Abstract
A 79-year-old man with elevated blood glucose was started on insulin therapy. IgG4 was as high as 1,830 mg/dL, and 18F-fluorodeoxyglucose-positron emission tomography/computed tomography (FDG-PET/CT) confirmed diffuse pancreatic enlargement and accumulation of FDG. Based on the above, autoimmune pancreatitis (AIP) was diagnosed, but steroid treatment was not performed. IgG4 later declined, and FDG accumulation in the pancreas disappeared on FDG-PET/CT at the age of 83 years. AIP was thought to have gradually remitted spontaneously over time. FDG-PET/CT is useful for evaluating AIP activity.
Keywords: autoimmune pancreatitis, IgG4, diabetes, FDG-PET/CT
Introduction
Autoimmune pancreatitis (AIP) was first reported by Sarles in 19681). In 1995, Yoshida et al. proposed diffuse pancreatic swelling and pancreatic ductal stenosis as the characteristics of AIP2). Serum IgG4 was later reported to be elevated in more than 90% of AIP patients and it is recognized as one phenotype of systemic disease associated with IgG43). AIP presents as a variety of lesions outside the pancreas and complications of diabetes are also frequent4). Here, I report a case of AIP diagnosed due to deterioration of diabetes, with spontaneous remission of imaging findings several years later.
Case Report
A 79-year-old man reported dry mouth and weight loss. He had a family history of pulmonary tuberculosis and angina pectoris. At the age of 67, the patient received coronary angioplasty and coronary artery bypass due to acute myocardial infarction. In the same year, he underwent clipping for an unruptured cerebral aneurysm. He was diagnosed with diabetes at age 68, and at 72, he developed high blood pressure and hyperlipidemia.
At the age of 73, he moved to Fukushima Prefecture and visited our hospital. He was taking aspirin 100 mg/day, amlodipine 5 mg/day, warfarin 2.75 mg/day, acarbose 0.6 mg/day, pioglitazone 15 mg/day, and ethyl icosapentate acid 1,800 mg/day. The HbA1c was 7.8%, with blood immunoreactive insulin level of 8 μU/mL. Since initial presentation, the HbA1c ranged from 6.6 to 8%. Total serum protein was initially normal at 8.0 g/dL, but a month later increased to 8.4 g/dL and remained high.
At the age of 79, he was hospitalized because his blood glucose control deteriorated. His height was 160 cm and he weighed 55.4 kg, with a body mass index of 21.6 kg/m2 and blood pressure of 131/81 mmHg. No heart murmur was heard, his abdomen was flat and soft, and no tenderness was noted. Aspirin 100 mg/day, warfarin 1 mg/day, sarpogrelate 100 mg/day, telmisartan 40 mg/hydrochlorothiazide 12.5 mg combination tablet/day, adenosine triphosphate 600 mg/day, mitiglinide 10 mg/voglibose 0.2 mg combination tablet (3 tablets/day), gliclazide 20 mg/day, mecobalamin 1,500 mg/day, ezetimibe 10 mg/day, and alogliptin 25 mg/pioglitazone 30 mg combination tablet/day were administered on admission.
Blood tests revealed a glucose level of 423 mg/dL and HbA1c of 13.9%, but ketone bodies and electrolytes were normal. Anti-glutamic acid decarboxylase antibody was negative. A glucagon load test was performed; blood C-peptide immunoreactivity (CPR) was 0.14 ng/mL before loading and 0.17 ng/mL after 6 minutes. Urinary CPR excretion was low at 22.2 μg/day. Serum total protein and γ-globulin levels were increased. IgG was increased in the γ-globulin fraction; IgG1, IgG 2, IgG 3, and IgG4 subtypes were increased, but IgG4 was particularly high at 1,830 mg/dL, comprising 33% of all IgG. Antinuclear antibody, anti-SSA/Ro antibody, and anti-SSB/La antibody were negative (Table 1).
Table 1. Laboratory data on admission.
Diabetic complications included simple retinopathy and mild neuropathy, but no nephropathy.
After hospitalization, mitiglinide/voglibose, gliclazide, and pioglitazone/alogliptin were discontinued, but other antihypertensive and antiplatelet agents were continued. The patient was prescribed 4 U of insulin aspart immediately before breakfast, just before lunch, and just before dinner. Before bed, 6 U of insulin detemir was prescribed. Insulin aspart was gradually increased to 13 U immediately before breakfast, 12 U just before lunch, and 11 U just before dinner. This regimen improved blood glucose control.
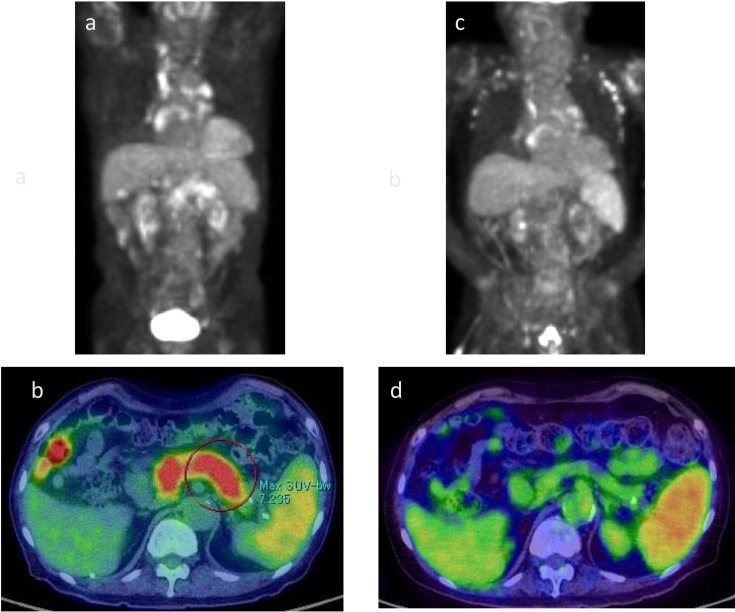
As IgG4 was high, autoimmune pancreatitis was suspected and imaging tests were performed. Abdominal computed tomography (CT) showed enlargement compared with pancreatic size at the age of 73 (Figure 1a, b). On magnetic resonance cholangiopancreatography (MRCP), the main pancreatic duct was unclear at the head of the pancreas, whereas the tail was normal (Figure 1c). Thus, stenosis of the main pancreatic duct at the head of the pancreas was suspected. The common bile duct narrowed at the area penetrating the pancreatic head (Figure 1c). Diffuse enlargement of the pancreas and accumulation of FDG were observed on 18F-fluorodeoxyglucose-positron emission tomography/computed tomography (FDG-PET/CT) (Figure 2 a, b). Right subclavian, abdominal periaortic, subdiaphragmatic, iliac, and inguinal lymph nodes were enlarged, and FDG accumulation was observed (Figure 2a). Imaging findings were consistent with autoimmune pancreatitis. Lymph nodes in the inguinal region were not palpated and lymph node biopsy was not performed. Endoscopic retrograde cholangiopancreatography (ERCP) was not performed because there was no jaundice, no increase of pancreatic enzymes, and no malignant findings on imaging.
Figure 1.
Changes in abdominal CT and MRCP findings.
a. At the age of 73 (traffic accident): There is no pancreas swelling (arrow). b. At the age of 79 (at the time of admission): Diffuse pancreatic swelling (arrow). c. At the age of 79 (when admitted) MRCP: The main pancreatic duct is unclear at the head of the pancreas, but is normal at the tail (arrow). The bile duct system is of normal thickness, but narrows at the area penetrating the pancreatic head (arrow). d. At the age of 83: Pancreatic swelling had reduced and improved (arrow).
Figure 2.
Changes on FDG-PET and FDG-PET/CT.
a, b. At the age of 79: Diffuse pancreatic enlargement is observed, and FDG accumulation with an SUV max of 5.4 to 7.2 is observed. Right subclavian, abdominal periaortic, subdiaphragmatic, iliac, and inguinal lymph nodes are also enlarged, and show FDG accumulation. c, d. At the age of 83: Cervical, subclavian, mediastinal, abdominal aortic, pelvic, and inguinal lymph node enlargement and accumulation of FDG were also observed, but accumulation of FDG in the pancreas disappeared.
Based on the above, steroid therapy was not required. He was discharged after receiving intensive insulin treatment because blood glucose control improved.
Post-discharge course
Swelling of the pancreas disappeared on abdominal CT at age 83, but abdominal periaortic, iliac, and inguinal lymph node enlargement persisted (Figure 1d). FDG accumulation in the pancreas disappeared but persisted in cervical, subclavian, mediastinal, abdominal aortic, pelvic, and inguinal lymph nodes (Figure 2c, d).
Serum IgG4 gradually decreased from 1,830 mg/dL to 623 mg/dL over the subsequent 4 years (Figure 3). Intensive insulin therapy was continued and HbA1c remained between 6.9 and 7.6% (Figure 3).
Figure 3.
Total insulin dose and changes in blood CPR, IgG4, and HbA1c.
olid line: IgG4, broken line: HbA1c.
The glucagon load test showed mildly improved blood CPR over a period of 4 years (Table 2).
Table 2. Changes in glucagon load test.
| Blood glucose (mg/dL) | CPR (ng/mL) | ΔCPR (ng/mL) | |||
|---|---|---|---|---|---|
| before | 6 minutes after | before | 6 minutes after | ||
| At the age 79 | 65 | 79 | 0.14 | 0.17 | 0.03 |
| At the age 83 | 92 | 115 | 0.34 | 0.69 | 0.35 |
Discussion
The patient was diagnosed with AIP at the age of 79 based on the diagnostic criteria5) of pancreatic swelling on abdominal CT and FDG-PET/CT, and extremely high serum IgG4.
Standard therapy for AIP includes steroids, which are indicated for patients with obstructive jaundice due to bile duct stricture, abdominal and back pain, and extrapancreatic lesions5). With steroid therapy, AIP remits at a high rate, and pancreatic swelling and ductal images improve on CT and ERCP6, 7). However, steroid therapy has a negative effect on glucose tolerance in older patients in particular8). In addition, spontaneous remission without steroid treatment has been reported in some cases of AIP9,10,11,12). Spontaneous remission cases have no biliary stenosis and serum IgG4 is often 135 mg/dL or less9, 10). In the present patient, serum IgG4 was high and lymphadenopathy was present, but liver dysfunction, jaundice, abdominal pain, and extrapancreatic lesions were absent. In addition, the patient was elderly and had severe diabetes mellitus; therefore, steroid therapy was not given.
FDG accumulates in the pancreas in active AIP, but declines when inflammation of the pancreas improves13). IgG4 is considered to reflect the activity of AIP14). During the disease course, the serum IgG4 decreased, pancreatic swelling improved on CT, and accumulation of FDG in the pancreas disappeared on FDG-PET/CT. Therefore, it was presumed that the inflammation of the pancreas caused by AIP had spontaneously remitted.
AIP impairs pancreatic endocrine function, thereby complicating diabetes15, 16). The patient was diagnosed with diabetes at age 68; thus, his glycemic control was thought to have deteriorated due to AIP onset. At the time of admission, his insulin secretion ability was markedly decreased; however, slight improvement at the age of 83 was not sufficient to avoid insulin treatment. As he had diabetes for approximately 10 years before the onset of AIP, his pancreatic β cells were assumed to be decreasing. In AIP, when ΔCPR in the glucagon load test before treatment is 0.6 ng/ml or less, it is often impossible to avoid insulin, even if steroid is given17).
This report described a case of AIP that remitted spontaneously without steroid therapy. AIP without jaundice, abdominal pain, or extra-pancreatic symptoms should be monitored without steroid treatment, even when serum IgG4 is high, because steroids have side effects and spontaneous remission of AIP is possible. Moreover, FDG-PET/CT is useful for evaluating AIP activity.
Conflict of Interest
The author reports no conflict of interest.
References
- 1.Sarles H, Sarles JC, Muratore R. Chronic inflammatory sclerosis of the pancreas—an autonomous pancreatic disease? Am J Dig Dis 1961; 6: 688–698. doi: 10.1007/BF02232341 [DOI] [PubMed] [Google Scholar]
- 2.Yoshida K, Toki F, Takeuchi T. Chronic pancreatitis caused by an autoimmune abnormality. Proposal of the concept of autoimmune pancreatitis. Dig Dis Sci 1995; 40: 1561–1568. doi: 10.1007/BF02285209 [DOI] [PubMed] [Google Scholar]
- 3.Hamano H, Kawa S, Horiuchi A. High serum IgG4 concentrations in patients with sclerosing pancreatitis. N Engl J Med 2001; 344: 732–738. doi: 10.1056/NEJM200103083441005 [DOI] [PubMed] [Google Scholar]
- 4.Ohara H, Nakazawa T, Sano H. Systemic extrapancreatic lesions associated with autoimmune pancreatitis. Pancreas 2005; 31: 232–237. doi: 10.1097/01.mpa.0000175178.85786.1d [DOI] [PubMed] [Google Scholar]
- 5.Ministry of Health Labor and Welfare Investigative Research Group for Refractory Pancreatic Disease•Japan Pancreas Society, Autoimmune Pancreatitis Clinical Practice Guideline 2013 Pancreas 2013; 28: 717–783 (in Japanese). [Google Scholar]
- 6.Sahani DV, Sainani NI, Deshpande V. Autoimmune pancreatitis: disease evolution, staging, response assessment, and CT features that predict response to corticosteroid therapy. Radiology 2009; 250: 118–129. doi: 10.1148/radiol.2493080279 [DOI] [PubMed] [Google Scholar]
- 7.Kamisawa T, Yoshiike M, Egawa N. Treating patients with autoimmune pancreatitis: results from a long-term follow-up study. Pancreatology 2005; 5: 234–238. doi: 10.1159/000085277 [DOI] [PubMed] [Google Scholar]
- 8.Ito T, Nishimori I, Inoue N. Treatment for autoimmune pancreatitis: consensus on the treatment for patients with autoimmune pancreatitis in Japan. J Gastroenterol 2007; 42 (Suppl 18): 50–58. doi: 10.1007/s00535-007-2051-y [DOI] [PubMed] [Google Scholar]
- 9.Kubota K, Iida H, Fujisawa T. Clinical factors predictive of spontaneous remission or relapse in cases of autoimmune pancreatitis. Gastrointest Endosc 2007; 66: 1142–1151. doi: 10.1016/j.gie.2007.06.059 [DOI] [PubMed] [Google Scholar]
- 10.Kubota K, Watanabe S, Uchiyama T. Factors predictive of relapse and spontaneous remission of autoimmune pancreatitis patients treated/not treated with corticosteroids. J Gastroenterol 2011; 46: 834–842. doi: 10.1007/s00535-011-0393-y [DOI] [PubMed] [Google Scholar]
- 11.Iizuka T, Sibusawa N, Yoshida S. A case of IgG4-associated autoimmune pancreatitis with hepatitis pseudo-tumor. Journal of Japanese Society of Internal Medicine 2012; 101: 468–471 (in Japanese). doi: 10.2169/naika.101.468 [DOI] [PubMed] [Google Scholar]
- 12.Yoshioka S, Kato J, Mori K. A case of autoimmune pancreatitis combined with type 2 diabetes mellitus and natural remission. J Japan Diab Soc 2006; 51: 445–449 (in Japanese). [Google Scholar]
- 13.Lee TY, Kim MH, Park DH. Utility of 18F-FDG PET/CT for differentiation of autoimmune pancreatitis with atypical pancreatic imaging findings from pancreatic cancer. AJR Am J Roentgenol 2009; 193: 343–348. doi: 10.2214/AJR.08.2297 [DOI] [PubMed] [Google Scholar]
- 14.Hamano H, Kawa S, Horiuchi A. High serum IgG4 concentrations in patients with sclerosing pancreatitis. N Engl J Med 2001; 344: 732–738. doi: 10.1056/NEJM200103083441005 [DOI] [PubMed] [Google Scholar]
- 15.Ito T, Kawabe K, Arita Y. Evaluation of pancreatic endocrine and exocrine function in patients with autoimmune pancreatitis. Pancreas 2007; 34: 254–259. doi: 10.1097/01.mpa.0000250127.18908.38 [DOI] [PubMed] [Google Scholar]
- 16.Nishimori I, Tamakoshi A, Kawa S. Research Committee on Intractable Pancreatic Diseases, the Ministry of Health and Welfare of JapanInfluence of steroid therapy on the course of diabetes mellitus in patients with autoimmune pancreatitis: findings from a nationwide survey in Japan. Pancreas 2006; 32: 244–248. doi: 10.1097/01.mpa.0000202950.02988.07 [DOI] [PubMed] [Google Scholar]
- 17.Hirano K, Isogawa A, Tada M. Long-term prognosis of autoimmune pancreatitis in terms of glucose tolerance. Pancreas 2012; 41: 691–695. [DOI] [PubMed] [Google Scholar]