Abstract
Objective
To investigate the expression level of TRAF3IP2 in psoriasis lesion, and to explore the functional roles of TRAF3IP2 on proliferation, apoptosis, cytokine expression and secretion of both keratinocytes and vascular endothelial cells in vitro.
Methods
The expression of TRAF3IP2 in skin samples of patients with psoriasis was analyzed by immunohistochemistry and qRT-PCR. To identify the effect of TRAF3IP2 knockdown on HaCaT and HUVEC cells, a plasmid vector expressing siRNA targeting TRAF3IP2 mRNA was designed and transfected into cells with Lipofectamine 2000. The levels of cytokines were identified using the ELISA Kits and qRT-PCR. Furthermore, cell proliferation, cell cycle and apoptosis were examined by using MTT, PI and Annexin V-FITC/7AAD assays, respectively. Furthermore, the expression of apoptosis-related proteins (Cleaved-Caspase 3, Caspase 3 and Bax) were detected by western blotting.
Results
TRAF3IP2 was significantly upregulated in psoriasis lesion. TRAF3IP2 knockdown reduced the expression of vascular endothelial growth factor A (VEGFA) and the release of IL-6, and IL-8, but had no effect on IL-23 in both HaCaT and HUVEC cells. In addition, knockdown of TRAF3IP2 significantly inhibited cell proliferation through blocking the cell cycle in the G2/M phase. Moreover, knockdown of TRAF3IP2 increased the expression of Caspase 3, Cleaved-Caspase 3 and Bax, which was supported by the increased apoptosis of both HaCaT and HUVEC cells.
Conclusion
Taken together, these results indicated that TRAF3IP2 might play a contributive role in the pathogenesis of psoriasis and may serve as a new target for the treatment of psoriasis. VEGF related pathways may be involved in the mechanism beneath.
Keywords: Cell biology, Genetics, Molecular biology, Physiology
1. Introduction
Psoriasis is a chronic inflammatory skin disorder characterized by histological features of hyper-proliferation of keratinocyte, inflammatory cell infiltration and vascular changes. Current evidence suggests that psoriasis is an immune-mediated systemic disorder with an increased risk of cardiovascular disease [1, 2, 3]. The pathogenesis of psoriasis associated with cardiovascular risk is complex and has not yet been fully elucidated. Therefore, it is desirable to find the optimal biologic and candidate gene predictors for the therapeutic response to individual patients.
Many genes such as HLA-C*06, ERAP1, IL23R, IL12B, TNFAIP3 and IFIH1 have been identified as susceptibility genes for psoriasis and are associated with adaptive or innate immunity as well as skin barrier function [4, 5, 6, 7, 8]. Among these, HLA-C*06 and the single-nucleotide polymorphisms (SNPs) in TNFAIP3 and IL23R are associated with therapeutic response to biologics [9, 10, 11, 12].
Recently, several reports have indicated that TNF receptor-associated factor 3 interacting protein 2 (TRAF3IP2) is a strong candidate gene for psoriasis and psoriasis arthritis. For example, genome-wide association studies have identified the SNP rs33980500l on TRAF3IP2 as a susceptible site for the risk of psoriasis [5, 13]. Therefore, it is speculated that TRAF3IP2 is involved in the pathogenesis of psoriasis, although there is no evidence that the polymorphism in TRAF3IP2 is associated with response to biologics in patients with psoriasis.
It is well known that biologics that target IL-17 and its receptors are very effective treatments for psoriasis. The TRAF3IP2 gene encodes a protein involved in regulation of IL-17-mediated signaling, which is recruited to the IL-17 receptor through SEFIR domain-mediated oligomerization [14]. Besides, TRAF3IP2 has been reported to play a causal role in the development and vulnerability of atherosclerotic plaque and may be a potential therapeutic target for atherosclerosis and vascular diseases [15]. Based on these observations, we hypothesized that TRAF3IP2 may play an important role in the response of genes expression in keratinocytes and vascular endothelial cells which are relevant to the pathogenesis of psoriasis. This hypothesis needs to be validated experimentally.
The aim of this study was to investigate the expression of TRAF3IP2 in psoriasis lesions and the effects of TRAF3IP2 knockdown on proliferation, apoptosis, and the expression and secretion of cytokines and VEGF in both HaCaT and HUVEC cells.
2. Materials and methods
2.1. Antibodies and plasmid constructs
Rabbit-anti-TRAF3IP2, rabbit-anti-Cleaved-Caspase 3, rabbit-anti-Caspase 3, rabbit-anti-Bax and rabbit-anti-β-actin antibodies were purchased from Sigma-Aldrich (USA). The TRAF3IP2 siRNA (siTRAF3IP2) and scramble siRNA (siNC) were purchased from Thermo Scientific Open Biosysterms and were cloned into the lentiviral vector GV248 (Jikai, Shanghai, China).
2.2. Human skin tissue collection
97 psoriasis tissues (53 males and 44 females, age range = 17–43 years) and 29 normal skin tissues (15 males and 14 females, age range = 15–56 years) were obtained. The collected fresh tissues were frozen in liquid nitrogen and stored at −80 °C until use. The study was performed in accordance with the declaration of Helsinki Principles and approved by the Research Ethics Board. The study was reviewed and approved by the Ethics Committee of Shandong Provincial Hospital Affiliated to Shandong University. All the patients or their families signed an informed consent.
2.3. Immunohistochemistry
Immunohistochemistry (IHC) was performed according to standard methods. Paraffin-embedded skin sections (5 μm) were deparaffinized in xylene and rehydrated through a graded ethanol series. For antigen retrieval, sections were boiled in 10 mM sodium citrate (pH 6.0) for 30 min at 95 °C. All sections were quenched with 3% H2O2 to reduce endogenous peroxidase activity, followed by a blocking buffer containing 10% normal goat serum was used to reduce nonspecific binding. The sections were incubated with rabbit anti-TRAF3IP2 antibody overnight at 4 °C, and followed by washing 3 times with PBS. Then horseradish peroxidase-labeled goat anti-rabbit IgG was added and incubated for 1 h at room temperature. Subsequently, the sections were stained with diaminobenzidine (DAB) reagent for 1 min. A rabbit IgG was used as a negative primary antibody control. The figures were captured by a Nikon 80i microscope. The IHC results were scored and quantified in a semi-quantitative manner based on the following scoring system as previously described [16, 17]. The ratio of positively stained cells was scored as follows: 0 (≤5%), 1 (6–25%), 2 (26–50%), 3 (51–75%), 4 (>75%). The staining intensity was graded as follows: 0 (colorless), 1 (light yellow), 2 (yellowish brown), 3 (chocolate brown). The score for each microscopic field was calculated by multiplying the two scores. The average scores of five fields were taken as the final immunoreactivity score.
2.4. Cell culture and transfection
HUVEC and HaCaT cell lines were obtained from American Tissue Culture Collection (ATCC). HUVEC and HaCaT cells were cultured in RPMI-1640 and DMEM medium (GIBCO), respectively, which were supplemented with 10% Fetal Calf Serum (FCS) (Capricorn Scientific) and 1% penicillin/streptomycin (Biowest). All cells were cultured in a humidified incubator containing 5% CO2 at 37 °C. Before transfection, the morphology of HUVEC and HaCaT cells was observed by using an inverted microscope (Nikon, Tokyo, Japan). For transfection, the control siRNA (siNC) or TRAF3IP2 siRNA (siTRAF3IP2) was transfected into cells using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. 4 h after transfection, the cells were replaced with the fresh RPMI-1640 or DMEM medium containing 10% FCS and cells were incubated in a humidified incubator until use. The siRNA transfection efficiency was verified by qRT-PCR.
2.5. MTT assays
The MTT assay was performed as previously described [18]. Before transfection, 1×104 cells/ml HUVEC and HaCaT cells were seeded into 24-well plates. After transfection, cells were incubated at 37 °C for 72 h, followed by addition of 1 mg/ml of MTT to each wells and incubation at 37 °C for 2 h. Thereafter, 500 μl of DMSO was added to dissolve the residual formazan crystals (Merck Millipore). The resultant absorbance was measured at 570 nm using a Microplate Reader (Bio-Rad, Hercules, USA).
2.6. Cell cycle analysis
Cultured HaCaT and HUVEC cells were harvested and fixed with cold 70% ethanol overnight at −20 °C. For the cell cycle analysis, PI staining solutions with 50 mg/ml PI and 100 mg/ml ribonuclease A were added to the cells and incubated for 30 min at 37 °C in the dark. Cell cycle was analyzed using the flow cytometry (BD Biosciences).
2.7. Cell apoptosis assay
Cell apoptosis was detected using the Annexin V Apoptosis Detection kit APC (single staining)according to the manufacturer's instructions (eBioscience; Thermo Fisher Scientific, Inc.) and as previously described [19, 20, 21]. Both HUVEC and HaCaT cells were seeded at a cell density of 2×106 cells/ml before transfection. After incubation at 37 °C for 72 h, cells were subjected to trypsin/EDTA and followed by washing with cold PBS. Cells were then centrifuged at 2,000 rpm for 5 min after which the pellets were resuspended in 1X annexin-binding buffer (BD Sciences). Thereafter, 10 μl of Annexin V solution was added to each cell suspension, and then incubation on ice for 15 min in the dark. Subsequently, 400 μl of ice-cold 1X annexin binding buffer was added to the samples for 30 min and all the obtained cell suspensions were examined using the BD Accuri C6 flow cytometer. The data analyses were performed using CellQuest software (version 5.1, BD Biosciences).
2.8. Quantitative real-time PCR
Total RNA was extracted with TRIzol reagent (Invitrogen) according to the manufacturer's instructions. The cDNA was synthesized with random hexamers (Applied Biosystems) using M-MLV reverse transcriptase (Promega). Gene expression level was calculated using the ΔΔCt method [22]. Primer sequences were as follows: GAPDH forward: 5′-TGACTTCAAC AGCGACACCCA-3′, reverse: 5′-CACCCTGTTGCTGTAGCCAAA-3’; TRAF3IP2 forward: 5′-CTGCGTCTGAGTCTGTGGTT-3′, reverse: 5′-TATCCCGTGTCTATGGTTGG-3’; IL-6 forward: 5′-GTGAAAGCAGCAAAGAGGC-3′, reverse: 5′-CATTTGTGGTTGGGTCAG G-3’; IL-8 forward: 5′-CCCCCATGGTTCAGAAGATTG-3′, reverse: 5′-TTGTCAGAAGCC AGCGTTCAC-3’; IL-23 forward: 5′-CTAAAAATAATGTGCCCCGT-3′, reverse: 5′-AGTC CTAGTAGGAGGTGT-3’; VEGFA forward: 5′-AGTGTGTGCCCACTGAGGA-3′, reverse: 5′-GTGCT GTAGGAAGCTCATCTC. GAPDH was used as the reference gene.
2.9. Western blotting
24 h after transfection, cells were harvested and lysed with 100 μl of RIPA lysate (Servicebio, Beijing, China) for 30 min on ice followed by centrifugation at 12000 rpm for 10 min at 4 °C, and subsequent collection of the supernatants. The concentration of protein was detected using the BCA Protein Quantification Kit (Servicebio, Beijing, China). About 30 μg of protein were separated by 10% sodium dodecyl sulphate polyacrylamide gels via electrophoresis (SDS-PAGE) (Bio-Rad) and electro-transferred to the PVDF membranes (Bio-Rad) (The PVDF membranes were activated with methanol prior to use). The membranes were then blocked in 3% BSA for 1 h. Subsequently, the membranes were incubated with the primary antibodies (TRAF3IP2, 1:3000; Cleaved-Caspase 3, 1:3000; Caspase 3, 1:3000; Bax, 1:3000; β-actin, 1:3000) overnight at 4 °C, and then washed three times in PBS-Tween followed by incubation in the appropriate secondary antibody (1:5000, Servicebio, Beijing, China) for 1 h at room temperature. After the membranes were washed three times with PBS-Tween, the chemiluminescent substrate (Biorad) was added to the membranes in order to detect proteins. Densitometric analysis was performed and protein levels were quantified using ImageJ software.
2.10. Enzyme-linked immunosorbent assay (ELISA)
HUVEC and HaCaT cells and the supernatant were collected in iced tubes, respectively. The cells were lysed with RIPA buffer and separated by centrifugation and stored at 80 °C until assayed. The secretion levels of cytokines VEGFA, IL-6, IL-8 and IL-23 were measured via the ELISA kit (Thermo Fisher Scientific, USA) according to the manufacturer's instructions. A multimode reader (Bio-Rad) was used to read the absorbance at 450 nm. Each measurement was performed in triplicate.
2.11. Statistical analysis
The significance of differences was determined by with the statistical software GraphPad prism for windows. T-test, one-way or two-way ANOVA was used adequately when needed and followed by Tukeys post-hoc multiple comparison test. All results are shown as mean and the standard deviation (mean ± SD). p < 0.05 was considered significant.
3. Results
3.1. Elevated expression of TRAF3IP2 in psoriasis
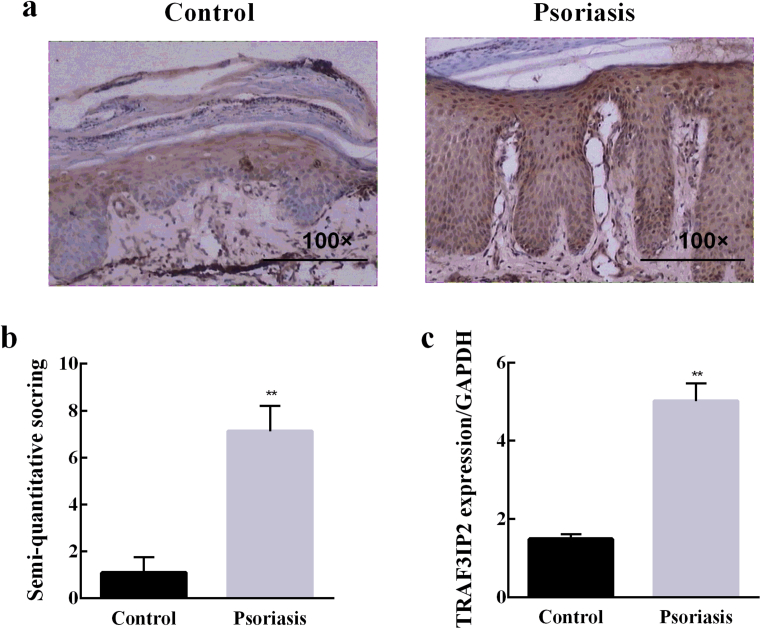
To detect the expression of TRAF3IP2 in psoriasis samples, we performed the immunohistochemistry experiments. The results showed that TRAF3IP2 was negatively expressed in the normal skin but up-regulated in the thickened epidermis of psoriasis skin (Fig. 1a). Semi-quantitative analysis of the immunohistochemistry results indicated that the expression of TRAF3IP2 was remarkably elevated in psoriasis skin compared with the normal controls (Fig. 1b). Furthermore, similar results were obtained in qRT-PCR (Fig. 1c). Taken together, these data demonstrate a significant increase in TRAF3IP2 expression in the psoriasis skin lesions.
Fig. 1.
TRAF3IP2 expression was upregulated in psoriatic lesions. (a) Representative Immunohistochemistry staining of healthy human skin and psoriatic human skin and positive expression of TRAF3IP2 in psoriatic dermal vascular endothelial cells. (b) Semi-quantitative analysis of TRAF3IP2 staining results from 29 healthy and 97 psoriatic skin samples. (c) qRT-PCR was used to detect the expression of TRAF3IP2 in healthy and psoriatic skin. **p < 0.01, vs the siNC group.
3.2. TRAF3IP2 knockdown affects the expression of cytokines in cultured HaCaT and HUVEC cells
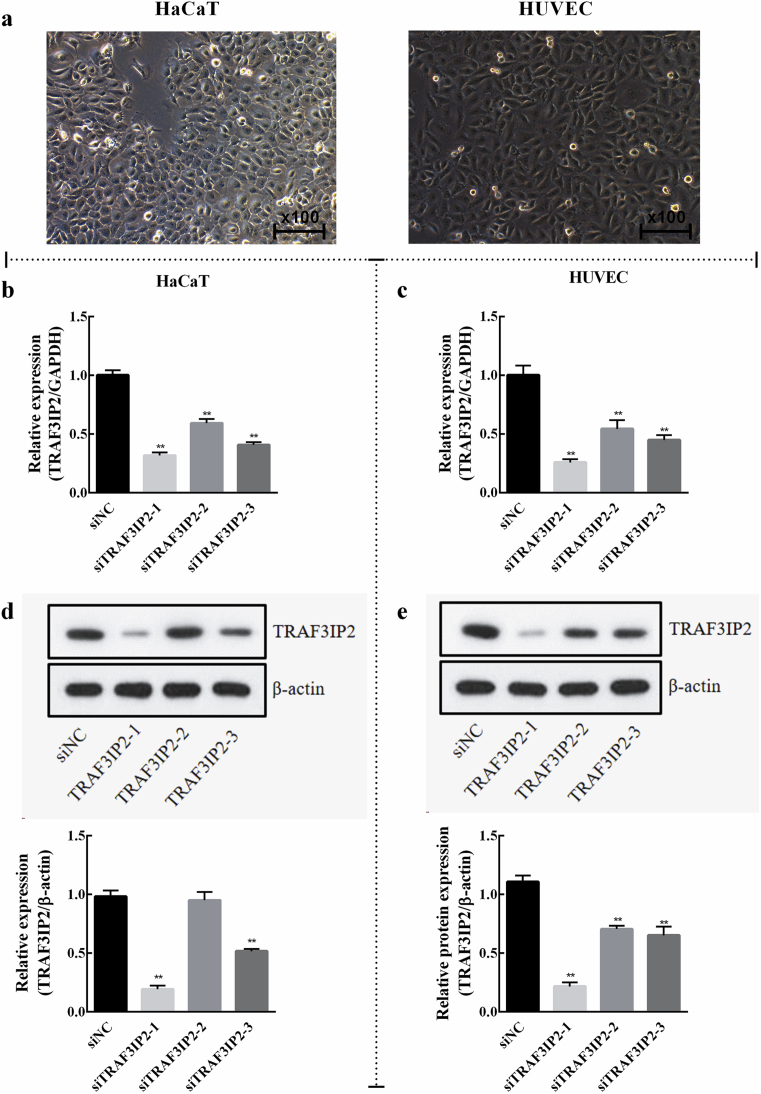
Since the above results indicated that TRAF3IP2 was highly expressed in the psoriasis skin lesions (Fig. 1), we performed in vitro studies to uncover the molecular mechanism of this protein. The HaCaT and HUVEC cells were used as experimental material because previous studies have pointed out their functional role in psoriasis. The morphology of the cells was observed by an inverted microscope, and it was found that HaCaT and HUVEC cells grew well in DMEM and RPIM-1640 medium, respectively, and the cells were arranged neatly and regularly (Fig. 2a). For this, siRNA interference technology was used to knockdown the expression of TRAF3IP2 in HaCaT and HUVEC cells. The efficiency of TRAF3IP2 knockdown was assessed by qRT-PCR and western blotting. As shown in Fig. 2, all three siRNAs significantly decreased the mRNA level of TRAF3IP2 in HaCaT cells (Fig. 2b) and HUVEC (Fig. 2c) cells. In addition, western blotting analysis also revealed that the protein expression level of TRAF3IP2 was obviously reduced by TRAF3IP2 siRNAs (Fig. 2d, e). Among them, siTRAF3IP2-1 had the highest knockout efficiency in both cell lines and was used in all subsequent experiments.
Fig. 2.
Efficiency of TRAF3IP2 knockdown in HaCaT and HUVEC cells. (a) Morphological characterization of HaCaT and HUVEC cells growing in DMEM and RPMI-1640 medium, respectively (x100). The efficiency of TRAF3IP2 knockdown in HaCaT and HUVEC cells was verified using qRT-PCR (b, c) and western blotting (d, e), respectively. Data are shown as mean ± SD. n = 3 independent experiments, *p < 0.05 and **p < 0.01, vs the siNC group. Note: The full, uncropped versions of the blots can be found as supplementary materials.
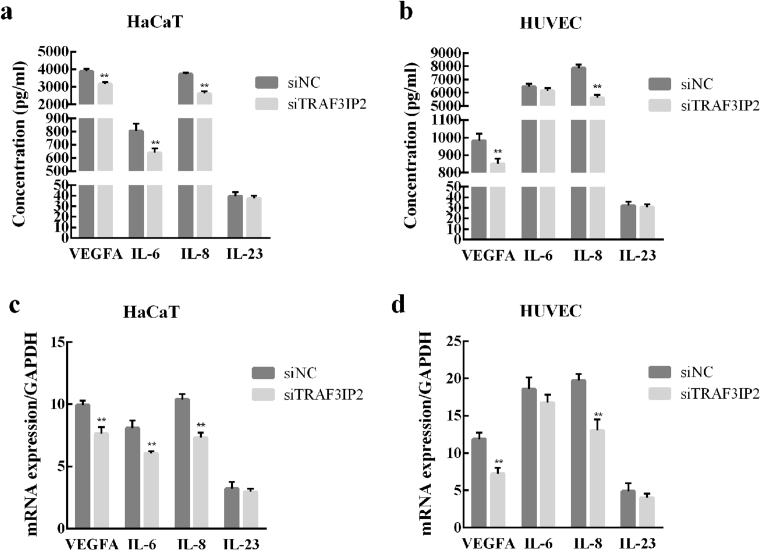
The effect of siTRAF3IP2 on the expression and secretion of cell cytokines in HaCaT and HUVEC cells were detected by using ELISA and qRT-PCR. The results showed that the TRAF3IP2 knockdown significantly reduced the secretion of VEGFA and IL-8, but had no effect on the secretion of IL-6 in HaCaT cells (Fig. 3a). In HUVEC cells, knockdown of TRAF3IP2 decreased the secretion of VEGF, IL-6, and IL-8 (Fig. 3b). However, knockdown of TRAF3IP2 had no effect on the secretion of IL-23 in both HaCaT and HUVEC cells. Additionally, similar results were obtained in qRT-PCR (Fig. 3c and d).
Fig. 3.
TRAF3IP2 knockdown affects the secretion and expression of cytokines in HUVEC and HaCaT cells. Secretion and expression levels of VEGFA, IL-6, IL-8 and IL-23 in HaCaT cells (a, c) and HUVEC cells (b, d) were measured by using ELISA and qRT-PCR, respectively. Data are shown as mean ± SD. n = 3 independent experiments, *p < 0.05, **p < 0.01, vs the siNC group.
3.3. Effect of TRAF3IP2 knockdown on proliferation, cell cycle and apoptosis of keratinocytes and vascular endothelial cells
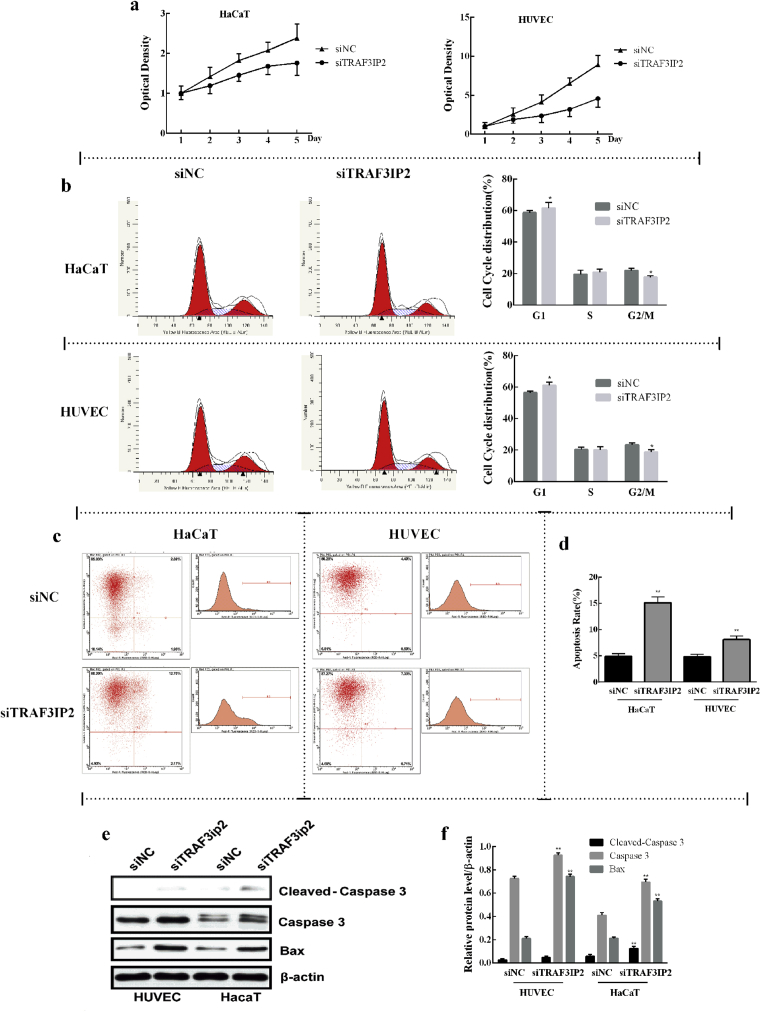
The effect of TRAF3IP2 knockdown on proliferation and apoptosis of HaCaT and HUVEC cells was detected by MTT assay and flow cytometry through Annexin-V/PI assays, respectively. When HUVEC and HaCaT cells were transfected with siTRAF3IP2, cell viability was significantly decreased compared with cells transfection with the control siNC (Fig. 4a). The cell cycle analysis was measured by using flow cytometry through PI staining. The results showed that TRAF3IP2 knockdown increased G1 phase cells and decreased G2/M phase cells, leading to a G1 growth arrest in HUVEC and HaCaT cells (Fig. 4b), indicating that TRAF3IP2 knockdown suppresses the proliferation of HUVEC and HaCaT cells by blocking the cycle at the G1 phase. In addition, the results showed that HUVEC cells transfected with siTRAF3IP2 revealed 7.33% of cells undergoing apoptosis, in contrast to 4.40% from cells that were transfected with control siNC (Fig. 4c). HaCaT cells transfected with siTRAF3IP2 showed 12.70% of cell apoptosis compared with 2.89% of cells transfected with control siNC (Fig. 4c). Further statistical analysis confirmed that cell apoptotis was significantly increased in both HUVEC and HaCaT cell lines after transfection with siTRAF3IP2 (Fig. 4d). Moreover, TRAF3IP2 knockdown upregulated the proteins expression levels of Caspase 3 and Bax compared with siNC group, suggesting that TRAF3IP2 promoted the process of apoptosis in HUVEC and HaCaT cells (Fig. 4e and f).
Fig. 4.
Effect of TRAF3IP2 knockdown on proliferation, cell cycle and apoptosis of HUVEC and HaCaT. (a) MTT assays were performed to assess HaCaT and HUVEC cell viability after treatment with TRAF3IP2 siRNA. It was found that HUVEC and HaCaT cells exhibited a significant decrease in cellular viability after transfection with siTRAF3IP2 compared with cells transfected with control siRNA. (b) Percentage of cells at different periods of cell cycle. It was found that TRAF3IP2 knockdown significantly increased the percentage of cells in G1 period. (c) It was revealed that most of the HUVEC and HaCaT cells fall into the upper left quadrant which is known to represent normal living cells. The upper right quadrant represented HUVEC and HaCaT cells were undergoing apoptosis and it was shown that, after transfected with siTRAF3IP2, a significantly increased in the percentage of HUVEC and HaCaT cells undergone apoptosis. (d) Quantification of the percentage of cells undergoing apoptosis in c. The expression of apoptosis-related proteins (Caspase 3 and Bax) were detected by western blotting (e), and the images were processed and analyzed by ImageJ software (f). Data are shown as mean ± SD. n = 3 independent experiments, *p < 0.05, **p < 0.01, vs the siNC group. Note: The full, uncropped versions of the blots can be found as supplementary materials.
4. Discussion
TRAF3IP2 encodes the protein Act1 (transcription factor NF-κB activator 1), which is essential for appropriate regulation of IL-17-mediated signaling [14]. The physiological importance of Act1 is reflected in its ability to interact with multiple signaling pathways downstream of the IL-17 receptor and its role in a variety of inflammatory pathologies. Recently studies have shown that TRAF3IP2 is present in susceptibility locus of known inflammatory diseases such as the psoriasis and psoriasis arthritis [5, 13]. For instance, studies have suggested TRAF3IP2 is a strong candidate gene for psoriasis due to the SNP rs33980500 located in the N-terminal region which possibly alters its interaction with the molecular chaperone HSP90 [23]. Therefore, it is of great interest to investigate the function of TRAF3IP2 in the pathogenesis of psoriasis. In this work, we demonstrated that the expression of TRAF3IP2 was significantly up-regulated in psoriatic lesions. In vitro, we observed that knockdown of TRAF3IP2 significantly decreased the proliferation of HaCaT and HUVEC cells, induced cell apotosis and blocked cell cycle at the G2/M phase. In addition, the levels of VEGFA and cytokines were decreased after TRAF3IP2 downregulation in HaCaT and HUVEC cells.
It is known that angiogenesis is a key pathogenic feature of psoriasis, and VEGF is an angiogenic growth factor that is overexpressed in both psoriatic and atherosclerotic lesions [24, 25]. A prospective controlled study also revealed the correlation between VEGF and subclinical atherosclerosis in patients with moderate to severe psoriasis [24]. Previous studies have shown that some patients with psoriasis may improve when exposed to VEGF inhibitors [26]. Our study further provides the insight that TRAF3IP2 is a positive regulator of VEGFA, which suggests that the monitoring of the TRAF3IP2/VEGFA axis for the treatment of psoriasis. Especially, since VEGFA is involved in angiogenesis, we hypothesize that the targeting of TRAF3IP2 will contribute in alleviating the angiogenesis involved in the pathogenesis of psoriasis and other angiogenesis-associated human diseases.
Inflammation is another important factor involved in the pathogenesis of a variety of human diseases. Here, we found that the levels of IL-6 and IL-8 were affected by TRAF3IP2 knockdown in HaCaT cells, but had no effect on IL-6 in HUVEC cells. This difference may be due to the difference in the sources of cells used, which suggests that TRAF3IP2 may govern different functions in different cell types. Additionally, TRAF3IP2 knockdown had no effect on IL-23 in both HaCaT and HUVEC cells, suggesting that TRAF3IP2 may not regulate IL23 in both cells. In summary, these results indicated that TRAF3IP2 regulates inflammation in HaCaT and HUVEC cells by controlling the release of IL-6 and IL-8. Thus, due to its effects in HaCaT and HUVEC cells which are determinant factors in psoriasis, we inferred that therapeutically targeting TRAF3IP2 can considerably mitigate the inflammation associated with psoriasis.
We also showed that TRAF3IP2 positively regulated the proliferation of keratinocytes and vascular endothelial cells, as downregulation of TRAF3IP2 inhibited proliferation of HaCaT and HUVEC cells by arresting the cell cycle in the G1 phase. Importantly, this result is consistent with the upregulated expression of TRAF3IP2 in psoriasis lesion. Paradoxically, however, previous studies indicated that Act1 (encoded by TPAF3IP2) has a growth-inhibitory effect through modulating Cx43 activity in breast cancer cells [27]. The proliferation of keratinocytes is a complex process, so it is not unusual that a protein performs opposite functions in the proliferation of two different epithelial cell lines. For instance, studies have shown that the S100A8/S100A9 complex suppresses keratinocyte proliferation, but promote the growth and metastasis of colorectal cancer cells [28, 29]. It is likely that TRAF3IP2 positively regulates the proliferation of keratinocytes and vascular endothelial cells in psoriasis through a distinct mechanism, which need further investigation. Our results also indicated that TRAF3IP2 regulated cell cycle in both cells, which further elucidated the effect of TRAF3IP2 on cell proliferation. Apoptosis is another key factor involved the physiology of keratinocyte and vascular endothelial cells both in normal and pathological conditions. Psoriatic keratinocytes exhibit enhanced ability to resist apoptosis, which may be one of the key factors in the pathogenesis of psoriasis [30]. Accordingly, we found that downregulation of TRAF3IP2 increased the apoptosis in both HaCaT and HUVEC cells. These results further suggested a potential contributive role of TRAF3IP2 in the psoriasis pathogenesis.
In summary, our results indicated that downregulation of TRAF3IP2 altered the expression and secretion of VEGF in keratinocytes and vascular endothelial cells. Furthermore, TRAF3IP2 knockdown reduced the proliferation of keratinocytes and vascular endothelial through promoting the apoptosis signaling pathway. Thus, TRAF3IP2 may be a potential therapeutic target for psoriasis and further studies are needed for effectively potentiating its application in the treatment of psoriasis.
Declarations
Author contribution statement
Yali Song, Li Zhang: Conceived and designed the experiments; Wrote the paper.
Lamei Chen, Qingxia Lin: Performed the experiments.
Yuanyuan Li: Analyzed and interpreted the data.
Wenmin Liu: Contributed reagents, materials, analysis tools or data.
Funding statement
This work was supported by the Youth Foundation of National Natural Science Foundation of China (grant number 81502729).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Fernandez-Armenteros J.M., Gomez-Arbones X., Buti-Soler M., Betriu-Bars A., Sanmartin-Novell V., Ortega-Bravo M. Psoriasis, metabolic syndrome and cardiovascular risk factors. A population-based study. J. Eur. Acad. Dermatol. Venereol. 2018 doi: 10.1111/jdv.15159. [DOI] [PubMed] [Google Scholar]
- 2.Owczarczyk-Saczonek A.B., Nowicki R.J. Prevalence of cardiovascular disease risk factors, and metabolic syndrome and its components in patients with psoriasis aged 30 to 49 years. Postepy Dermatol. Alergol. 2015;32(4):290–295. doi: 10.5114/pdia.2014.40966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim G.W., Park H.J., Kim H.S., Kim S.H., Ko H.C., Kim B.S. Analysis of cardiovascular risk factors and metabolic syndrome in Korean patients with psoriasis. Ann. Dermatol. 2012;24(1):11–15. doi: 10.5021/ad.2012.24.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nair R.P., Stuart P.E., Nistor I., Hiremagalore R., Chia N.V., Jenisch S. Sequence and haplotype analysis supports HLA-C as the psoriasis susceptibility 1 gene. Am. J. Hum. Genet. 2006;78(5):827–851. doi: 10.1086/503821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Genetic Analysis of Psoriasis C., the Wellcome Trust Case Control C., Strange A., Capon F., Spencer C.C., Knight J. A genome-wide association study identifies new psoriasis susceptibility loci and an interaction between HLA-C and ERAP1. Nat. Genet. 2010;42(11):985–990. doi: 10.1038/ng.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cargill M., Schrodi S.J., Chang M., Garcia V.E., Brandon R., Callis K.P. A large-scale genetic association study confirms IL12B and leads to the identification of IL23R as psoriasis-risk genes. Am. J. Hum. Genet. 2007;80(2):273–290. doi: 10.1086/511051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao B., Li L., Lei Q., Guan K.L. The Hippo-YAP pathway in organ size control and tumorigenesis: an updated version. Genes Dev. 2010;24(9):862–874. doi: 10.1101/gad.1909210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van den Reek J., Coenen M.J.H., van de L'Isle Arias M., Zweegers J., Rodijk-Olthuis D., Schalkwijk J. Polymorphisms in CD84, IL12B and TNFAIP3 are associated with response to biologics in patients with psoriasis. Br. J. Dermatol. 2017;176(5):1288–1296. doi: 10.1111/bjd.15005. [DOI] [PubMed] [Google Scholar]
- 9.Talamonti M., Botti E., Galluzzo M., Teoli M., Spallone G., Bavetta M. Pharmacogenetics of psoriasis: HLA-Cw6 but not LCE3B/3C deletion nor TNFAIP3 polymorphism predisposes to clinical response to interleukin 12/23 blocker ustekinumab. Br. J. Dermatol. 2013;169(2):458–463. doi: 10.1111/bjd.12331. [DOI] [PubMed] [Google Scholar]
- 10.Tejasvi T., Stuart P.E., Chandran V., Voorhees J.J., Gladman D.D., Rahman P. TNFAIP3 gene polymorphisms are associated with response to TNF blockade in psoriasis. J. Investig. Dermatol. 2012;132(3 Pt 1):593–600. doi: 10.1038/jid.2011.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gallo E., Cabaleiro T., Roman M., Solano-Lopez G., Abad-Santos F., Garcia-Diez A. The relationship between tumour necrosis factor (TNF)-alpha promoter and IL12B/IL-23R genes polymorphisms and the efficacy of anti-TNF-alpha therapy in psoriasis: a case-control study. Br. J. Dermatol. 2013;169(4):819–829. doi: 10.1111/bjd.12425. [DOI] [PubMed] [Google Scholar]
- 12.Cabaleiro T., Roman M., Gallo E., Ochoa D., Tudelilla F., Talegon M. Association between psoriasis and polymorphisms in the TNF, IL12B, and IL23R genes in Spanish patients. Eur. J. Dermatol. EJD. 2013;23(5):640–645. doi: 10.1684/ejd.2013.2144. [DOI] [PubMed] [Google Scholar]
- 13.Huffmeier U., Uebe S., Ekici A.B., Bowes J., Giardina E., Korendowych E. Common variants at TRAF3IP2 are associated with susceptibility to psoriatic arthritis and psoriasis. Nat. Genet. 2010;42(11):996–999. doi: 10.1038/ng.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lambert S., Swindell W.R., Tsoi L.C., Stoll S.W., Elder J.T. Dual role of Act1 in keratinocyte differentiation and host defense: TRAF3IP2 silencing alters keratinocyte differentiation and inhibits IL-17 responses. J. Investig. Dermatol. 2017;137(7):1501–1511. doi: 10.1016/j.jid.2016.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sakamuri S., Higashi Y., Sukhanov S., Siddesha J.M., Delafontaine P., Siebenlist U. TRAF3IP2 mediates atherosclerotic plaque development and vulnerability in ApoE(-/-) mice. Atherosclerosis. 2016;252:153–160. doi: 10.1016/j.atherosclerosis.2016.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen W., Gong Y., Zhang X., Tong Y., Wang X., Fei C. Decreased expression of IL-27 in moderate-to-severe psoriasis and its anti-inflammation role in imiquimod-induced psoriasis-like mouse model. J. Dermatol. Sci. 2017;85(2):115–123. doi: 10.1016/j.jdermsci.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 17.Jia J., Li C., Luo S., Liu-Smith F., Yang J., Wang X. Yes-associated protein contributes to the development of human cutaneous squamous cell carcinoma via activation of RAS. J. Investig. Dermatol. 2016;136(6):1267–1277. doi: 10.1016/j.jid.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 18.Vania L., Rebelo T.M., Ferreira E., Weiss S.F.T. Knock-down of LRP/LR promotes apoptosis in early and late stage colorectal carcinoma cells via caspase activation. BMC Cancer. 2018;18(1):602. doi: 10.1186/s12885-018-4531-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu Y., Chen J., Zhu X., Tang L., Luo X., Shi Y. Role of miR-449b-3p in endometriosis via effects on endometrial stromal cell proliferation and angiogenesis. Mol. Med. Rep. 2018;18(3):3359–3365. doi: 10.3892/mmr.2018.9341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu X.W., Yang X.M., Zhao W.J., Zhou L., Li D.C., Zheng Y.H. DNM1L, a key prognostic predictor for gastric adenocarcinoma, is involved in cell proliferation, invasion, and apoptosis. Oncology letters. 2018;16(3):3635–3641. doi: 10.3892/ol.2018.9138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xie J., Yan Y., Liu F., Kang H., Xu F., Xiao W. Knockdown of Rab7a suppresses the proliferation, migration, and xenograft tumor growth of breast cancer cells. Biosci. Rep. 2019;39(2) doi: 10.1042/BSR20180480. BSR20180480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shun-Wu Y.U., Liu H.Y., Luo L.J. Analysis of relative gene expression using different real-time quantitative PCR. Acta Agron. Sin. 2007 [Google Scholar]
- 23.Wang C., Wu L., Bulek K., Martin B.N., Zepp J.A., Kang Z. The psoriasis-associated D10N variant of the adaptor Act1 with impaired regulation by the molecular chaperone hsp90. Nat. Immunol. 2013;14(1):72–81. doi: 10.1038/ni.2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shahidi-Dadras M., Haghighatkhah H.R., Abdollahimajd F., Younespour S., Partovi Kia M., Zargari O. Correlation between vascular endothelial growth factor and subclinical atherosclerosis in patients with psoriasis. Int. J. Dermatol. 2016;55(1):52–59. doi: 10.1111/ijd.12842. [DOI] [PubMed] [Google Scholar]
- 25.Leong T.T., Fearon U., Veale D.J. Angiogenesis in psoriasis and psoriatic arthritis: clues to disease pathogenesis. Curr. Rheumatol. Rep. 2005;7(4):325–329. doi: 10.1007/s11926-005-0044-5. [DOI] [PubMed] [Google Scholar]
- 26.Weidemann A.K., Crawshaw A.A., Byrne E., Young H.S. Vascular endothelial growth factor inhibitors: investigational therapies for the treatment of psoriasis. Clin. Cosmet. Investig. Dermatol. 2013;6:233–244. doi: 10.2147/CCID.S35312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grek C.L., Rhett J.M., Bruce J.S., Abt M.A., Ghatnekar G.S., Yeh E.S. Targeting connexin 43 with alpha-connexin carboxyl-terminal (ACT1) peptide enhances the activity of the targeted inhibitors, tamoxifen and lapatinib, in breast cancer: clinical implication for ACT1. BMC Cancer. 2015;15:296. doi: 10.1186/s12885-015-1229-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bresnick A.R., Weber D.J., Zimmer D.B. S100 proteins in cancer. Nat. Rev. Cancer. 2015;15(2):96–109. doi: 10.1038/nrc3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bertheloot D., Latz E. HMGB1, IL-1alpha, IL-33 and S100 proteins: dual-function alarmins. Cell. Mol. Immunol. 2017;14(1):43–64. doi: 10.1038/cmi.2016.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Y., Tu C., Zhang D., Zheng Y., Peng Z., Feng Y. Wnt/beta-Catenin and Wnt5a/Ca pathways regulate proliferation and apoptosis of keratinocytes in psoriasis lesions. Cell. Physiol. Biochem. 2015;36(5):1890–1902. doi: 10.1159/000430158. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.