Abstract
Hepatitis C virus (HCV) infection represents one of the major public health challenges worldwide. HCV is a blood-borne pathogen associated with a high rate of mortality and imposes a dramatic societal and economic burden on health systems. Untreated chronic HCV infection can progress to liver cirrhosis and cancer. Lessons can be learned from countries such as Egypt and Georgia that are considered to be ‘on-track’ for the World Health Organization HCV elimination targets, as well as countries such as Iran that are ‘working towards elimination’. This article compares HCV-related policies and strategies in Iran, Egypt and Georgia to identify programme strengths and limitations that could inform policy and decision makers in Iran. Controlling and eliminating HCV remain a serious public health challenge. The rising HCV incidence could generate a dramatic economic burden in the coming years. Therefore, Iran requires a strategic plan to fight HCV. Adequate cultural and social infrastructures are needed. Centres specifically devoted to the diagnosis and management of this infection should be used for screening and delivery of inexpensive and high-quality testing. Quick initiation of treatment should take place at lower costs to facilitate access to treatment.
Keywords: hepatitis C virus, content analysis, health policies, Iran, Egypt, Georgia
Introduction
Hepatitis C virus (HCV) is a blood-borne pathogen responsible for significant morbidity and mortality, and imposes an important societal and economic burden for health systems [1]. Untreated chronic HCV infection can progress to liver cirrhosis in 20%–30% of cases [2,3] and to cancer with a rate of approximately 3.5% per year, and a cumulative risk ranging from 5% to 30% within 5 years [4–6]. The current HCV standard of care includes regimens based on direct-acting antivirals (DAAs), which not only are safer but also, importantly, can achieve cure rates above 95% with a shorter treatment duration (usually 12 weeks) compared with other conventional therapies (pegylated interferon and ribavirin). However, their high costs are a major barrier to access.
The Global Burden of Disease of the Institute for Health Metrics and Evaluation (IHME) reported viral hepatitis to be the 10th cause of death in 1990 and the 7th in 2013 [7]. HCV accounts for 48% of global viral hepatitis-related mortality.
The HCV economic burden worldwide is significantly increasing. For example, HCV-related costs in the USA have been estimated to be more than $10 billion per year [8]. Costs related to HCV include treatment costs, as well as indirect ones, such as reduced productivity, unemployment and poor quality of life [9,10]. In a systematic review, incremental annual indirect costs associated with HCV in untreated individuals compared with non-HCV ones were estimated to be €4209 in the USA and ranged from €280 to €659 [11] in five European countries, depending on the type of healthcare system.
The World Health Organization (WHO) report released in April 2017 on the status of hepatitis worldwide documented that 1.75 million new cases of HCV infection occurred in 2015, with about 71 million people already infected. According to this report, the Eastern Mediterranean region has the highest HCV prevalence rate in the world [12]. Prevention, screening and treatment are essential for managing HCV since they improve quality of life and lead to lower costs. Globally, health policy and decision makers are working towards improving the health and quality of life of people by means of policies, strategies and effective plans to reduce the incidence of illnesses [13,14].
To achieve HCV elimination, the health sector must use its full potential to control the disease. Despite efforts made by many countries, the incidence of this disease is increasing worldwide. Unfortunately, the Millennium Development Goals (MDGs) – a collection of eight international development goals for the year 2015 established after the Millennium Summit of the United Nations (UN) in 2000 – did not pay particular attention to viral hepatitis [15], whereas in the Sustainable Development Goals (SDGs) – a collection of 17 global goals set by the UN General Assembly in 2015 – HCV was taken into full consideration. According to SDGs, countries should eliminate HCV by 2030, utilising appropriate policies and strategies [16,17]. Between 2015 and 2030, WHO targets include curbing new HCV infections by 80% and mortality by 65%, and increasing HCV diagnoses to 90% and the number of eligible persons under treatment to 80%. Countries have been implementing various policies in the three above-mentioned areas: prevention, screening and treatment [18–20]. Iran is one of the countries that aim to reach the ambitious goal of HCV elimination.
Each country has specific policies to control HCV, with strengths and weaknesses, which other countries can learn from. In this article, we chose to focus on Egypt and Georgia, which are examples of two countries considered to be ‘on-track’ for WHO HCV elimination targets (the other 10 ones being Australia, Brazil, Germany, Iceland, Japan, the Netherlands, Qatar, Italy, Spain, Switzerland, the UK and Mongolia), and on Iran, which is ‘working towards elimination’ [21]. These three countries present similar healthcare systems and socio-economic status.
This article compares HCV-related policies and strategies in Iran, Egypt and Georgia to assess the strengths and limitations of these programmes.
Methods
To review policies and strategies, we conducted a content analysis based on policy documents available in Iran, Egypt and Georgia from January 2000 to May 2018. We searched different scholarly electronic databases (Embase, PubMed/MEDLINE, Scopus and ISI/Web of Science), as well as the websites of the Ministries of Health, the WHO and Google. Our search strategy was as follows: (‘hepatitis’ OR ‘viral hepatitis’ OR ‘hepatitis C virus’ OR ‘hepatitis C’ OR ‘HCV’) AND (‘policy’ OR ‘policies’ OR ‘health policy’ OR ‘strategy’ OR ‘strategies’ OR ‘health plans’ OR ‘programmes’) AND (‘Iran’ OR ‘Egypt’ OR ‘Georgia’). Policy documents were included for examining and assessing policies and strategies related to HCV in these countries, taking into account the three dimensions of prevention, screening and treatment. Specifically, the following items were considered in the policy documents: policies related to disease prevention, education and awareness, individuals’ treatment, evidence-based guidelines and documents related to financial issues. There were also policy documents that did not refer to HCV programmes or duplicate items, which were subsequently excluded. A collection of 73 documents was retrieved, and, finally, 16 of them were included. Two authors (MB and NLB) independently searched and selected relevant documents. Policy document content was reviewed by three authors (HAG, AR and MB). Disagreement concerning the content of documents was resolved through discussion. The content of the documents was extracted and synthesised according to three sections: prevention, screening and treatment, carried out in connection with them, including the outcome of the programmes.
Results
Epidemiological overview
The various policies and programmes adopted by Iran, Egypt and Georgia regarding the three HCV-related areas of prevention, screening and treatment are listed in Tables 1 and 2.
Table 1.
The policies related to HCV infection adopted in Iran, Egypt and Georgia
| Policies and programmes | Iran | Egypt | Georgia |
|---|---|---|---|
| National policy plan for HCV | No | Yes | No |
| Availability of epidemiological data | Yes | Yes | Yes |
| Evidence-based policy and data suitable for action | Yes | Yes | No |
| Estimate of economic burden of HCV | Yes | Yes | Yes |
| Knowledge and awareness of HCV among the general population and high-risk groups | Yes | Yes | Yes |
| Screening for high-risk groups | Yes | Yes | Yes |
| Prevention strategies for PWID | Yes | Yes | Yes |
| Treatment inclusion of PWID | Yes | Yes | Yes |
| Treatment guidelines for HCV | Yes | Yes | Yes |
| Strategies for harm reduction | Yes | Yes | Yes |
| Promoting partnerships | No | Yes | Yes |
| Mobilising resources | No | Yes | Yes |
| Use of financial resources of international organisations | Yes | Yes | Yes |
| Collaboration with international organisations | No | Yes | Yes |
| National policy on injection safety | Yes | Yes | Yes |
| National infection control policy for blood banks | Yes | Yes | Yes |
| Publicly funded treatment | No | Yes | Yes |
| National essential medicines list or HCV drugs subsidised by the government | Yes | Yes | Yes |
| Surveillance system for HCV | Yes | Yes | Yes |
| Harm reduction policy for high-risk groups | No | Yes | Yes |
| HCV prevalence among males (2016) | 233,868.3 | 4,002,706.5 | 118,827.9 |
| HCV prevalence among females (2016) | 195,453.9 | 3,757,236 | 129,653.1 |
| Overall prevalence rate among the general population (%) | 0.6 | 11.9 | 7.7 |
HCV, hepatitis C virus; PWID, people who inject drugs.
Table 2.
Prevention, screening and treatment strategies related to HCV infection adopted in Iran, Egypt and Georgia
| Strategy | Georgia | Egypt | Iran |
|---|---|---|---|
| Prevention |
|
|
|
| Screening |
|
|
|
| Treatment |
|
|
|
DCV, daclatasvir; HCV, hepatitis C virus; NGO, non-governmental organization; SOF, sofosbuvir.
Iran
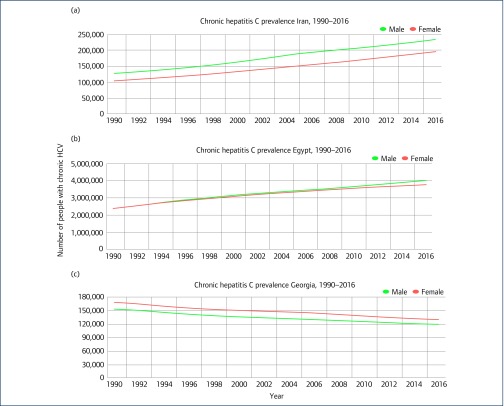
Iran is located in the Middle East/Asia and is a middle-income country. According to the IHME in 2016, there were 233,868 men and 195,453 women with chronic HCV [22]. Figure 1a shows the trend of people who lived with chronic HCV in the country between 1996 and 2016. According to a meta-analysis published in 2017 pooling studies conducted between 2001 and 2015, the HCV rate in the general population was 0.6% [23]. In another meta-analysis among blood donors published in 2013, summarising the literature for the period of 1996–2011, HCV prevalence was estimated at 0.5% [24]. In a systematic review and meta-analysis of HCV prevalence in 2000 and 2017 in individuals with thalassemia, the HCV rate was 19% [25], while in another meta-analysis, the HCV prevalence among Iranian prisoners was 28% [26]. Finally, in a further meta-analysis, its prevalence in people who inject drugs (PWID) was reported to be 45% [27].
Figure 1.
The numbers of people living with chronic HCV in Iran, Egypt and Georgia between 1996 and 2016
Egypt
Egypt is located in Africa and is a low- to middle-income country. According to WHO, in 2017 Egypt had the highest HCV prevalence rate in the world [12]. According to the IHME in 2016, there were 4,002,706 men and 3,757,236 women with chronic HCV [22]. Figure 1b shows the trend of people who live with chronic HCV in the country between 1996 and 2016. Based on the results of a meta-analysis, HCV prevalence was estimated to be 11.9% among the general population (populations at relatively low risk of exposure to HCV, such as healthy children, blood donors, antenatal clinic attendees and pregnant women), 55.6% among populations at high risk (PWID, hemodialysis, multitransfused individuals and hemophiliacs), 14.3% among populations at intermediate risk (household contacts of HCV-infected individuals, prisoners, individuals with diabetes and healthcare workers), 56.0% among populations with liver-related conditions (acute viral hepatitis, liver cirrhosis, chronic liver disease, hepatocellular carcinoma and non-Hodgkin's lymphoma) and 35.0% among special clinical populations (dermatological manifestations, rheumatologic disorders and non-liver-related malignancies) [28].
Georgia
The country is located in the Caucasus region of Eurasia, at the crossroads of Western Asia and Eastern Europe, and is classified as a low- to middle-income country. Its HCV prevalence is 7.7% (5.4% tested positive for active infection by PCR) [29]. According to the IHME in 2016, there were 118,827 men and 129,653 women with chronic HCV [22]. Figure 1c shows the trend of people who lived with chronic HCV in the country between 1996 and 2016. In a study in 2015, the prevalence of HCV in PWID was 92% [30]. According to the Ministry of Health, the prevalence of HCV in prisoners was 52%. In another study, prevalence of HCV in HIV-positive individuals was 48.57% [31].
Discussion
This article compares the HCV prevalence and burden in Iran, Egypt and Georgia, as well as their strategies for management and treatment of the infection [12].
Inadequate health policies can negatively affect health in a community. Furthermore, many individuals have inadequate information about HCV [32]. One of the best policies to reduce its incidence includes programmes to increase public information, especially among high-risk target populations [33,34]. Increasing HCV awareness may reduce its stigmatisation in communities [35].
Policy and decision makers in Georgia were faced with a high prevalence of HCV and decided in 2015 to put it at the top of the country's public health agenda [36]. The Ministry of Labor, Health and Social Affairs (MOLHSA) initiated evidence-based policies and programmes to diagnose HCV in at-risk groups (men aged 30 to 59, PWID, prisoners and people with a history of receiving blood products) [37]. One of the most important activities was to raise HCV awareness for all people and service providers, as well as to reduce stigma and change cultural beliefs [36]. In Egypt, all relevant stakeholders [non-governmental organizations (NGOs), healthcare professionals and allied health personnel] were involved, in addition to the Ministry of Health and Population (MOHP) as main custodian of the HCV elimination programme. The MOHP in Egypt trained physicians, nurses and health providers and educated the general population. University students were informed of the infection and took part in education programmes aimed at the general population. The media also played a very important role in increasing awareness in the country [38].
Public education about HCV in Iran, as in many other countries, is ongoing, but cultural issues and stigma are still widespread [39]. The Iranian Ministry of Health and Medical Education (MoHME) has been implementing HCV training programmes on prevention at various levels. Moreover, Iran's primary healthcare network has established relevant information campaigns and initiatives, such as the Hepatitis Prevention and Healing Week [40]. One of the most important educational activities for HCV prevention is the Iranian Hepatitis Network, an NGO and research centre that has made a very valuable contribution for HCV advocacy, such as information dissemination to high-risk groups, pupils, university students and other lay people, as well as physicians, nurses and other healthcare service provider groups [41]. Besides the role of media in educating and informing the public, the potential of this valuable lever is still not fully exploited [41,42]. The role of the media in reducing HCV infections in Egypt could serve as a model for Iran.
Diagnosing the infection remains one of the major challenges associated with HCV in many developing countries, as many infected people are unaware of their disease [43]. One of the most important policies in Georgia was free HCV screening, which started in 2015 [37]. In Egypt, screening centres were established, and experimental programmes were launched to partially subsidise/reduce costs [38], most of which (up to 45%) were generated by laboratory tests [44]. For people with low income and those without insurance coverage, there were also centres in some cities that offered tests free of charge. In implementing screening programmes, health policy and decision makers should prioritise risk groups such as PWIDs and prisoners [45]. Insurers should support individuals with HCV and reduce costs [46].
Treatment for HCV should start as soon as possible as timely management prevents complications [47]. In Georgia, at the beginning of the HCV implementation of the elimination policy, only four centres in large cities were providing treatment. Following the full programme implementation, 27 centres offered a wide range of treatment options [36,37]. A major strength of the programme lay in the free access to highly effective DAA combinations (ledipasvir/sofosbuvir) [48].
In Egypt, about 54 centres of HCV treatment were established between 2007 and 2016 [38]. HCV care and treatment costs were estimated to be around US$80 million per year, with the government subsidising only 40% of them [49], the remaining costs being paid by insurance companies and individuals [38]. To further reduce drug prices, the country's health ministry has partnered with pharmaceutical companies, resulting in significant reduction in drug pricing [50]. Some pharmaceutical companies have also advocated for in-country generic drug production to increase affordability [51]. However, a significant proportion of individuals continue to encounter difficulties in accessing these life-saving medications [38,49].
Iranian pharmaceutical companies have been manufacturing generic HCV drugs [45]. In the case of imported medicines, insurance would only cover the cheapest brand names. Having specific centres for HCV treatment has improved the number of people treated and the quality of treatment, even though a gap in access to services still persists [52].
Cooperation with international organisations and institutions regarding HCV is essential. Transferring experiences can lead to improved policies and strategies for controlling the infection [53]. The Georgian MOLHSA has extensive collaboration with the US Centers for Disease Control and Prevention [36,37,54]. In Egypt, collaborations were established with organisations such as the WHO, the United Nations Children's Fund and Pasteur Institute [49]. Scientific and information capacities of these organisations were used to improve in-country expertise in Egypt. In terms of individual treatment, healthcare providers have been using foreign drug companies to support domestic pharmacists, importing drugs from abroad and obtaining discounted prizes. This has made treatment cheaper [38]. Iran has international cooperation in the field of HCV-related research with other countries of the Eastern Mediterranean Regional Office (EMRO) region, but due to political sanctions, drug companies may not be able to cooperate with Iran [55].
Control policies in high-risk individuals (PWID and prisoners, among others) are one of the most basic activities that can play a decisive role in HCV elimination [56]. Georgia has adopted harm reduction policies, such as needle syringe exchange programmes and opioid substitution therapy, to reduce harm in at-risk groups [36,37]. In Egypt, similar policies have been implemented to reduce HCV transmission [57].
In Iran, most activities are related to improving community health with the MoHME, whereas prisoner protection and treatment are the responsibility of the prison organisation. The support and treatment of PWIDs require the attention of the Ministry of Cooperatives, Labor, and Social Welfare. Unfortunately, Iran lacks a suitable harm reduction policy.
Egypt and Georgia are successful examples of countries where healthcare policy and decision makers have put HCV on their agenda. Support for individuals and high-risk groups is one of the key elements to succeed in HCV elimination [58]. These two countries have initially faced a high HCV-related incidence of illnesses but have managed to control it [59].
Despite the fact that HCV incidence in Iran is increasing [45], it has not yet been put on the health agenda as a serious priority. The attitude of both the community and the policy and decision makers towards it still remains negative.
Furthermore, HCV screening and treatment activities require adequate funding. The MoHME is not able to allocate the necessary financial resources, and a lack of cooperation with other organisations can cause serious problems for individuals who are unable to afford diagnostic monitoring and treatment costs.
Conclusion
Controlling and eliminating HCV are a difficult public health challenge. Its increasing incidence in recent years has generated a dramatic economic burden. Iran needs a comprehensive strategy to fight HCV with adequate cultural and social infrastructures. Dedicated centres to HCV diagnosis and management should be used for screening and delivery of inexpensive and high-quality tests with individual rapid access to treatment within a context of reduced costs to improve access.
Acknowledgement
This study was part of a PhD thesis (MB) supported by the School of Health Management, Iran University of Medical Sciences (IUMS/SHMIS_1396/9423557001).
Conflict of interest
The authors declare no conflict of interest.
References
- 1. Myers RP, Krajden M, Bilodeau M et al. Burden of disease and cost of chronic hepatitis C virus infection in Canada. Can J Gastroenterol Hepatol 2014; 28: 243– 250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Thein HH, Yi Q, Dore GJ et al. Estimation of stage-specific fibrosis progression rates in chronic hepatitis C virus infection: a meta-analysis and meta-regression. Hepatology 2008; 48: 418– 431. [DOI] [PubMed] [Google Scholar]
- 3. Seeff LB. Natural history of chronic hepatitis C. Hepatology 2002; 36: S35– S46. [DOI] [PubMed] [Google Scholar]
- 4. El-Serag HB. Hepatocellular carcinoma. N Engl J Med 2011; 365: 1118– 1127. [DOI] [PubMed] [Google Scholar]
- 5. Fattovich G, Stroffolini T, Zagni I et al. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology 2004; 127: S35– S50. [DOI] [PubMed] [Google Scholar]
- 6. Pinter M, Trauner M, Peck-Radosavljevic M et al. Cancer and liver cirrhosis: implications on prognosis and management. ESMO Open 2016; 1: e000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stanaway JD, Flaxman AD, Naghavi M et al. The global burden of viral hepatitis from 1990 to 2013: findings from the Global Burden of Disease Study 2013. Lancet 2016; 388: 1081– 1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stepanova M, Younossi ZM. Economic burden of hepatitis C infection. Clin Liver Dis 2017; 21: 579– 594. [DOI] [PubMed] [Google Scholar]
- 9. Kim do Y, Yoon KT, Kim W et al. Estimation of direct medical cost related to the management of chronic hepatitis C and its complications in South Korea. Medicine (Baltimore) 2016; 95: e3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Su J, Brook RA, Kleinman NL et al. The impact of hepatitis C virus infection on work absence, productivity, and healthcare benefit costs. Hepatology 2010; 52: 436– 442. [DOI] [PubMed] [Google Scholar]
- 11. Pascual-Argente N, Puig-Junoy J, Llagostera-Punzano A. Non-healthcare costs of hepatitis C: a systematic review. Expert Rev Gastroenterol Hepatol 2018; 12: 19– 30. [DOI] [PubMed] [Google Scholar]
- 12. WHO Global hepatitis report, 2017. 2017. Available at: www.who.int/hepatitis/publications/global-hepatitis-report2017/en/ ( accessed March 2019).
- 13. Resnik DB. Responsibility for health: personal, social, and environmental. J Med Ethics 2007; 33: 444– 445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ali A, Katz DL. Disease prevention and health promotion: how integrative medicine fits. Am J Prev Med 2015; 49: S230– S240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kumar S, Kumar N, Vivekadhish S. Millennium development goals (MDGs) to sustainable development goals (SDGs): addressing unfinished agenda and strengthening sustainable development and partnership. Indian J Community Med 2016; 41: 1– 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hutin Y, Low-Beer D, Bergeri I et al. Viral hepatitis strategic information to achieve elimination by 2030: key elements for HIV program managers. JMIR Public Health Surveill 2017; 3: e91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Waheed Y. Transition from millennium development goals to sustainable development goals and hepatitis. Pathog Glob Health 2015; 109: 353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nasrullah M, Sergeenko D, Gamkrelidze A et al. HCV elimination – lessons learned from a small Eurasian country, Georgia. Nat Rev Gastroenterol Hepatol 2017; 14: 447– 448. [DOI] [PubMed] [Google Scholar]
- 19. Goldberg D, Brown G, Hutchinson S et al. Hepatitis C action plan for Scotland: phase II (May 2008–March 2011). Euro Surveill 2008; 13: pii: 18876. [DOI] [PubMed] [Google Scholar]
- 20. Guriev V, Spinu C, Sajen O et al. Epidemiology of hepatitis C in the Republic of Moldova: achievements and remaining challenges in prevention and control. J Infect Dev Ctries 2016; 10: 1162– 1167. [DOI] [PubMed] [Google Scholar]
- 21. Center for Disease Analysis Polaris Observatory. Countries on track to achieve WHO elimination targets. 2018. Available at: http://cdafound.org/polaris/ ( accessed March 2019).
- 22. Institute for Health Metrics and Evaluation (IHME) IHME Hepatitis facts. 2018. Available at: http://hepatitis.ihme.services/trends ( accessed March 2019).
- 23. Mirminachi B, Mohammadi Z, Merat S et al. Update on the prevalence of hepatitis C virus infection among Iranian general population: a systematic review and meta-analysis. Hepat Mon 2017; 17: e42291. [Google Scholar]
- 24. Khodabandehloo M, Roshani D, Sayehmiri K. Prevalence and trend of hepatitis C virus infection among blood donors in Iran: a systematic review and meta-analysis. J Res Med Sci 2013; 18: 674– 682. [PMC free article] [PubMed] [Google Scholar]
- 25. Behzadifar M, Gorji HA, Bragazzi NL. The prevalence of hepatitis C virus infection in thalassemia patients in Iran from 2000 to 2017: a systematic review and meta-analysis. Arch Virol 2018; 163: 1131– 1140. [DOI] [PubMed] [Google Scholar]
- 26. Behzadifar M, Gorji HA, Rezapour A et al. Prevalence of hepatitis C virus infection among prisoners in Iran: a systematic review and meta-analysis. Harm Reduct J 2018; 15: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Malekinejad M, Navadeh S, Lotfizadeh A et al. High hepatitis C virus prevalence among drug users in Iran: systematic review and meta-analysis of epidemiological evidence (2001–2012). Int J Infect Dis 2015; 40: 116– 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kouyoumjian SP, Chemaitelly H, Abu-Raddad LJ. Characterizing hepatitis C virus epidemiology in Egypt: systematic reviews, meta-analyses, and meta-regressions. Sci Rep 2018; 8: 1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gvinjilia L, Nasrullah M, Sergeenko D et al. National progress toward hepatitis C elimination. Georgia, 2015–2016. MMWR Morb Mortal Wkly Rep 2016; 65: 1132– 1135. [DOI] [PubMed] [Google Scholar]
- 30. Luhmann N, Champagnat J, Golovin S et al. Access to hepatitis C treatment for people who inject drugs in low and middle income settings: evidence from five countries in Eastern Europe and Asia. Int J Drug Policy 2015; 26: 1081– 1087. [DOI] [PubMed] [Google Scholar]
- 31. Badridze N, Chkhartishvili N, Abutidze A et al. Prevalence of hepatitis B and C among HIV positive patients in Georgia and its associated risk factors. Georgian Med News 2008; 12: 54– 60. [PubMed] [Google Scholar]
- 32. WHO Prevention and control of viral hepatitis infection: framework for global action. 2012. Available at: www.who.int/hiv/pub/hepatitis/Framework/en/ ( accessed March 2019).
- 33. Andriulli A, Stroffolini T, Mariano A et al. Declining prevalence and increasing awareness of HCV infection in Italy: a population-based survey in five metropolitan areas. Eur J Intern Med 2018; 53: 79– 84. [DOI] [PubMed] [Google Scholar]
- 34. Iakunchykova O, Meteliuk A, Zelenev A et al. Hepatitis C virus status awareness and test results confirmation among people who inject drugs in Ukraine. Int J Drug Policy 2018; 57: 11– 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Northrop JM. A dirty little secret: stigma, shame and hepatitis C in the health setting. Med Humanit 2017; 43: 218– 224. [DOI] [PubMed] [Google Scholar]
- 36. Mitruka K, Tsertsvadze T, Butsashvili M et al. Launch of a nationwide hepatitis C elimination program. Georgia, April 2015. MMWR Morb Mortal Wkly Rep 2015; 64: 753– 757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nasrullah M, Sergeenko D, Gvinjilia L et al. The role of screening and treatment in national progress toward hepatitis C elimination. Georgia, 2015–2016. MMWR Morb Mortal Wkly Rep 2017; 66: 773– 776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. El-Akel W, El-Sayed MH, El Kassas M et al. National treatment programme of hepatitis C in Egypt: hepatitis C virus model of care. J Viral Hepat 2017; 24: 262– 267. [DOI] [PubMed] [Google Scholar]
- 39. Masoudnia E, Chenaninasab H. Impact of perceived social stigma on self-esteem in patients with acquired immunodeficiency syndrome. J Holist Nurs Midwifery 2016; 26: 80– 89. [Google Scholar]
- 40. Behzadifar M, Gorji HA, Rezapour A et al. The role of the primary healthcare network in Iran in hepatitis C virus elimination by 2030. J Virus Erad 2018; 4: 186– 188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. World Hepatitis Alliance Iran Hepatitis Network (IHN). 2018. Available at: www.worldhepatitisalliance.org/member/iran-hepatitis-network-ihn ( accessed March 2019).
- 42. Nima M, Karimi-Sari H, Alavian SM. Art and viral hepatitis elimination programmes. J Virus Erad 2018; 4: 59– 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dore GJ, Ward J, Thursz M. Hepatitis C disease burden and strategies to manage the burden (guest editors Mark Thursz, Gregory Dore and John Ward). J Viral Hepat 2014; 21 ( suppl 1): 1– 4. [DOI] [PubMed] [Google Scholar]
- 44. Ashtari S, Vahedi M, Karkhaneh M et al. Estimation of direct medical costs of hepatitis C. Med Sci 2014; 23: 21– 27. [Google Scholar]
- 45. Taherkhani R, Farshadpour F. Lurking epidemic of hepatitis C virus infection in Iran: a call to action. World J Hepatol 2017; 9: 1040– 1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Millman AJ, Ntiri-Reid B, Irvin R et al. Barriers to treatment access for chronic hepatitis C virus infection: a case series. Top Antivir Med 2017; 25: 110– 113. [PMC free article] [PubMed] [Google Scholar]
- 47. Arora S, Thornton K, Murata G et al. Outcomes of treatment for hepatitis C virus infection by primary care providers. N Engl J Med 2011; 364: 2199– 2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pawlotsky JM, Feld JJ, Zeuzem S et al. From non-A, non-B hepatitis to hepatitis C virus cure. J Hepatol 2015; 62: S87– S99. [DOI] [PubMed] [Google Scholar]
- 49. Centers for Disease Control and Prevention (CDC) Progress toward prevention and control of hepatitis C virus infection. Egypt, 2001–2012. MMWR Morb Mortal Wkly Rep 2012; 61: 545– 549. [PubMed] [Google Scholar]
- 50. Elgharably A, Gomaa AI, Crossey MM et al. Hepatitis C in Egypt – past, present, and future. Int J Gen Med 2017; 10: 1– 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gomaa A, Allam N, Elsharkawy A et al. Hepatitis C infection in Egypt: prevalence, impact and management strategies. Hepat Med 2017; 9: 17– 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hajarizadeh B. Generic direct acting antiviral treatment: the first step towards elimination of hepatitis C in Iran. Hepat Mon 2017; 17: e45788. [Google Scholar]
- 53. Hagan LM, Schinazi RF. Best strategies for global HCV eradication. Liver Int 2013; 33: 68– 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Smith BD, Jorgensen C, Zibbell JE et al. Centers for Disease Control and Prevention initiatives to prevent hepatitis C virus infection: a selective update. Clin Infect Dis 2012; 55 ( suppl 1): S49– S53. [DOI] [PubMed] [Google Scholar]
- 55. Gorji A. Sanctions against Iran: the impact on health services. Iran J Public Health 2014; 43: 381– 382. [PMC free article] [PubMed] [Google Scholar]
- 56. Chen Y, Tang Z, Tang S et al. Decreasing HIV, syphilis, and hepatitis C infection after a decade of harm reduction implementation among drug users in southwestern areas of China. J Stud Alcohol Drugs 2018; 79: 248– 257. [PubMed] [Google Scholar]
- 57. Oraby D. Harm reduction approach in Egypt: the insight of injecting drug users. Harm Reduct J 2013; 10: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lavis JN, Wilson MG, Oxman AD et al. SUPPORT tools for evidence-informed health policymaking (STP) 4: using research evidence to clarify a problem. Health Res Policy Syst 2009; 7 ( suppl 1): S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Di Ruggiero E, Cohen JE, Cole DC et al. Public health agenda setting in a global context: the international labor organization's decent work agenda. Am J Public Health 2015; 105: e58– e61. [DOI] [PMC free article] [PubMed] [Google Scholar]