Abstract
High dietary salt intake is an important risk factor for cardiovascular and renal diseases. However, sexual disparity exists in the response of target organs to high salt diet (HSD). To determine how sex affects cardiac and renal functions’ response to HSD, 20 weanling Sprague-Dawley rats (10 males and 10 females) were divided into 4 groups of 5 rats each. The rats were fed a normal diet (0.3% NaCl) or HSD (8% NaCl) for 12 weeks. Fluid balance (FB) was determined from 24 hrs water intake and voided urine. Blood pressure (BP) was measured via arterial cannulation under anesthesia (25% w/v urethane and 1% w/v α-chloralose; 5 ml/kg, i.p). Serum levels of troponin I, aminotransaminases, creatinine, urea, uric acid and electrolytes as well as urinary concentration of albumin, creatinine, and electrolytes were measured using appropriate assay kits. Values are presented as mean ± S.E.M, compared by two-way ANOVA and Bonferroni post Hoc test. In the male rat, HSD significantly increased BP, serum: Troponin I, LDH and sodium (p < 0.05), urinary: albumin, sodium, potassium and FB (p < 0.05). In the female rat, HSD increased BP, serum: troponin I, LDH, sodium and creatinine clearance (p < 0.05), urinary: albumin, sodium and potassium (p < 0.01). However, HSD increased more, the BP, serum: Troponin I, LDH, urinary albumin and FB in male rats, while HSD increased urinary sodium more in female rats. Basal values in male vs. female of serum LDH and urinary albumin were significantly different. Thus, sex plays an important role in the response of the heart and kidney to salt stress.
Keywords: Physiology, Pathology
1. Introduction
Hypertension is an important contributor to cardiovascular and renal disease. However, hypertension can be influenced by a number of risk factors such as genetic, environmental, lifestyle and sex. An excess of dietary salt is the most common environmental factor that contributes to the development of hypertension leading to cardiovascular and renal pathologies [1, 2]. Salt-sensitive hypertension is associated with higher morbidity and mortality from cardiovascular diseases than hypertension caused by other factors [3, 4]. In fact, salt sensitivity increases the risk of death independent of elevated blood pressure (BP) [3].
The mechanisms involved in BP elevating effect of a high salt diet are not fully understood. However, experimental studies have suggested impairment of cardiovascular and renal functions as well as dysregulation of fluid volume and autonomic system [5, 6]. In addition to raising BP, a high salt diet poses other threats to human health. For instance, a high salt diet has been shown to increase the left ventricular mass, thicken and stiffen the aorta while thickening and narrowing the resistance arteries such as mesenteric, coronary and renal arteries [5, 6, 7]. We have previously shown that high salt diet thickens and narrows the mesenteric artery by promoting vascular smooth muscle cells hyperplasia and extracellular matrix proteins deposition [8] and that, high salt diet also impairs both endothelium - dependent and independent relaxation response to agonist [9, 10]. Other harmful effects of a high salt diet aside BP elevation include, cardiac and vascular hypertrophy as well as renal dysfunction which, may be independent of the arterial pressure [6]. However, these harmful effects appear to be as important as the arterial blood pressure elevating effect of a high salt diet.
Cardiovascular diseases and their risk factors exhibit sex differences. Men are reported to be more prone to, and are more likely to die of, heart diseases when compared with females of similar age [11]. Even at later period of life, when age cancels out the sex disparity in the prevalence and severity of CVDs and heart diseases between male and females, females still exhibit some peculiar specific traits in the dynamics and pathophysiology of CVDs and heart diseases. For example, while the prevalence of heart failure is comparable between men and women post 75 years of age, women have a greater prevalence of heart failure with preserved ejection fraction (HFpEF) compared to men [12]. In HFpEF, the ejection fraction remains the same yet the ventricle is failing. The higher prevalence of HFpEF in women is due to basic sex differences in the physiology of men and women cardiovascular system. For instance, women possess a smaller left ventricular chamber, lower stroke volume, yet a comparable cardiac output to male because they have a higher resting heart rate when compared with men [12, 13]. Likewise, in women, the effect of aging on cardiomyocytes decline is attenuated while LV concentric remodeling and diastolic dysfunction are more pronounced [12].
Like other cardiovascular diseases and its risk factors, sensitivity to high dietary salt also exhibit sexual disparity as male rats show a greater increase in BP when compared with female rats after a high salt diet [14, 15]. The reason for this sex differences in CVDs, its risk factors, as well as BP sensitivity to dietary salt is multifactorial and not properly understood. However, several hypotheses have been propounded for this sexual disparity. Such include, the potential role of sex hormones (estrogen and testosterone), the renin–angiotensin system (RAS), oxidative stress, endothelin, and sympathetic system activation [16]. Specifically, high androgen and lower estrogen levels are reported to be associated with risk factors for heart diseases in post-menopausal women and high testosterone to estrogen ratio is said to be associated with elevated risk for incident CVDs, coronary heart diseases and heart failure [17].
The sex differences in the sensitivity of BP to a high salt diet is quite clear, however, not much has been done with regards to sex differences in other harmful effects of high dietary salt that are independent of, or additive to blood pressure elevation. Therefore, this study was designed to investigate the sex differences in the cardiac and renal response to high salt diet by measuring the biomarkers of the cardiac and renal functions in Sprague-Dawley rats exposed to a long term high dietary salt.
2. Materials and methods
2.1. Animal groupings
All animal procedures in this study were performed in accordance with the guidelines of the research and ethics committee of college of medicine, university of Lagos for the use of laboratory animals.
Twenty (10 males; 10 females) 6 weeks old weanling Sprague-Dawley rats weighing between 90-120 grams were acquired from Animal Care Facility (ACF) of the College of Medicine, University of Lagos. They were housed in ventilated cages on a 12:12 hour light-dark cycle and acclimatized for a week. The rats were divided into 4 groups along sex and diet lines: male plus normal Salt (Male + NS); Male plus high salt (Male + HS); Female plus normal salt (Female + NS) and female plus high salt (Female + HS). Normal salt groups were fed a diet containing 0.3% and high salt groups were fed diet containing 8% salt. The rats chow was in pelleted form and purchased from an animal feed company (Ratsmattaz, Lagos, Nigeria). The high salt diet contains 7.7% NaCl w/w in addition to the normal 0.3% w/w NaCl that is originally present in the normal diet. Food and water were provided ad libitum.
2.2. Body weight, food intake and fluid balance determination
Body weight and the amount of food intake were determined weekly throughout the duration of the experiment using weighing balance (Ed4000 Symmetry Cole-Parmer USA). Percent weight change was calculated as the difference between the final and initial body weight divided by initial body weight. Food consumption was evaluated daily, as known amount of food was given to each animal in a cage. After 24 hrs, the remaining food was taken from the cage and weighed. To determine the quantity of feed consumed, the left-over was deducted from the total amount initially provided. Food intake was calculated as g/day. During the last week of the experimental period each rat was housed separately for 24 hours in a metabolic cage for collection of urine and measurement of daily water intake. Fluid balance was determined as the difference between 24-hour –urine volume and water intake. An aliquot of the collected urine was kept in refrigerator for further analysis.
2.3. Blood pressure measurement
At the end of the 12-week experimental period, blood pressure of animals was measured via arterial cannulation as previously described [9]. Briefly, under 25% urethane and 1% α-chloralose anesthesia (5 ml/kg bodyweight i.p), an incision was made on the trachea and a trachea tube PE250 was inserted for air passage. Two loops of thread were then placed around the trachea to hold in place the trachea tube. The carotid artery was carefully separated from the Vagus nerve, exteriorized and cannulated using a PE50 cannula filled with 1% normal saline, and the cannula was connected to a pressure transducer (model 7d, grass instrument Massachusetts USA) by a 3-way tap which was in turn connected to a grass polygraph via a preamplifier and a driver amplifier to an ink writing stylus. The animal was allowed to rest for 10–15 minutes before blood pressure readings were taken. Mean arterial pressure was calculated as the sum of the diastolic pressure (DP) and a third of pulse pressure (PP) (DP+1/3PP). Pulse pressure is the difference between systolic pressure (SP) and diastolic pressure in mmHg. Rate pressure product (RPP) which is an indicator of myocardial oxygen demand (MOD) was determined from the product of systolic pressure and heart rate.
2.4. Biochemical assays
Blood was collected into plain bottles from the cannulated carotid artery of anaesthetized rats immediately after blood pressure measurement. Collected blood was centrifuged for 15 min at 3000 rev./min, serum was used for the blood chemistry assay. After sacrifice, the heart and kidneys of the animals were harvested and carefully cleared of connective tissues, dried between filter paper and weighed on (Ohaus scout pro balance 400 × 0.01g). The organ weight index was taken as the division of each organ by the body weight multiplied by 100.
Serum cardiac troponin I (cTn-I) values was determined using enzyme-linked immunosorbent assay (ELISA) based commercially available kit (Diagnostic Automation, Inc. Calabasas) according to the manufacturer's instructions. Serum lactate dehydrogenase (LDH) levels were measured by colorimetric-based commercially available kit (Cypress Diagnostics, Belgium) according to the manufacturer's instructions.
Serum aspartate aminotransaminase (AST) and alanine aminotransaminase (ALT) levels were measured using colorimetric based commercially available kits (Randox Laboratories Ltd, UK) according to the manufacturer's instructions.
Urinary and serum creatinine; urinary albumin, serum uric acid and serum urea concentrations were determined using commercially available kits (Biolabo Reagents, France), according to the manufacturer's instruction. Creatinine clearance was determined from the effective urinary and serum creatinine concentrations.
2.5. Urinary and serum electrolyte concentration
Urinary and serum concentrations of sodium and potassium were determined using the gas flame method. Using compressed air, diluted samples were sprayed as fine droplets into a luminous gas flame which becomes colored by the characteristic emission of sodium or potassium ions in the sample. Using a light filter, the light wavelength corresponds to that of the metal concentrations being determined. The amount of light emitted depends on the concentration of the ions present in the sample. The flame photometer was first adjusted to zero using deionised water. Thereafter a standard sample was prepared. The samples were diluted with deionized water to 1 in 200 dilutions. The standard knob for potassium was adjusted to 5.0 mEq/L while that of sodium was set at 140 mEq/L. The various sample dilutions were subjected to the flame and their concentration recorded.
2.6. Statistical analysis
Data were presented as Mean ± SEM and analyzed using two-way analysis of variance (2- way ANOVA) and Bonferroni post hoc test. The columns were defined by the treatment (normal and high salt diet) and the rows were defined by the biological variable - sex (male and female). Confidence interval was placed at 95%, so that in all cases probability values less than 0.05 (p ≤ 0.05) were considered significant. GraphPad Prism version 5.0 for windows was used for these statistical analyses (GraphPad software, San Diego California USA).
3. Results
3.1. Effect of high salt diet on body weight, organs weight indices and fluid balance in male and female Sprague-Dawley rats
Table 1 shows the percent body weight gain, heart and kidney weight indices and fluid balance of male and female Sprague-Dawley rats fed a normal or high salt diet for 12 weeks. High salt diet reduced the magnitude of the increase in percent weight gain of both male and female. However, the magnitude of percent weight gain in males fed a high salt diet were significantly less (p < 0.05) when compared with that of females fed a high salt diet. Neither the male nor female rats showed apathy to the high salt diet as there was no difference in food consumption between animals placed on normal or high salt diet (not shown). A high salt diet increased the weight indices of both the heart and kidney only in the male rats. However, female fed a high salt diet had a significantly higher (p < 0.05) renal weight index when compared with female fed a normal salt diet. Fluid retention was higher in male rats fed a high salt diet when compared with the controls that are placed on normal salt diet. Likewise, male rats fed a high salt diet retained more fluid when compared with females fed a high salt diet.
Table 1.
Effect of a high salt diet on the per cent weight change, heart and kidney weight indices and fluid balance in male and female Sprague-Dawley rats.
| Groups | %weight change | Heart Index | Kidney Index | Fluid Balance (ml) |
|---|---|---|---|---|
| Male + NS | 152.9 ± 4.4 | 0.26 ± 0.03 | 0.51 ± 0.04 | 4.34 ± 0.7 |
| Male + HS | 120.09 ± 3.3∗∗∗ | 0.37 ± 0.01Ϯ | 0.68 ± 0.03ϯϯ | 10.21 ± 1.19ϮϮ |
| Female + NS | 135.47 ± 4.45 | 0.33 ± 0.02 | 0.52 ± 0.03 | 4.1 ± 1.69 |
| Female + HS | 122.17 ± 4.72∗∗ | 0.44 ± 0.04Ϯ | 0.57 ± 0.07 | 6.1 ± 0.55# |
Data presented as mean ± S.E.M. Significant reduction (*p < 0.05; **p < 0.01; ***p < 0.001) when compared with corresponding normal salt diet groups. Significant increase (Ϯp<0.05; ϮϮp<0.01) when compared with corresponding control on a normal salt diet. Significant decrease (#p < 0.05) when compared with male high salt group, N = (5).
3.2. Effect of high salt diet on mean arterial blood pressure and rate pressure product (RPP) in male and female Sprague-Dawley rats
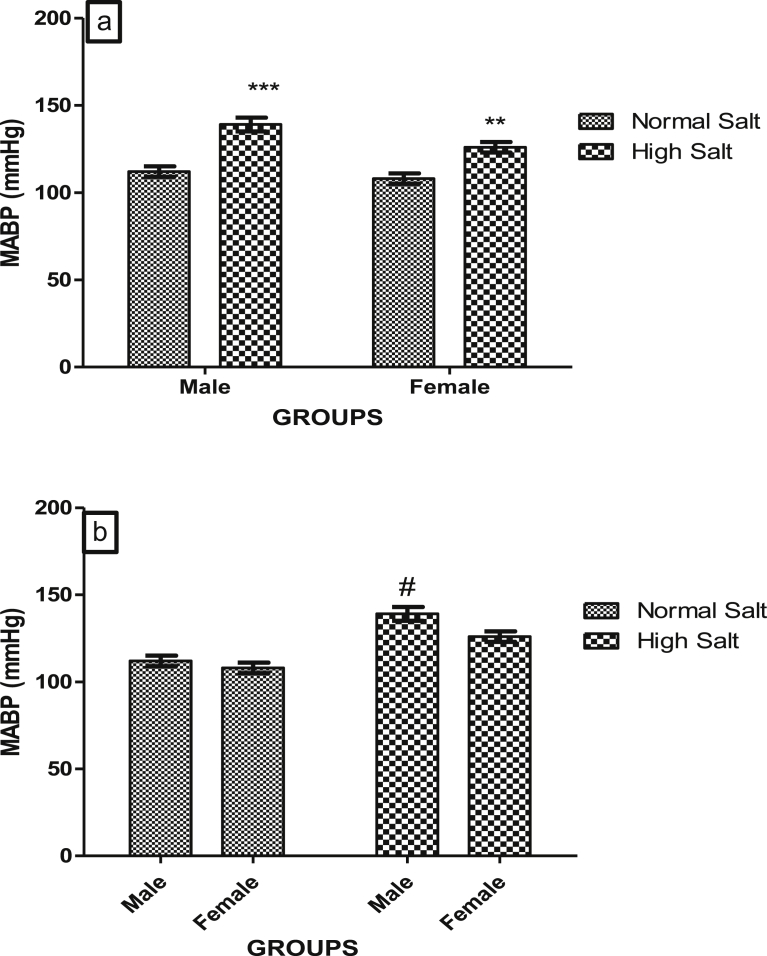
High salt diet increased blood pressure both in the male and female rats when compared with their corresponding control which were fed a normal salt diet (Fig. 1a). However, when fed a high salt diet, male rats develop a higher arterial blood pressure compared to the females. Likewise, the magnitude of BP elevating effect of high salt diet was higher in the male compared to the female: p < 0.001 Male + HS vs. Male + NS compared to p < 0.01 female + HS vs. female + NS (Fig. 1b).
Fig. 1.
(a). Effect of high salt diet on Mean Arterial Blood Pressure (MABP) of male and female Sprague – Dawley rats. Significant increase (**p < 0.01; ***p < 0.001) when compared with corresponding controls. (b). Role of sex on the effect of high salt diet on MABP in Sprague-Dawley rats. #Significant increase (p < 0.05) when compared with female high salt diet. N = 5.
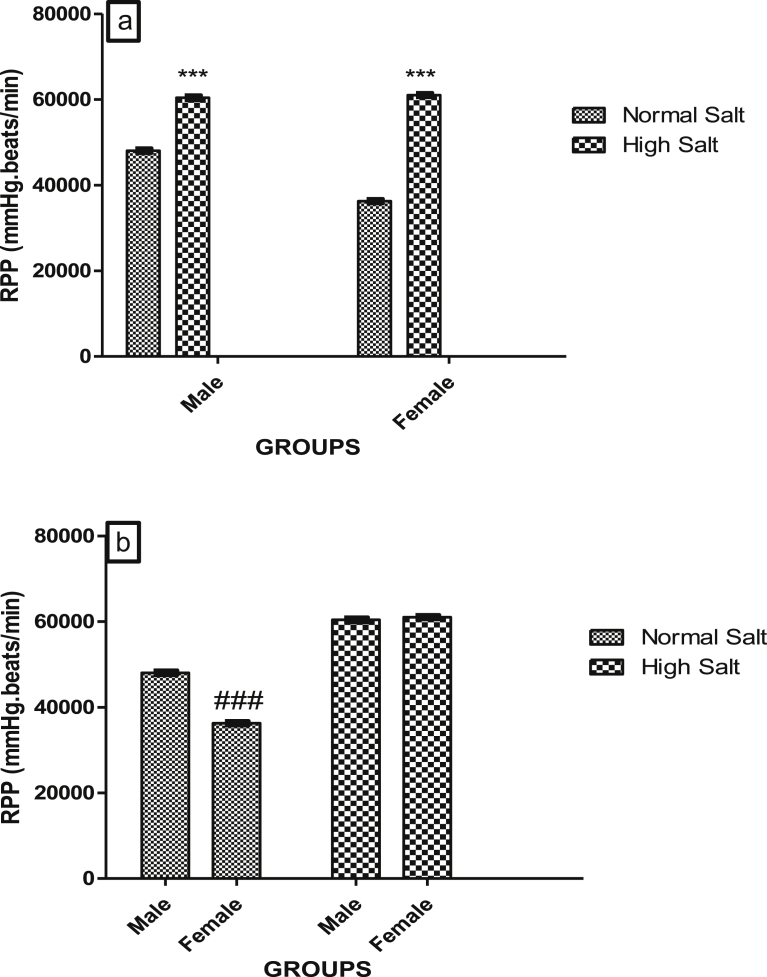
In the control rats that were fed a normal salt diet, RPP was significantly higher in the male when compare with the female rat (Fig. 2b). However, RPP in both male and female rat was elevated by a high salt diet to a similar level (Fig. 2a), abolishing the sex difference that exists between the RPP at the basal level in the rats (Fig. 2b).
Fig. 2.
(a). Effect of high salt diet on Rate Pressure Product (RPP) of male and female Sprague – Dawley rats. Significant increase (***p < 0.001) when compared with corresponding controls, (b). Role of sex on the effect of high salt diet on RPP in Sprague-Dawley rats. #Significant decrease when compared with male normal salt diet. N = 5.
3.3. Effect of high salt diet on biomarkers of cardiac function in male and female Sprague-Dawley rats
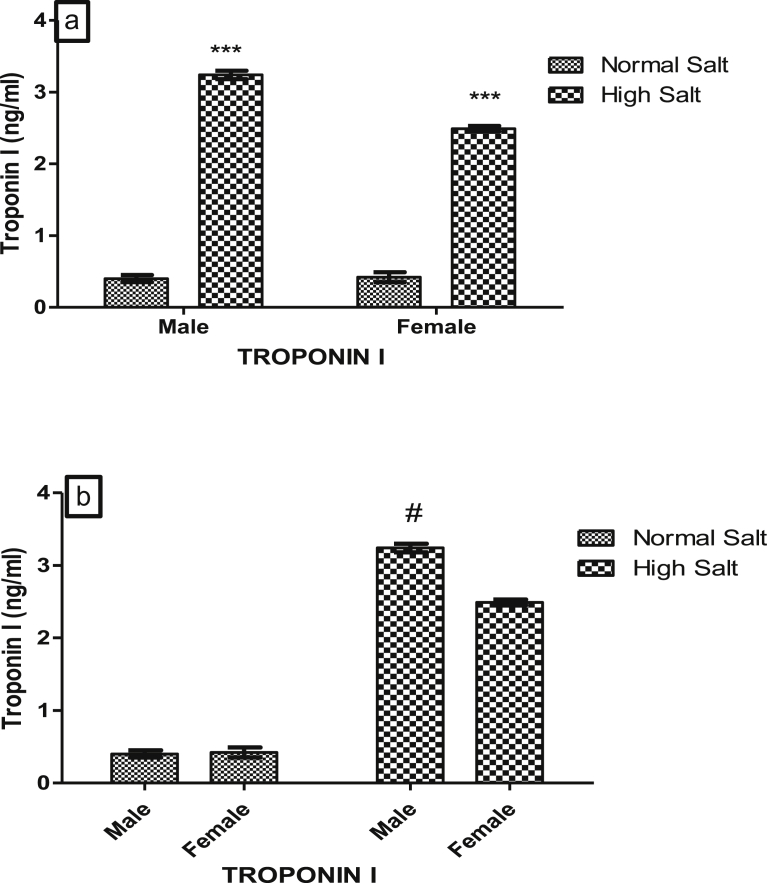
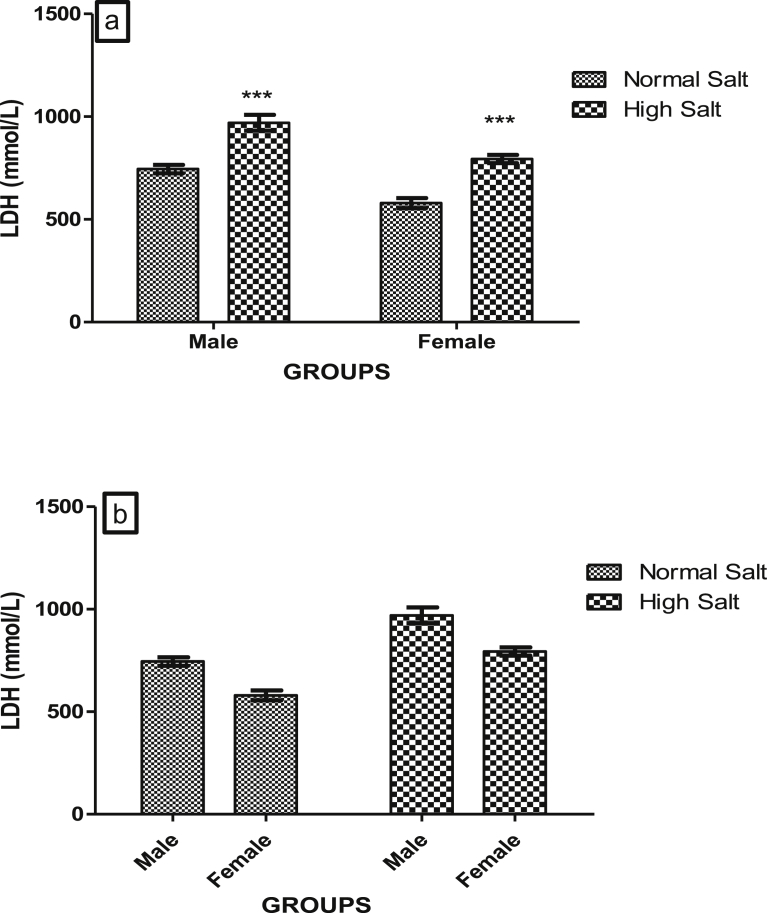
Figs. 3a and b, 4a and b, 5a and b and 6a and b show the serum concentrations of cardiac troponin I (cTn-I), lactate dehydrogenase (LDH), aspartate amino transferase (AST) and alanine amino transferase (ALT) of male and female rats fed normal (0.3%) or high (8%) salt diet respectively.
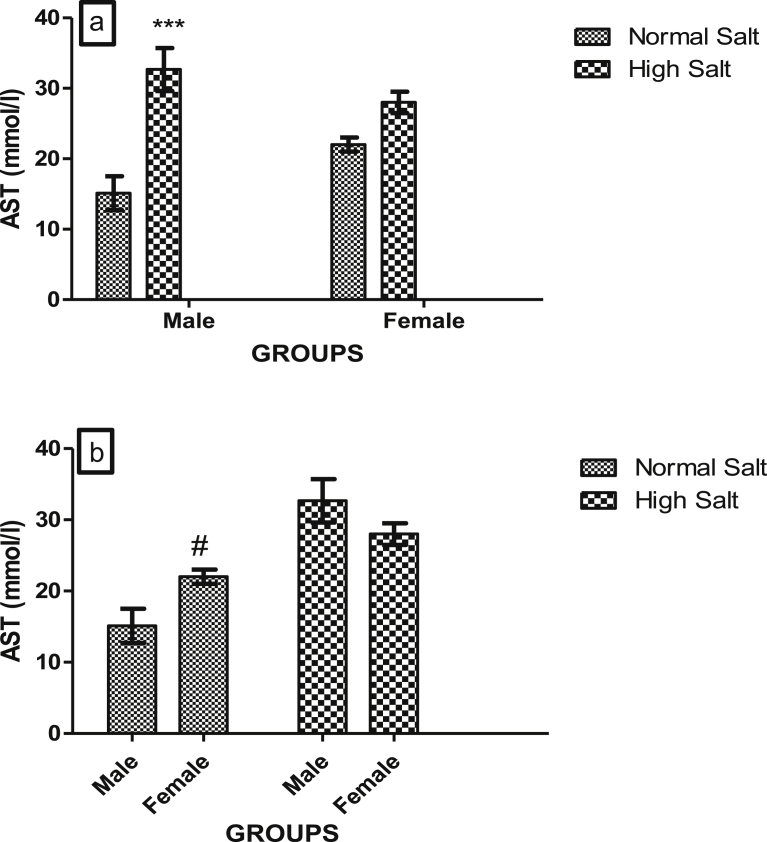
Fig. 5.
(a). Effect of a high salt diet on AST concentration in male and female Sprague-Dawley rats. Significant increase (***p < 0.001) when compared with corresponding controls. (b). Role of sex on the effect of high salt diet on serum AST concentration in Sprague-Dawley rats. #Significant increase (p < 0.05) when compared with male on normal salt diet (control). N = 5.
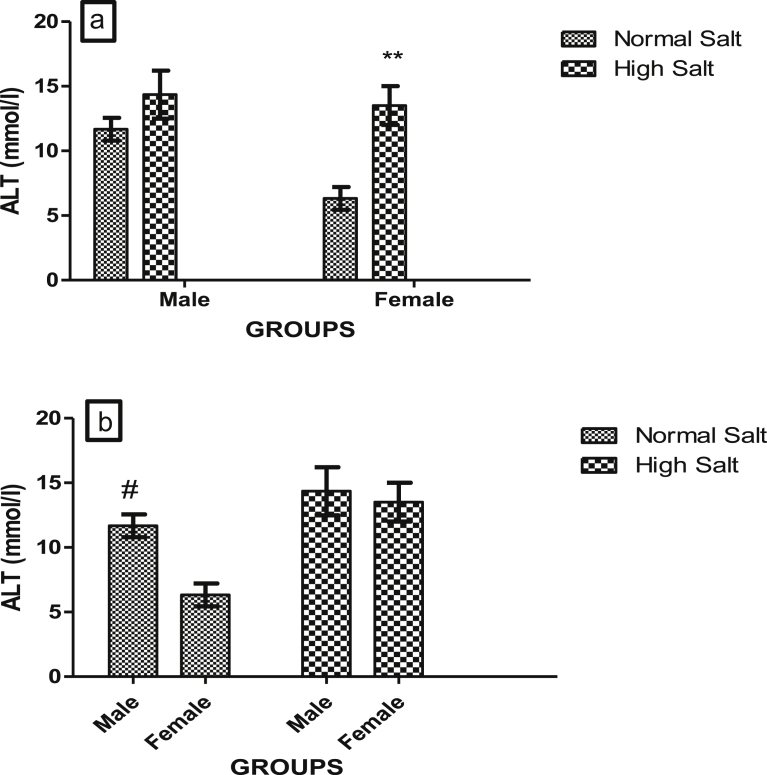
Fig. 6.
(a). Effect of a high salt diet on serum concentration of ALT in male and female Sprague-Dawley rats. Significant increase (**p < 0.01) when compared with female on normal salt diet (control). (b). Role of sex on the effect of high salt diet on serum ALT concentration. Significantly higher (#p < 0.05) when compared with corresponding female controls. N = 5.
There was a significant increase (p < 0.05) in cTn-I levels of male and female rats fed a high salt diet when compared with those on normal diet (Fig. 3a). However, the effect of a high salt diet on cTn-I exhibit sexual disparity as cTn-I concentrations in male rat fed a high salt diet was significantly higher (p < 0.05) than that of female fed a high salt diet but there was no difference in the serum cTn-I concentrations of both male and female rats fed a normal salt diet (Fig. 3b).
Fig. 3.
(a). Effect of high salt diet on serum Troponin I concentration in male and female Sprague – Dawley rats. Significant increase (***p < 0.001) when compared with corresponding controls, (b). Role of sex on the effect of high salt diet on serum Troponin I concentration in Sprague-Dawley. Significant increase #(p < 0.05) when compared with female high salt diet. N = 5.
High salt diet significantly increased (p < 0.001) the serum concentrations of LDH in both the male and female rat when compared with their corresponding control groups that were on normal salt diet (Fig. 4a). But there was no significant difference in the serum concentration of LDH between male and female on either normal or high salt diet when compared with each other (Fig. 4b). This suggests that the effect of a high salt diet on serum concentration of LDH in Sprague-Dawley rat does not exhibit sexual dimorphism.
Fig. 4.
(a). Effect of a high salt diet on serum LDH concentration in male and female Sprague-Dawley rats. Significant increase (***p < 0.001) when compared with corresponding controls. (b). Role of sex on the effect of high salt diet on serum LDH concentration. No significant difference between male and female (p > 0.05) N = 5.
High salt diet significantly increased (p < 0.001) serum level of AST in male but not in female rats (Fig. 5a). However, there was a significant increase in the serum concentration of AST in female rat fed a normal salt diet when compared with male fed a normal salt diet, suggesting that the normal basal serum level of AST exhibit sexual disparity in Sprague-Dawley rats (Fig. 5b).
There was a significant increase (p < 0.01) in the serum concentration of ALT in female rat that were fed a high salt diet when compared with the control female rats that were fed a normal salt diet (Fig. 6a). However, there was no significant difference in the serum concentration of ALT in male rat when compared with their control, but there was a significant elevation in the serum ALT concentration of male rat fed a normal salt diet when compared with that of female rat fed a normal salt diet (Fig. 6b).
3.4. Effect of high salt diet on creatinine clearance, concentrations of serum urea, serum uric acid and urinary albumin in male and female Sprague-Dawley rats
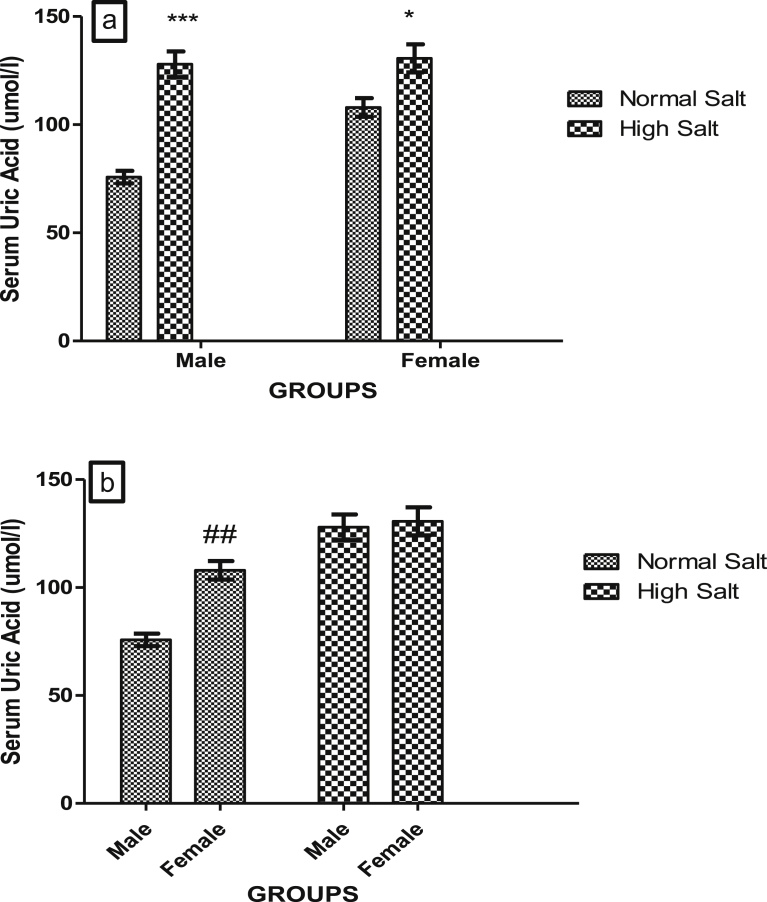
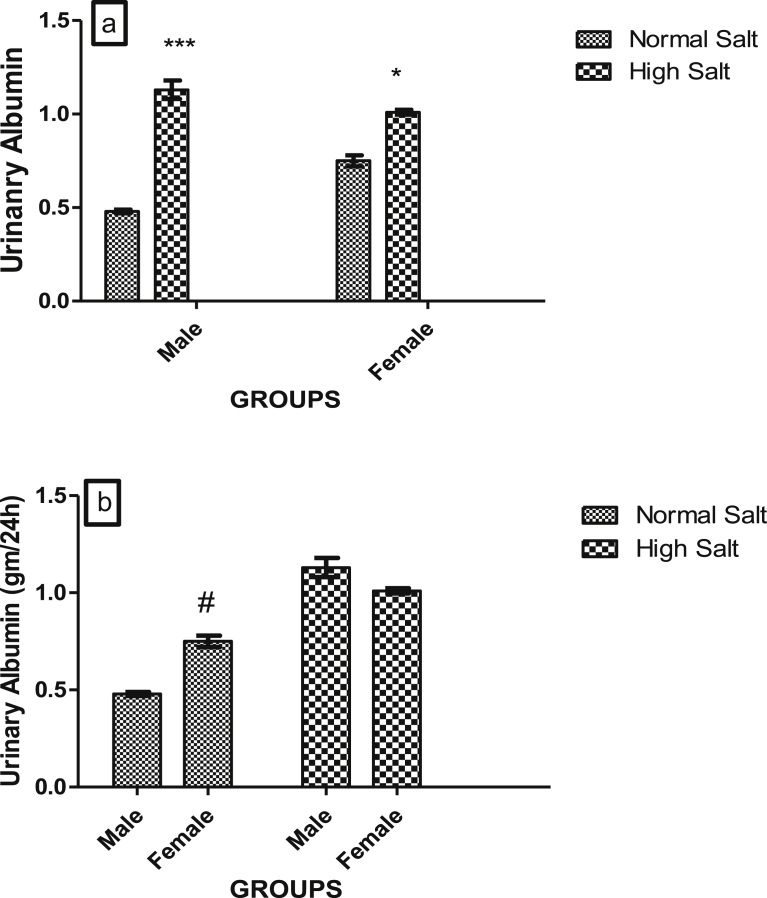
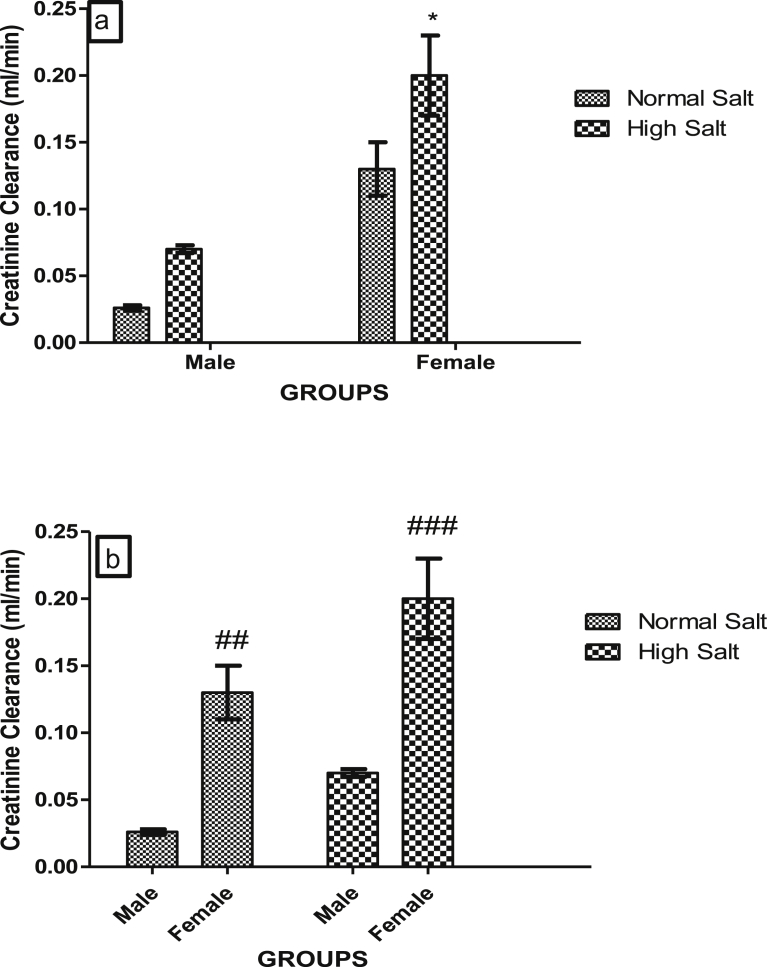
Figs. 7a and b, 8a and b, 9a and b and 10a and b show the effect of a high salt diet on creatinine clearance, serum concentrations of urea, uric acid and urinary concentration of albumin in male and female Sprague-Dawley rats respectively. The clearance of creatinine was significantly higher (p < 0.05) in female rats fed a high salt diet when compared with its control (Fig. 7a). There was no significant difference in the creatinine clearance of male rats fed a high salt diet when compared with male fed a normal salt diet (Fig. 7a). However, creatinine clearances were significantly higher in females fed a normal or high salt diet when compared with male rats fed a corresponding normal or high salt diet (Fig. 7b).
Fig. 9.
(a). Effect of high salt diet on Serum concentrations of uric acid in male and female Sprague Dawley rats. Significant increase (*p < 0.05; ***p < 0.001) when compared with corresponding controls. (b). Role of sex on the effect of high salt diet on serum uric acid concentration in Sprague-Dawley rats. Significant increase (##p < 0.01) when compared with control male. N = 5.
Fig. 10.
(a). Effect of high salt diet on urinary concentration of albumin in male and female Sprague-Dawley rats. Significant increase (*p < 0.05; ***p < 0.001) when compared with corresponding controls. (b). Role of sex on the effect of high salt diet on urinary albumin concentration in Sprague-Dawley rats. Significant increase (#p < 0.05) when compared with control male. N = 5.
Fig. 7.
(a). Effect of a high salt diet on creatinine clearance in male and female Sprague-Dawley rats. Significant increase (*p < 0.05) when compared with female control. (b). Role of sex on the effect of high salt diet on creatinine clearance in Sprague-Dawley rats. #Significant increase (##p < 0.01; ###p < 0.001) when compared with corresponding male control on the same diets (normal or high salt diet). N = 5.
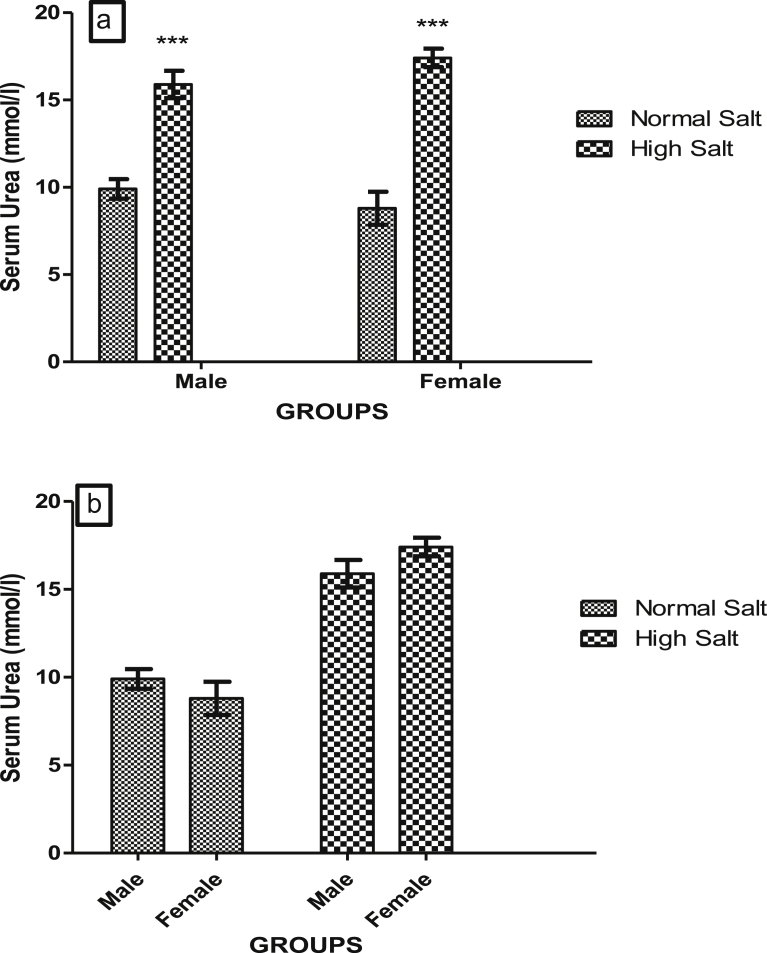
High salt diet significantly increased (p < 0.001) serum concentrations of urea in both male and female rats when compared with their corresponding control (Fig. 8a). Serum concentrations of urea were similar in both male and female rats fed a high salt diet (Fig. 8b) suggesting that there was no sex disparity in the effect of a high salt diet on serum concentration of urea.
Fig. 8.
(a). Effect of high salt diet on Serum concentration of Urea in male and female Sprague Dawley rats. Significant increase (***p < 0.001) when compared with corresponding controls. (b). Role of sex on the effect of high salt diet on serum urea concentration in Sprague-Dawley rats. No significant difference (p > 0.05) between male and female N = 5.
There was a significant increase in the serum concentration of uric acid in male (p < 0.001) and female (p < 0.05) fed a high salt diet when compared with their corresponding control (Fig. 9a). However, there was a significant increase (p < 0.01) in the concentration of uric acid in the serum of female rat on normal salt diet when compared with male rat on a normal salt diet (Fig. 9b).
High salt diet significantly elevated urinary concentration of albumin in both male (p < 0.001) and female (p < 0.05) when compared with their corresponding controls (Fig. 10a). But the effect of a high salt diet was greater in the male when compared with the female, as there was a significant decrease in the urinary concentration of albumin in the female fed a high salt diet when compared with male on the same diet (Fig. 10b).
3.5. Effect of high salt diet on urinary and serum electrolytes concentrations
Table 2 shows the effect of a high salt diet on concentrations of serum and urinary sodium and potassium in male and female rats. At the end of the 12 weeks salt-loading, there was a significant elevation in both serum and urinary concentrations of sodium in both male and female rats (p < 0.01). There was no significant difference in the concentrations of potassium in the serum of both male and female rats on either normal or high salt diet when compared with each other. However, a high salt diet increased urinary excretion of potassium in both the male (p < 0.05) and the female (p < 0.01) rat when compared with their corresponding controls.
Table 2.
Effect of a high salt diet on Serum and urinary concentrations of sodium and potassium in male and female Sprague – Dawley rats.
| Groups | Serum Sodium (mmol/l) | Serum potassium (mmol/l) | Urinary Sodium (mmol/l) | Urinary Potassium (mmol/l) |
|---|---|---|---|---|
| Male + NS | 131.4 ± 2.40 | 4.79 ± 0.19 | 0.24 ± 0.01 | 0.10 ± 0.001 |
| Male + HS | 142.0 ± 2.33∗∗ | 4.78 ± 0.14 | 1.12 ± 0.06∗∗ | 0.24 ± 0.06∗ |
| Female + NS | 133.6 ± 1.44 | 4.69 ± 0.10 | 0.39 ± 0.04 | 0.14 ± 0.001 |
| Female + HS | 143.6 ± 1.50∗∗ | 4.98 ± 0.25 | 2.4 ± 0.18∗∗ | 0.32 ± 0.002∗∗ |
Significant increase (*p < 0.05; **p < 0.01) when compared with corresponding controls. There is no significant difference (p > 0.05) between male and female rats' serum and urinary electrolyte concentration. N = (5).
4. Discussion
The major findings of this study are that: 1) in both male and female rats, a high salt diet increased BP, RPP, heart weight index, the serum concentrations of troponin I, LDH, urea, uric acid and sodium ions as well as urinary concentrations of sodium, potassium and albumin; 2) in only the male rats, a high salt diet increased the kidney weight index, fluid retention and serum concentration of AST; 3) in only the female rats, high salt diet increased serum concentration of ALT and creatinine clearance; 4) The effect of a high salt diet on BP, fluid retention, serum concentration of Troponin I, urinary concentration of albumin and creatinine clearance exhibits sex disparity. With the exception of creatinine clearance where the effect of high salt diet was more pronounced in female rats, the magnitude of the effect of a high salt diet were higher on all the aforementioned parameters in males; 5) in the control rats fed a normal salt diet, RPP, serum concentrations of AST, ALT, uric acid and creatinine clearance exhibit sexual dimorphism. Except RPP and serum concentration of ALT which were lower in female, serum concentrations of AST, and uric acid and creatinine clearance were higher in the female rats.
The decrease in percent body weight gain observed in the salt loaded group agrees with the previous study in which observations showed that intake of high salt diet decreased the percentage body weight gain of rats fed high salt diet [18]. Salt intake has been shown to stimulate appetite, increase food intake and body metabolism, which then causes an increase in energy expenditure and leads to a decrease in body weight [18]. This observation of a reduction in the magnitude of gain in body weight of rats fed a high salt diet may also be related to an increase in body water loss through excessive urination observed during the experiment with the intact plus high-salt diet groups.
Increased organ body weight index is an indication of swelling or hypertrophy in the particular organ [19]. However, the decrease in the magnitude of weight gain in the high salt fed rats ruled out the possibility of swelling as being responsible for the increase in the organ weight indices because, swelling would have concomitantly increased the total animal's body weight as well as the organs' weight indices. The increase in the heart weight index of the rats fed a high salt diet may be due to hypertrophy of the heart. This is consistent with other findings which shows that high salt intake is strongly implicated in cardiac hypertrophy hence increase in heart weight index [6, 20, 21]. The increase in kidney weight index of rat observed in this study may be as a result of renal hypertrophy and/or hyperplasia as studies have shown that pronounced changes in reabsorptive abilities and cytoarchitecture of kidney follows a high dietary salt intake in order to maintain sodium balance [22]. In addition, dietary salt intake has been implicated in the progression of renal injury, increases proteinuria and renal growth in rats [23].
The increase in blood pressure in rats fed a high salt diet in this study is consistent with our previous findings [8, 9, 24] and that of others [25]. In this study, myocardial oxygen demand (MOD) was calculated as the rate pressure product (RPP) from the product of the heart rate and the systolic blood pressure. This is indicative of the work done by the cardiac muscle. An increase in the RPP of the rats fed a high salt diet suggests an increase in the work of the heart in these animals. And this finding is consistent with the elevated BP that was observed in the same group of rats fed a high salt diet, since an increase in the systemic BP increases the work done by the heart in pumping blood out of the left ventricle up and above the systemic BP. The increase in RPP and the BP is also consistent with the observed increase in cardiac weight indices in rats fed a high salt diet as an increase in the work of the heart will lead to cardiac hypertrophy. In addition to raising BP, a high salt diet has been shown to increase the tissue demand for oxygen [26], a finding that supports our result on the increase in RPP in the group of rats fed a high salt diet. Abolition of sex disparity that exist in the RPP of control male and female rats fed by a high salt diet suggests that female rats respond more to an increase in tissue oxygen demand effect of a high salt diet.
Cardiac troponins are regulatory proteins within the myocardium that are released into the circulation in the event of damage to the myocyte. Therefore, serum troponin is an exquisitely sensitive marker of myocardial injury and is necessary for establishing the diagnosis of myocardial infarction (MI). Troponins are protein components of striated muscle. There are three different troponins: troponin C, troponin T and troponin I. Troponin-I exists in three isoforms; one in fast-twitch skeletal muscle, one in slow-twitch skeletal muscle, and one in cardiac muscle. Troponin I have been found to increase in acute myocardial infarction after 3–6 hours of the onset of chest pain, peak in about 12 hours and remain elevated for 3–10 days because of continuous release of myofilament components from the injured cardiac muscle [27, 28].
The observation in this study that a high salt diet significantly increased troponin I of male and female rats when compared with those on normal salt diet may be due to leakage of troponin I (cTnI) from viable cardiomyocytes of the rats. After muscle injury by trauma or ischemia, troponin is released into the blood stream and its levels correlate well with tissue injury [29]. Left ventricular hypertrophy [30] and inflammatory conditions such as myocarditis and pericarditis [31] has been found to correlate with elevated troponin release in stable physical conditions. The increase in the cTnI of rats fed a high salt diet is consistent with the elevated MOD and BP of the same group of rats in this study, as an increase in BP, MOD and cardiac hypertrophy could predispose the cardiac muscle to damage and hence the release of cardiac troponin I into blood stream of the rats. The greater increase in troponin I of male rats when compared with female rat in this study may be due to the protective effects of estrogens in the cardiovascular system [16, 32]. Estrogen has been shown to attenuate the development of cardiac hypertrophy and fibrosis through activation of steroid receptors located on myocytes, fibroblasts, and the extracellular matrix [33] and delays the progression of hypertension [34], development of cardiac hypertrophy [35], reduces activation of the renin-angiotensin system [36] and limits fibrosis, in salt-sensitive experimental models [17, 21] and in humans [37].
Serum levels of LDH, AST and ALT provide an excellent tool for the diagnosis of tissue damage. LDH has isoforms in the heart and other organs such as the liver with the H-type isoenzyme predominating in the heart [38]. Elevated levels of AST, ALT and LDH observed in rats fed a high dietary salt when compared with those on normal diet may be due to the disruption or damage of cell membrane that led to the leakage of these enzymes from the heart tissue to the serum. In myocardial stress such as infarction, LDH level in the serum was found to increase and the enzyme level was found to be proportional to the level of the damage to the heart muscles and in serious cases the elevation can even be threefold [38, 39]. Likewise, the determination of AST is used to diagnose acute myocardial infarction, the serum level of AST has been shown to relates directly to the number of cells affected by disease or injury [15]. Elevation of LDH, AST and ALT in the present study indicates an increased insult to the myocardium consequent of salt stress as marked elevation of serum AST is indicative of myocardial stress [40]. Our finding of sex disparity in the serum concentrations of ALT in the control animals is consistent with other studies which have reported a consistently higher concentration of serum ALT in males when compared with females [41, 42]. This may account for the basis on which elevated ALT is considered a more important predictor of cardiovascular risk in males [43].
The results of this study demonstrate that salt intake is an important factor in the control of urinary volume and fluid intake. In both groups of male and female animals, high dietary salt intake resulted in comparable increases in urinary output with an increase in water intake and fluid balance in the males. According to Rhoades and Bell [44], an increase in serum sodium and serum osmolarity stimulate thirst and anti-diuretic hormone which results in an increase in fluid intake, reduced serum osmolarity and increased urine volume [44].
In this study, rats fed with high dietary salt had significantly increased serum urea. Serum urea has been reported to increase in acute and chronic intrinsic renal disease and also when there is decreased effective circulating blood volume with decreased renal perfusion [45]. A lower rate of secretion of urea into urine resulting from renal insufficiency would cause its concentration in serum to increase. However, in the current study, rats fed a high dietary salt for 12 weeks were also found to have increased urinary urea concentration. The result observed in this study may be as a result of increased feed intake and increased metabolic rate associated with high salt intake [18], hence increased urinary excretion of metabolites such as urea.
Creatinine is formed in muscle from creatine phosphate by irreversible, non-enzymatic dehydration and loss of phosphate. The serum level of creatinine as well as its renal clearance is indicative of renal function as creatinine is excreted only via the kidney [46]. The result of this study showed an increase in creatinine clearance in females in high salt group when compared with the normal. These may be due to the renal protective role of estrogen in females [16].
Studies have found that proteinuria is a major risk factor for the progression of renal disease [47]. The increase in the urinary albumin concentration of high salt fed animals in this study may be a manifestation of renal dysfunction. It is possible that the high sodium diet is harmful to the selective permeability of the glomerular basement membrane and worsen the urinary excretion of albumin in high salt animals [17, 48] as high sodium intake has been associated with high serum uric acid and urinary albumin excretion levels in human [49].
Findings from this study show higher uric acid concentration in serum of rats fed with high salt diet when compared with those fed with normal diet. Elevated serum uric acid (SUA) levels can result from a number of factors, including both acute and chronic causes. Hyperuricemia can result from conditions that cause a reduction in the glomerular filtration rate (GFR), a decrease in the excretion of uric acid, or an increase in overall tubular absorption [50]. Hyperuricemia has been shown to be linked to a number of diseases and conditions, including gout, hypertension, cardiovascular disease, myocardial infarction, stroke, and renal disease [51, 52].
The increase in the serum concentration of sodium ion in the salt loaded rats is consistent with increased fluid retention and elevated BP that were observed in the same group of rats in this study. These findings are also consistent with earlier reports that salt loading leads to elevated serum levels of sodium ion [53], fluid retention [2] and elevated BP [24]. Salt is not readily excreted from the body when ingested. Rather, it indirectly increases the extracellular fluid volume via two basic mechanisms, it increases the osmolarity of the body fluids which stimulate the thirst center causing increased water intake and also stimulates the hypothalamic posterior pituitary gland secretion mechanism to secrete increase quantities of anti-diuretic hormone [53]. In order to excrete the excess sodium ions in the body, the systemic blood pressure is elevated leading to an increase in BP via a mechanism referred to as pressure natriuresis [54, 55]. It is already known that sodium ion and potassium ion work and operate inversely as a result of the activity of sodium potassium pump [56, 57]. Increased excretion of sodium ion would have resulted in a concomitant reabsorption of potassium ion from the renal tubules. However, in this study, there was no significant net change in serum potassium concentration in rats fed on high salt diet when compared with those on normal diet.
These observed sex differences in cardiac and renal functions in this study may be due to differences in sex hormones in male and females. It has been established that female sex hormones affect renal excretion. Studies in rats have shown that whole kidney GFR and single nephron GFR are higher in males than females due to a higher renal blood flow and lower renal vascular resistance, that result in sustained hyperfiltration in males [58]. Such effects of the different sex hormones could be explained by the observed sex differences in renal pathology between male and female, as studies have shown that while the number of glomeruli is the same between males and females, renal vascular resistance is much higher in females and thus the male kidney is vasodilated relative to the female hence higher rate of metabolite excretion in males than in females [58].
Thus, findings from this study suggest that effect of a high salt diet on blood pressure and biomarkers of cardiac and renal functions exhibit sex disparity which is greater in male when compared with female Sprague-Dawley rats. A better understanding of potential sex-specific differences in cardiac and renal functions and pathologies may provide and important insight for the effective prevention and/or management of salt-sensitive hypertension and its attending end organ or target organ damage.
Declarations
Author contribution statement
Ahmed Kolade Oloyo: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Ngozi O. A. Imaga: Perfomed the experiments; Contributed reagents, materials, analysis tools or data.
Fatope Yemisi: Performed the experiments; Contributed reagents, materials, analysis tools or data.
Olusoga A. Sofola: Conceived and designed the expereiments; Analyzed and interpreted the data; Wrote the paper.
Funding statement
Ahmed Kolade Oloyo is a beneficiary of the Medical Education Partnership Initiative in Nigeria (MEPIN) Mentored Research Grant. This work was supported by a grant from the Fogarty International Center (FIC), of the National Institutes of Health (R24TW008878).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.D’Elia L., Galletti F., La Fata E., Sabino P., Strazzullo P. Effect of dietary sodium restriction on arterial stiffness: systematic review and meta-analysis of the randomized controlled trials. J. Hypertens. 2018;36(4):734–743. doi: 10.1097/HJH.0000000000001604. [DOI] [PubMed] [Google Scholar]
- 2.Meneton P., Jeunemaitre X., de Wardener H.E., Macgregor G.A. Links between dietary salt intake, renal salt handling, blood pressure, and cardiovascular diseases. Physiol. Rev. 2005;85(2):679–715. doi: 10.1152/physrev.00056.2003. [DOI] [PubMed] [Google Scholar]
- 3.Weinberger M.H., Fineberg N.S., Fineberg S.E., Weinberger M. Salt sensitivity, pulse pressure, and death in normal and hypertensive humans. Hypertension. 2001;37(2):429–432. doi: 10.1161/01.hyp.37.2.429. [DOI] [PubMed] [Google Scholar]
- 4.Felder R.A., White M.J., Williams S.M., Jose P.A. Diagnostic tools for Hypertension and salt Sensitivity testing”. Curr. Opin. Nephrol. Hypertens. 2013;22(1):65–76. doi: 10.1097/MNH.0b013e32835b3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farquhar B., Edwards D.G., Jurkovitz C.T., Weintraub W.S. Dietary sodium and health: more than just blood pressure. J. Am. Coll. Cardiol. 2015;65(10):1042–1050. doi: 10.1016/j.jacc.2014.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kotchen T.A., Theodore A., Cowley A.W., Jr., Frohlich E.D. Salt in health and disease—a delicate balance. N. Engl. J. Med. 2013;368(13):1229–1237. doi: 10.1056/NEJMra1212606. [DOI] [PubMed] [Google Scholar]
- 7.De Wardener H.E., MacGregor G.A. Harmful effects of dietary salt in addition to hypertension. J. Hum. Hypertens. 2002;16(4):213. doi: 10.1038/sj.jhh.1001374. [DOI] [PubMed] [Google Scholar]
- 8.Oloyo A.K., Sofola O.A., Anigbogu C.N., Nair R.R., Vijayakumar H.S., Fernandez A.C. Testosterone reduces vascular relaxation by altering cyclic adenosine monophosphate pathway and potassium channel activation in male Sprague Dawley rats fed a high-salt diet. Ther. Adv. Cardiovasc. Dis. 2013;7(2):75–85. doi: 10.1177/1753944713479996. [DOI] [PubMed] [Google Scholar]
- 9.Oloyo A.K., Sofola O.A., Anigbogu C.N. Orchidectomy attenuates impaired endothelial effects of a high-salt diet in Sprague–Dawley rats. Can. J. Physiol. Pharmacol. 2011;89(4):295–304. doi: 10.1139/y11-023. [DOI] [PubMed] [Google Scholar]
- 10.Oloyo A.K., Vineetha V.P., Anigbogu C.N., Sofola O.A. Orchidectomy ameliorates the vascular hypertrophic effect of a high salt diet in Sprague-Dawley rats. J. Afr. Assoc. Physiol. Sci. 2013;1(1):37–45. [Google Scholar]
- 11.Liu P.Y., Death A.K., Handelsman D.J. Androgens and cardiovascular disease. Endocr. Rev. 2003;24(3):313–340. doi: 10.1210/er.2003-0005. [DOI] [PubMed] [Google Scholar]
- 12.Beale A.L., Nanayakkara S., Segan L., Mariani J.A., Maeder M.T., van Empel V., Kaye D.M. Sex differences in heart failure with preserved ejection fraction pathophysiology: a detailed invasive hemodynamic and echocardiographic analysis. JACC (J. Am. Coll. Cardiol.): Heart Fail. 2019;7(3):239–249. doi: 10.1016/j.jchf.2019.01.004. [DOI] [PubMed] [Google Scholar]
- 13.Duca F., Zotter-Tufaro C., Kammerlander A.A., Aschauer S., Binder C., Mascherbauer J., Bonderman D. Gender-related differences in heart failure with preserved ejection fraction. Sci. Rep. 2018;8(1):1080. doi: 10.1038/s41598-018-19507-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hinojosa-Laborde C., Craig T., Zheng W., Ji H., Haywood J.R., Sandberg K. Ovariectomy augments hypertension in aging female Dahl salt-sensitive rats. Hypertension. 2004;44(4):405–409. doi: 10.1161/01.HYP.0000142893.08655.96. [DOI] [PubMed] [Google Scholar]
- 15.Chappell M.C., Westwood B.M., Yamaleyeva L.M. Differential effects of sex steroids in young and aged female mRen2. Lewis rats: a model of estrogen and salt-sensitive hypertension. Gend. Med. 2008;5:S65–S75. doi: 10.1016/j.genm.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blenck C.L., Harvey P.A., Reckelhoff J.F., Leinwand L.A. The importance of biological sex and estrogen in rodent models of cardiovascular health and disease. Circ. Res. 2016;118(8):1294–1312. doi: 10.1161/CIRCRESAHA.116.307509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao D., Guallar E., Ouyang P., Subramanya V., Vaidya D., Ndumele C.E., Budoff M.J. Endogenous sex hormones and incident cardiovascular disease in post-menopausal women. J. Am. Coll. Cardiol. 2018;71(22):2555–2566. doi: 10.1016/j.jacc.2018.01.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coêlho M.S., Passadore M.D., Gasparetti A.L., Bibancos T., Prada P.O., Furukawa L.L., Furukawa L.N.S. High-or low-salt diet from weaning to adulthood: effect on body weight, food intake and energy balance in rats. Nutr. Metabol. Cardiovasc. Dis. 2006;16(2):148–155. doi: 10.1016/j.numecd.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 19.Amresh G., Paras N.S., Chandana V.R. Toxicological screening of traditional medicine Laghupatha (Cissampelos pareira) in experimental animals. J. Ethnopharmacol. 2008;116(3):454–460. doi: 10.1016/j.jep.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 20.Simon G., Jaeckel M., Illyes G. Development of structural vascular changes in salt-fed rats. Am. J. Hypertens. 2003;16(6):488–493. doi: 10.1016/s0895-7061(03)00568-5. [DOI] [PubMed] [Google Scholar]
- 21.Jessup J.A., Lindsey S.H., Wang H., Chappell M.C., Groban L. Attenuation of salt-induced cardiac remodeling and diastolic dysfunction by the GPER agonist G-1 in female mRen2. Lewis rats. PLoS One. 2010;5(11) doi: 10.1371/journal.pone.0015433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qasem A.A., Farag S.E., Hamed E., Emara M., Bihery A., Pasha H. Urinary biomarkers of acute kidney injury in patients with liver cirrhosis. Med. Arch. 2014;68(2):132. doi: 10.1155/2014/376795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bienaimé F., Canaud G., El Karoui K., Gallazzini M., Terzi F. Molecular pathways of chronic kidney disease progression. Néphrol. Thérapeutique. 2016;12:S35–S38. doi: 10.1016/j.nephro.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 24.Oloyo A.K., Sofola O.A., Yakubu M.A. Orchidectomy attenuates high-salt diet-induced increases in blood pressure, renovascular resistance, and hind limb vascular dysfunction: role of testosterone. Clin. Exp. Pharmacol. Physiol. 2016;43(9):825–833. doi: 10.1111/1440-1681.12595. [DOI] [PubMed] [Google Scholar]
- 25.Walkowska A., Pawlak M., Jane S.M., Kompanowska-Jezierska E., Wilanowski T. Effects of high and low sodium diet on blood pressure and heart rate in mice lacking the functional Grainyhead-like 1 gene. Physiol. Res. 2017;66(1):163. doi: 10.33549/physiolres.933298. [DOI] [PubMed] [Google Scholar]
- 26.Kanbay M., Chen Y., Solak Y., Sanders P.W. Mechanisms and consequences of salt sensitivity and dietary salt intake. Curr. Opin. Nephrol. Hypertens. 2011;20(1):37. doi: 10.1097/MNH.0b013e32834122f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shah A.S.V., Anand A., Sandoval Y., Lee K.K., Smith S.W., Adamson P.D., Chapman A.R. High-sensitivity cardiac troponin I at presentation in patients with suspected acute coronary syndrome: a cohort study. Lancet. 2015;386(10012):2481–2488. doi: 10.1016/S0140-6736(15)00391-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li X., Luo R., Jiang R., Kong H., Tang Y., Shu Y., Hua W. The prognostic use of serum concentrations of cardiac troponin-I, CK-MB and myoglobin in patients with idiopathic dilated cardiomyopathy. Heart & Lung J. Acute Crit. Care. 2014;43(3):219–224. doi: 10.1016/j.hrtlng.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 29.Rubini Gimenez M., Twerenbold R., Reichlin T., Wildi K., Haaf P., Schaefer M., Zellweger C. Direct comparison of high-sensitivity-cardiac troponin I vs. T for the early diagnosis of acute myocardial infarction. Eur. Heart J. 2014;35(34):2303–2311. doi: 10.1093/eurheartj/ehu188. [DOI] [PubMed] [Google Scholar]
- 30.Konishi M., Sugiyama S., Sugamura K., Nozaki T., Ohba K., Matsubara J., Sakamoto K. Basal and ischemia-induced transcardiac troponin release into the coronary circulation in patients with suspected coronary artery disease. PLoS One. 2013;8(4) doi: 10.1371/journal.pone.0060163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Urhausen A., ürgen Scharhag J., Herrmann M., Kindermann W. Clinical significance of increased cardiac troponins T and I in participants of ultra-endurance events. Am. J. Cardiol. 2004;94(5):696–698. doi: 10.1016/j.amjcard.2004.05.050. [DOI] [PubMed] [Google Scholar]
- 32.Mendelsohn M.E., Karas R.H. Molecular and cellular basis of cardiovascular gender differences. Science. 2005;308(5728):1583–1587. doi: 10.1126/science.1112062. [DOI] [PubMed] [Google Scholar]
- 33.Regitz-Zagrosek V., Kararigas G. Mechanistic pathways of sex differences in cardiovascular disease. Physiol. Rev. 2016;97(1):1–37. doi: 10.1152/physrev.00021.2015. [DOI] [PubMed] [Google Scholar]
- 34.Krzesiński P., Stańczyk A., Gielerak G., Uziębło-Życzkowska B., Kurpaska M., Piotrowicz K., Skrobowski A. Sex determines cardiovascular hemodynamics in hypertension. J. Hum. Hypertens. 2015;29(10):610. doi: 10.1038/jhh.2014.134. [DOI] [PubMed] [Google Scholar]
- 35.Masoudi F.A., Havranek E.P., Smith G., Fish R.H., Steiner J.F., Ordin D.L., Krumholz H.M. Gender, age, and heart failure with preserved left ventricular systolic function. J. Am. Coll. Cardiol. 2003;41(2):217–223. doi: 10.1016/s0735-1097(02)02696-7. [DOI] [PubMed] [Google Scholar]
- 36.Lopez-Pier M.A., Lipovka Y., Koppinger M.P., Harris P.R., Konhilas J.P. The clinical impact of estrogen loss on cardiovascular disease in menopausal females. Med. Res. Arch. 2018;6(2) [PMC free article] [PubMed] [Google Scholar]
- 37.Li S., Gupte A.A. The role of estrogen in cardiac metabolism and diastolic function”. Methodist DeBakey Cardiovasc. J. 2017;13(1):4. doi: 10.14797/mdcj-13-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Butt A.A., Michaels S., Greer D., Clark R., Kissinger P., Martin D.H. Serum LDH level as a clue to the diagnosis of histoplasmosis. AIDS Read. 2002;12(7):317–321. PMID:12161854. [PubMed] [Google Scholar]
- 39.Zarlasht F., Ramadan M., Almoadhen M., Lin K., Khaja M., Basir R. Lactate dehydrogenase and ferritin levels: a clinical clue for early diagnosis of disseminated histoplasmosis in HIV patients. J. Med. Cases. 2016;7(3):81–83. [Google Scholar]
- 40.Nigam P.K. Biochemical markers of myocardial injury. Indian J. Clin. Biochem. 2007;22(1):10–17. doi: 10.1007/BF02912874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen E.Q., Huang F.J., He L.L., Bai L., Wang L.C., Zhou T.Y., Tang H. Histological changes in Chinese chronic hepatitis B patients with ALT lower than two times upper limits of normal. Dig. Dis. Sci. 2010;55(2):432–437. doi: 10.1007/s10620-009-0724-5. [DOI] [PubMed] [Google Scholar]
- 42.Saxena S., Korula J., Fong T.L., Shulman I.A. Are gender-specific ALT cutoff values necessary? Lab. Med. 1995;26(10):682–686. [Google Scholar]
- 43.Feitosa M.F., Reiner A.P., Wojczynski M.K., Graff M., North K.E., Carr J.J., Borecki I.B. Sex-influenced association of nonalcoholic fatty liver disease with coronary heart disease. Atherosclerosis. 2013;227(2):420–424. doi: 10.1016/j.atherosclerosis.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rhoades R.A., Bell D.R., editors. Medical Physiology: Principles for Clinical Medicine. Lippincott Williams & Wilkins; 2012. pp. 428–435. Chapter 23. [Google Scholar]
- 45.Ocampo H.J., Torres Rosales A., Rodríguez Castellanos F. Comparison of four methods for measuring glomerular filtration rate by inulin clearance in healthy individuals and patients with renal failure. Nefrologia. 2010;30(3):324–330. doi: 10.3265/Nefrologia.pre2010.Mar.10238. [DOI] [PubMed] [Google Scholar]
- 46.Abdelhalim M.A.K., Moussa S.A.A. The gold nanoparticle size and exposure duration effect on the liver and kidney function of rats: in vivo. Saudi J. Biol. Sci. 2013;20(2):177–181. doi: 10.1016/j.sjbs.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Atkins R.C., Briganti E.M., Lewis J.B., Hunsicker L.G., Braden G., de Crespigny P.J.C., DeFerrari G. Proteinuria reduction and progression to renal failure in patients with type 2 diabetes mellitus and overt nephropathy. Am. J. Kidney Dis. 2005;45(2):281–287. doi: 10.1053/j.ajkd.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 48.Russo L.M., Wayne D.C., Tanya M.O. Mechanism of albuminuria associated with cardiovascular disease and kidney disease. Kidney Int. 2004;66:S67–S68. doi: 10.1111/j.1523-1755.2004.09218.x. [DOI] [PubMed] [Google Scholar]
- 49.Forman J.P., Scheven L., de Jong P.E., Bakker S.J.L., Curhan G.C., Gansevoort R.T. Association between sodium intake and change in uric acid, urine albumin excretion, and the risk of developing hypertension. Circulation. 2012 doi: 10.1161/CIRCULATIONAHA.112.096115. CIRCULATIONAHA-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Johnson R.J., Rodriguez-Iturbe B., Nakagawa T., Kang D., Feig D.I., Herrera-Acosta J. Subtle renal injury is likely a common mechanism for salt-sensitive essential hypertension. Hypertension. 2005;45(3):326–330. doi: 10.1161/01.HYP.0000154784.14018.5f. [DOI] [PubMed] [Google Scholar]
- 51.Kang D., Nakagawa T., Feng L., Watanabe S., Han L., Mazzali M., Truong L., Harris R., Johnson R.J. A role for uric acid in the progression of renal disease. J. Am. Soc. Nephrol. 2002;13(12):2888–2897. doi: 10.1097/01.asn.0000034910.58454.fd. [DOI] [PubMed] [Google Scholar]
- 52.Bos M.J., Koudstaal P.J., Hofman A., Witteman J.C.M., Breteler M.M.B. Uric acid is a risk factor for myocardial infarction and stroke: the Rotterdam study. Stroke. 2006;37(6):1503–1507. doi: 10.1161/01.STR.0000221716.55088.d4. [DOI] [PubMed] [Google Scholar]
- 53.Guyton A.C., Hall J.E. tenth ed. Saunders; 2002. Text Book of Medical Physiology; pp. 345–356. [Google Scholar]
- 54.Granger J.P., Alexander B.T., Llinas M. Mechanisms of pressure natriuresis. Curr. Hypertens. Rep. 2002;4(2):152–159. doi: 10.1007/s11906-002-0040-3. [DOI] [PubMed] [Google Scholar]
- 55.Ivy J.R., Bailey M.A. Pressure natriuresis and the renal control of arterial blood pressure. J. Physiol. 2014;592(18):3955–3967. doi: 10.1113/jphysiol.2014.271676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Davidson B.A. tenth ed. Churchill Livingstone; 2005. Principles and Practice of Medicine. [Google Scholar]
- 57.Mayne D.P. sixth ed. Book Power USA; 2005. Clinical Chemistry in Diagnosis and Treatment. [Google Scholar]
- 58.Sabolić I., Abdul R.A., Wolfgang E.B., Wanke C., Bahn A., Burckhardt G. Gender differences in kidney function. Pflueg. Arch. Eur. J. Physiol. 2007;455(3):397. doi: 10.1007/s00424-007-0308-1. [DOI] [PubMed] [Google Scholar]