Abstract
Port site recurrence is a rare but well-documented adverse event peculiar to laparoscopic surgery. We report an unusual outcome of unexpected early stage ovarian cancer in which port site recurrence occurred after laparoscopic surgery and was followed by diffuse subcutaneous metastases. A 31-year-old Japanese woman with a large tumor in her abdomen visited our hospital. Because no intratumoral solid component was detected on diagnostic imaging, the tumor was diagnosed as a benign ovarian tumor and the patient underwent left ovarian laparoscopic cystectomy. Contrary to our expectations, however, the ovarian tumor was a mucinous carcinoma. We performed additional surgery, but the tumor recurred in the umbilical area, and multiple subcutaneous metastases later appeared. The curative effect of chemotherapy and radiation was limited. This atypical metastatic distribution of an extremely small amount of cancer might have been caused by the laparoscopic procedure. Protection against tumor cell dissemination is necessary during all forms of laparoscopic surgery.
Keywords: laparoscopy, ovarian cancer, port site recurrence, subcutaneous metastases, umbilicus
Introduction
Although laparoscopic surgery is now widely used to treat various gynecological diseases owing to its safety and less invasive nature, ovarian cancer is an exception to this trend from the viewpoint of oncologic outcomes. The potential risk of port site recurrence (PSR) is one of the most important concerns. However, the precise mechanism of PSR has not been fully elucidated.
We found several published reports discussing PSR in the PubMed database, all of which reported a low probability of PSR occurrence1,2,3,4,5). Almost all of the cases described demonstrated simultaneous carcinomatosis or metastases to other sites2,3,4,5), suggesting that PSR may, in general, not be related to prognosis3,4,5).
Herein we report a case of PSR followed by diffuse subcutaneous metastases subsequent to laparoscopic ovarian cystectomy performed for unexpected early stage ovarian cancer. This is the first report demonstrating that even an extremely small amount of cancer tissue is associated with a risk of PSR, and this may be a hallmark of poor prognosis, especially in the absence of carcinomatosis or other advanced stages of cancer.
Case Report
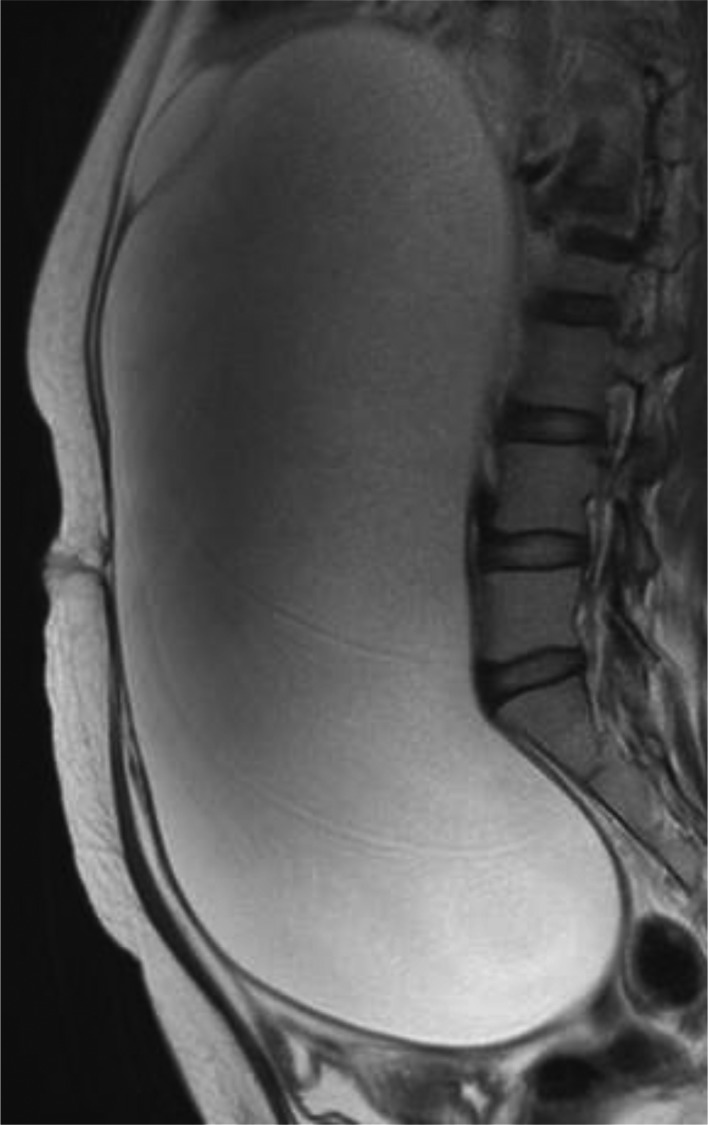
A 31-year-old Japanese woman, gravida 0, with no remarkable medical history, presented with the complaint of abdominal distension. Ultrasound at the time of initial examination revealed a large tumor with no solid component in the abdominal cavity. Initial laboratory tests including tumor marker profile demonstrated no remarkable findings: carcinoembryonic antigen (CEA) 1.0 ng/mL, cancer antigen 125 (CA125) 32.0 U/mL, and cancer antigen 19-9 (CA19-9) 15.4 U/mL. Contrast-enhanced magnetic resonance imaging (MRI) of the pelvis revealed a 30 × 24 × 13-cm polycystic tumor with no intratumoral solid component (Figure 1a). The tumor showed no contrast enhancement. Based on the absence of malignant findings, we diagnosed a benign ovarian tumor. In the following month, we performed a laparoscopic left ovarian cystectomy. The operative time was 135 minutes and the blood loss was 850 mL, including intratumoral fluid. At the beginning of the operation, we inflated the abdomen using CO2 gas, maintaining intra-abdominal pressure during pneumoperitoneum below 10 mmHg. A 3-cm incision was made at the umbilical site, through which 5 liters of intratumoral mucinous fluid were aspirated by rupturing directly without any wound protection (Figure 2). Some of the intratumoral fluid leaked into the abdomen. Afterwards, a 5-mm trocar was inserted into the lower right abdomen, a SILSTM Port (Covidien, Mansfield, MA, USA) was used in the umbilical site, and the tumor was resected laparoscopically. The excised tumor was removed through the umbilical incision site and appeared to be benign on macroscopic examination. No other abnormal findings were observed in the abdominal cavity.
Figure 1.
Image of the tumor. Sagittal T2-weighted image revealed a 30 × 24 × 13-cm tumor with no intratumoral solid component. Only one cyst was visible in upper part of tumor.
Figure 2.
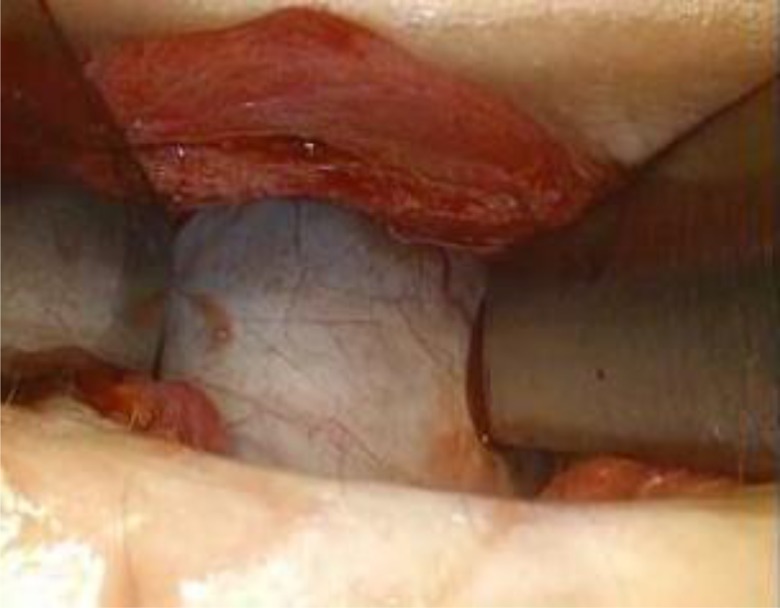
Laparoscopic left ovarian cystectomy procedure. Five liters of intratumoral mucinous fluid were aspirated by rupturing directly, while umbilical incision was left unprotected.
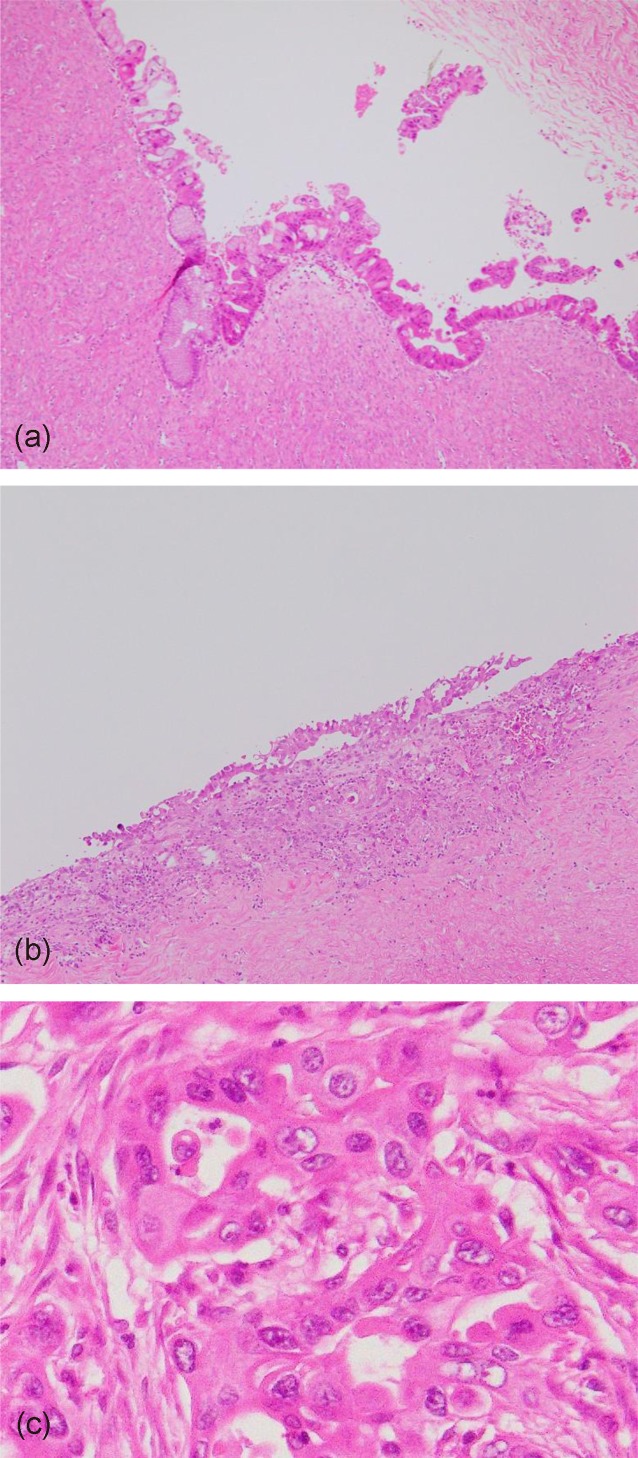
Contrary to our expectations, the tumor was diagnosed as a mucinous carcinoma rather than a benign tumor. Macroscopically, no malignant findings, including in the solid component, could be seen in the tumor. Examination of the entire specimen (109 segments in total) and histopathological analysis revealed that the majority of the tumor consisted of an adenoma, but a small number of individual cells with severe atypia had invaded 1 mm into the stroma (Figure 3a–3c). These were 3, 4, and 10 mm in size.
Figure 3.
Histopathological analysis of tumor. (a) Mucinous adenoma. Majority of tumor consisted of adenoma without atypia. (Original magnification ×100, H&E stain.) (b) Mucinous carcinoma with infiltrative invasion. Small number of tumor cells with severe atypia had invaded 1 mm into stroma in area 10 mm in size. (Original magnification ×100, H&E stain.) (c) The invasive tumor cells. Tumor cells had nonuniformly large-sized nuclei with prominent nucleoli. (Original magnification ×400, H&E stain.)
Additional tests and treatments were performed. Contrast-enhanced computed tomography (CT) and endoscopy of the gastrointestinal tract and colon demonstrated no remarkable findings. We excised the residual left adnexa, partial omentum, and appendix via open laparotomy on postoperative day 59. The operative time was 71 minutes and the blood loss was 32 mL. Only a few, solitary, atypical cells were found in the histopathological specimen of the residual left ovary. Cytology of the ascites was negative. Stage 1C1 ovarian cancer was finally diagnosed. We discussed the potential risks of ovarian cancer with the patient and her family and decided to pursue a course of follow-up without any additional treatment.
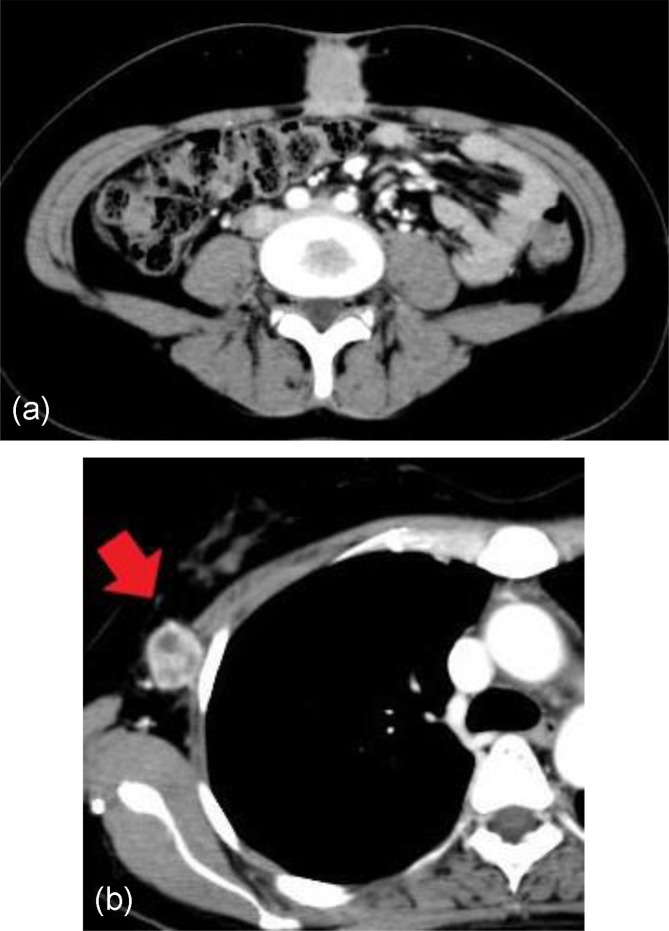
Eleven months after the second surgery, a 2-cm tumor in the umbilical area was identified on follow-up CT (Figure 4a), and PSR was diagnosed. The tumor marker profiles were unremarkable: CEA 0.2 ng/mL and CA125 21.4 U/mL. The CA19-9 value of 51.9 U/mL was slightly higher than normal. No other tumor was observed on diagnostic imaging, and the recurrent tumor was resected in the following month. The operative time was 90 minutes and the blood loss was 10 mL. Histopathological analysis indicated a recurrence of the ovarian cancer. The incised margin of the specimen contained no cancer cells. Cytology of the ascites was negative. Adjuvant combined chemotherapy with paclitaxel and carboplatin was initiated. However, multiple subcutaneous metastases appeared around the right axilla during chemotherapy (Figure 4b). The patient was treated with a combination of chemotherapy and radiation, but the curative effect was limited, and the subcutaneous metastases in the region of the right axilla emerged repeatedly. The subcutaneous metastases gradually spread to other parts of the body and later appeared in bone and the adrenal glands, which were the atypical places for the spread of ovarian cancer. Eventually, the cancer metastasized to the systemic lymph nodes, liver, and thorax. The patient died 36 months after the first surgery. The patient and her family agreed to the publication of the present report.
Figure 4.
Images of recurrent tumor. (a) Axial CT revealed recurrence in umbilical area. No other findings were observed in distant areas. (b) Axial CT revealed subcutaneous metastasis in right axilla (red arrow).
Discussion
Because laparoscopic tumor resection is now the first choice in the treatment of benign ovarian tumors, preoperative differentiation between benign and malignant ovarian tumors is crucial. However, even with state-of-the-art diagnostic imaging techniques, an accurate diagnosis is particularly challenging when the cancer cells exist only within a limited area in a large ovarian tumor. A laparoscopic procedure performed for early ovarian cancer that cannot be detected preoperatively might result in a poor outcome, as in the present case.
This is the first report of an unusual manifestation of PSR of ovarian cancer. This case has two important implications. First, even in early stage ovarian cancer, and even as a result of an extremely small amount of cancer, PSR can occur after laparoscopic surgery. Our literature search of the PubMed database identified reports of PSR1,2,3,4,5), but there were no reports of the occurrence of PSR in early stage ovarian cancer. All previous studies reported that PSR occurred in advanced stages, including carcinomatosis and metastases2,3,4,5). In the present case, we strongly suspect a relationship between PSR and the laparoscopic procedure because the case involved early stage ovarian cancer that could not be detected preoperatively owing to the extremely small size of the cancer. Moreover, the procedure of rupturing the tumor to reduce its volume and the lack of wound protection during the tumorectomy are both suspected to have increased the risk of PSR. To prevent unexpected tumor cell dissemination, a no-touch isolation technique should be employed, in which the tumor is ruptured in a bag to prevent spillage into the abdomen.
Second, PSR might be a hallmark of poor prognosis in the absence of carcinomatosis or other advanced stage cancer, as evidenced by the atypical metastatic distribution observed in the present case. Some reports of PSR have indicated that PSR itself did not influence the prognosis of ovarian cancer3,4,5). In most cases, PSR occurs in advanced stage cancer, and patients who are at risk of PSR have a poor prognosis owing to their advanced cancer status. We therefore believe that there is no relationship between the PSR itself and the prognosis of advanced cancer. However, the same cannot be said in early ovarian cancer, such as in the present case, which demonstrated that PSR was a risk factor for atypical metastatic distribution, i.e., multiple, recurrent, subcutaneous metastases.
The occurrence of subcutaneous metastases without any other metastases caused by internal cancer is unusual6). The physiology of the atypical metastatic distribution in this case was similar to that observed in the case of umbilical nodules sometimes referred to as “Sister Mary Joseph’s nodules” (SMJN)7, 8). SMJN is a result of the metastasis of cancer in the pelvis or abdomen, and the umbilicus is at risk in the spread of cancer cells owing to several factors including hematogenous or lymphatic metastasis, transperitoneal invasion, and remnant structures. The exact metastatic mechanism in SMJN remains unclear, but SMJN is sometimes known as the spread to subcutaneous tissue. It is likely that the atypical metastatic distribution in this case was caused by a mechanism related to SMJN. In the present case, SMJN may unfortunately have been primarily iatrogenic.
From a laparoscopic perspective, CO2-pneumoperitoneum might be related to the atypical metastatic distribution observed in the present case. Volz et al. demonstrated in an animal model that pneumoperitoneum provoked cancer cell implantation and growth9). Some reports have also suggested that pneumoperitoneum might trigger the proliferation of ovarian cancer cells in subcutaneous tissue10, 11). Although other reports have indicated that pneumoperitoneum did not influence the overall survival of ovarian cancer12, 13), these studies focused on advanced stages of cancer. CO2-pneumoperitoneum may also have triggered atypical metastatic distribution, i.e., multiple subcutaneous metastases, in the present case.
Conclusion
Our findings suggest that PSR occurred following laparoscopic surgery for early stage ovarian cancer in the present case. An atypical metastatic distribution following PSR indicated that PSR might be a hallmark of poor prognosis. We strongly recommend that tumor cell dissemination be prevented during all types of laparoscopic surgery, given the difficulty of making an accurate, preoperative diagnosis of ovarian tumors. Further research is needed to confirm the precise mechanism of the PSR for prevention.
Conflict of Interest
There are no conflicts of interest to disclose.
References
- 1.Childers JM, Aqua KA, Surwit EA. Abdominal-wall tumor implantation after laparoscopy for malignant conditions. Obstet Gynecol 1994; 84: 765–769. [PubMed] [Google Scholar]
- 2.Zivanovic O, Sonoda Y, Diaz JP. The rate of port-site metastases after 2251 laparoscopic procedures in women with underlying malignant disease. Gynecol Oncol 2008; 111: 431–437. doi: 10.1016/j.ygyno.2008.08.024 [DOI] [PubMed] [Google Scholar]
- 3.Kruitwagen RF, Swinkels BM, Keyser KG. Incidence and effect on survival of abdominal wall metastases at trocar or puncture sites following laparoscopy or paracentesis in women with ovarian cancer. Gynecol Oncol 1996; 60: 233–237. doi: 10.1006/gyno.1996.0031 [DOI] [PubMed] [Google Scholar]
- 4.Vergote I, Marquette S, Amant F. Port-site metastases after open laparoscopy: a study in 173 patients with advanced ovarian carcinoma. Int J Gynecol Cancer 2005; 15: 776–779. doi: 10.1111/j.1525-1438.2005.00135.x [DOI] [PubMed] [Google Scholar]
- 5.Ataseven B, Grimm C, Harter P. Prognostic impact of port-site metastasis after diagnostic laparoscopy for epithelial ovarian cancer. Ann Surg Oncol 2016; 23(Suppl 5): 834–840. doi: 10.1245/s10434-016-5415-9 [DOI] [PubMed] [Google Scholar]
- 6.Lookingbill DP, Spangler N, Sexton FM. Skin involvement as the presenting sign of internal carcinoma. A retrospective study of 7316 cancer patients. J Am Acad Dermatol 1990; 22: 19–26. doi: 10.1016/0190-9622(90)70002-Y [DOI] [PubMed] [Google Scholar]
- 7.Gabriele R, Conte M, Egidi F. Umbilical metastases: current viewpoint. World J Surg Oncol 2005; 3: 13. doi: 10.1186/1477-7819-3-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsai HW, Yuan CC, Wang PH. Umbilicus as the only site of metastasis in recurrent ovarian cancer. J Chin Med Assoc 2006; 69: 233–235. doi: 10.1016/S1726-4901(09)70225-4 [DOI] [PubMed] [Google Scholar]
- 9.Volz J, Köster S, Spacek Z. The influence of pneumoperitoneum used in laparoscopic surgery on an intraabdominal tumor growth. Cancer 1999; 86: 770–774. doi: [DOI] [PubMed] [Google Scholar]
- 10.Lee JW, Park YA, Cho YJ. The effect of surgical wound on ovarian carcinoma growth in an animal model. Anticancer Res 2013; 33: 3177–3184. [PubMed] [Google Scholar]
- 11.Zhang Y, Luo X, Fan B. Effect of CO2 pneumoperitoneum on the proliferation of human ovarian cancer cell line SKOV-3 and the expression of NM23-H1 and MMP-2. Arch Gynecol Obstet 2015; 291: 403–411. doi: 10.1007/s00404-014-3414-2 [DOI] [PubMed] [Google Scholar]
- 12.Lécuru F, Agostini A, Camatte S. Impact of pneumoperitoneum on tumor growth. Surg Endosc 2002; 16: 1170–1174. doi: 10.1007/s00464-001-9226-z [DOI] [PubMed] [Google Scholar]
- 13.Abu-Rustum NR, Sonoda Y, Chi DS. The effects of CO2 pneumoperitoneum on the survival of women with persistent metastatic ovarian cancer. Gynecol Oncol 2003; 90: 431–434. doi: 10.1016/S0090-8258(03)00330-5 [DOI] [PubMed] [Google Scholar]