Abstract
Background
Heart rate variability (HRV) has proven to be a powerful non-invasive tool to investigate cardiac autonomic control and, seems to be influenced by nutritional status and exercise practice. However, the acute effects of fed or fasting states on HRV and blood pressure (BP) during low-to-moderate intensity aerobic exercise are currently unknown. Therefore, we investigated the baseline values and behavior of HRV, BP, and heart rate (HR) before and after low-to-moderate intensity aerobic exercise in fed and fasted states in healthy adults.
Methods
12 healthy individuals with mean age (SD) 59.0 (9.1) years performed two tests on a treadmill at 80% of the mean velocity of the 6-min walking test separated by 48 h: 12 h fasted (FST) or 1 h fed (FED). HRV, BP and HR were analyzed at rest, posttest, and at the third, fifth, and seventh minutes of recovery.
Results
HRV and HR presented no significant alterations between nutritional conditions. HR at baseline was not different between nutritional conditions. Diastolic blood pressure was increased during the fasted baseline state.
Conclusions
The results of the current study provide that 12 h overnight fasting does not seem to be enough to affect significant changes in the autonomic modulation in healthy adults submitted to low-to-moderate intensity aerobic exercise.
Keywords: Fasting, Autonomic modulation, Exercise, Nutrition, Aging process
1. Introduction
Heart rate and blood pressure are carefully regulated by the autonomic nervous system. Heart rate variability is a non-invasive method used to assess the effects of the autonomic nervous system [1] and is related to lifestyle in adults [2]. Beat-to-beat variation in heart rate variability is considered a non-invasive measurement of the autonomic nervous system balance, providing a quantitative evaluation of neuroautonomic function [1].
Regular physical exercise, smoking, caloric restriction, and disturbed sleeping patterns can present significant effects on heart rate variability in adults. It was reported that reduced dietary fat intake increased parasympathetic activity in women [3] and reduced blood pressure in individuals with abdominal obesity [4]. Additionally, effects of 12 h of fasting on heart rate variability and blood pressure during the menstrual phases in women were previously demonstrated [5]. Furthermore, de Jonge et al. [6] reported, in overweight individuals, improved vagal activity and reduced sympathetic activity after six months of caloric restriction combined with exercise, despite weight loss comparable to caloric restriction only.
Regarding the massive speculation about the potential benefits of exercise training in the fasting condition, our hypothesis is that cardiac autonomic parameters might be influenced by nutritional states. Aerobic exercise performed at low-to-moderate intensity may be a useful strategy to induce a significant increase in fat oxidation while exercise is being performed in the fasted state compared to the fed state [7]. The anti-stress effects of fasting were demonstrated in men, whereby short-term fasting (12 h) increased parasympathetic domain activity while meal intake activated sympathetic domain activity [8]. However, there is no study comparing the acute effects of the fed and fasted states on heart rate variability and blood pressure during low-to-moderate intensity aerobic exercise. Thus, the aims of the current study were to compare the baseline values and behavior of heart rate variability, blood pressure, and heart rate before and after low-to-moderate intensity aerobic exercise in the fed and fasted states in healthy adults individuals.
2. Materials and methods
2.1. Participants
Twelve healthy participants (five men and seven post-menopausal women) with mean age (SD) 59.0 (9.1) years, body mass index 27.5 (3.0) kg/m2, and height: 1.61 (0.1) m participated in this study. The study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures were approved by the Ethics Committee of the University (code 37519014.5.0000.5659). All participants provided written informed consent prior to participation in the study. The participants reported performing aerobic exercise at least 150 min per week for at least six months being considered physically active. In this study participants using beta-blocker medication or with any known illness that alters heart rhythm were excluded [1].
2.2. General design
Participants attended three visits. During the first visit, maximal walking distance performed in 6 min was assessed (i.e., six min walk test; 6MWT) [9], allowing determination of mean speed. On the remaining two visits, after 48 h interval between each visit, participants performed a 15-min effort at 80% of the mean speed observed during the first visit, in a fed state (FED) or fasted state (FST). The condition of the second visit was randomized and counterbalanced at the third visit (Fig. 1).
Fig. 1.
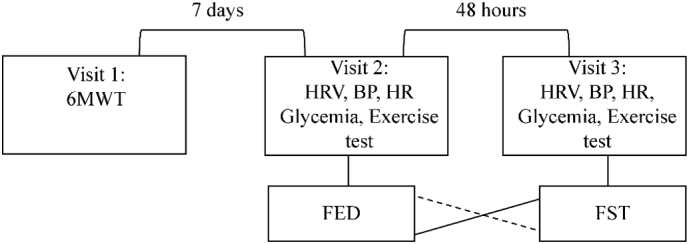
Experimental design. 6MWT: six minutes' walk test; FST: after 12 h of overnight fasting condition; FED: after 1 h after a regular breakfast; HRV: heart rate variability; BP: blood pressure; HR: heart rate.
2.3. Blood analysis
All tests were performed in the morning (7:00–8:30 am) after 12 h overnight fasting or 1 h after a regular breakfast (bread, butter, orange juice). Prior to all tests, subjects were advised to abstain from drinking any beverages containing caffeine for at least 12 h. Before the tests all volunteers remained seated for approximately 5 min after which a venous blood sample was performed to verify serum glucose level. The analysis was performed in the clinical laboratory of the Faculty of Pharmaceutical Sciences of Ribeirão Preto (University of São Paulo), by means of an enzymatic analysis kit (Wiener Lab, Argentina) on an automatic device (Konelab 600i, Wiener Lab, Argentina).
2.4. Six minute walk test and exercise sessions
Aerobic capacity was evaluated using the six-minute walk test (6MWT) that consisted of walking as quickly as possible in a rectangular area (45.7 m) for 6 min, with every 4.57 m demarcated by a cone. This test is specific for older and elderly adults, validated and with normative reference values. The total number of laps was counted and when the time ended, the participants remained standing in their finishing position to measure the total distance performed [[9], [10], [11]].
After the 6MWT, each participant performed two efforts separated by 48 h, in the FED or FST condition, using a calibrated treadmill (Imbramed Super ATL; Porto Alegre, Brasil). The effort had a duration of 15 min, with a constant load, corresponding to 80% of the mean speed observed in the 6MWT [9]. This mean velocity was chosen to characterize the exercise of low-to- moderate intensity. Before the test, immediately after, and during recovery (i.e. third, fifth, and seventh minutes), blood pressure (BP) and heart rate (HR) were measured. Heart rate variability (HRV) was measured continuously from 5 min preceding the efforts until the seventh minute of recovery (Polar Team [2], Finland).
2.5. Cardiovascular responses
Noninvasive systolic (SBP) and diastolic (DBP) blood pressure were measured in the upper arm using a digital manometer (OMRON, HEM-7113, Brazil) after sitting for 5 min at rest [12,13]. Participants remained seated in a chair with eyes open and directed toward the wall, not speaking, while the measurements were performed under appropriate lighting and a comfortable temperature [14].
2.6. Heart rate variability
Heart rate variability analysis was carried out based on the guidelines of the Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology [1] in spontaneously breathing patients. In a quiet room at a comfortable temperature, participants were equipped with a strap around the chest with a Polar Team [2] heart rate transmitter (Polar Electro Oy, Kempele, Finland) and then rested in the sitting position for approximately 15 min for measurement in the baseline conditions. Time and frequency domain parameters of heart rate variability were obtained from 10 min recordings of R-R intervals, discarding the initial 5 min [14]. For analysis of R-R intervals during the exercise was used the 15 min of the test period. In the recovery, was used the 7 min of the consecutive R-R intervals after the interruption of the low-to-moderate intensity aerobic exercise with the participants in the sitting position.
The register was performed using custom computer software (CardioSeries v2.0, http://sites.google.com/site/cardioseries) developed by Dias, DPM of the University of São Paulo, Brazil [15]. The RR values were redesigned in 3 Hz cubic spline interpolation, to normalize time interval between the beats. The interpolated RR series were divided into half-overlapping sets of 256 data points, overlapping 50% (Welch Protocol). The stationary segment was visually inspected and those with artifacts or transients were excluded. Each RR stationary segment was submitted to the spectral analysis by Fast Fourier Transform (FFT), after Hanning window. The RR time series were integrated in bands of low frequency (LF; 0.04–0.15 Hz) and high frequency (HF; 0.15–0.5 Hz) and the results are expressed in absolute values (ms2) and normalized units (nu).
2.7. Statistics
Data normality was tested and confirmed by the Kolmogorov-Smirnov test, allowing the description of values as mean (standard deviation). Mauchly's test confirmed the sphericity of data, enabling the use of parametric statistics for comparisons. Thus, responses during exercise and recovery periods were compared between the FED and FST conditions using a mixed model for repeated measurements. Sidaks's test was applied to determine the differences between conditions and time points. Considering our mixed sample and the range of participants' age, the gender and the total years were considered as covariates in these analyses. The sample size of at least 10 in each group presented an 80% power to detect a difference between HF power means of 1 ms with a level of 0.05 (two-tailed), based on an SD of 0.78 ms obtained from time HRV recordings in a previous cohort of healthy female subjects [17]. The Statistical Package for Social Science software, version 17.0 (SPSS Inc., Chicago, Illinois) was used and the level of significance was set at p-value <0.05.
3. Results
Table 1 shows the baseline, exercise, and recovery data for heart rate variability in the participants in the FST and FED conditions. In both conditions, during the exercise and recovery, very low frequency (VLF) presented lower values than baseline (p < 0.05). During the exercise the values of low frequency (LF) and high frequency (HF) were decreased (p < 0.05), while the standard deviation of normal to normal R-R intervals (SDNN) values increase significantly (p < 0.05). No differences were observed between conditions (p > 0.05). In addition, no changes were found between the FED 508 (71) m or FST 498 (49) conditions for the traveled distance on the 6MWT.
Table 1.
Heart rate variability parameters in 12 healthy adults in fasting and fed conditions.
| FED |
FST |
|||||
|---|---|---|---|---|---|---|
| Baseline | Exercise | Recovery | Baseline | Exercise | Recovery | |
| Frequency domain | ||||||
| List indices | ||||||
| VLF (ms2) | 273.8 (174.1) | 8.8 (6.3)a | 103.3 (96.0)a | 395.4 (230.0) | 9.3 (5.5)a |
123.3 (100.2)a |
| LF (ms2) | 176.3 (128.5) | 14.0 (12.0)a | 132.8 (117.5) | 179.9 (113.4) | 12.5 (12.7)a | 179.9 (216.6) |
| HF (ms2) | 103.8 (99.3) | 18.2 (25.1)a | 185.2 (252.4) | 110.4 (78.6) | 15.3 (20.8)a | 194.7 (308.9) |
| LF/HF (a.u.) | 3.2 (3.9) | 1.5 (1.0) | 2.2 (2.4) | 3.0 (2.8) | 2.0 (2.3) | 2.2 (2.1) |
| Time domain | ||||||
| List indices | ||||||
| RMSSD (ms) | 16.0 (8.6) | 6.9 (3.5) | 17.6 (14.4) | 16.4 (6.5) | 7.1 (5.3) | 17.6 (12.8) |
| SDNN (ms) | 17.6 (14.4) | 80.7 (35.7)a | 24.7 (9.9) | 31.0 (15.0) | 85.2 (44.6)a | 25.6 (13.8) |
FST: fasting condition; FED: fed condition; VLF: very low frequency; LF: low frequency; HF: high frequency; LF/HF: low-to-high frequency ratio; RMSSD: root mean square of successive differences; SDNN: standard deviation of intervals; Mean (SD). Mixed model analysis, p < 0.05.
Difference from baseline at the same situation.
The blood glucose levels were significantly reduced in the FST condition at baseline compared to the FED condition 3.96 (0.67) versus 5.83 (1.92 mmol/L) (p < 0.05), respectively, which was expected to prove the fasting state.
The heart rate response is shown in Fig. 2. A significant increase from baseline were observed after exercise (p < 0.05), which lasted until 3 min of recovery (p < 0.05), independently of condition. No differences were observed between conditions for heart rate values (p > 0.05).
Fig. 2.

Heart rate response at baseline, after exercise and during recovery. Closed symbols represent the fed condition, while open symbols represent the fasted condition. *Significant difference from baseline (p < 0.05).
The systolic blood pressure increased after exercise, as expected, although not statistically significant difference between the nutritional condition (p > 0.05) (Fig. 3). Diastolic blood pressure was different at baseline between conditions, increasing after exercise from baseline (p < 0.05), only at the FST condition, without a clinical significant change. No effects of exercise were observed at FED condition (p > 0.05) (Fig. 4).
Fig. 3.
Systolic blood pressure at baseline, after exercise and during recovery. Closed symbols represent the fed condition, while open symbols represent the fasted condition.
Fig. 4.
Diastolic blood pressure at baseline, after exercise and during recovery. Closed symbols represent the fed condition, while open symbols represent the fasted condition. *Significant difference from baseline. #Significant difference from fed condition (p < 0.05).
4. Discussion
This study aimed to investigate the differences between FED and FST conditions in heart rate variability, heart rate, and blood pressure in healthy adults submitted to low-to-moderate intensity aerobic exercise. The main results were as follows: [1] heart rate variability parameters were unchanged between conditions; [2] diastolic blood pressure was increased at baseline in the fasted state, however, without a clinical significant change.
Our results showed that the 12 h fasted state does not induce significant improvement in cardiac autonomic modulation in healthy adults (Table 1). Previously, Mazurak et al. [16] reported the influence of 48 h of total fasting on autonomic regulation of heart rhythm measured through heart rate variability and on salivary cortisol levels in healthy non-obese women mean age: 21.4 (2.1 years). The authors suggested a decrease in total heart rate variability as well as a decrease in parasympathetic and baroreceptor regulation of heart rhythm under baseline conditions as a result of fasting. Interestingly, Cansel et al. [17] demonstrated increased heart rate variability parameters in the fasting month of Ramadan. Fasting during Ramadan is a radical change in lifestyle for the period of a lunar month. In summer months, the fasting can last up to 18 h or more. The authors reported increased heart rate variability in 40 healthy individuals mean age: 29.3 (5.9 years). In addition, Kuwahara et al. [8] reported that in male subjects mean age: 23.0 (0.9 years) 12 h of fasting resulted in dominance of parasympathetic activity compared with food intake groups, one of which ingested a high energy/high fat meal and the other a moderate energy/high carbohydrate meal.
The results of unchanged heart rate variability in the present study might be related to the different ages of participants compared with previous studies. It is known that aging may decrease vagal modulation [[18], [19], [20], [21]] and possibly reduces the sensitivity to change. Gehart et al. [19] demonstrated that in older women mean age: 59.0 (6.0 years) compared to younger women mean age: 21.0 (2.0 years) submitted to 12 weeks of resistance training there was no significant effect on any autonomic parameter. Recently we showed that the presence of metabolic syndrome was not sufficient to induce changes in heart rate variability at baseline, during a cardiopulmonary exercise test, or in recovery, when patients were compared to healthy individuals mean age: 42.8 (10.7 years) [14]. In the current study we suggest that 12 h of fasting may not have been a sufficient stimulus to alter heart rate variability in healthy adults.
Additionally, aerobic exercise training promotes several cardiovascular adjustments that are influenced by the central areas involved in the output of the autonomic nervous system [[22], [23], [24]] and these adjustments perhaps depend of the nutritional status. In the current study, we showed that low-to-moderate aerobic exercise did not cause changes in heart rate variability in either nutritional condition. In agreement with our results, Lima-Silva et al. [25] demonstrated that low carbohydrate availability altered heart rate variability parameters during severe, but not moderate-intensity exercise. They studied six healthy males mean age: 26.5 (6.7 years) undergoing moderate and high intensity exercise in two different nutritional conditions, low availability and moderate availability of carbohydrate. The results suggested that the respiratory mechanics might affect sinus node activity, modifying the beat-to-beat oscillations over time but without modification of the mean heart rate during only severe-intensity exercise.
Moreover, there is an increase in plasma catecholamines levels in a fasting condition that are known to influence the energy substrate mobilization and use at rest and in response to exercise [26]. Solianik et al. [27] reported the effects of a 48 h, zero-calorie diet on autonomic function, brain activity, cognition, and mood in nine amateur weight lifters mean age: 25.9 (4.1 years). The authors observed that heart rate and systolic blood pressure were reduced after fasting, whereas all 2 min of the heart rate variability indices were unchanged. Nevertheless, Cao et al. [28], demonstrated that sympathetic and vagal responses were sex specific to both carbohydrate rich meal intake and postural stress in healthy older men and women mean age: 65.0 (2.1 years) with normal insulin sensitivity. Following carbohydrate ingestion, a cardiac vagal reduction was evident in apparently healthy older women, but not in older men. The authors highlight that the precise mechanism by which a carbohydrate diet mediates efferent vagal inhibition of cardiovascular risk remains to be determined.
In the present study, which was expected, the blood glucose levels in the FST and FED conditions at baseline were different. It is noteworthy that ketones are produced in response to a low glucose level [29] and that the major ketone body, β-hydroxybutyrate, inhibits the sympathetic nervous system [30]. Our results demonstrated that there was no difference in heart rate in the FST group compared to the FED group (Fig. 2) at baseline. After moderate intensity exercise, which was expected, the heart rate increased in both conditions compared from baseline and after 3 min of recovery the heart rate remained high compared to baseline in both conditions. Furthermore, diastolic blood pressure was increased in the FST group at baseline (Fig. 4) and no difference for systolic blood pressure between the nutritional conditions was found (Fig. 3). Blood pressure is a dynamic variable whose values naturally change over time and within different regions of the body [31]. Following 30 min of meal ingestion, there is a fall in splanchnic vascular resistance, which results in an overall fall in total peripheral resistance [32] and this fact might explain the high diastolic blood pressure in the FST state at baseline compared to the FED state, although this change presents no clinical significance.
4.1. Limitations
Regarding the measurement instruments, it is difficult to compare research findings due to the different methodologies used to evaluate nutritional status. Finally, it is important to state that no individual experienced any discomfort throughout the intervention period. In addition, one of the limitations of the present study was the lack of control of the water drinking volume during the fasting state and to ensure the compliance of the participants.
5. Conclusions
The present study aimed for the first time to investigate heart rate variability in different nutritional conditions in healthy adults submitted to low-to-moderate intensity aerobic exercise. Regarding the benefits of the practice of physical exercise in fasting, it is of significant importance to know the influence of this nutritional pattern on the behavior of hemodynamic and cardiovascular variables before, during and after physical exercise. Thus, despite the limited number of participants, the current study adds important conclusions regarding this nutritional practice, especially if HRV or HR is used for the prescription of physical exercise for example. In conclusion, we observed that 12 h overnight fasting does not seem to be enough to affect significant and clinical changes in the autonomic nervous system activity in healthy adults submitted to low-to-moderate intensity aerobic exercise.
Funding
The present study was funded by FAPESP (2013/21159-8) and CNPq (485045/2013-3).
Declaration of Competing Interest
None declared.
Acknowledgements
The authors would like to thank all the participants for agreeing to participate in the study.
References
- 1.Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Eur. Heart J. 1996;17:354–381. [PubMed] [Google Scholar]; Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Eur Heart J. 1996; 17: 354–81. [PubMed]
- 2.Kanaley J.A., Baynard T., Franklin R.M., Weinstock R.S., Goulopoulou S., Carhart R. The effects of a glucose load and sympathetic challenge on autonomic function in obese women with and without type 2 diabetes mellitus. Metabolism. 2007;56:778–785. doi: 10.1016/j.metabol.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]; Kanaley JA, Baynard T, Franklin RM, Weinstock RS, Goulopoulou S, Carhart R, et al. The effects of a glucose load and sympathetic challenge on autonomic function in obese women with and without type 2 diabetes mellitus. Metabolism. 2007; 56: 778–85. [DOI] [PMC free article] [PubMed]
- 3.Pellizzer A.M., Straznicky N.E., Lim S., Kamen P.W., Krum H. Reduced dietary fat intake increases parasympathetic activity in healthy premenopausal women. Clin. Exp. Pharmacol. Physiol. 1999;26:656–660. doi: 10.1046/j.1440-1681.1999.03103.x. [DOI] [PubMed] [Google Scholar]; Pellizzer AM, Straznicky NE, Lim S, Kamen PW, Krum H. Reduced dietary fat intake increases parasympathetic activity in healthy premenopausal women. Clin Exp Pharmacol Physiol. 1999; 26: 656–60. [DOI] [PubMed]
- 4.Sosner P., Bosquet L., Herpin D., Guilbeault V., Latour E., Paquette-Tannir L. Net blood pressure reduction following 9 months of lifestyle and high-intensity interval training intervention in individuals with abdominal obesity. J. Clin. Hypertens. 2016;00:1–7. doi: 10.1111/jch.12829. [DOI] [PMC free article] [PubMed] [Google Scholar]; Sosner P, Bosquet L, Herpin D, Guilbeault V, Latour E, Paquette-Tannir L, et al. Net Blood Pressure Reduction Following 9 Months of Lifestyle and High-Intensity Interval Training Intervention in Individuals With Abdominal Obesity. J Clin Hypertens. 2016; 00: 1–7. [DOI] [PMC free article] [PubMed]
- 5.Ohara K., Okita Y., Kouda K., Mase T., Miyawaki C., Nakamura H. Cardiovascular response to short-term fasting in menstrual phases in young women: an observational study. BMC Womens Health. 2015;15:67. doi: 10.1186/s12905-015-0224-z. [DOI] [PMC free article] [PubMed] [Google Scholar]; Ohara K, Okita Y, Kouda K, Mase T, Miyawaki C, Nakamura H. Cardiovascular response to short-term fasting in menstrual phases in young women: an observational study. BMC Womens Health. 2015; 15: 67. [DOI] [PMC free article] [PubMed]
- 6.de Jonge L., Moreira E.A.M., Martin C.K., Ravussin E. Impact of 6-month caloric restriction on autonomic nervous system activity in healthy, overweight, individuals. Obesity (Silver Spring) 2010;18:414–416. doi: 10.1038/oby.2009.408. [DOI] [PMC free article] [PubMed] [Google Scholar]; de Jonge L, Moreira EAM, Martin CK, Ravussin E. Impact of 6-month caloric restriction on autonomic nervous system activity in healthy, overweight, individuals. Obesity (Silver Spring). 2010; 18: 414–6. [DOI] [PMC free article] [PubMed]
- 7.Vieira A.F., Costa R.R., Macedo R.C.O., Coconcelli L., Kruel L.F.M., Longo V.D. Effects of aerobic exercise performed in fasted v. fed state on fat and carbohydrate metabolism in adults: a systematic review and meta-analysis. Br. J. Nutr. 2016;116:1153–1164. doi: 10.1017/S0007114516003160. [DOI] [PubMed] [Google Scholar]; Vieira AF, Costa RR, Macedo RCO, Coconcelli L, Kruel LFM, Longo VD, et al. Effects of aerobic exercise performed in fasted v. fed state on fat and carbohydrate metabolism in adults: a systematic review and meta-analysis. Br J Nutr. 2016; 116: 1153–64. [DOI] [PubMed]
- 8.Kuwahara K., Okita Y., Kouda K., Nakamura H. Effects of modern eating patterns on the cardiac autonomic nervous system in young Japanese males. J. Physiol. Anthropol. 2011;30:223–231. doi: 10.2114/jpa2.30.223. [DOI] [PubMed] [Google Scholar]; Kuwahara K, Okita Y, Kouda K, Nakamura H. Effects of modern eating patterns on the cardiac autonomic nervous system in young Japanese males. J Physiol Anthropol. 2011; 30: 223–31. [DOI] [PubMed]
- 9.Jenkins S.C. 6-Minute walk test in patients with COPD: clinical applications in pulmonary rehabilitation. Physiotherapy. 2007;93:175–182. [Google Scholar]; Jenkins SC. 6-Minute walk test in patients with COPD: clinical applications in pulmonary rehabilitation. Physiotherapy. 2007; 93: 175–82.
- 10.Rikli R.E., Jones C.J. Development and validation of a functional fitness test for community-residing older adults. J. Aging Phys. Act. 1999;7:129–161. [Google Scholar]; Rikli RE, Jones CJ. Development and Validation of a Functional Fitness Test for Community-Residing Older Adults. Journal of Aging and Physical Activity. 1999; 7: 129–61.
- 11.Trapé A.A., Lizzi E.A.S., Gonçalves T.C.P., Rodrigues J.A.L., Tavares S.S., Lacchini R. Effect of multicomponent training on blood pressure, nitric oxide, redox status, and physical fitness in older adult women: influence of endothelial nitric oxide syntahse (NOS3) haplotypes. Oxidative Med. Cell. Longev. 2017:1–12. doi: 10.1155/2017/2578950. [DOI] [PMC free article] [PubMed] [Google Scholar]; Trapé AA, Lizzi EAS, Gonçalves TCP, Rodrigues JAL, Tavares SS, Lacchini R, et al. Effect of multicomponent training on blood pressure, nitric oxide, redox status, and physical fitness in older adult women: Influence of endothelial nitric oxide syntahse (NOS3) haplotypes. Oxid Med Cell Longev. 2017; 1-12. [DOI] [PMC free article] [PubMed]
- 12.Pickering T.G., Hall J.E., Appel L.J., Falkner B.E., Graves J., Hill M.N. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Cou. Circulation. 2005;111:697–716. doi: 10.1161/01.CIR.0000154900.76284.F6. [DOI] [PubMed] [Google Scholar]; Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN, et al. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Cou. Circulation. 2005; 111: 697-716. [DOI] [PubMed]
- 13.Malachias M.V.B., Souza W.K.S.B., Plavnik F.L., Rodrigues C.I.S., Brandão A.A., Neves M.F.T. 7a Diretriz Brasileira De Hipertensão Arterial. Arq. Bras. Cardiol. 2016;107:1–83. 3Supl.3. [Google Scholar]; Malachias MVB, Souza WKSB, Plavnik FL, Rodrigues CIS, Brandão AA, Neves MFT, et al. 7a Diretriz Brasileira De Hipertensão Arterial. Arq Bras Cardiol 2016; 107 (3Supl.3): 1-83.
- 14.Rodrigues J.A.L., Ferrari G.D., Fernandes I.A., Ferezin L.P., Trapé Á.A., Bueno Júnior C.R. Characterization of the heart rate variability in individuals with metabolic syndrome. Rev. Bras. Med. Esporte. 2017;23:208–210. [Google Scholar]; Rodrigues JAL, Ferrari GD, Fernandes IA, Ferezin LP, Trapé ÁA, Bueno Júnior CR. Characterization of the heart rate variability in individuals with metabolic syndrome. Rev Bras Med do Esporte. 2017; 23: 208-210.
- 15.Silva A.S., Ariza D., Dias D.P.M., Crestani C.C., Martins-Pinge M.C. Cardiovascular and autonomic alterations in rats with Parkinsonism induced by 6-OHDA and treated with L-DOPA. Life Sci. 2015;127:82–89. doi: 10.1016/j.lfs.2015.01.032. [DOI] [PubMed] [Google Scholar]; Silva AS, Ariza D, Dias DPM, Crestani CC, Martins-Pinge MC. Cardiovascular and autonomic alterations in rats with Parkinsonism induced by 6-OHDA and treated with L-DOPA. Life Sci. 2015; 127: 82–9. [DOI] [PubMed]
- 16.Mazurak N., Gü Nther A., Grau F., Muth E., Pustovoyt M., Bischoff S. Effects of a 48-h fast on heart rate variability and cortisol levels in healthy female subjects. Eur. J. Clin. Nutr. 2013;6732:401–406. doi: 10.1038/ejcn.2013.32. [DOI] [PubMed] [Google Scholar]; Mazurak N, Gü Nther A, Grau F, Muth E, Pustovoyt M, Bischoff S, et al. Effects of a 48-h fast on heart rate variability and cortisol levels in healthy female subjects. Eur J Clin Nutr. 2013; 6732: 401–6. [DOI] [PubMed]
- 17.Cansel M., Taşolar H., Yağmur J., Ermiş N., Açıkgöz N., Eyyüpkoca F. The effects of Ramadan fasting on heart rate variability in healthy individuals: a prospective study. Anadolu Kardiyol. Derg. 2014;14:413–416. doi: 10.5152/akd.2014.5108. [DOI] [PubMed] [Google Scholar]; Cansel M, Taşolar H, Yağmur J, Ermiş N, Açıkgöz N, Eyyüpkoca F, et al. The effects of Ramadan fasting on heart rate variability in healthy individuals: a prospective study. Anadolu Kardiyol Derg. 2014; 14: 413–6. [DOI] [PubMed]
- 18.Ahn J.H., Kong M. The relationship among pulse wave velocity, ankle-brachial pressure index and heart rate variability in adult males. Korean J. Fam. Med. 2011;32:406–411. doi: 10.4082/kjfm.2011.32.7.406. [DOI] [PMC free article] [PubMed] [Google Scholar]; Ahn JH, Kong M. The relationship among pulse wave velocity, ankle-brachial pressure index and heart rate variability in adult males. Korean J Fam Med. 2011; 32: 406–11. [DOI] [PMC free article] [PubMed]
- 19.Gerhart H., Tai Y.L., Fennell C., Mayo X., Kingsley J.D. Autonomic modulation in older women: using resistance exercise as a countermeasure. Int. J. Exerc. Sci. 2017:1–10. doi: 10.70252/VJFF5546. [DOI] [PMC free article] [PubMed] [Google Scholar]; Gerhart H, Tai YL, Fennell C, Mayo X, Kingsley JD. Autonomic modulation in older women: using resistance exercise as a countermeasure. Int J Exerc Sci. 2017;:1–10. [DOI] [PMC free article] [PubMed]
- 20.Zulfiqar U., Jurivich D.A., Gao W., Singer D.H. Relation of high heart rate variability to healthy longevity. Am. J. Cardiol. 2010;105:1181–1185. doi: 10.1016/j.amjcard.2009.12.022. [DOI] [PubMed] [Google Scholar]; Zulfiqar U, Jurivich DA, Gao W, Singer DH. Relation of High Heart Rate Variability to Healthy Longevity. Am J Cardiol. 2010; 105: 1181–5. [DOI] [PubMed]
- 21.Soares-Miranda L., Sattelmair J., Chaves P., Duncan G.E., Siscovick D.S., Stein P.K. Physical activity and heart rate variability in older adults: the cardiovascular health study. Circulation. 2014;129:2100–2110. doi: 10.1161/CIRCULATIONAHA.113.005361. [DOI] [PMC free article] [PubMed] [Google Scholar]; Soares-Miranda L, Sattelmair J, Chaves P, Duncan GE, Siscovick DS, Stein PK, et al. Physical activity and heart rate variability in older adults: The cardiovascular health study. Circulation. 2014; 129: 2100–10. [DOI] [PMC free article] [PubMed]
- 22.Martins-Pinge M.C. Cardiovascular and autonomic modulation by the central nervous system after aerobic exercise training. Braz. J. Med. Biol. Res. 2011;44:848–854. doi: 10.1590/s0100-879x2011007500102. [DOI] [PubMed] [Google Scholar]; Martins-Pinge MC. Cardiovascular and autonomic modulation by the central nervous system after aerobic exercise training. Brazilian J Med Biol Res. 2011; 44: 848–54. [DOI] [PubMed]
- 23.Rodrigues JAL, Santos BC, Medeiros LH, Gonçalves TCP, Júnior CRB. Effects of different periodization strategies of combined aerobic and strength training on heart rate variability in older women. J. Strength Cond. Res. 2019. Feb 6. DOI: 10.1519/JSC.0000000000003013 [(Epub ahead of print)]. [DOI] [PubMed]
- 24.Cipryan L., Laursen P.B., Plews D.J. Cardiac autonomic response following high-intensity running work-to-rest interval manipulation. Eur. J. Sport Sci. 2016;16:808–817. doi: 10.1080/17461391.2015.1103317. [DOI] [PubMed] [Google Scholar]; Cipryan L, Laursen PB, Plews DJ. Cardiac autonomic response following high-intensity running work-to-rest interval manipulation. Eur J Sport Sci. 2016; 16: 808–17. [DOI] [PubMed]
- 25.Lima-Silva A.E., Bertuzzi R.C.M., Pires F.O., Fronchetti L., Gevaerd M.S., De-Oliveira F.R. A low carbohydrate diet affects autonomic modulation during heavy but not moderate exercise. Eur. J. Appl. Physiol. 2010;108:1133–1140. doi: 10.1007/s00421-009-1329-6. [DOI] [PubMed] [Google Scholar]; Lima-Silva AE, Bertuzzi RCM, Pires FO, Fronchetti L, Gevaerd MS, De-Oliveira FR. A low carbohydrate diet affects autonomic modulation during heavy but not moderate exercise. Eur J Appl Physiol. 2010; 108: 1133–40. [DOI] [PubMed]
- 26.Zouhal H., Jacob C., Delamarche P., Gratas-Delamarche A. Catecholamines and the effects of exercise, training and gender. Sports Med. 2008;38:401–423. doi: 10.2165/00007256-200838050-00004. [DOI] [PubMed] [Google Scholar]; Zouhal H, Jacob C, Delamarche P, Gratas-Delamarche A. Catecholamines and the effects of exercise, training and gender. Sports Med. 2008; 38: 401–23. [DOI] [PubMed]
- 27.Solianik R., Sujeta A., Terentjevienė A., Skurvydas A. Effect of 48 h fasting on autonomic function, brain activity, cognition, and mood in amateur weight lifters. Biomed. Res. Int. 2016;2016:1–8. doi: 10.1155/2016/1503956. [DOI] [PMC free article] [PubMed] [Google Scholar]; Solianik R, Sujeta A, Terentjevienė A, Skurvydas A. Effect of 48 h Fasting on Autonomic Function, Brain Activity, Cognition, and Mood in Amateur Weight Lifters. Biomed Res Int. 2016; 2016: 1–8. [DOI] [PMC free article] [PubMed]
- 28.Cao L., Graham S.L., Pilowsky X.P.M. Carbohydrate ingestion induces sex-specific cardiac vagal inhibition, but not vascular sympathetic modulation, in healthy older women. Am. J. Phys. Regul. Integr. Comp. Phys. 2016;311:49–56. doi: 10.1152/ajpregu.00486.2015. [DOI] [PubMed] [Google Scholar]; Cao L, Graham SL, Pilowsky XPM. Carbohydrate ingestion induces sex-specific cardiac vagal inhibition , but not vascular sympathetic modulation, in healthy older women. Am J Physiol Regul Integr Comp Physiol. 2016; 311: 49–56. [DOI] [PubMed]
- 29.Cunnane S., Nugent S., Roy M., Courchesne-Loyer A., Croteau E., Tremblay S. Brain fuel metabolism, aging, and Alzheimer's disease. Nutrition. 2011;27:3–20. doi: 10.1016/j.nut.2010.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]; Cunnane S, Nugent S, Roy M, Courchesne-Loyer A, Croteau E, Tremblay S, et al. Brain fuel metabolism, aging, and Alzheimer's disease. Nutrition. 2011; 27: 3–20. [DOI] [PMC free article] [PubMed]
- 30.Kimura I., Inoue D., Maeda T., Hara T., Ichimura A., Miyauchi S. Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein-coupled receptor 41 (GPR41) Proc. Natl. Acad. Sci. U. S. A. 2011;108:8030–8035. doi: 10.1073/pnas.1016088108. [DOI] [PMC free article] [PubMed] [Google Scholar]; Kimura I, Inoue D, Maeda T, Hara T, Ichimura A, Miyauchi S, et al. Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein-coupled receptor 41 (GPR41). Proc Natl Acad Sci U S A. 2011; 108: 8030–5. [DOI] [PMC free article] [PubMed]
- 31.Kallioinen N., Hill A., Horswill M.S., Ward H.E., Watson M.O. Sources of inaccuracy in the measurement of adult patients' resting blood pressure in clinical settings: a systematic review. J. Hypertens. 2017;35:421–441. doi: 10.1097/HJH.0000000000001197. [DOI] [PMC free article] [PubMed] [Google Scholar]; Kallioinen N, Hill A, Horswill MS, Ward HE, Watson MO. Sources of inaccuracy in the measurement of adult patients' resting blood pressure in clinical settings: a systematic review. J Hypertens. 2017; 35: 421–41. [DOI] [PMC free article] [PubMed]
- 32.Kearney M.T., Stubbs T.A., Cowley A.J., Macdonald I.A. A carbohydrate meal attenuates the forearm vasoconstrictor response to lower body subatmospheric pressure in healthy young adults. Clin. Auton. Res. 1997;7:285–291. doi: 10.1007/BF02267719. [DOI] [PubMed] [Google Scholar]; Kearney MT, Stubbs TA, Cowley AJ, Macdonald IA. A carbohydrate meal attenuates the forearm vasoconstrictor response to lower body subatmospheric pressure in healthy young adults. Clin Auton Res. 1997; 7: 285–91. [DOI] [PubMed]




