Abstract
To evaluate the changes in the blood flow on retina and the optic nerve head (ONH) after conventional laser treatment and to compare it to that after patterned scanning laser (PASCAL) treatment in patients with severe nonproliferative diabetic retinopathy (S-NPDR).
In this prospective, cross-sectional study, the blood flow on retina and the ONH was assessed by laser speckle flowgraphy using the mean blur rate (MBR) in 39 eyes with S-NPDR before, 1, 4, 8, 12 weeks after panretinal photocoagulation (PRP). Of 39 eyes, 17eyes with 17 patients treated by conventional laser and 22 eyes with 22 patients treated by PASCAL.
The mean age was 55.5 ± 11.5 years in the conventional laser group, 55.6 ± 11.8 years in the PASCAL group. The MBR-vessel, which can be dominantly expressed as retinal blood flow, was significantly reduced after PRP treated by conventional laser (P < .001), but did not change after PRP treated by PASCAL. The ratio of MBR-vessel to the baseline was significantly lower in the conventional laser group only at Week 1 (P = .045). The MBR-tissue, which can be dominantly expressed as the ONH blood flow, did not significantly change after PRP in the both group. The multiple stepwise regression analysis revealed that the laser burns was an independent factor significantly correlated with the ratio of MBR-vessel at Week 1 to the baseline (β = −0.550, P = .012).
The retinal blood flow was significantly reduced during the 12 weeks only after completion of PRP by conventional laser treatment. Our results indicate that short pulse on PRP treatment performed by the PASCAL would not significantly reduce the retinal blood flow.
Keywords: laser speckle flowgraphy, mean blur rate, panretinal photocoagulation, patterned scanning laser, retinal blood flow
1. Introduction
Diabetic retinopathy is one of the leading causes of blindness in the industrialized world. In 1976, the diabetic retinopathy study showed that panretinal photocoagulation (PRP) for proliferative diabetic retinopathy (PDR) with high-risk characteristics decreased the risk of severe vision loss by over 50% out to 4 years of follow-up.[1] Thereafter, studies have demonstrated that PRP decreases the incidence of blindness in patients with PDR.[2,3] A 5-stage disease severity classification has been proposed for eyes with diabetic retinopathy; 3 stages of low risk, a fourth stage of severe non-PDR (S-NPDR), and a fifth stage of PDR.[4,5] The Early Treatment Diabetic Retinopathy Study reported that PRP treatment was associated with good long-term visual acuity in most patients with S-NPDR and PDR.[6]
There has been reported that a significant reduction in the retinal blood flow after PRP in eyes with PDR.[7–9] Grunwald et al demonstrated there were decreased venous diameter, flow velocity, and total blood flow following PRP.[7,8] Fujio et al demonstrated that after PRP treatment of half of the fundus, there were statistically significant regional decreases in the retinal blood flow (50%–78%) and decreased vessel diameters, ranging from 1% to 9%.[9]
Many techniques have been developed for measuring retinal flow including fluorescein angiography,[10] radioactive microspheres, hydrogen clearance, bidirectional laser Doppler velocimetry,[7,8] color Doppler ultrasonography,[11] and a pulsatile technique.[12] However, the change in retinal blood flow in detail after PRP has not been fully understood, presumably because of time-intensiveness and poor reproducibility in many techniques for measuring retinal blood flow.
Laser speckle flowgraphy (LSFG) is a noninvasive, real-time method that can measure the relative blood flow without the use of contrast agents,[13,14] and performs repeated measurements of defined retinal regions, which makes it possible to quantitatively estimate blood flow changes in retina and the optic nerve head (ONH), in vivo. Blood flow can be quantified as the mean blur rate (MBR) through the use of the LSFG-NAVI (Softcare Ltd, Fukuoka, Japan).
Blumenkranz et al reported a patterned scanning laser (PASCAL) treatment technique[15] that enabled a rapid application of multiple laser spots in an array with short pulse durations which decreases the width and the axial extent of the laser lesions of the retinal pigment epithelium (RPE) and outer retinal layer.[16] Accordingly, PASCAL treatment would have an advantage over conventional laser therapy on the ocular blood flow. However, there have been no publications showing the effect of PASCAL treatment for DR on the retinal blood flow compared with conventional laser treatment in a search of Pubmed. Therefore, a comparison of the benefits of PASCAL and conventional laser treatment on the retinal blood flow requires to be determined for eyes with S-NPDR or PDR.
Thus, the aim of this study was to evaluate the changes in the blood flow determined by LSFG on retina and the ONH after conventional laser treatment and to compare it to that after PASCAL treatment in patients with S-NPDR.
2. Methods
2.1. Ethics statement
This was a prospective, single-center, cross-sectional study, and the procedures were approved by the Institutional Review Board and the Ethics Committee of the Nagoya University Graduate School of Medicine and registered with the University Hospital Medical Network-clinical trials registry (UMIN000028453). The procedures conformed to the tenets of the Declaration of Helsinki. We explained the nature of the study to all patients and obtained a signed informed consent.
2.2. Subjects
This study was conducted at the Nagoya University Hospital between July 2016 and December 2017. Patients were randomly assigned to conventional laser treatment or PASCAL treatment by an independent investigator (YU) with an allocation ratio of 1:1. All patients had a comprehensive ophthalmic examination including measurements of axial length and intraocular pressure (IOP), optical coherence tomography (OCT), slit-lamp examinations, and fundus examinations before and 1, 4, 8, and 12 week(s) after the PRP treatment. All patients were asked to abstain from alcoholic and caffeinated beverages on the morning of the examination. The pupil was dilated 30 minutes before the examinations with tropicamide phenylephrine eye drops (Mydrin-P, Santen Pharmaceutical, Osaka, Japan). The patients rested for 15 to 20 minutes in a dark quiet room before the examinations. We performed all examinations in the sitting position at approximately 12:00 hours to avoid diurnal variations.[17–19]
The IOP was measured with a handheld tonometer (Icare; TiolatOy, Helsinki, Finland) and the axial lengths were measured with a partial optical coherence interferometry (IOLMaster; Carl Zeiss Meditec, La Jolla, CA). The diastolic blood pressure (DBP) and systolic blood pressure (SBP) were measured with an automatic sphygmomanometer (CH-483C; Citizen, Tokyo, Japan). The mean arterial blood pressure (MAP) and the ocular perfusion pressure (OPP) were calculated as follows: MAP = DBP + 1/3 (SBP − DBP), and OPP = 2/3 MAP − IOP.
2.3. Exclusion criteria
The exclusion criteria included the presence of severe cataract, vitreous hemorrhage, history of photocoagulation or intraocular surgery, retinochoroidal pathology, for example, choroidal neovascularization, age-related macular degeneration, and medical conditions that could influence the hemodynamics of the eye other than diabetes, such as vascular diseases, hypertension, and arrhythmia.
2.4. PRP parameters
Fluorescein fundus angiography (FFA) demonstrated that all patients had nonperfused retinal areas in 3 or more quadrants. According to the recommendations of the Early Treatment Diabetic Retinopathy Study group, PRP was performed in the eyes with S-NPDR.[20] Topical anesthesia was used on all eyes before using contact lens. The PRP was performed through a widefield contact lens (Ocular Mainster PRP 165; Ocular Instruments, Bellevue, WA).
The same surgeon (YM) performed all PRPs with a spot size of 200 μm for both types of PRPs. A pulse duration of 20 ms was used with the PASCAL treatment and 200 ms with the conventional laser treatment.
In the conventional laser treatment, a 532-nm wavelength argon laser device (NOVUS Varia; Lumenis, San Jose, CA) was used. The power was set at 170 to 230 mW and the exposure was maintained until a grayish-white lesion was seen to create an effective retinal burn. The spots were placed at 1 spot distance apart in the conventional group. The number of burns applied during each session ranged from 300 to 400 spots and a total of 1200 to 1600 burns were applied to each eye. PRP was performed in 4 sessions, the inferior, nasal, superior, and temporal quadrants in that order at an interval of 1 week between treatments.
A 5 × 5 square grid was used to treat 25 spots simultaneously with PASCAL SlimLine (Topcon, Tokyo, Japan). We set the power to 300 to 650 mW and performed PRP in 2 sessions at precisely 1 week between the treatments.
2.5. Laser speckle flowgraphy
We used the LSFG-NAVI (Softcare Co, Ltd) instrument to determine the ONH blood flow. The principles of LSFG have been described in detail.[21–24] The MBR is a measure of the relative blood flow velocity, and it is determined by examining the pattern of the speckle contrast produced by the erythrocytes in the ocular blood vessels.[13] The MBR images are acquired at a rate of 30 frames/s over a 4-second period. The same site can be measured by using the auto-tracking system. To evaluate the circulation on the ONH, a circular marker was set surrounding the ONH (Fig. 1A). The “vessel extraction” function of the software then identified the vessel and tissue areas on the ONH so that the MBR of each could be assessed separately (Fig. 1B). The vessel area can be used to evaluate the blood flow in the retinal vessels excluding the choroidal blood vessels.[25] The LSFG was measured 3 times at each time point in all eyes. The average of the variables derived from the LSFG device was calculated. The ratio of MBR-vessel and -tissue to the baseline was used to compare the effect of PRP between the conventional laser group and the PASCAL group.
Figure 1.
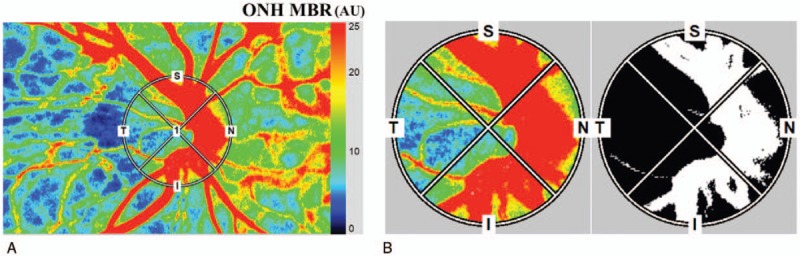
Representative composite color maps of the MBR as measured by LSFG. (A) Red color indicates a high MBR and the blue color indicates a low MBR. To measure the MBR of the blood flow on the ONH a circle was set around the ONH. (B) A binary formatted image for segmentation between the vessel (white area) and tissue (black area) areas. LSFG = laser speckle flowgraphy, MBR = mean blur rate, ONH = optic nerve head.
2.6. Statistical analyses
Sample size was calculated with ONH MBR determined by LSFG in previous report, which showed 20% reduction of ONH MBR after conventional PRP.[26] At 90% power and α level of 0.05, a supposition that mean reduction of the ONH MBR after PRP is 20% would need 17 subjects in each group, based on the reported standard deviation. Assuming a dropout rate of 20%, 21 subjects in each group should be enrolled.
The values are presented as the mean ± standard deviation. Independent t test was used to compare normally distributed data. One way analysis of variance was used to evaluate changes in BCVA, CFT, MBR-vessel, -tissue, IOP, and OPP over time. Pearson correlation coefficient test was used to evaluate the association between the reduction ratio of MBR-vessel at Week 1 to the baseline and other variables. Multiple linear regression analysis was used to evaluate the association between reduction ratio of MBR-vessel at Week 1 and independent variables, including laser burns, insulin/oral antidiabetic drugs, BCVA, eGFR, body mass index, creatinine, duration of diabetes, HbA1c, and age.
We performed all statistical analyses with SAS9.4 (SAS Inc, Cary). A P value < .05 was considered statistically significant.
3. Results
3.1. Patient demographics
A total of 46 patients with type II diabetes and S-NPDR without macula edema was screened and 43 patients were enrolled, 3 patients did not meet inclusion criteria. Of 43 patients, 21 patients were assigned to the conventional laser group and 22 patients to the PASCAL group. In the conventional laser group, 4 of 21 patients dropped out during PRP treatment. As a result, 17 eyes with 17 patients in the conventional laser group and 22 eyes with 22 patients in the PASCAL group were studied. No significant differences were observed in the systemic and ocular variables between the 2 groups (Table 1).
Table 1.
Clinical characteristics of all subject.

The mean number of the photocoagulation burns was 1524 ± 157 in the conventional laser group (Fig. 2) and 4959 ± 582 in the PASCAL group (Fig. 3). None of the patients developed any adverse events related to the PRP. The stage of the DR had not worsened as determined by the FFA findings at 12 weeks after the PRP.
Figure 2.
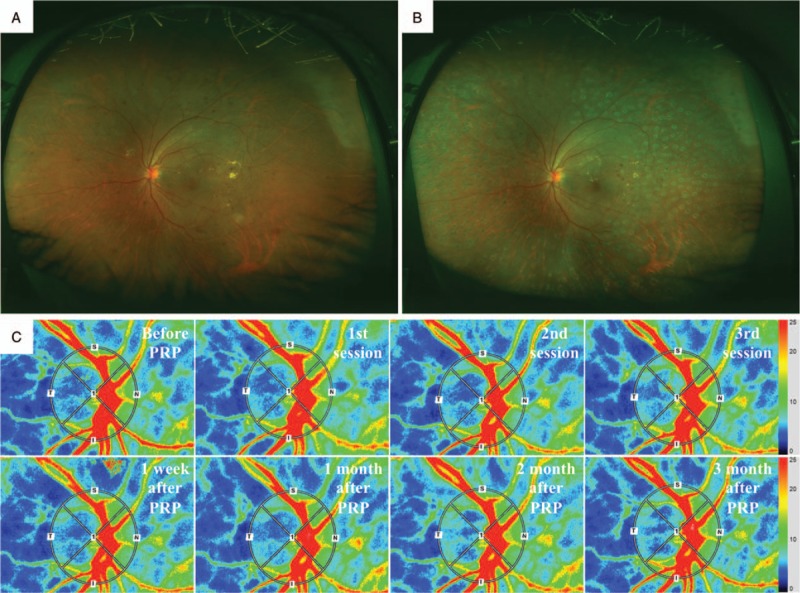
Fundus photographs and composite color maps of a xx-year-old woman with S-NPDR. Fundus photograph taken with Optos 200TX showing S-NPDR before (A) and after PRP treated by conventional laser (B). Representative composite color maps of the MBR as determined by LSFG before, after first, second and third session, and 1, 4, 8, and 12 wk after PRP treatment on the ONH (C). There was a significant reduction in the MBR-vessel after the PRP. LSFG = laser speckle flowgraphy, MBR = mean blur rate, ONH = optic nerve head, PRP = panretinal photocoagulation, S-NPDR = severe nonproliferative diabetic retinopathy.
Figure 3.
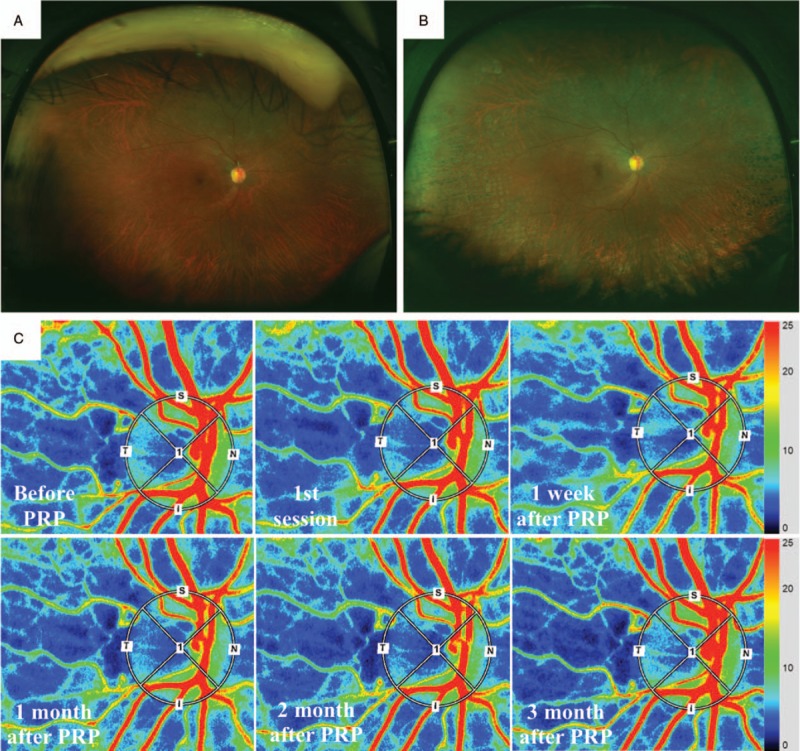
Fundus photographs and composite color maps of a xx-year-old woman with severe S-NPDR. Fundus photograph taken with Optos 200TX showing S-NPDR before (A) and after PRP treated by PASCAL (B). Representative composite color maps before, after first session, and 1, 4, 8, and 12 wk after PRP treatment on the ONH (C). There was no significant reduction in the MBR-vessel after the PRP. MBR = mean blur rate, ONH = optic nerve head, PASCAL = patterned scanning laser, PRP = panretinal photocoagulation, S-NPDR = severe nonproliferative diabetic retinopathy.
3.2. Changes in ONH MBR
In the conventional laser group, the mean MBR-vessel was 39.5 ± 8.2 arbitrary units (AU) before PRP, 36.5 ± 10.1 AU (92%) after the first session, 34.6 ± 9.0 AU (87%) after the second session, 33.1 ± 7.4 AU (84%) after the third session of the PRP, 29.0 ± 5.6 AU (76%) at 1 week, 34.3 ± 11.5 AU (87%) at 4 weeks, 34.3 ± 9.5 AU (87%) at 8 weeks, and 36.9 ± 9.5 AU (92%) at 12 weeks after the completion of PRP (Table 2). The MBR-vessel was significantly decreased after PRP in the conventional group (P < .001) (Week 1, P < .001; Week 4, P = .029; Week 8, P = .015) (Fig. 4).
Table 2.
Change in variable parameters with time.

Figure 4.
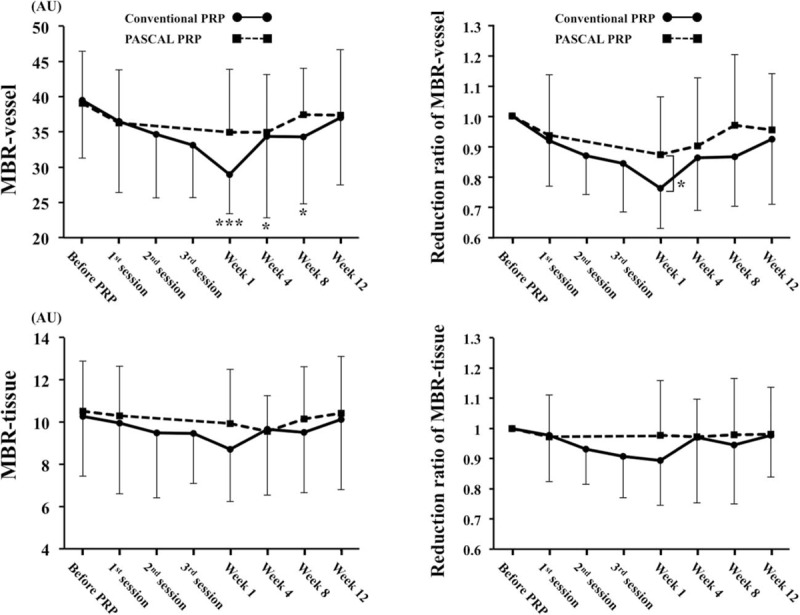
There was a significant reduction in the MBR-vessel after the PRP in the conventional laser group (A) and the ratio of MBR-vessel to the baseline in the conventional group was significantly lower than that in the PASCAL group at 1 wk after completion of PRP (B). There was not significantly reduction in the MBR-tissue in the both groups (C) and no significant difference was observed in the ratio of the MBR-tissue throughout the post-PRP period (D). ∗∗∗P < .001, ∗∗P < .01, ∗P < .05. MBR = mean blur rate, PASCAL = patterned scanning laser, PRP = panretinal photocoagulation.
In the PASCAL group, the mean MBR-vessel was 39.1 ± 7.4 AU before PRP, 36.3 ± 7.5 AU (93%) after the first session, 34.9 ± 8.9 AU (87%) at 1 week, 34.9 ± 8.2 AU (87%) at 4 weeks, 37.4 ± 6.6 AU (96%) at 8 weeks, and 37.3 ± 9.3AU (96%) at 12 weeks after PRP (Table 2). The MBR-vessel did not significantly change after PRP treatment in the PASCAL group.
The ratio of MBR-vessel to the baseline was significantly lower in the conventional laser group compared to the PASCAL group only at Week 1 (P = .045) (Fig. 4).
In the conventional laser group, the mean MBR-tissue was 10.3 ± 2.8 AU before PRP, 10.0 ± 3.4 AU (97%) after the first session, 9.5 ± 3.1 AU (93%) after the second session, 9.5 ± 2.4 AU (92%) after the third session of the PRP, 8.7 ± 2.5 AU (89%) at 1 week, 9.7 ± 3.1 AU (94%) at 4 weeks, 9.5 ± 2.9 AU (93%) at 8 weeks, and 10.1 ± 3.3 AU (98%) at 12 weeks following PRP (Table 2). The MBR-tissue did not significantly change after PRP treatment in the conventional laser group (Fig. 5).
Figure 5.
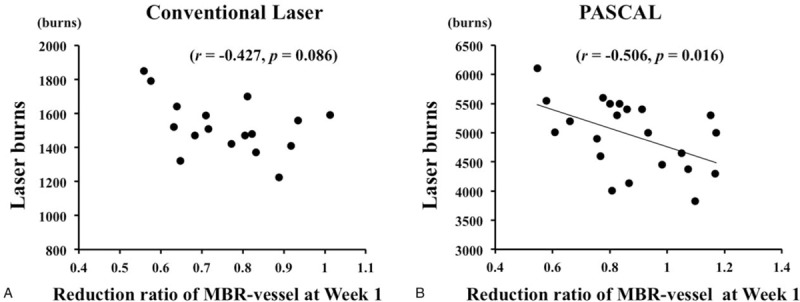
Scatterplot of the ratio of MBR-vessel at Wk 1 to the baseline versus the number of laser photocoagulation burns. The ratio of MBR-vessel at Wk 1 to the baseline was significantly correlated with the number of laser photocoagulation burns in the conventional laser group (r = −0.427, P = .086) (A) and the PASCAL group (r = −0.506, P = .016) (B). MBR = mean blur rate, PASCAL = patterned scanning laser.
In the PASCAL group, the mean MBR-tissue was 10.5 ± 2.3 AU before PRP, 10.3 ± 2.3 AU (98%) at the first session, 9.9 ± 2.5 AU (94%) at 1 week, 9.6 ± 1.7 AU (91%) at 4 weeks, 10.2 ± 2.4 AU (97%) at 8 weeks, and 10.4 ± 2.7 AU (99%) at 12 weeks following PRP (Table 2). The MBR- tissue did not significantly change after PRP treatment in the PASCAL group (Fig. 5).
The ratio of MBR-tissue to the baseline was not significantly different between the conventional laser group and the PASCAL group at any time (Fig. 1C).
3.3. Changes in other parameters
Although the BCVA, subfoveal choroidal thickness (SFCT), IOP, MAP, and OPP did not significantly change after PRP treatment in the both groups, the CFT significantly changed after PRP treatment in the conventional group (P < .001) and the PASCAL group (P = .001) (Table 2).
3.4. Correlations between ONH MBR and other parameters
Pearson correlation coefficient analyses showed that the ratio of MBR-vessel at Week 1 to the baseline was significantly correlated with the laser burns in the PASCAL group (r = −0.506, P < .016) (Table 3) (Fig. 5).
Table 3.
Result of Pearson correlation coefficient between the reduction ratio of MBR-vessel at Wk 1 to the baseline and other variables.

The multiple stepwise regression analysis revealed that the laser burns was independent factors indicating the ratio of MBR-vessel at Week 1 to the baseline (β = −0.550, P = .012) (Table 4).
Table 4.
Result of multiple stepwise regression analysis for factors independency contributing to the reduction ratio of MBR-vessel at Wk 1 to the baseline in the PASCAL group.

The trend of the change in MBR-vessel and -tissue were not significantly correlated with that in the BCVA, SFCT, CFT, IOP, MAP, and OPP.
4. Discussion
Our results demonstrated a significant reduction of the MBR-vessel after the PRP only by the conventional laser treatment in eyes with S-NPDR. The reduction ratio of the MBR-vessel in the conventional laser group was significant lower than that in the PASCAL group at 1 week following PRP. There were no significant correlations in the trend between the MBR-vessel or -tissue and other variables. The number of the laser burns was correlated with the ONH-MBR- vessel at 1 week after PRP.
Although many techniques have been developed for measuring retinal blood flow,[7,8,10–12] it is not easy to measure the retinal blood flow at the exact same area and evaluate the change in it after PRP treatment because of the shortcoming of time-intensiveness and poor reproducibility. Accordingly, we evaluated the retinal blood flow for the vascular areas of the ONH in eyes with S-NPDR and evaluated the change in it following PRP treatment. MBR-vessel on the ONH is separated from MBR using the “vessel extraction” function of the software, that is, which can be dominantly expressed as retinal blood flow.
There have been many reports that show a significant reduction in the retinal blood flow after PRP in eyes with PDR.[7–9,26–29] Grunwald et al demonstrated a reduction in venous diameter, flow velocity, and total blood flow following PRP.[7,8] Fujio et al demonstrated that there were statistically significant regional decreases in the retinal blood flow (50%–78%) and decreased vessel diameters, ranging from 1% to 9% after PRP treatment of half of the fundus.[9] It has been demonstrated that the blood flow in severe NPDR with PRP on the ONH was significantly lower than that of severe NPDR without PRP using LSFG.[26]
There have been reported about the reasons for the reduced retinal blood flow after PRP as follows.[30–36] PRP improves the oxygenation of the ischemic inner retinal layers by destroying some of the metabolically highly active photoreceptor cells which would then lead to a greater flow of oxygen from the choriocapillaris to the inner layers of the retina.[30–32] Experimental studies show the improved oxygenation after retinal laser treatment,[33] and much higher retinal oxygen tension in laser treated areas than in untreated areas of the same retina.[34–36] When the changes in the oxygen flux reach the inner retina, the retinal arteries constrict, causing a reduction in the blood flow.[37–39]
In our study, the retinal blood flow was gradually decreased after every session of PRP in the both groups, and was significantly reduced after completion of PRP in the conventional group. In addition, the number of laser burns was significantly correlated with the reduction rate of retinal blood flow. These results indicate that PRP reduce the retinal blood flow and corroborate the previous reports[30–36] and the above mechanism.
The retinal blood flow was slightly recovered after the completion of PRP, which results that the retinal blood flow at Week 12 was not significantly different from the baseline in the conventional laser group. Recently, the similar tendency has been reported using LSFG.[40] Diddie et al found initial increase in retinal oxygen tension following retinal laser treatment but this effect was short-lived.[41] Photoreceptor outer segments damaged during photocoagulation in rabbits have been shown to recover by 4 weeks after laser irradiation.[42] The restoration of the photoreceptor layer in rats was more rapid, reaching its maximum extent by 3 weeks. The restoration can be observed in human eye using high-resolution OCT or OCT angiography.[43] In addition, Hiroshiba et al reported that the leukocyte velocities in the retinal capillaries were significantly decreased immediately after photocoagulation and then gradually recovered to normal as time elapsed.[29] Those results suggest that the retinal blood flow is reduced immediately after laser photocoagulation as the above mechanism, but it can be recovered gradually with the restoration of the outer retinal layer, probably the increased retinal oxygen tension is reduced because of restoration of outer retina. On the other hand, there has been a study that evaluated 76 eyes with S-NPDR at 9 years following the PRP, and it was reported that the MBR determined on the ONH is significantly decreased at 70% of that of normal subjects.[26] Furthermore, the retinal vessels progressively constrict for several years following laser treatment.[44] Taken together, the possibility is not denied that the retinal blood flow is transiently recovered just following PRP treatment, but decreases gradually for a long period after PRP.
The photocoagulation burns extended outside the laser lesioned area by at least 1 to 2 mm following the conventional laser treatment.[45] PASCAL treatment has several advantages over conventional laser treatment including the short-pulse duration which decreases the axial extent and the width in the laser burns of the outer retinal layer and RPE.[16] On the other hand, the effect of PASCAL treatment appears to be less than conventional laser treatment with the same number of laser spots.[46] It has been reported that 6924 laser burns in an area of 836 mm2 is required in eyes with severe PDR and 3998 laser burns and an area of 456 mm2 is required in eyes with PDR to accomplish a complete regression of the disease using PASCAL treatment.[47] Accordingly, a much larger number of laser burns with 4959 burns in the PASCAL treatment group was performed compared with 1524 laser burns in the conventional laser group.
Our results showed that the retinal blood flow was not significantly decreased during the 12 weeks following PRP by PASCAL. In addition, the reduction rate of retinal blood flow in the PASCAL group was significantly lower than that in the conventional group. These findings indicate that PASCAL therapy has a less effect on the retinal blood flow, even with enough number of photocoagulation burns until 12 weeks following PRP. Furthermore, the photocoagulation spot by PASCAL will not expand due to the short-pulse duration, which may then cause more differences in the retinal blood flow in the later period after PRP. On the other hand, Yamada et al found that retinal blood flow was significantly reduced during and after PRP treatments using PASCAL.[40] The reason for this discrepancy between our and Yamada's study was not determined. However, the response to PRP treatments varies among individuals, and difference of laser spot or power might have contributed to the lack of significant correlations.
The MBR-tissue was not significantly changed in the both groups. However, it has been reported that the MBR-tissue is significantly decreased at 79% of that of normal subjects at 9 years following the PRP.[26] Also, studies have noted ONH pallor in diabetic eyes with PRP treatment,[48,49] implying that blood flow is decreased in the tissue of the ONH. Taken together, it might be that blood flow in the tissue of the ONH does not reduce rapidly but continues to reduce for a long time after the PRP.
Limitations of this study included small sample size. In the conventional group, although the reduction ratio was not statistically significantly correlated with the laser burns probably because of small number, the correlation coefficient was −0.427 and those were though to be weak correlation. Second, the follow-up was short-term and we do not know about the change in retinal or ONH blood flow after 12 weeks after PRP. Third, the first post-laser examination was at 1 week in our study, and the change in retinal blood flow may have been occurred at an earlier e period than 1 week. Further longitudinal studies using a larger number of subjects will be necessary for clarification.
In conclusion, the retinal blood flow was significantly reduced during the 12 weeks only after completion of PRP by conventional laser treatment. Our results indicate that short pulse on PRP treatment performed by the PASCAL would not significantly reduce the retinal blood flow.
Author contributions
Conceptualization: Takeshi Iwase.
Data curation: Takeshi Iwase, Yuji Mikoshiba.
Formal analysis: Takeshi Iwase, Kentaro Yamamoto.
Methodology: Takeshi Iwase.
Software: Eimei Ra.
Supervision: Takeshi Iwase.
Validation: Takeshi Iwase, Yuji Mikoshiba, Eimei Ra, Kentaro Yamamoto, Yoshitaka Ueno, Hiroko Terasaki.
Footnotes
Abbreviations: AU = arbitrary units, BCVA = best-corrected visual acuity, DBP = diastolic blood pressure, FFA = fluorescein fundus angiography, IOP = intraocular pressure, LSFG = laser speckle flowgraphy, MAP = mean arterial blood pressure, MBR = mean blur rate, NO = nitric oxide, OCT = optical coherence tomography, ONH = optic nerve head, OPP = ocular perfusion pressure, PASCAL = patterned scanning laser, PDR = proliferative diabetic retinopathy, PRP = panretinal photocoagulation, SBP = systolic blood pressure, SFCT = subfoveal choroidal thickness.
The authors have no funding and conflicts of interest to disclose.
References
- [1].The Diabetic Retinopathy Study Research Group. Preliminary report on effects of photocoagulation therapy. Am J Ophthalmol 1976;81:383–96. [DOI] [PubMed] [Google Scholar]
- [2].The Diabetic Retinopathy Study Research Group. Photocoagulation treatment of proliferative diabetic retinopathy. Clinical application of Diabetic Retinopathy Study (DRS) findings, DRS Report Number 8. Ophthalmology 1981;88:583–600. [PubMed] [Google Scholar]
- [3].Early Treatment Diabetic Retinopathy Study Research Group. Early photocoagulation for diabetic retinopathy ETDRS report number 9. Ophthalmology 1991;98:766–85. [PubMed] [Google Scholar]
- [4].Viswanath K, McGavin DD. Diabetic retinopathy: clinical findings and management. Community Eye Health 2003;16:21–4. [PMC free article] [PubMed] [Google Scholar]
- [5].Wilkinson CP, Ferris FL, 3rd, Klein RE, et al. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology 2003;110:1677–82. [DOI] [PubMed] [Google Scholar]
- [6].Chew EY, Ferris FL, 3rd, Csaky KG, et al. The long-term effects of laser photocoagulation treatment in patients with diabetic retinopathy: the early treatment diabetic retinopathy follow-up study. Ophthalmology 2003;110:1683–9. [DOI] [PubMed] [Google Scholar]
- [7].Grunwald JE, Brucker AJ, Petrig BL, et al. Retinal blood flow regulation and the clinical response to panretinal photocoagulation in proliferative diabetic retinopathy. Ophthalmology 1989;96:1518–22. [DOI] [PubMed] [Google Scholar]
- [8].Grunwald JE, Riva CE, Brucker AJ, et al. Effect of panretinal photocoagulation on retinal blood flow in proliferative diabetic retinopathy. Ophthalmology 1986;93:590–5. [DOI] [PubMed] [Google Scholar]
- [9].Fujio N, Feke GT, Goger DG, et al. Regional retinal blood flow reduction following half fundus photocoagulation treatment. Br J Ophthalmol 1994;78:335–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Bursell SE, Clermont AC, Kinsley BT, et al. Retinal blood flow changes in patients with insulin-dependent diabetes mellitus and no diabetic retinopathy. Invest Ophthalmol Vis Sci 1996;37:886–97. [PubMed] [Google Scholar]
- [11].Baydar S, Adapinar B, Kebapci N, et al. Colour Doppler ultrasound evaluation of orbital vessels in diabetic retinopathy. Australas Radiol 2007;51:230–5. [DOI] [PubMed] [Google Scholar]
- [12].Savage HI, Hendrix JW, Peterson DC, et al. Differences in pulsatile ocular blood flow among three classifications of diabetic retinopathy. Invest Ophthalmol Vis Sci 2004;45:4504–9. [DOI] [PubMed] [Google Scholar]
- [13].Sugiyama T, Araie M, Riva CE, et al. Use of laser speckle flowgraphy in ocular blood flow research. Acta Ophthalmol 2010;88:723–9. [DOI] [PubMed] [Google Scholar]
- [14].Nagahara M, Tamaki Y, Tomidokoro A, et al. In vivo measurement of blood velocity in human major retinal vessels using the laser speckle method. Invest Ophthalmol Vis Sci 2011;52:87–92. [DOI] [PubMed] [Google Scholar]
- [15].Blumenkranz MS, Yellachich D, Andersen DE, et al. Semiautomated patterned scanning laser for retinal photocoagulation. Retina 2006;26:370–6. [DOI] [PubMed] [Google Scholar]
- [16].Jain A, Blumenkranz MS, Paulus Y, et al. Effect of pulse duration on size and character of the lesion in retinal photocoagulation. Arch Ophthalmol 2008;126:78–85. [DOI] [PubMed] [Google Scholar]
- [17].Kimura M, Nishimura A, Yokogawa H, et al. Subfoveal choroidal thickness change following segmental scleral buckling for rhegmatogenous retinal detachment. Am J Ophthalmol 2012;154:893–900. [DOI] [PubMed] [Google Scholar]
- [18].Usui S, Ikuno Y, Akiba M, et al. Circadian changes in subfoveal choroidal thickness and the relationship with circulatory factors in healthy subjects. Invest Ophthalmol Vis Sci 2012;53:2300–7. [DOI] [PubMed] [Google Scholar]
- [19].Iwase T, Yamamoto K, Ra E, et al. Diurnal variations in blood flow at optic nerve head and choroid in healthy eyes: diurnal variations in blood flow. Medicine (Baltimore) 2015;94:e519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].The Early Treatment Diabetic Retinopathy Study Research Group. Techniques for scatter and local photocoagulation treatment of diabetic retinopathy: early treatment diabetic retinopathy study report no. 3. Int Ophthalmol Clin 1987;27:254–64. [DOI] [PubMed] [Google Scholar]
- [21].Fujii H. Visualisation of retinal blood flow by laser speckle flow-graphy. Med Biol Eng Comput 1994;32:302–4. [DOI] [PubMed] [Google Scholar]
- [22].Sugiyama T, Utsumi T, Azuma I, et al. Measurement of optic nerve head circulation: comparison of laser speckle and hydrogen clearance methods. Jpn J Ophthalmol 1996;40:339–43. [PubMed] [Google Scholar]
- [23].Tamaki Y, Araie M, Kawamoto E, et al. Non-contact, two-dimensional measurement of tissue circulation in choroid and optic nerve head using laser speckle phenomenon. Exp Eye Res 1995;60:373–83. [DOI] [PubMed] [Google Scholar]
- [24].Tamaki Y, Araie M, Tomita K, et al. Real-time measurement of human optic nerve head and choroid circulation, using the laser speckle phenomenon. Jpn J Ophthalmol 1997;41:49–54. [DOI] [PubMed] [Google Scholar]
- [25].Yamada Y, Suzuma K, Matsumoto M, et al. Retinal blood flow correlates to aqueous vascular endothelial growth factor in central retinal vein occlusion. Retina 2015;35:2037–42. [DOI] [PubMed] [Google Scholar]
- [26].Iwase T, Kobayashi M, Yamamoto K, et al. Effects of photocoagulation on ocular blood flow in patients with severe non-proliferative diabetic retinopathy. PLoS One 2017;12:e0174427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Mendivil A, Cuartero V. Ocular blood flow velocities in patients with proliferative diabetic retinopathy after scatter photocoagulation. Two years of follow-up. Retina 1996;16:222–7. [DOI] [PubMed] [Google Scholar]
- [28].Hessemer V, Schmidt KG. Influence of panretinal photocoagulation on the ocular pulse curve. Am J Ophthalmol 1997;123:748–52. [DOI] [PubMed] [Google Scholar]
- [29].Hiroshiba N, Ogura Y, Nishiwaki H, et al. Alterations of retinal microcirculation in response to scatter photocoagulation. Invest Ophthalmol Vis Sci 1998;39:769–76. [PubMed] [Google Scholar]
- [30].Apple DJ, Goldberg MF, Wyhinny G. Histopathology and ultrastructure of the argon laser lesion in human retinal and choroidal vasculatures. Am J Ophthalmol 1973;75:595–609. [DOI] [PubMed] [Google Scholar]
- [31].Wilson DJ, Green WR. Argon laser panretinal photocoagulation for diabetic retinopathy. Scanning electron microscopy of human choroidal vascular casts. Arch Ophthalmol 1987;105:239–42. [DOI] [PubMed] [Google Scholar]
- [32].Stitt AW, Gardiner TA, Archer DB. Retinal and choroidal responses to panretinal photocoagulation: an ultrastructural perspective. Graefes Arch Clin Exp Ophthalmol 1995;233:699–705. [DOI] [PubMed] [Google Scholar]
- [33].Stefansson E, Landers MB, 3rd, Wolbarsht ML. Increased retinal oxygen supply following pan-retinal photocoagulation and vitrectomy and lensectomy. Trans Am Ophthalmol Soc 1981;79:307–34. [PMC free article] [PubMed] [Google Scholar]
- [34].Stefansson E, Landers MB, 3rd, Wolbarsht ML. Vitrectomy, lensectomy, and ocular oxygenation. Retina 1982;2:159–66. [DOI] [PubMed] [Google Scholar]
- [35].Feke GT, Tagawa H, Deupree DM, et al. Blood flow in the normal human retina. Invest Ophthalmol Vis Sci 1989;30:58–65. [PubMed] [Google Scholar]
- [36].Novack RL, Stefansson E, Hatchell DL. The effect of photocoagulation on the oxygenation and ultrastructure of avascular retina. Exp Eye Res 1990;50:289–96. [DOI] [PubMed] [Google Scholar]
- [37].Wolbarsht ML, Landers MB., 3rd The rationale of photocoagulation therapy for proliferative diabetic retinopathy: a review and a model. Ophthalmic Surg 1980;11:235–45. [PubMed] [Google Scholar]
- [38].Wolbarsht ML, Landers MB, 3rd, Stefansson E. Vasodilation and the etiology of diabetic retinopathy: a new model. Ophthalmic Surg 1981;12:104–7. [PubMed] [Google Scholar]
- [39].Feke GT, Green GJ, Goger DG, et al. Laser Doppler measurements of the effect of panretinal photocoagulation on retinal blood flow. Ophthalmology 1982;89:757–62. [DOI] [PubMed] [Google Scholar]
- [40].Yamada Y, Suzuma K, Onizuka N, et al. Evaluation of retinal blood flow before and after panretinal photocoagulation using pattern scan laser for diabetic retinopathy. Curr Eye Res 2017;42:1707–12. [DOI] [PubMed] [Google Scholar]
- [41].Diddie KR, Ernest JT. The effect of photocoagulation on the choroidal vasculature and retinal oxygen tension. Am J Ophthalmol 1977;84:62–6. [DOI] [PubMed] [Google Scholar]
- [42].Roider J, Michaud NA, Flotte TJ, et al. Response of the retinal pigment epithelium to selective photocoagulation. Arch Ophthalmol 1992;110:1786–92. [DOI] [PubMed] [Google Scholar]
- [43].Iwase T, Ueno Y, Ra E, et al. Changes in choriocapillaris and retinal morphology after laser photocoagulation by OCT angiography: a case report. Medicine (Baltimore) 2018;97:e13278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Kristinsson JK, Gottfredsdottir MS, Stefansson E. Retinal vessel dilatation and elongation precedes diabetic macular oedema. Br J Ophthalmol 1997;81:274–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Lee CJ, Smith JH, Kang-Mieler JJ, et al. Decreased circulation in the feline choriocapillaris underlying retinal photocoagulation lesions. Invest Ophthalmol Vis Sci 2011;52:3398–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Chappelow AV, Tan K, Waheed NK, et al. Panretinal photocoagulation for proliferative diabetic retinopathy: pattern scan laser versus argon laser. Am J Ophthalmol 2012;153:137–42. [DOI] [PubMed] [Google Scholar]
- [47].Muqit MM, Marcellino GR, Henson DB, et al. Pascal panretinal laser ablation and regression analysis in proliferative diabetic retinopathy: Manchester Pascal Study Report 4. Eye (Lond) 2011;25:1447–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Johns KJ, Leonard-Martin T, Feman SS. The effect of panretinal photocoagulation on optic nerve cupping. Ophthalmology 1989;96:211–6. [DOI] [PubMed] [Google Scholar]
- [49].Lim MC, Tanimoto SA, Furlani BA, et al. Effect of diabetic retinopathy and panretinal photocoagulation on retinal nerve fiber layer and optic nerve appearance. Arch Ophthalmol 2009;127:857–62. [DOI] [PubMed] [Google Scholar]


