Highlights
-
•
Campylobacter primarily accounts for outbreaks in humans via consumption of contaminated poultry products or water.
-
•
Campylobacter jejuni is a commensal of the gastrointestinal tract.
-
•
C. jejuni is a zoonotic pathogen with global distribution.
-
•
C. jejuni is the most common cause of bacterial foodborne diseases.
-
•
Campylobacter should be considered if spiral rod-shaped gramnegative bacilli are detected.
Keywords: Campylobacter jejuni, Bacteremia, Enteritis, Asplenia
Abstract
Campylobacter bacteremia is an unusual presentation of a common infectious disease such as enteritis. We provide key teaching points about its presentation, risk factors, diagnosis and treatment.
Introduction
Campylobacter spp. comprises spiral, rod-shaped or curved gram-negative bacteria, which are commensals in the gastrointestinal tract of many wild and domesticated animals as well as all avian species fit for human consumption. Campylobacter is a zoonotic pathogen with global distribution and is the most common cause of bacterial foodborne diseases. The documented environmental reservoirs and sources of human infection are tap, bore, and pond water, and the recognized routes of transmission are consumption of undercooked or contaminated food, mainly chicken, or water; contact with infected animals; and person-to-person transmission (fecal–oral or via fomites). Several Campylobacter species have been implicated in human infection, with 95% of infections due to C. jejuni, C. coli, and C. fetus [1].
Case report
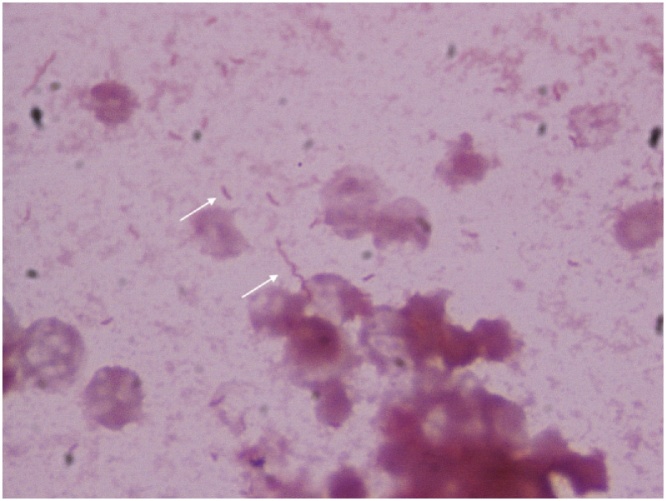
A 39-year-old woman was referred to our clinic with the complaint of multiple episodes of watery diarrhea accompanied with fever, chills, myalgia, and abdominal bloating for 72 h. She had a history of portal hypertension due to cavernous degeneration of the portal vein, and she underwent a splenorenal shunt with splenectomy at the age of 13 years. On examination, the patient was found to be febrile (39.2° C); with tachycardia (118 bpm) and hypotension (88/55 mmHg), along with ascites and abdominal distension, and increased bowel sounds. Her blood test results revealed a hemoglobin level of 8.7 g/ dL and a white blood cell count of 8.1 × 103 cells/μL with 77% neutrophils and 10% lymphocytes. The patient’s liver enzyme levels and kidney function were within normal limits. Her bloodstream cultures were positive for multiple curved and spiral gram-negative bacilli after 16 h of incubation, and was finally identified as Campylobacter jejuni using MALDI-TOF MS (Fig. 1, arrows). Biochemical tests indicated an oxidase, catalase and hippurate negative, and indoxyl acetate-positive bacterial species, corresponding to Campylobacter jejuni. Bacterial isolation from stool was not possible due to lack of appropriate media at our institute. She received imipenem 500 mg every 6 h for 14 days because ciprofloxacin, cefotaxime and cefepime resistance. She responded well to this treatment regimen. Although our patient was not receiving immunosuppression, her portal hypertension and asplenic condition could have led to C. jejuni bacteremia.
Fig. 1.
Gram stain of blood culture at ×100 magnification, with multiple curved and spiral Gram-negative rods with phenotypic testing positive for Campylobacter jejuni.
Discussion
Campylobacteriosis is the most frequently notified foodborne illness in Europe and the second most notified one in the United States [2]. In Latin America, C. jejuni and C. coli are the causative agents in up to 25% of cases of diarrhea, with asymptomatic carrier status being common [3]. Other emerging species capable of causing the disease in immunosuppressed patients are C. lari, C. upsaliensis, C. ureolyticus, and C. hyointestinalis [1]. A significant risk factor reported in the United States and Europe for campylobacteriosis is international travel [4], appearing to be mainly associated with travel to Southeast and South Asia, Africa, and Latin America [5].
Campylobacteriosis is usually characterized by a self-limiting enteric disease with an incubation period of 2–5 days, and on average, the symptoms last 7 days, similar to that of other enteric pathogens; diarrhea may be watery, is typically inflammatory, and may be associated with bleeding. Campylobacteriosis rarely causes bacteremia (in <1% cases of intestinal infections), with C. jejuni and C. coli found be the cause in most cases [1]. However, according to some studies, Campylobacter bacteremia is historically related to C. fetus and less frequently associated with enteritis; up to 93% of cases are related to well-recognized risk factors such as advanced age, chronic liver disease, human immunodeficiency virus infection, malignancy, and humoral immunodeficiency [6], with a mortality rate of 4% – 16%. In a French study, C. fetus was the causative pathogen in up to 53% of Campylobacter bacteremia cases, causing cellulitis (19%), endovascular infection (13%), and medical device-related infections (7%); additionally, this study found no identified causes of immunosuppression in up to 21% of the cases [7]. However, a later study in Spain found that C. jejuni was the most frequently isolated species (63%) in patients with underlying diseases and in abdominal and extraintestinal sources of community-acquired infections [8]. Conversely, a nationwide study of C. jejuni and C. coli bacteremia in Finland found that the disease occurred in young individuals of domestic origin and without any significant underlying disease [9], suggesting an etiological variation and presence of different risk factors according to the geographic location. Meningitis, extraoral abscesses, myocarditis, hepatitis, and cholecystitis are the well-recognized extraintestinal manifestations of the disease [1]. Interestingly, despite treatment, some bacteremia patients develop sequelae after infection, such as Guillain-Barre syndrome, reactive arthritis, and irritable bowel syndrome; furthermore, there is some evidence linking with other gastrointestinal disorders such as functional dyspepsia [10].
Campylobacter infection is diagnosed through cultures, and the identification is confirmed using phenotypic methods that include dark-field microscopy to detect motility and morphology, Gram staining and biochemical methods to detect oxidase and catalase production, hydrolysis of sodium hippurate, hydrogen sulfide production on triple sugar iron agar in the presence of 1% cysteine hydrochloride, and detectable growth on brain heart infusion agar plates incubated at 25 °C and 42 °C [1]. Molecular methods are also useful for rapid diagnosis, using sequencing techniques or genus- or species-specific PCR amplification of the 16S rRNA gen, and multiplex PCR analysis [11]. Many cases of C. jejuni gastroenteritis are self-limiting and do not require antibacterial treatment, but bacteremia-associated cases are generally treated with antimicrobials. Antibacterial susceptibility is determined using the Kirby-Bauer disk diffusion method or E-test, with increasing C. jejuni resistance to tetracycline, ampicillin, macrolides and quinolones; this is at least partially related to veterinary use of quinolones [12,13]. Additionally, bactericidal activity of cefotaxime is lower than that of amoxicillin or imipenem, and treatment failures with third-generation cephalosporins have been reported [14].
Macrolides are the preferred choice of therapy, and ciprofloxacin has been recommended for the treatment of human infections caused by macrolide-resistant Campylobacter species [1]. Inappropriate or delayed appropriate antimicrobial treatment does not seem to be associated with an increased mortality [8,9]. A mortality rate of 15% is associated with C. fetus bacteremia cases, which is most likely due to an immunosuppressed population [7]. Measures to prevent Campylobacter zoonotic infections include vaccinating poultry animals, improving hygiene practices throughout the poultry production chain processes, controlling the slaughtering procedures, and educating the public [1].
In summary, campylobacteriosis is an established cause of enteritis, and secondary bacteremia is unusual. However, bacteremia may manifest due to underlying diseases and/or risk factors; thus, it should be considered in patients presenting with enteritis and an underlying disease, especially if spiral rod-shaped gram-negative bacilli are detected in the bloodstream.
Author contributions
The author contributed to design, drafting and revising the paper, and approval of the submitted and final versions.
Declarations of interest
The author declare having no conflict of interests.
Compliance with ethical standards
This article does not contain any studies with human participants or animals performed by any of the authors
Informed consent
Informed consent was obtained from the patient to publish the case and accompanying images.
Funding source
This study did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethical approval
This report was approved by the ethics and research committees of the Institutions.
CRediT authorship contribution statement
Ramírez Isabel: Conceptualization, Data curation, Investigation, Methodology, Writing - original draft, Writing - review & editing.
References
- 1.Kaakoush N.O., Castaño-Rodriguez N., Mitchell H.M., Man S.M. Global epidemiology of Campylobacter infection. Clin Microbiol Rev. 2015;28(3):687–720. doi: 10.1128/CMR.00006-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Facciolà A., Riso R., Avventuroso E., Visalli G., Delia S.A., Laganà P. Campylobacter: from micobiology to prevention. J Prev Med Hyg. 2017;58(2):E79–E92. [PMC free article] [PubMed] [Google Scholar]
- 3.Fernández H. Campylobacter and campylobacteriosis: a view from South America. Rev Peru Med Exp Salud Publica. 2011;28(1):121–127. doi: 10.1590/s1726-46342011000100019. [DOI] [PubMed] [Google Scholar]
- 4.Domingues A.R., Pires S.M., Halasa T., Hald T. Source attribution of human campylobacteriosis using a meta-analysis of case-control studies of sporadic infections. Epidemiol Infect. 2012;140:970–981. doi: 10.1017/S0950268811002676. [DOI] [PubMed] [Google Scholar]
- 5.Mughini-Gras L., Smid J.H., Wagenaar J.A., De Boer A., Havelaar A.H., Friesema I.H. Campylobacteriosis in returning travelers and potential secondary transmission of exotic strains. Epidemiol Infect. 2014;142 doi: 10.1017/S0950268813002069. 1277–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pigrau C., Bartolome R., Almirante B., Planes A.M., Gavalda J., Pahissa A. Bacteremia due to Campylobacter species: clinical findings and antimicrobial susceptibility patterns. Clin Infect Dis. 1997;25:1414–1420. doi: 10.1086/516127. [DOI] [PubMed] [Google Scholar]
- 7.Pacanowski J., Lalande V., Lacombe K., Boudraa C., Lesprit P., Legrand P. Campylobacter bacteremia: clinical features and factors associated with fatal outcome. Clin Infect Dis. 2008;47(6):790–796. doi: 10.1086/591530. [DOI] [PubMed] [Google Scholar]
- 8.Fernández-Cruz A., Muñoz P., Mohedano R., Valerio M., Marin M., Alcalá L. Campylobacter bacteremia: clinical characteristics, incidence, and outcome over 23 years. Medicine. 2010;89(5):319–330. doi: 10.1097/MD.0b013e3181f2638d. [DOI] [PubMed] [Google Scholar]
- 9.Feodoroff B., Lauhio A., Ellström P., Rautelin H. A nationwide study of Campylobacter jejuni and Campylobacter coli bacteremia in Finland over a 10-year period, 1998-2007, with special reference to clinical characteristics and antimicrobial susceptibility. Clin Infect Dis. 2011;53(8):e99–e106. doi: 10.1093/cid/cir509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Porter C.K., Gormley R., Tribble D.R., Cash B.D., Riddle M.S. The incidence and gastrointestinal infectious risk of functional gastrointestinal disorders in a healthy U.S. Adult population. Am J Gastroenterol. 2011;106(1):130–138. doi: 10.1038/ajg.2010.371. [DOI] [PubMed] [Google Scholar]
- 11.Spina A., Kerr K.G., Cormican M., Barbut F., Eigentler A., Zerva L. Spectrum of enteropathogens detected by the FilmArray GI panel in a multicentre study of community-acquired gastroenteritis. Clin Microbiol Infect. 2015;21(8):719–728. doi: 10.1016/j.cmi.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 12.Luangtongkum T., Jeon B., Han J., Plummer P., Logue C.M., Zhang Q. Antibiotic resistance in Campylobacter: emergence, transmission and persistence. Future Microbiol. 2009;4(2):189–200. doi: 10.2217/17460913.4.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Belanger A.E., Shryock T. Macrolide-resistant Campylobacter: the meat of the matter. J Antimicrob Chemother. 2007;60:715–723. doi: 10.1093/jac/dkm300. [DOI] [PubMed] [Google Scholar]
- 14.Francioli P., Herzstein J., Grob J.P., Vallotton J.J., Mombelli G., Glauser M.P. Campylobacter fetus subspecies fetus bacteremia. Arch Intern Med. 1985;145:289–292. [PubMed] [Google Scholar]