Abstract
Objectives
Current antiretroviral therapy can suppress HIV replication, increase CD4 count and result in increased lifespan. However, it cannot eradicate the virus due to the presence of latent provirus in cellular reservoirs, such as resting CD4+ T cells. Using combination latency-reversing agents to shock the virus out of latency for elimination through immune clearance or viral cytopathic effect is one of the most promising strategies for HIV eradication. Specifically, recent evidence shows that isoform-selective histone deacetylase inhibitors may be more effective than their non-selective counterparts. Therefore, identification and characterisation of new isoform-selective compounds are of prime importance. Here, we sought to determine the ability of two new isoform-targeted hydroxamic acid derivatives to reactivate HIV from latency.
Methods
We used cell lines and infected primary resting CD4+ T cells. These were treated with these compounds with HIV reactivation measured using fluorescence-activated cell sorting, Western blots and luciferase luminescence. Isoform selectivity and acetylation of the HIV promoter were measured by Western blotting and chromatic immunoprecipitation.
Results and conclusions
The two new hydroxamic acid derivatives, MC2625 and MC1742, potently reactivate HIV from latency. These compounds are isoform-selective histone deacetylate inhibitors that increase the levels of histone acetylation at the HIV promoter. In addition, they synergise effectively with the protein kinase C modulators bryostatin-1 and INDY, an inhibitor of the dual-specificity tyrosine phosphorylation regulated kinase 1A. We conclude that the combinations of new hydroxamic acid derivatives and bryostatin-1 or INDY could be a new tool for HIV reactivation in the cure efforts.
Keywords: HIV, latency, isoform-targeted, histone deacetylase inhibitors
Introduction
Modern antiretroviral therapy successfully controls HIV replication to undetectable levels in compliant individuals. Despite its efficacy, medication has to be taken lifelong and does not eliminate a latent reservoir of provirus identified in memory CD4+ T cells and, possibly, other reservoirs [1–3]. Several strategies have been proposed to eliminate this reservoir, most importantly, the ‘shock-and-kill’ approach, whereby reactivation of the latent provirus might lead to the death of infected resting CD4+ T cells via viral cytopathic effects or immune clearance [4–6]. One of the promising molecules towards ‘shock and kill’ is histone deacetylase inhibitor (HDACi). These molecules reactivate viral production by facilitating histone acetylation, leading to chromatin unwinding and transcription. The most studied HDACi is the hydroxamic acid derivative vorinostat [suberoylanilide hydroxamic acid (SAHA)] [4,7–9].
However, an important drawback of SAHA and other HDACis currently in preclinical testing is their lack of efficacy in clinical studies. Isoform-selective HDACis could be made more potent, especially in combination with other latency-reversing agents (LRAs) [10,11]. In addition, they are less likely to be prone to off-target effects. While there are at least four different classes of histone deacetylases (HDACs) with over 15 distinct proteins, only a subset of class I HDACs (HDAC 1, 2 and 3) seem critical in terms of HIV latency [12,13]. Compounds such as SAHA inhibit most classes of HDACs. We and others have recently demonstrated that isoform-selective compounds can reactivate HIV and can be even more potent than their pan-HDACi counterparts [10,11]. The search for more specified HDACi is of importance as these molecules promise to disrupt fewer cellular processes than SAHA, potentially leading to fewer side effects. More importantly, multiple clinical studies have shown that a single-agent HDACi is unlikely to reduce the reservoir size [4,14,15]. Combination with other agents, such as protein kinase C (PKC) analogues, which are much more potent in vitro, is yet to go into human trials [16,17]. Here, we have characterised two novel molecules, MC2625 and MC1742, initially identified through virtual screening with enhanced comparative binding energy analysis [18] to be class I isoform-selective compounds. We selected these two compounds as they were predicted to be isoform selective, even though, like SAHA, they are of the hydroxamic acid family. Both compounds reactivate HIV from latency and increase histone acetylation at the level of the HIV promoter. Unlike SAHA, both MC2625 and MC1742 were shown to be relatively isoform specific. Furthermore, we have shown that they synergise with INDY, an inhibitor of the dual-specificity tyrosine phosphorylation-regulated kinase 1A (DYRK1A), and also with the PKC modulator, bryostatin-1.
Materials and methods
Primary cell studies
The Washington University Institutional Review Board (IRB) approved the study. All blood donors signed the IRB-approved informed consent.
Cell lines
The J-Lat 10.6 cells, a derivative of Jurkat T cells, were maintained in Roswell Park Memorial Institute (RPMI) 1640 medium plus 10% heat-inactivated fetal bovine serum (FBS), 200 nM L- glutamine and penicillin/streptomycin. They were obtained from the National Institutes of Health (NIH) AIDS Reagent Program and have been well described and extensively used in HIV reactivation studies [19,20]. Briefly, they are a clone of Jurkat cells infected with HIV-1R7E-GFP that express GFP at low levels at baseline. Upon stimulation with LRAs, cells express high GFP levels that can be measured by FACS. Cells were treated for 24 hours with the various LRAs, and FACS analysis was performed for GFP+ cells using the Becton Dickinson (Franklin Lakes, NJ, USA) FACS system. Cells typically diverged into two groups. Healthier cells to the right of the scatter plot were selected across the various treatments for the analysis.
Cellular viability assays
The MTT assay was used for all toxicity assays to check cell viability in response to the tested compounds [21]. The assay is based on the reduction of MTT dye to formazan by mitochondrial enzymes present in viable cells. Subsequent absorbance reading is proportional to the number of viable cells per well. J-Lat 10.6 cells or, where indicated, primary CD4+ T cells were incubated with compounds for 24 hours, after which they were exposed to MTT dye (Sigma, Saint Louis, MO, USA) for 3 hours. Cells were then lysed with a solubilisation buffer. The absorbance of each sample was measured at 570-nm wavelength using the Tecan microplate reader with the Magellan software (TECAN, Männedorf, Switzerland).
Western blots
J-Lat 10.6 cells or primary T cells were incubated with compounds at the indicated time points. Cells were lysed with 0.2% NP40 with protease/phosphatase inhibitors. Twenty to forty micrograms of protein was run on 12.5% agarose gel and then transferred to nitrocellulose for 1 hour. Membranes were blocked with 5% milk in 0.05% phosphate-buffered saline Tween 20 for 1 hour. Primary antibodies were incubated overnight at 4°C, followed by wash and horseradish peroxidase enzyme (HRP)-conjugated secondary antibodies incubated for 1 hour at room temperature. Signal was detected with Femto Super Signal (Thermo Fisher Scientific, Waltham, MA, USA). Actin was used as loading control.
Quantitative reverse-transcriptase polymerase chain reaction and chromatin immunoprecipitation
Total cell RNA extraction was performed with RNeasy Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. Real-time qRT-PCR was done with the one-step RT-PCR kit (Bio-Rad, Hercules, CA, USA) using the Bio-Rad CFX Connect system (Bio-Rad) with SYBR Green I. All reactions included a negative control in which no RT was added. Gene expression was calculated by the comparative CT method (2−ΔΔCt) using GAPDH as control. The gag primers used were f (5′-GACGCCTCGCACCCATCTC-3′) and r (5′-CTGAAGCGCGCACGGCAA-3′). ChIP was performed using a kit from Abcam (ab500; Cambridge, UK) according to the manufacturer's protocol. Briefly, J-Lat 10.6 cells were incubated with compounds for 24 hours. Immunoprecipitation was performed with monoclonal acetylated histone H3 antibody or monoclonal IgG antibody as isotype control. HIV-1 LTR was amplified by quantitative PCR from total chromatin (input) or immunoprecipitated DNA using the following primers: f: 5′-AGCCCTCAGATG CTACATATAAGCA-3′ and r: 5′-TAG CCAGAGAGCTCCCAGGCTCAG A-3′. GAPDH promoter primers were f: 5′-ATGGTTGCCACTGGGGATCT-3’ and r: 5′-TGCCAAAGCCTAGGGGAAGA-3′. The amount of amplified DNA was expressed as percent of the input. Data sets were normalised to input values using threshold numbers of cycles as follows: % input = 2(CTinput−CTIP) × 100.
Primary cell latency model
We have used the model described by Greene et al. [22]. Resting primary T cell were isolated and infected for 2 hours with HIV-1NL4-3 luciferase virus (a gift from Warner Greene) using spinoculation for 2 hours at 37°C. Cells were then washed three times, pulled together from the 96-well plates and incubated with 10 μM of darunavir for 48 hours. Cells were again washed, divided into a million cells each and incubated with the LRAs for 48 hours. This was done in the presence of the integrase inhibitor raltegravir to prevent new integration events. HIV reactivation was measured as luciferase luminescence or Western blots of cell lysates.
Primary cell isolation and culture
Peripheral blood mononuclear cells were isolated from blood donated by HIV seronegative participants using a Ficoll-Hypaque gradient (GE Healthcare, Chicago, IL, USA). The EasySep human CD4+ T cell Enrichment Kit (STEMCELL Technologies, Vancouver, BC, Canada) was used for CD4+ T cell isolation. Cells were further purified to resting CD4+ T cells where needed by depleting cells expressing CD25 using a negative selection kit from STEMCELL Technologies according to the manufacturer's instructions. Cells were maintained in RPMI with 10% FBS after isolation. The purity of CD4+ T cells was verified by flow cytometry and was generally found to be 98%.
Chemicals and antibodies
The following chemicals were obtained from Sigma: bryostatin-1 and SAHA (vorinostat). CD3/CD28 beads were from Thermo Fisher Scientific (Waltham, MA, USA). Fluorescein isothiocyanate (FITC)-conjugated anti-human CD69 (clone FN50, cat # 11-0699-41) and allophycocyanin (APC)-conjugated anti-human CD25 (clone BC96, cat # 17-0259-41) were from eBioscience (San Diego, CA, USA). Mouse isotype control IgG (5415S), acetylated tubulin Lys 40 (#6-11B-1), rabbit monoclonal NF-κB p65 (D14E12) and acetylated histone H3 Lys9/Lys14 (#9677) antibodies were obtained from Cell Signaling (Danvers, MA, USA). Goat polyclonal actin (SC1615) and donkey anti-goat IgG HRP (SC-2020) were from Santa Cruz Biotechnology Inc (Dallas, TX, USA). Monoclonal p24 gag antibody was obtained through the NIH AIDS Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases, NIH: anti-HIV-1 p24 hybridoma (183-H12-5C) from Dr Bruce Chesebro [19]. The HDACis were designed with a computational ligand screening analysis based on comparative binding energy [20]. The organic synthesis of MC1742 and MC2625 has been previously described [22].
Bliss independence model and isobole analysis
We used the Bliss independence model, which is one of the ways to differentiate between additive and synergistic effects of two combined compounds, which has been previously described [10,23]. The second method for evaluating drug synergism that we used was isobole analysis [24]. With this method, a separate response curve is generated for the drug A effect at each of several drug B concentrations. The drug B concentration at which drug A achieves 50% of the maximum response is calculated according to each response curve. These values are then plotted on a graph of (concentration of drug A, concentration of drug B) with a new curve along which 50% of the maximum response consistently arises; this new curve is called an isobole. A straight or nearly straight line is generated if the two drugs are additive, and a concave curve is generated if the two are synergistic.
Statistics
Student's t-test was used for pairwise comparisons and one-way analysis of variance was performed for multiple comparisons using the GraphPad Prism software (GraphPad Software Inc, La Jolla, CA, USA). A P-value of <0.05 was considered significant.
Results
New hydroxamic acid derivatives reactivate HIV from latency
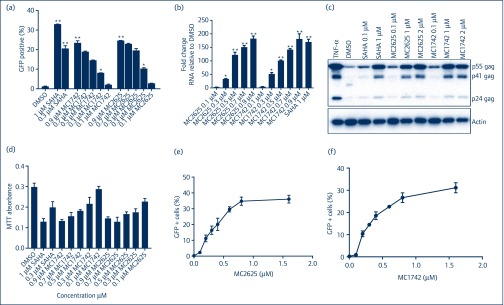
To test the ability of these compounds (Figure 1) to reactivate HIV from latency, we used the well-described JLAT 10.6 cells [19,20]. These Jurkat-derived cells have HIVΔenvGFP integrated at one position in the genome with GFP in place of the Nef gene. At baseline, HIV is latent and GFP expression is undetectable. Upon activation with LRAs, HIV reactivation can be measured by GFP+ cells using fluorescence-activated cell sorting (FACS). As shown in Figure 2a, there was a dose-dependent increase in HIV reactivation for both MC1742 and MC2625, with HIV reactivation increasing from baseline below 3%–25%. To use a different method to measure HIV reactivation, we measured HIV-1 gag mRNA by quantitative reverse-transcriptase polymerase chain reaction (qRT-PCR) in the JLAT 10.6 cells. Figure 2b shows that HIV mRNA was increased up to 180-fold in the presence of MC2625 or MC1742. To determine if the HIV reactivation observed via FACS and qRT-PCR correlated with HIV products, we have measured HIV-1 Gag protein using Western blots. Again, there was a dose-dependent increase of the protein upon treatment with these compounds (Figure 2c). Toxicity evaluation in the JLAT 10.6 cells produced levels similar to those of SAHA (Figure 2d). EC50 determinations (Figure 2e,f) showed that MC2625 and MC1742 were of similar potency (EC50, 350nM). This data confirmed that these two new hydroxamic compounds are HIV latency-reversing agents comparable to SAHA.
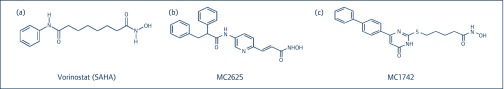
Figure 1.
Structure of (a) SAHA (vorinostat) compared to the new hydroxamic acid derivatives (b) MC2625 and (c) MC1742 presented in this paper. SAHA: suberoylanilide hydroxamic acid
Figure 2.
MC2625 and MC1742 reactivate HIV from latency. (a) J-Lat 10.6 cells were incubated with compounds at indicated concentrations for 24 hours. Percent green fluorescent protein positive (GFP+) GFP+ cells were measured by FACS and were used as a measure of HIV reactivation from latency. SAHA and DMSO were used as positive and negative controls, respectively. (b) J-Lat 10.6 cells were incubated with the indicated compounds for 24 hours, and HIV-1 mRNA was quantified by quantitative reverse-transcriptase polymerase chain reaction with primers for gag, using GAPDH as internal control. Fold change is relative to DMSO. (c) J-Lat 10.6 cells were incubated with the compounds for 24 hours, and Western blots were performed for HIV-1 gag and actin. (d) J-Lat 10.6 cells were incubated with the indicated compounds for 24 hours, and MTT toxicity assay was performed as described in Materials and methods. (e,f) Measurement of EC50 for MC2625 and MC1742. J-Lat 10.6 cells were incubated with increasing concentrations of compounds and FACS for GFP+ cells performed previously. Data show means, and error bars indicate ±SEM, n = 3. **P < 0.001, *P < 0.05. Student's t-test was used for pairwise comparisons with DMSO. DMSO: dimethylsulphoxide; FACS: fluorescence-activated cell sorting; MTT: 3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide; SAHA: suberoylanilide hydroxamic acid; TNF-α:, tumour necrosis factor-α
MC1742 and MC2625 reactivate latent HIV in a primary cell model of latency
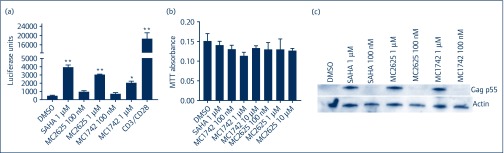
Next, we examined if these compounds could reactivate HIV in a primary cell model of latency. For this, we used the model described by Greene et al. [21]. Briefly, resting CD4+ T cells were infected with full-length HIV-Luc by spinoculation for 2 hours, washed and allowed to incubate with protease inhibitor (darunavir) for 48 hours. Cells were washed again and treated with various LRAs for 24 hours in the presence of integrase inhibitor raltegravir. HIV reactivation was measured by luciferase luminescence in cell lysates. As shown in Figure 3a, there was minimal background luciferase detected with dimethylsulphoxide, while global activation of the T cells using CD3/CD28 beads led to extensive reactivation. Both MC2625 and MC1742 showed significant dose-dependent reactivation of HIV in the primary cell latency model with 6.4- and 4.3-fold increases, respectively (Figure 3a). Notably, these compounds were non-toxic to primary CD4+ T cells up to 10 μM in concentration, which showed about 20% cell loss (Figure 3b). To confirm the production of HIV products in the primary cell latency system, we measured HIV Gag by Western blots. As shown in Figure 3c, HIV gag p55 could be detected with SAHA and the new compounds at 1 μM concentration. We could not detect Gag products p44 and p24 as we did in Figure 2c, most likely due to the low amounts of Gag produced in the primary cell system. Together, the data show that these hydroxamic acid-derived HDACis are potent LRAs.
Figure 3.
MC1742 and MC2625 induce HIV reactivation in a primary cell latency model (Greene model). (a) Resting CD4+ T cells were infected with replication-competent HIV-Luc and were treated with the protease inhibitor darunavir for 2 days. Cells were incubated with the compounds shown for 24 hours in the presence of raltegravir (see Materials and methods for details). HIV reactivation was measured using luciferase luminescence in infected cell lysates normalised to total protein concentration. (b) Toxicity profile of MC1742 and MC2625 in resting T cells. Primary resting T cells were incubated with the indicated compounds for 48 hours, and the MTT toxicity assay was performed as described in Materials and methods. (c) Primary resting CD4+ T cells prepared as in (a) were reactivated for 24 hours, and Western blots were performed for gag and actin. Data indicate means, and error bars indicate ±SEM, n = 3. **P < 0.001, *P < 0.05. Student's t-test was used for pairwise comparisons with DMSO. DMSO: dimethylsulphoxide; MTT: 3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide; SAHA: suberoylanilide hydroxamic acid
MC1742 and MC2625 are isoform selective and enhance histone H3 acetylation at the HIV promoter
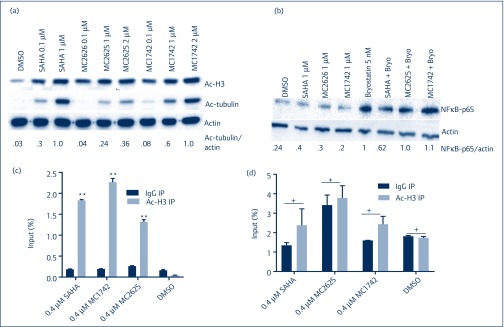
We then proceeded to test the HDAC class selectivity for these compounds. We used acetylation of histone H3 (class I HDAC) and acetylation of tubulin, which measures HDAC 6 (class II HDAC) inhibition. We treated primary resting CD4+ T cells with increasing concentrations of compounds for 8 hours and measured acetylated H3 and tubulin. At a dose of 1 μM, SAHA treatment resulted in the acetylation of both histone H3 and tubulin, as expected for a pan-HDACi (Figure 4a). At the same concentration, both MC2625 and MC1742 increased levels of acetylated histone H3 without significant increases in tubulin. When the dose was doubled to 2 μM, MC1742, but not MC2625, showed tubulin acetylation. These data demonstrate that while both compounds are class I isoform targeted at concentrations that can induce HIV reactivation, MC2625 is more selective than MC1742 in resting CD+ T cells (Figure 4a). Since SAHA has been shown to inhibit nuclear factor-kappa B (NF-κB) activation when combined with bryostatin-1 [11], we tested these compounds for their effect on NF-κB p65 activation. Incubation of resting T cells with SAHA and byrostatin-1 showed about 40% reduction in active p65 levels (Figure 4b). However, combination of MC2625 or MC1742 with bryostatin-1 showed no difference in active p65 levels similar to that with entinostat, a benzamide isoform-selective HDACi [11]. This showed that the more selective hydroxamic acid derivatives do not inhibit NF-κB when combined with bryostatin-1. To determine if the compounds influenced HIV promoter acetylation, we performed chromatin immunoprecipitation (ChIP) using specific antibody for acetylated histone H3 and HIV-1 long-terminal repeat (LTR) primers. Figure 4c shows that both compounds, like SAHA, enriched the portion of Ac-H3 present at the HIV LTR without increasing acetylation at the glyceraldehyde-3 phosphate dehydrogenase (GAPDH) promoter (Figure 4d). Together, these data confirm the new hydroxamic compounds as isoform-selective HDACis that increase histone acetylation at the HIV promoter.
Figure 4.
Isoform selectivity for hydroxamic acid compounds and accumulation of acetylated histone H3 at the HIV promoter. (a) Compounds were incubated with resting primary CD4+ T cells for 8 hours. Acetylated histone H3 (a measure of class I HDAC) and acetylated tubulin (a measure of class II HDAC) were resolved on WBs. Quantification of acetylated tubulin over actin was normalised to SAHA. (b) MC2625 and MC1742 do not inhibit NF-κB activation. Primary CD4+ T cells were incubated with the indicated compounds with or without 5 nM of byrostatin-1 for 24 hours, and WB was performed for NF-κB p65 and actin. Quantification of p65 over actin was normalised to byrostatin-1. (c,d) Chromatin immunoprecipitation using Ac-H3 antibody for HIV LTR (c) or glyceraldehyde-3 phosphate dehydrogenase promoter (d). J-Lat 10.6 cells were incubated with DMSO or the indicated compounds for 24 hours, and ChIP was performed using specific antibody for acetylated histone H3 or IgG. Data indicate means, and error bars indicate ±SEM, n = 3. **P < 0.001. Student's t-test was used for pairwise comparisons with IgG. DMSO: dimethylsulphoxide; HDAC: histone deacetylase; NF-κB: nuclear factor-kappa B; SAHA: suberoylanilide hydroxamic acid; WB: Western blot
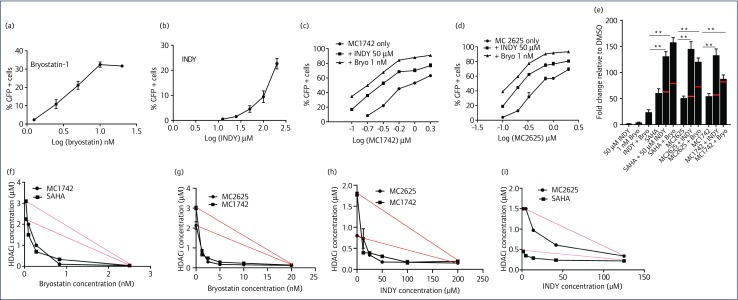
MC1742 and MC2625 synergise with bryostatin-1 and INDY
Single-compound activation of latent HIV is unlikely to reactivate enough virus to reduce the HIV reservoir size. It has been suggested that a combination of several agents is more likely to be successful. So far, some of the most potent reactivation agents are combinations of HDACis and PKC agonists like bryostatin-1 [10,16,25]. Therefore, we determined if the new compounds had synergy with bryostatin-1 and the DYRK1A inhibitor INDY, found previously to reactivate HIV from latency and prime T cells for reactivation by bryostatin-1 [26,27]. High doses of bryostatin-1 result in excellent reactivation of HIV from latency, but dose is limited by cellular proliferation and toxicity. We have performed a titration of the new HDACis with constant doses of bryostatin-1 or INDY and measured HIV reactivation with percentages of GFP+ cells. Since byrostain-1 and INDY do not show much reactivation at the concentrations of 1 nM and 50 μM, respectively (Figure 5a,b), the responses we observed looked synergistic rather than additive (Figure 5c,d). To determine if this effect was synergistic, we used the Bliss independence model (see Materials and methods). We treated J-Lat 10.6 cells with a constant dose of MC2625 or MC1742 in the presence or absence of bryostatin-1 and INDY and measured HIV reactivation using qRT-PCR. As shown in Figure 5e, experimental results for the combined treatment exceeded those predicted by the Bliss independence model (red lines), indicating a synergistic rather than additive effect. To evaluate synergy in an even more robust manner, we used an isobole analysis [24,28]. With J-Lat 10.6 cell activation as the output, we generated individual response curves to SAHA and MC2625 at five different INDY concentrations and similarly for SAHA and MC1742 individually using different bryostatin-1 concentrations. For each concentration of INDY or bryostatin-1, we calculated the concentration of HDACi at which 45% of JLAT 10.6 cells were activated (=50% of maximum J-Lat 10.6 activation, assuming maximum JLAT 10.6 activation to be 90%) along the curve and then plotted this concentration against INDY or bryostatin-1 concentration on a new graph to generate the isobole. If two drugs are additive, the isobole is predicted to be linear or nearly linear (red lines) and concave if synergistic. For all the four combinations evaluated, we observed concavity underneath the predicted isobole for additive action (Figure 5f–i). This confirms that these combinations were synergistic.
Figure 5.
New hydroxamic acid-derived compounds synergise with bryostatin-1 and dual-specificity tyrosine phosphorylation-regulated kinase 1A inhibitor INDY. (a,b) Dose response for byrostatin-1 and INDY in J-Lat 10.6 cells. (c,d) J-Lat 10.6 cells were incubated with increasing HDACi concentration and constant concentration of either 1 nm of bryostatin-1 or 50 μM of INDY. The reactivation of HIV was measured by fluorescence-activated cell sorting of green fluorescent protein (GFP+) cells. (e) J-Lat 10.6 cells were incubated with the indicated compounds, and HIV reactivation was measured by qRT-PCR of gag mRNA. HDACi concentrations were 0.33 μM for SAHA and 0.60 μM for MC2625 and MC1742. Red lines indicate predicted effect by the Bliss independence model. (f–i) Isobole curves for synergy HDAC inhibitors and INDY or bryostatin-1. Red lines are predictions from the model if the compounds were additive. Data indicate means, and error bars indicate ±SEM, n = 3. **P < 0.001, analysis of variance. DMSO, dimethylsulphoxide; HDACi, histone deacetylase inhibitor; SAHA, suberoylanilide hydroxamic acid
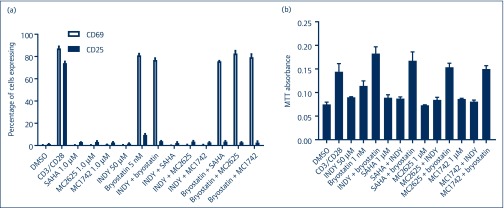
MC1742 and MC2625 do not activate resting T cells
An important attribute of a good LRA is HIV reactivation from latency without global T cell activation. To investigate if these compounds influence T cell activation, we used primary CD4+ T cells depleted of activated CD25+ cells. After incubation with the various compounds, FACS was performed for CD25+ (as a marker for highly activated T cells) and CD69 (an early activation marker) to quantify the cellular activation status. As shown in Figure 6a, these compounds did not activate resting T cells at concentrations that reactivated HIV from latency in resting cells. In combination with 1 nM of bryostatin-1, activation of CD69 was observed as previously observed [16]. Also, these combinations did not have a toxic effect on resting CD4+ T cells as measured by the 3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide (MTT) assay (Figure 6b). However, proliferative effects of CD3/CD28 and bryostatin-1 were observed (Figure 5b). Therefore, MC2625 and MC1742 reactivate HIV from latency without activating T cells, making them potentially useful LRAs.
Figure 6.
Combinations of hydroxamic acid derivatives with INDY or bryostatin-1 do not activate primary resting T cells. (a) Primary resting CD4+CD25− cells were isolated from HIV-negative blood donors and incubated with the indicated compounds for 24 hours. Cells were incubated with CD25 or CD69 antibody, and fluorescence-activated cell sorting was performed. (b) Toxicity profile of the latency-reversing agent combinations in primary T cells. Resting T cells were incubated with the indicated compounds, and MTT toxicity assay was performed as in Materials and methods. DMSO: dimethylsulphoxide; MTT: 3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide; SAHA: suberoylanilide hydroxamic acid
Discussion
In terms of the present HIV cure efforts, the search is ongoing for a potent LRA that could reactivate HIV from latency in an efficient manner with minimal off-target effects. Novel compounds used in different combinations need to be identified and tested to increase the chances of finding an ideal LRA that will work in the clinical setting. Here, we have shown that two new hydroxamic acid derivatives, MC2625 and MC1742, are isoform-selective LRAs that enhance histone H3 acetylation at the HIV promoter but do not activate resting T cells. Moreover, these compounds show synergy with bryostatin-1 and INDY, the DRYK1A inhibitor.
Although these compounds were slightly less potent than SAHA, the advantage is their relative isoform selectivity. The induction of class I HDACs, which are more relevant for HIV, is expected to result in fewer side effects when tested in humans. In support of this, Zaikos et al. have shown that SAHA can inhibit transcription factors that promote HIV reactivation when combined with bryostatin-1 [11]. We recently identified largazoles as isoform-selective class I specific HDACis, which are more potent than SAHA with excellent synergy with bryostatin-1 and its analogues [10]. Here, we have added the two hydroxamic acid derivatives as potent LRAs, thus increasing the repertoire of compounds that could be clinically tested for HIV reactivation. Although PKC agonists are more potent as LRAs, their use remain limited due to perceived dose-dependent side effects and potential cellular proliferation [29]. The fact that the two compounds described here synergised with very low doses of bryostatin-1 (1 nM) without inhibiting NF-κB activation is therefore reassuring.
We have also observed a synergy between these new compounds and INDY. The role that DYRK1A plays in HIV latency is just beginning to be explored. Although its inhibition alone has little effect on HIV reactivation at low doses, cells respond exponentially when combined with DYRK1A. In addition, DYRK1A inhibition has been shown to prime resting T cells for reactivation by a mechanism that is still undetermined [27]. The finding that DYRK1A depletion helps T cells transition to a more active phenotype may offer clues as to their effect on HIV latency [30]. Ongoing work is looking at the role DYRK1A plays in HIV-infected resting T cells. Since a single agent is unlikely to make a significant dent in the HIV reservoir, the search for new combinations of compounds with different mechanisms of action on HIV latency should continue. The identification of MC2625 and MC1742, in combination with bryostatin-1 and INDY, is a step in this direction.
Acknowledgements
Funding
GBK is a recipient of the Harold Amos Medical Faculty Development grant from the Robert Woods Johnson Foundation. This work is supported by NIH K08 grant 1K08 AI120854-01 awarded to GBK. The funders had no role in the experimental design, data analysis or writing of the paper.
References
- 1. Chun TW, Engel D, Berrey MM et al. Early establishment of a pool of latently infected, resting CD4(+) T cells during primary HIV-1 infection. Proc Natl Acad Sci U S A 1998; 95: 8869– 8873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lorenzo-Redondo R, Fryer HR, Bedford T et al. Persistent HIV-1 replication maintains the tissue reservoir during therapy. Nature 2016; 530: 51– 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Davey RT Jr, Bhat N, Yoder C et al. HIV-1 and T cell dynamics after interruption of highly active antiretroviral therapy (HAART) in patients with a history of sustained viral suppression. Proc Natl Acad Sci U S A 1999; 96: 15109– 15114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Archin NM, Liberty AL, Kashuba AD et al. Administration of vorinostat disrupts HIV-1 latency in patients on antiretroviral therapy. Nature 2012; 487: 482– 485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Deeks SG, Lewin SR, Ross AL et al. International AIDS Society global scientific strategy: towards an HIV cure 2016. Nat Med 2016; 22: 839– 850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Xing S, Siliciano RF. Targeting HIV latency: pharmacologic strategies toward eradication. Drug Discov Today 2013; 18: 541– 551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Archin NM, Bateson R, Tripathy MK et al. HIV-1 expression within resting CD4+ T cells after multiple doses of vorinostat. J Infect Dis 2014; 210: 728– 735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Archin NM, Kirchherr JL, Sung JA et al. Interval dosing with the HDAC inhibitor vorinostat effectively reverses HIV latency. J Clin Invest 2017; 127: 3126– 3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sung JA, Sholtis K, Kirchherr J et al. Vorinostat renders the replication-competent latent reservoir of human immunodeficiency virus (HIV) vulnerable to clearance by CD8 T cells. EBioMedicine 2017; 23: 52– 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Albert BJ, Niu A, Ramani R et al. Combinations of isoform-targeted histone deacetylase inhibitors and bryostatin analogues display remarkable potency to activate latent HIV without global T-cell activation. Sci Rep 2017; 7: 7456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zaikos TD, Painter MM, Sebastian Kettinger NT et al. Class 1-selective histone deacetylase (HDAC) inhibitors enhance HIV latency reversal while preserving the activity of HDAC isoforms necessary for maximal HIV gene expression. J Virol 2018; 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Barton KM, Archin NM, Keedy KS et al. Selective HDAC inhibition for the disruption of latent HIV-1 infection. PLoS One 2014; 9: e102684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Huber K, Doyon G, Plaks J et al. Inhibitors of histone deacetylases: correlation between isoform specificity and reactivation of HIV type 1 (HIV-1) from latently infected cells. J Biol Chem 2011; 286: 22211– 22218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Harper KN. Romidepsin reverses HIV-1 latency in vivo. AIDS 2016; 30: N3. [DOI] [PubMed] [Google Scholar]
- 15. Sogaard OS, Graversen ME, Leth S et al. The depsipeptide romidepsin reverses HIV-1 latency in vivo. PLoS Pathog 2015; 11: e1005142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bullen CK, Laird GM, Durand CM et al. New ex vivo approaches distinguish effective and ineffective single agents for reversing HIV-1 latency in vivo. Nat Med 2014; 20: 425– 429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Marsden MD, Wu X, Navab SM et al. Characterization of designed, synthetically accessible bryostatin analog HIV latency reversing agents. Virology 2018; 520: 83– 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Silvestri L, Ballante F, Mai A et al. Histone deacetylase inhibitors: structure-based modeling and isoform-selectivity prediction. J Chem Inf Model 2012; 52: 2215– 2235. [DOI] [PubMed] [Google Scholar]
- 19. Jordan A, Bisgrove D, Verdin E. HIV reproducibly establishes a latent infection after acute infection of T cells in vitro. EMBO J 2003; 22: 1868– 1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Spina CA, Anderson J, Archin NM et al. An in-depth comparison of latent HIV-1 reactivation in multiple cell model systems and resting CD4+ T cells from aviremic patients. PLoS Pathog 2013; 9: e1003834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 1983; 65: 55– 63. [DOI] [PubMed] [Google Scholar]
- 22. Lassen KG, Hebbeler AM, Bhattacharyya D et al. A flexible model of HIV-1 latency permitting evaluation of many primary CD4 T-cell reservoirs. PLoS One 2012; 7: e30176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhao W, Sachsenmeier K, Zhang L et al. A new Bliss independence model to analyze drug combination data. J Biomol Screen 2014; 19: 817– 821. [DOI] [PubMed] [Google Scholar]
- 24. Tallarida RJ. Revisiting the isobole and related quantitative methods for assessing drug synergism. J Pharmacol Exp Ther 2012; 342: 2– 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Archin NM, Margolis DM. Emerging strategies to deplete the HIV reservoir. Curr Opin Infect Dis 2014; 27: 29– 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Booiman T, Loukachov VV, Dort KA et al. DYRK1A controls HIV-1 replication at a transcriptional level in an NFAT dependent manner. PLoS One 2015; 10: e0144229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Seu L, Sabbaj S, Duverger A et al. Stable phenotypic changes of the host T cells are essential to the long-term stability of latent HIV-1 infection. J Virol 2015; 89: 6656– 6672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tallarida RJ. Quantitative methods for assessing drug synergism. Genes Cancer 2011; 2: 1003– 1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Marsden MD, Loy BA, Wu X et al. In vivo activation of latent HIV with a synthetic bryostatin analog effects both latent cell ‘kick’ and ‘kill’ in strategy for virus eradication. PLoS Pathog 2017; 13: e1006575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Thompson BJ, Bhansali R, Diebold L et al. DYRK1A controls the transition from proliferation to quiescence during lymphoid development by destabilizing Cyclin D3. J Exp Med 2015; 212: 953– 970. [DOI] [PMC free article] [PubMed] [Google Scholar]