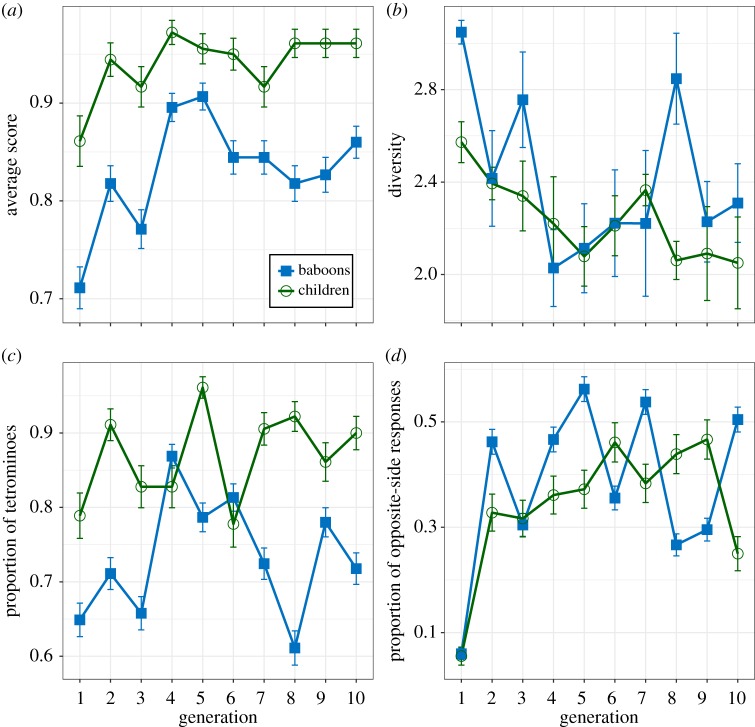
A visual inspection of the data obtained from the transmission chain experiments with children reveals strikingly similar tendencies to those found in baboons (figure 3). Using the analyses described in §2c(ii), we found a clear increase in task performance over generations (β = 0.124, s.e. = 0.046, z = 2.719, p = 0.007), a significant decrease in the diversity of the sets of responses (β = −0.046, s.e. = 0.019, t = −2.433, p = 0.016), a stable high proportion of tetrominoes over generations (intercept: β = 1.718, s.e. = 0.246, z = 6.979, p < 0.001; generation: β = 0.059, s.e. = 0.048, z = 1.249, p = 0.212) and a significant increase in the proportion of opposite-side responses (β = 0.102, s.e. = 0.04, z = 2.538, p = 0.011). The analyses further suggest no difference in the effect of generation across species in all these tendencies; we did not find a single significant interaction between generation and primate species (score, z = −0.924, p = 0.355; diversity, t = 0.186, p = 0.853; tetrominoes, z = −0.636, p = 0.525; opposite-side responses, z = −0.565, p = 0.572). However, we found differences across species in overall score as well as in the overall production of tetrominoes: baboons scored lower (β = −0.962, s.e. = 0.250, z = −3.844, p < 0.001) and produced less tetrominoes than children (β = −0.748, s.e. = 0.328, z = −2.277, p = 0.023), confirming the differences observed in figure 3a,c, respectively. Results therefore suggest that the general tendencies found in children are very similar to those found in baboons.
Figure 3.
Results from the transmission chains with baboons (blue squares) and children (green circles): (a) average score defined by the proportion of successful trials; (b) average Shannon's diversity index within the set of responses; (c) average proportion of tetrominoes produced; and (d) average increase in opposite-side responses. Error bars represent s.e. (Online version in colour.)
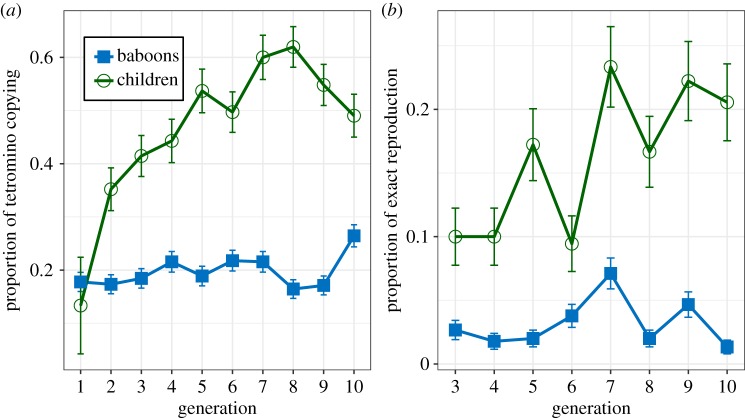
However, the inspection of the specific patterns produced (see figure 2) suggested that children tended to copy the overall shape of the response of the previous individual but shifted its position to avoid direct copying of the observed pattern—which was possible because the non-copying task only prevented them from copying both shape and location of the input patterns. Figure 4a shows the proportion of input tetrominoes whose shape was copied (in a different location) in the response, and figure 4b shows the proportion of trials in which the tetromino produced at a given generation is the exact reproduction (shares the same shape and location) of the one produced two generations ago in the same chain. We observe that while baboons tend not to copy the overall shape of input tetrominoes in their responses, children seem to do so increasingly over generations. A logistic mixed-effects model confirms that children copy input tetrominoes increasingly over generations (β = 0.099, s.e. = 0.025, z = 3.923, p < 0.001) and significantly more than baboons (as suggested by the interaction between generation and primate species, β = −0.082, s.e. = 0.034, z = −2.374, p = 0.018). Another model further confirms that the proportion of reproduction of the exact same response as the one produced two generations ago also increased in children (β = 0.099, s.e. = 0.030, z = 3.282, p = 0.001), and significantly more than in baboons (β = −0.042, s.e. = 0.035, z = −2.371, p = 0.018).
Figure 4.
(a) Average proportion of tetrominoes that are copied from one generation to the next. (b) Proportion of responses that are identical between every other generation. (Online version in colour.)
We further explored the difference in tetromino copying between children and baboons by examining specific tetromino shapes, because the inspection of the patterns also suggested that children tended to produce many lines and that they copied them more so than any other pattern. An inspection of the average number of tetrominoes produced as well as the proportion of tetromino-copying subset by each of the five possible tetromino shapes reveals a clear preference for lines over other tetrominoes in children (see electronic supplementary material, §C). A logistic mixed-effects regression model (detailed in the electronic supplementary material) shows that lines are the most copied tetrominoes (β = 0.803, s.e. = 0.206, z = 3.905, p < 0.001; the smallest difference is shown with square tetrominoes: β = −1.342, s.e. = 0.316, z = −4.250, p < 0.001) but that this tendency to copy lines does not increase over time (β = −0.012, s.e. = 0.036, z = −0.324, p = 0.746). Nonetheless, a further logistic mixed-effects model excluding line tetrominoes suggests that this constant tendency to copy lines is not the sole driver of the effect of generation on the overall proportion of copied tetrominoes; children still copy the shape of other input tetrominoes increasingly over generations (β = 0.009, s.e. = 0.003, z = 2.921, p = 0.003).
4. Discussion
The idea that faithful copying is essential to CCE is both intuitive and appealing: if socially learned behaviours are not faithfully transmitted, modifications to what is being transmitted will not be passed on to other individuals and will therefore be lost [14]. In a process closely similar to biological replication, faithful copying could guarantee the transmission of modifications and therefore naturally lead to CCE.
The purpose of this study was to test this fundamental hypothesis by examining the possibility of finding the essential properties of CCE with what was set up as a non-copying task. We used a cultural transmission task similar to the copying task used in [26] but in which the participants had to avoid what was produced by the previous individual in the chain. The results from the transmission chain experiments with baboons exhibited all three fundamental properties of CCE examined: (i) an increase in score linked to (ii) the emergence of systematic structure and (iii) lineage specificity. Despite the presence of a large evolutionary space (1820 possible responses) and a 27% chance of being correct by chance, we found the emergence of systematic responses alternating in position from one side of the response grid to another. The results from baboons thus show that the three fundamental properties of CCE examined are possible without copying.
Next, we aimed at testing the generalizability of our results to children. Interestingly, children's results were very similar to the baboons' regarding CCE: we also found an increase in score linked to the emergence of systematic structures. However, unlike baboons, children introduced copying mechanisms into the non-copying task by copying the shape of the input pattern in a different location, which was not prevented in the task (the non-copying task only forbid them from copying the exact grid pattern in the input, which included both the shape and location of the stimulus). This strategy adopted by children might in turn potentially explain their higher scores and tetromino production in comparison to baboons.
The observed copying strategy could be in line with children's tendency to high-fidelity copy even when not required in the task [41,42]. Complementarily, it could also be partly explained by the fact that children, unlike baboons, only saw grids of two and four squares during training before the target grid of 16, and in these grids, the rewarded output is necessarily the mirror image of the input. However, we only observe high-fidelity copying of specific shapes (i.e. tetrominoes), which are potentially already preferred by children because they are easier to produce and/or remember than more scattered grid patterns (around 80% of responses are tetrominoes in the first generation of children's chains). Once these preferred shapes are in the system, they are maintained. Results thus suggest that the observed bias is not solely a copying bias, but a bias towards tetromino shapes which results in a behaviour that can appear as high-fidelity copying once these patterns are introduced. Further support for this conclusion comes from the lack of lineage specificity in children's results, which reveals a shared prior bias in children's performance: all transmission chains converge on the same behaviour, constituted mainly of tetromino responses, and in particular, of lines.
However, in spite of the large number of lines, we also found evidence of an increase in a general tendency to copy, suggesting that the more the systems became structured, the more likely specific structures were to be copied (figure 4a).
The fact that the children copied the pattern they saw while at the same time trying to avoid its location created a remarkable situation in which the responses of the individuals separated by one generation became more likely to be exactly the same (both in shape and position; figure 4b). A tendency to avoid what the previous individual did may be conceived as a reproduction of behaviour over two steps when the number of possible behaviours is limited, an interesting illustration of the theoretical example of reconstruction given in [33].
Social learning is usually defined as a broad notion that encompasses any form of transmission of information between individuals [43]; however, studies of social learning tend to focus on the observational learning of technological problems. Our study broadens the experimental perspective on social learning and CCE in several ways. First, we focus on the tendency to avoid doing what others have done before, a clear but understudied case of social learning. Furthermore, our experiment lacks observational learning because it is based on the indirect transmission of visual patterns through a network of computers, a common feature of human social learning. Lastly, individuals in our task are trying to best respond to each other's inputs, rather than collectively improve an artefact. From that perspective, our results also speak to the relationship between CCE and collective intelligence, which also suggests that repeated interactions among individuals can improve group performance without the need for copying [44].
Finally, the purpose of this experiment was to address a theoretical question concerning the possibility of observing defining properties of CCE with a non-copying task, not to assess the importance or relevance of this phenomenon in nature. Nevertheless, are there natural examples of the type of transmission studied here? In animals, for example, when resources are scarce, the observation of others going to a (e.g. food or nesting) patch could promote the search of a different patch. In humans there is also an often-explicit search for innovation, for instance, in art and science. In conclusion, our results suggest that CCE does not necessarily depend on high-fidelity copying and that there is a broad spectrum of possible transmission mechanisms that will lead to CCE; these mechanisms that are not based solely, or even mainly, on indiscriminate high-fidelity copying remain to be further explored.
Supplementary Material
Supplementary Material
Acknowledgements
The authors thank the staff at the Rousset-sur-Arc Primate Center (CNRS-UPS846, France) and at the Edinburgh Zoo's Budongo Trail/Living Links (UK) for technical support, and Julie Gullstrand and Marieke Woensdregt for helping in data collection. All authors discussed the results and their implications and commented on the manuscript at all stages. J.F. developed the ALDM test systems. C.S., J.F., S.K. and N.C. coded the software for the experiments. C.S., J.F. and N.C. collected the data. C.S. and N.C. analysed the results.
Footnotes
We decided to change the colour of the squares in the input patterns to follow the (human) Western colour convention in which red is associated with prohibition.
For linear regression models, we obtained p-values using the lmerTest [45] package where p-values are calculated based on Satterthwaite's approximation for degrees of freedom. For logistic models, we followed the standard practice and obtained p-values based on asymptotic Wald tests.
Transmission trials were the test trials in which the baboons’ input was the output of the previous baboon in the transmission chain, and the random trials were those 50 trials that the same baboons produced before the transmission trials.
Ethics
The research with baboons was carried out in accordance with French and EU standards and received approval from the French Ministère de l'Education Nationale et de la Recherche (approval no. APAFIS-2717-2015111708173794-V3). Procedures were also consistent with the guidelines of the Association for the Study of Animal Behaviour. The experiment with children was carried out in accordance with the research ethics procedures of the Edinburgh Zoo's Bundongo Trail and approved by the ethics committee of the School of Philosophy, Psychology and Language Sciences at The University of Edinburgh (ref no. 325-1718).
Data accessibility
The data that support the findings of this study are openly available in the Open Science Foundation repository at https://doi.org/10.17605/OSF.IO/ZA265.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by the Agence Nationale de la Recherche ANR-13-PDOC-0004 (ASCE), ANR-16-CONV-0002 (ILCB) and ANR-11-LABX-0036 (BLRI). This project has also received funding from the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation programme (grant agreement 681942), held by K.S. The funders had no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript.
References
- 1.Tehrani JJ. 2013. The phylogeny of Little Red Riding Hood. PLoS ONE 8, e78871 ( 10.1371/journal.pone.0078871) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morin O. 2013. How portraits turned their eyes upon us: visual preferences and demographic change in cultural evolution. Evol. Hum. Behav. 34, 222–229. ( 10.1016/j.evolhumbehav.2013.01.004) [DOI] [Google Scholar]
- 3.Nichols S. 2002. On the genealogy of norms: a case for the role of emotion in cultural evolution. Phil. Sci. 69, 234–255. ( 10.1086/341051) [DOI] [Google Scholar]
- 4.Keller R. 2005. On language change: the invisible hand in language. London, UK: Routledge. [Google Scholar]
- 5.Garland EC, Goldizen AW, Rekdahl ML, Constantine R, Garrigue C, Hauser ND, Poole MM, Robbins J, Noad MJ. 2011. Dynamic horizontal cultural transmission of humpback whale song at the ocean basin scale. Curr. Biol. 21, 687–691. ( 10.1016/j.cub.2011.03.019) [DOI] [PubMed] [Google Scholar]
- 6.Grant BR, Grant PR. 2010. Songs of Darwin's finches diverge when a new species enters the community. Proc. Natl Acad. Sci. USA 107, 20 156–20 163. ( 10.1073/pnas.1015115107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grant PR, Grant BR. 2009. The secondary contact phase of allopatric speciation in Darwin's finches. Proc. Natl Acad. Sci. USA 106, 20 141–20 148. ( 10.1073/pnas.0911761106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grant PR, Grant BR. 1997. Hybridization, sexual imprinting, and mate choice. Am. Nat. 149, 1–28. ( 10.1086/285976) [DOI] [Google Scholar]
- 9.Grant BR, Grant PR. 1996. Cultural inheritance of song and its role in the evolution of Darwin's finches. Evolution 50, 2471–2487. ( 10.1111/j.1558-5646.1996.tb03633.x) [DOI] [PubMed] [Google Scholar]
- 10.Dean LG, Kendal RL, Schapiro SJ, Thierry B, Laland KN. 2012. Identification of the social and cognitive processes underlying human cumulative culture. Science 335, 1114–1118. ( 10.1126/science.1213969) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sasaki T, Biro D. 2017. Cumulative culture can emerge from collective intelligence in animal groups. Nat. Commun. 8, 15049 ( 10.1038/ncomms15049) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feher O, Wang H, Saar S, Mitra PP, Tchernichovski O. 2009. De novo establishment of wild-type song culture in the zebra finch. Nature 459, 564–568. ( 10.1038/nature07994) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tennie C, Call J, Tomasello M. 2009. Ratcheting up the ratchet: on the evolution of cumulative culture. Phil. Trans. R Soc. B 364, 2405–2415. ( 10.1098/rstb.2009.0052) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tomasello M, Kruger AC, Ratner HH. 1993. Cultural learning. Behav. Brain Sci. 16, 495–552. ( 10.1017/S0140525X0003123X) [DOI] [Google Scholar]
- 15.Kempe M, Lycett SJ, Mesoudi A. 2014. From cultural traditions to cumulative culture: parameterizing the differences between human and nonhuman culture. J. Theor. Biol. 359, 29–36. ( 10.1016/j.jtbi.2014.05.046) [DOI] [PubMed] [Google Scholar]
- 16.Mesoudi A, Whiten A, Laland KN. 2006. Towards a unified science of cultural evolution. Behav. Brain Sci. 29, 329–383. ( 10.1017/S0140525X06009083) [DOI] [PubMed] [Google Scholar]
- 17.Mesoudi A, Whiten A, Laland KN. 2004. Is human cultural evolution Darwinian? Evidence reviewed from the perspective of the origin of species. Evolution 58, 1–11. ( 10.1111/j.0014-3820.2004.tb01568.x) [DOI] [PubMed] [Google Scholar]
- 18.Richerson PJ, Boyd R. 2005. Not by genes alone: how culture transformed human evolution. Chicago, IL: University of Chicago Press. [Google Scholar]
- 19.Lewis HM, Laland KN. 2012. Transmission fidelity is the key to the build-up of cumulative culture. Phil. Trans. R Soc. B 367, 2171–2180. ( 10.1098/rstb.2012.0119) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Charbonneau M. In press Understanding cultural fidelity. Br. J. Phil. Sci. ( 10.1093/bjps/axy052) [DOI] [Google Scholar]
- 21.Claidière N, Sperber D. 2010. Imitation explains the propagation, not the stability of animal culture. Proc. R. Soc. B 277, 651–659. ( 10.1098/rspb.2009.1615) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pagel M, Atkinson QD, Calude AS, Meade A. 2013. Ultraconserved words point to deep language ancestry across Eurasia. Proc. Natl Acad. Sci. USA 110, 8471–8476. ( 10.1073/pnas.1218726110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Truskanov N, Prat Y. 2018. Cultural transmission in an ever-changing world: trial-and-error copying may be more robust than precise imitation. Phil. Trans. R Soc. B 373, 20170050 ( 10.1098/rstb.2017.0050) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caldwell CA, Millen AE. 2008. Experimental models for testing hypotheses about cumulative cultural evolution. Evol. Hum. Behav. 29, 165–171. ( 10.1016/j.evolhumbehav.2007.12.001) [DOI] [Google Scholar]
- 25.Zwirner E, Thornton A. 2015. Cognitive requirements of cumulative culture: teaching is useful but not essential. Sci. Rep. 5, 16781 ( 10.1038/srep16781) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Claidière N, Smith K, Kirby S, Fagot J. 2014. Cultural evolution of systematically structured behaviour in a non-human primate. Proc. R. Soc. Lond. B 281, 20141541 ( 10.1098/rspb.2014.1541) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Caldwell C, Millen A. 2008. Studying cumulative cultural evolution in the laboratory. Phil. Trans. R Soc. B 363, 3529 ( 10.1098/rstb.2008.0133) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kirby S, Cornish H, Smith K. 2008. Cumulative cultural evolution in the laboratory: an experimental approach to the origins of structure in human language. Proc. Natl Acad. Sci. USA 105, 10 681–10 686. ( 10.1073/pnas.0707835105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beckner C, Pierrehumbert JB, Hay J. 2017. The emergence of linguistic structure in an online iterated learning task. J. Lang. Evol. 2, 160–176. ( 10.1093/jole/lzx001) [DOI] [Google Scholar]
- 30.Mesoudi A, Whiten A. 2008. The multiple roles of cultural transmission experiments in understanding human cultural evolution. Phil. Trans. R Soc. B 363, 3489–3501. ( 10.1098/rstb.2008.0129) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Whiten A, Mesoudi A. 2008. Establishing an experimental science of culture: animal social diffusion experiments. Phil. Trans. R Soc. B 363, 3477–3488. ( 10.1098/rstb.2008.0134) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mesoudi A, Thornton A. 2018. What is cumulative cultural evolution? Proc. R. Soc. B 285, 20180712 ( 10.1098/rspb.2018.0712) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Claidière N, Scott-Phillips TC, Sperber D. 2014. How Darwinian is cultural evolution? Phil. Trans. Soc. B Biol. Sci. 369, 20130368 ( 10.1098/rstb.2013.0368) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fagot J, Marzouki Y, Huguet P, Gullstrand J, Claidière N. 2015. Assessment of social cognition in non-human primates using a network of computerized automated learning device (ALDM) test systems. J. Vis. Exp. 99, e52798 ( 10.3791/52798) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fagot J, Gullstrand J, Kemp C, Defilles C, Mekaouche M. 2014. Effects of freely accessible computerized test systems on the spontaneous behaviors and stress level of Guinea baboons (Papio papio). Am. J. Primatol. 76, 56–64. ( 10.1002/ajp.22193) [DOI] [PubMed] [Google Scholar]
- 36.Fagot J, Bonté E. 2010. Automated testing of cognitive performance in monkeys: use of a battery of computerized test systems by a troop of semi-free-ranging baboons (Papio papio). Behav. Res. Methods 42, 507–516. ( 10.3758/BRM.42.2.507) [DOI] [PubMed] [Google Scholar]
- 37.Fagot J, Paleressompoulle D. 2009. Automatic testing of cognitive performance in baboons maintained in social groups. Behav. Res. Methods 41, 396–404. ( 10.3758/BRM.41.2.396) [DOI] [PubMed] [Google Scholar]
- 38.R Core Team. 2015. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 39.Bates D, Maechler M, Bolker B, Walker S. 2015. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. ( 10.18637/jss.v067.i01) [DOI] [Google Scholar]
- 40.Shannon CE. 1948. A mathematical theory of communication. Bell Syst. Tech. J. 27, 379–423. ( 10.1002/j.1538-7305.1948.tb01338.x) [DOI] [Google Scholar]
- 41.Lyons DE, Young AG, Keil FC. 2007. The hidden structure of overimitation. Proc. Natl Acad. Sci. USA 104, 19 751–19 756. ( 10.1073/pnas.0704452104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Whiten A, McGuigan N, Marshall-Pescini S, Hopper LM. 2009. Emulation, imitation, over-imitation and the scope of culture for child and chimpanzee. Phil. Trans. R Soc. B 364, 2417–2428. ( 10.1098/rstb.2009.0069) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heyes CM. 1994. Social learning in animals: categories and mechanisms. Biological Reviews 69, 207–231. ( 10.1111/j.1469-185X.1994.tb01506.x) [DOI] [PubMed] [Google Scholar]
- 44.Biro D, Sasaki T, Portugal SJ. 2016. Bringing a time–depth perspective to collective animal behaviour. Trends Ecol. Evol. 31, 550–562. ( 10.1016/j.tree.2016.03.018) [DOI] [PubMed] [Google Scholar]
- 45.Kuznetsova A, Brockhoff PB, Christensen RHB. 2017. lmerTest package: tests in linear mixed effects models. J. Stat. Softw. 82, 1–26. ( 10.18637/jss.v082.i13) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are openly available in the Open Science Foundation repository at https://doi.org/10.17605/OSF.IO/ZA265.