Abstract
Objective
Soluble Tumor Necrosis Factor Receptor II (sTNFR2) is used as a biomarker to study cardiovascular disease (CVD) in diverse populations. TNF inhibitors (TNFi's) are a common treatment for inflammatory conditions. The objective of this study was to examine whether TNFi use impacts measured sTNFR2 levels.
Methods
We studied blood samples from a cohort of RA patients with clinical data and high sensitivity-C-reactive protein (hsCRP) measurements. To assess for interference, we tested the entire cohort for the expected positive correlation between sTNFR2 and TNFi using Pearson correlations. We then performed Pearson correlations between sTNFR2 and TNFi and sequentially removed subjects on adalimumab, etanercept, and infliximab; if interference was occurring, no correlation would be observed between hsCRP and sTNFR2, and correlation would be restored by removing subjects on the treatment causing the interference.
Results
We studied 190 subjects, 84.2% female, 73.4% anti-CCP positive. All subjects with sTNFR2 level exceeding measurable level were on etanercept. The expected positive correlation between hsCRP and sTNFR2 was not observed when assessing the entire cohort, r = 0.05, p = 0.51. However, the expected correlation was restored only after excluding subjects on etanercept, r = 0.46, p < 0.0001, and not adalimumab or infliximab. ELISA for sTNFR2 was performed using etanercept only and demonstrated direct binding to sTNFR2.
Conclusions
Our data identified interference between etanercept and the TNFR2 assay. Of the TNFi's, only etanercept has a TNF-binding domain modeled after TNFR2. These data should be considered when designing studies using sTNFR2 in populations where etanercept is a treatment option.
Keywords: Rheumatoid arthritis, Cardiovascular, Inflammation, Tumor necrosis factor inhibitor (TNFi), High sensitivity C-reactive protein (hsCRP), Biomarker
1. Introduction
Soluble tumor necrosis factor receptor II (sTNFR2) has been widely studied as a biomarker of inflammation to assess cardiovascular (CV) risk in the general population, and to study inflammatory conditions such as rheumatoid arthritis [[1], [2], [3]]. TNF-alpha, a ligand of TNFR2, plays an important role in the upregulation leukocyte adhesion molecules on the endothelium, which causes enhanced interactions with leukocyte and contribute to inflammatory effects [4]. TNFR2, whose expression is also upregulated in synovial membrane of RA patients, is also found to promote T-cell co-stimulation, which is thought to be an important factor in the pathogenesis of RA [2,4,5]. Effective RA therapies target TNF-alpha, with five TNF inhibitors (TNFi's) available on the market. In the vasculature, TNF-alpha is associated with plaque vulnerability [6] and elevated TNF-alpha levels are associated with increased CV risk as measured by coronary artery calcification, independent of traditional risk factors [7].
TNF-alpha degrades rapidly in the serum, and thus sTNFR2, which is more stable, has been the biomarker of choice to approximate TNF-alpha levels [8] for studies of cardiovascular and inflammatory conditions. Soluble TNFR2 expression is correlated with TNF-alpha levels and can be used as a proxy for inflammation [3,9,10]. As this marker is increasingly being used in studies for both diagnosis and prognosis of both CVD and RA, it is important to understand factors that can affect levels of TNFR2. The purpose of this study is to determine whether specific TNFi therapies may interfere with the level of measured sTNFR2 in RA.
2. Methods
2.1. Study population
We performed a cross-sectional study using samples from the Brigham and Women's Hospital Rheumatoid Arthritis Sequential Study (BRASS). BRASS is a prospective cohort study of RA with detailed clinical data, collected every 6 months; high sensitivity C-reactive protein (hsCRP) is measured annually [11]. Peripheral blood samples are also collected annually, and plasma are isolated using standard clinical testing protocols and stored at −80 °C [11]. Since a focus of BRASS is to study treatment response, RA treatment data are collected at each visit from the electronic health records, the treating rheumatologist, and the patient. The study population included in this study comprise of 190 subjects who were part of a cardiovascular sub-study of RA subjects.
2.2. HsCRP and sTNFR2 measurements
HsCRP was measured in all subjects at the clinical laboratory of Boston Children's Hospital, Boston, MA using standard methods [12]. sTNFR2 levels were measured using the Quantikine ELISA Human TNF RII/TNFRSF1B Immunoassay (R&D Systems, Inc., Minneapolis, MN).
2.3. Statistical analysis
To first determine whether TNFi may interfere with sTNFR2 levels, we tested the correlations between hsCRP and sTNFR2 in all subjects. The expected relationship is a positive correlation between hsCRP and sTNFR2. We hypothesized that this known correlation would be absent or attenuated if there was interference by a TNFi. To determine which treatment was driving interference, we performed a Pearson correlation between hsCRP and sTNFR2 in the entire population, and the same population with patients on specific TNFi's excluded. We performed the analysis by subtracting out the 3 main TNFi's used in our study population: etanercept, adalimumab, and infliximab. If a treatment was associated with interference, when subjects on the treatment were removed from the analysis, we anticipated restoration of the expected positive correlation between hsCRP and sTNFR2.
SolubleTNFR2 level was categorized into three groups: (1) >46 and ≤ 10,000 pg/mL, typical range; (2) >10,000 pg/mL and ≤100,000 pg/mL, samples requiring 200-fold dilution; (3) >100,000 pg/mL, levels exceeding measurable values. The distribution of subjects on the different TNFi's in each group was compared using a chi-square test.
Based on correlation and chi square analysis above, we identified that one TNFi was associated with interference. To directly test for cross-reactivity, we performed the sTNFR2 ELISA in increasing dilutions of the drug etanercept, from 50 mg/mL, the concentration in the syringe, to concentrations within the assay range of 5–500 pg/mL. The correlation between sTNFR2 concentration with concentration of TNFi was tested using the Pearson correlation.
All programming analyses were performed using SAS 9.4 (SAS Institute Inc., Cary, NC) and Rstudio Version 1.0.143 (2009–2016 RStudio, Inc., Boston, MA). P-value of less than 0.05 represents statistical significance. All aspects of this study were approved by the Partners Healthcare Institutional Review Board.
3. Results
We studied 190 RA subjects, mean age 59.6 years, 84.2% female, mean disease duration 18.9 years, and median DAS28 of 3.65; 73.5% of subjects were RF positive, 73.4% anti-CCP positive, and median hsCRP 17.9 mg/dL. At time of blood draw, subjects were on the following treatments: methotrexate (96%), sulfasalazine (3.7%), leflunomide (5.7%), and hydroxychloroquine (8.9%). Biologic DMARD use at time of blood draw include 13.7% on adalimumab, 26.3% on etanercept, 7.9% on infliximab, 0.5% on certolizumab, 1.1% on tocilizumab, 6.8% on abatacept, and 2.6% on rituximab.
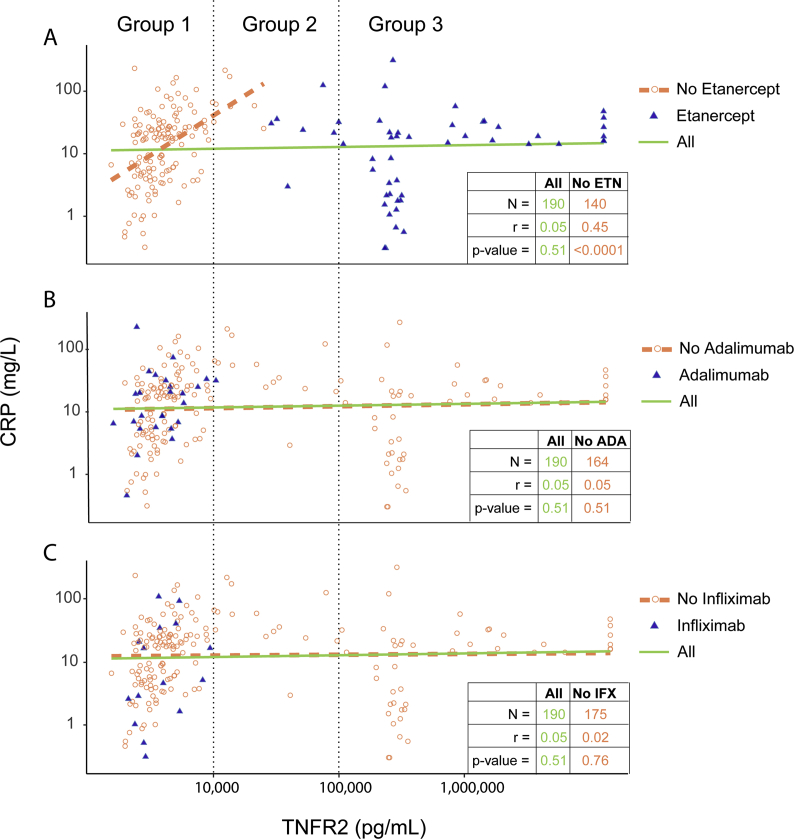
All subjects with sTNFR2 level exceeding measurable level (Group 3) were on etanercept therapy, and in some despite low levels of hsCRP (Fig. 1A). We did not observe the expected correlation between hsCRP and sTNFR2 among in the total study population, (green line), r = 0.05, p = 0.51 (Fig. 1A). After excluding subjects on etanercept the expected correlation between hsCRP and sTNFR2 was observed, r = 0.46, p < 0.0001 (Fig. 1A). No significant change in the correlation between hsCRP and sTNFR2 was observed after excluding subjects on adalimumab (r = 0.05, p = 0.51) or infliximab (r = 0.02, p = 0.76) (Fig. 1B and C, respectively). Excluding other DMARDs including abatacept, methotrexate, leflunomide, and hydroxychloroquine also did not result in a significant change in the correlation.
Fig. 1.
Correlation between plasma sTNFR2, hsCRP, and from subjects on different TNFi's. Scatter plots of the 190 RA subjects illustrate influence of etanercept (A), adalimumab (B), and infliximab (C) on the correlation between hsCRP and sTNFR2. Both hsCRP and sTNFR2 levels are on a natural logarithm scale. (A–C) Blue triangles and orange circles represent subjects on and not on the corresponding TNFi. Green solid lines represent linear regression smooth for all subjects; Count (N), Pearson correlation (r) and p-value are shown in the bottom right table in green. Orange dotted lines represent linear regression smooth for subjects not on the corresponding TNFi; Count (N), Pearson correlation (r) and p-value are shown in the bottom right table in orange.
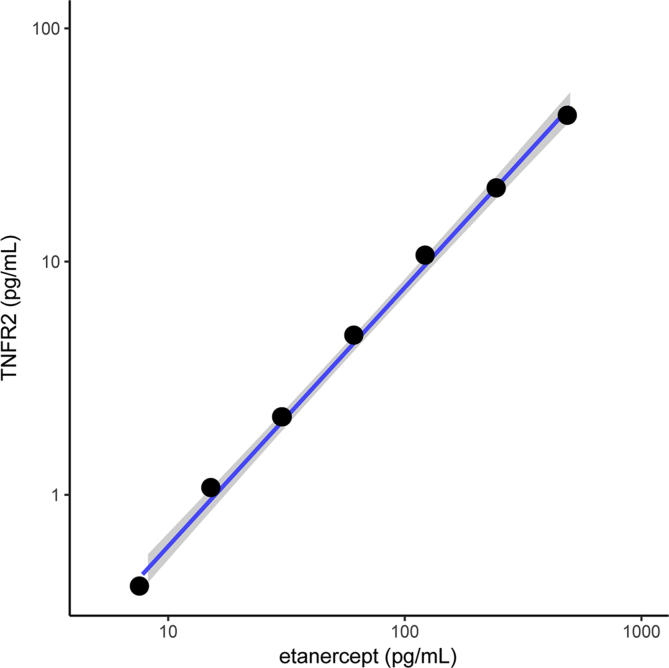
Since etanercept appeared to be the cause of interference, we next directly performed the sTNFR2 ELISA with increasing dilutions of etanercept. As undiluted etanercept yielded sTNFR2 levels beyond the standard calibration curve, serial dilutions were performed to obtain interpretable sTNFR2 levels within the calibration curve ranging from 5 pg/mL to 500 pg/mL. A linear relationship was observed between etanercept concentration and sTNFR2 levels measured by ELISA (Fig. 2).
Fig. 2.
Correlation between etanercept concentration and ELISA results for sTNFR2 detection, log10 transformed.
4. Discussions
We observed that etanercept can lead to spuriously high measured sTNFR2 values in subjects with RA. We demonstrated this phenomenon in two ways. First, when studying the correlation between a standard marker of inflammation, hsCRP with sTNFR2, we did not observe the expected positive correlation. However, when subjects on etanercept were excluded from the analysis, we observed the expected positive correlation between the two inflammatory markers. Excluding other TNFi's did not restore the expected correlation between hsCRP and sTNFR2. Second, using only etanercept in the sTNFR2 ELISA showed evidence of binding. In fact, a 1 × 107-fold dilution was required to achieve levels in the measurable range.
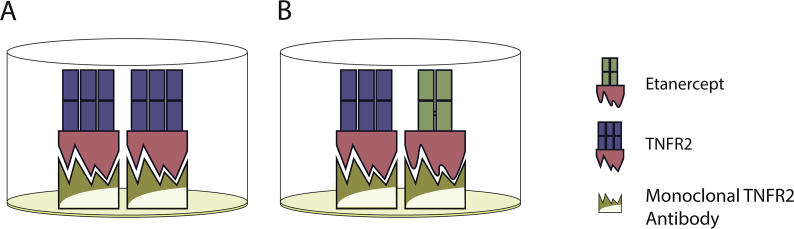
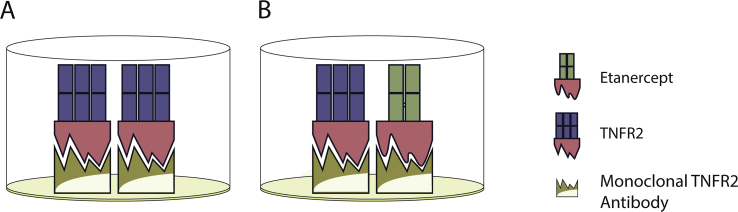
TNF-alpha has also been shown to play a key role in the pathogenesis and amplified inflammatory response characteristic of RA [3]. TNFR1 and TNFR2 are two types of TNF receptors that are immunologically distinct and mediate different signal transduction pathways. TNFR2 (also known as p75 or TNFRSF1B) expression has a higher correlation with autoimmune conditions than TNFR1 [10]. Soluble TNF binding proteins, found in human serum, were found to detect and bind to (thus neutralizing biological activities of) TNF-alpha (Fig. 3A).
Fig. 3.
Schematic illustration of interaction between sTNFR2 and etanercept binding to the sTNFR2 assay. ELISA test showing A) sTNFR2 and B) etanercept binding to the monoclonal TNFR2 antibody. sTNFR2 and etanercept share the same soluble extracellular TNFR2 portion (colored in red), which is recognized by the monoclonal TNFR2 antibody.
Etanercept is a dimeric fusion protein of the soluble TNFR linked to a Fc portion of an immunoglobulin gamma1 (IgG1) (Fig. 3B). With a TNF-binding domain modeled after sTNFR2, etanercept binds to the monoclonal TNFR2 antibody found in commercial ELISA kit, in a similar fashion as sTNFR2 (Supplementary Fig. 1B). This study focused on sTNFR2 because of its use in prior studies of rheumatic disease and CV risk, and thus we cannot comment on potential interference on sTNFR1.
There is a growing body of evidence showing that inflammation as measured by biomarkers, such as sTNFR2, is a major driving factor underlying cardiovascular disease in the general population, as well as in RA [3,13]. Our study demonstrates the need to consider etanercept treatment when designing studies using sTNFR2.
Funding
The study was funded by National Heart, Lung, and Blood Institute R01 HL127118, and the Harold and DuVal Bowen Fund.
Competing interests
NY, JH, MF, CI, MEW, NR, NS, GB, and KPL have no disclosures.
Declaration of interest
None.
Acknowledgement
We would like to thank Patricia Greene, RN and Frances Griffin, RN for their assistance with the study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.plabm.2019.e00122.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Fig. s1.
References
- 1.Vasan R.S. Biomarkers of cardiovascular disease: molecular basis and practical considerations. Circulation. 2010;113:2335–2362. doi: 10.1161/CIRCULATIONAHA.104.482570. [DOI] [PubMed] [Google Scholar]
- 2.Bluml S., Scheinecker C., Smolen J.S., Redlich K. Targeting TNF receptors in rheumatoid arthritis. Int. Immunol. 2012;24:275–281. doi: 10.1093/intimm/dxs047. [DOI] [PubMed] [Google Scholar]
- 3.Karlson E.W., Chibnik L.B., Tworoger S.S., Lee I.M., Buring J.E., Shadick N.A. Biomarkers of inflammation and development of rheumatoid arthritis in women from two prospective cohort studies. Arthritis Rheum. 2009;60:641–652. doi: 10.1002/art.24350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chakrabarti S., Davidge S.T. Estradiol modulates tumor necrosis factor-induced endothelial inflammation: role of tumor necrosis factor receptor 2. J. Vasc. Res. 2013;50:21–34. doi: 10.1159/000342736. [DOI] [PubMed] [Google Scholar]
- 5.Doss G.P., Agoramoorthy G., Chakraborty C. TNF/TNFR: drug target for autoimmune diseases and immune-mediated inflammatory diseases. Front Biosci (Landmark Ed) 2014;19:1028–1040. doi: 10.2741/4265. [DOI] [PubMed] [Google Scholar]
- 6.Welsh P., Grassia G., Bota S., Sattar N., Maffia P. Targeting inflammation to reduce cardiovascular disease risk: a realistic clinical prospect? Br. J. Pharmacol. 2017;174:3898–3913. doi: 10.1111/bph.13818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rho Y.H., Chung C.P., Oeser A., Solus J., Asanuma Y., Sokka T. Inflammatory mediators and premature coronary atherosclerosis in rheumatoid arthritis. Arthritis Rheum. 2009;61:1580–1585. doi: 10.1002/art.25009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dibbs Z., Thornby J., White B.G., Mann D.L. Natural variability of circulating levels of cytokines and cytokine receptors in patients with heart failure: implications for clinical trials. J. Am. Coll. Cardiol. 1999;33:1935–1942. doi: 10.1016/s0735-1097(99)00130-8. [DOI] [PubMed] [Google Scholar]
- 9.Cope A.P., Aderka D., Doherty M., Engelmann H., Gibbons D., Jones A.C. Increased levels of soluble tumor necrosis factor receptors in the sera and synovial fluid of patients with rheumatic diseases. Arthritis Rheum. 1992;35:1160–1169. doi: 10.1002/art.1780351008. [DOI] [PubMed] [Google Scholar]
- 10.Steiner G., Studnicka-Benke A., Witzmann G., Hofler E., Smolen J. Soluble receptors for tumor necrosis factor and interleukin-2 in serum and synovial fluid of patients with rheumatoid arthritis, reactive arthritis and osteoarthritis. J. Rheumatol. 1995;22:406–412. [PubMed] [Google Scholar]
- 11.Iannaccone C.K., Lee Y.C., Cui J., Frits M.L., Glass R.J., Plenge R.M. Using genetic and clinical data to understand response to disease-modifying anti-rheumatic drug therapy: data from the Brigham and Women's Hospital Rheumatoid Arthritis Sequential Study. Rheumatology. 2011;50:40–46. doi: 10.1093/rheumatology/keq263. [DOI] [PubMed] [Google Scholar]
- 12.Aziz N., Fahey J.L., Detels R., Butch A.W. Analytical performance of a highly sensitive C-reactive protein-based Immunoassay and the effects of laboratory variables on levels of protein in blood. Clin. Diagn. Lab. Immunol. 2003;10:652–657. doi: 10.1128/CDLI.10.4.652-657.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zakynthinos E., Pappa N. Inflammatory biomarkers in coronary artery disease. J. Cardiol. 2009;53:317–333. doi: 10.1016/j.jjcc.2008.12.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.