Abstract
Mycoplasma pneumoniae infection most commonly manifests as a mild respiratory illness and headache. Pneumonia occurs in approximately 10% of patients with respiratory symptoms. M. pneumoniae infection can also cause neurological and other extrapulmonary complications. In this case report we describe a 33-year-old Caucasian man presenting with headache and raised intracranial pressure, found to be a para-infectious complication of M. pneumoniae infection. Nasopharyngeal PCR was highly useful in facilitating early diagnosis, as IgM antibodies were negative during the early stages of illness. Azithromycin is the preferred agent for M. pneumoniae treatment. The addition of M. pneumoniae PCR to hospitals' rapid respiratory viral PCR panels could promote early directed therapy and antimicrobial stewardship.
Keywords: Mycoplasma pneumoniae, Atypical pneumonia, Raised intracranial pressure
1. Introduction
Mycoplasma pneumoniae is a common cause of atypical pneumonia that requires directed treatment. Diagnosis may be delayed in patients presenting with extrapulmonary symptoms, with potential for long-term morbidity and mortality. In this report we describe an unusual clinical manifestation of M. pneumoniae infection, initially presenting a diagnostic dilemma that was able to be resolved through multiplex PCR techniques.
2. Case
A 33-year-old Caucasian man presented to the Royal Darwin Hospital Emergency Department with a 4-day history of frontal headache, fever, dry cough and myalgia.
His medical history included viral meningitis at age 30 and a penicillin allergy (urticarial rash). He was on no regular medications and had not recently travelled outside Australia. No sick contacts were identified.
On examination the patient was persistently febrile to 40 °C. He maintained a Glasgow Coma Score of 15/15 but was clinically meningitic and unable to tolerate fundoscopy due to photophobia. There were no focal neurologic deficits and no rash, lymphadenopathy or hepatosplenomegaly. Chest auscultation revealed bronchial breath sounds in the right lower lung field. Otoscopy was unremarkable.
The white cell differential showed lymphopenia (0.5 × 109/L; reference interval [RI] 1.5–4.0 × 109/L). Multiple blood cultures, nasopharyngeal polymerase chain reaction (PCR) (for influenza A, B and respiratory syncytial virus), serologies for atypical pneumonia, melioidosis, flaviviruses and HIV, and urinary Legionella and Streptococcus pneumoniae antigen tests were negative.
Initial chest radiography demonstrated right lower zone opacity suggestive of consolidation (Fig. 1A). Non-contrast computed tomography of the brain was unremarkable.
Fig. 1.
A. Chest radiograph on day 1 of hospital admission, demonstrating consolidation in the posterior basal segment of the right lower lobe. B: Chest radiograph on day 3 of admission showing progressive, diffuse consolidation surrounding the right hilum and extending into the right lower lobe, with lesser consolidation surrounding the left hilum extending into the medial aspect of the left lower lobe.
Lumbar puncture revealed an elevated opening pressure of 270 mm H2O (RI less than 150 mm H2O) and was followed by a transient improvement in the headache. Cerebrospinal fluid (CSF) leukocyte count was 1 × 106/L and erythrocyte count 0 × 106/L, with a normal protein (0.26 g/L; RI 0.15–0.45 g/L) and glucose level (4.1 mmol/L; RI 2.7–4.2 mmol/L). CSF gram stain, multiplex PCR (FilmArray Meningitis/Encephalitis) and cryptococcal antigen tests were negative.
The patient received intravenous ceftriaxone and oral doxycycline as per the local community-acquired pneumonia protocol. However, over 72 hours he remained febrile with worsening headache, photophobia and hypoxia (requiring 6L oxygen via Hudson mask). The C-reactive protein level rose from 81 to 225 mg/L (RI 0–5 mg/L). Repeat chest radiography now showed bilateral opacities, extensive in the right lower zone (Fig. 1B). Antibiotics were escalated to intravenous azithromycin, meropenem and vancomycin.
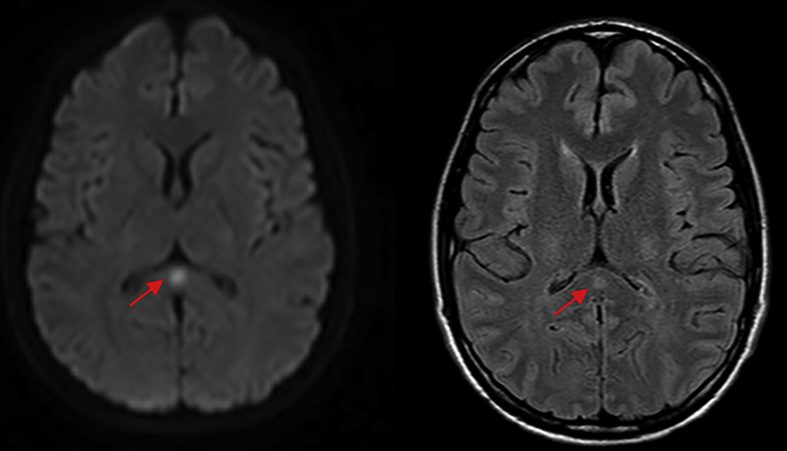
Magnetic resonance imaging of the brain revealed an isolated lesion in the splenium of the corpus callosum, with T2 hyperintensity and diffusion restriction (Fig. 2). The impression was of a ‘transient lesion of the splenium’, of possible para-infective aetiology. Nasopharyngeal swabs were then analysed more broadly using multiplex PCR (FilmArray Respiratory Panel), which flagged a positive M. pneumoniae PCR result.
Fig. 2.
Axial diffusion-weighted (left) and FLAIR magnetic resonance imaging of the brain, demonstrating a focal zone of diffusion restriction and hyperintensity occupying the central splenium of the corpus callosum, measuring approximately 7 × 5 mm (red arrows). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
The patient's condition improved following antibiotic change. He received a total of 5 days of intravenous azithromycin. He was discharged home after an 11-day hospital admission. Convalescent M. pneumoniae serology taken 10 days after admission demonstrated seroconversion to positive IgM (with negative IgG), confirming a diagnosis of acute M. pneumoniae infection. CSF PCR for M. pneumoniae was negative, favouring an immune-mediated meningitis over direct CSF infection. CSF oligoclonal bands and anti-myelin associated glycoprotein antibodies were also negative. The patient made a full recovery within 6 weeks.
3. Discussion
M. pneumoniae is a common cause of atypical pneumonia. Mycoplasmas lack a cell wall and are bound only by a cell membrane. The bacterium is therefore invisible on gram stain and beta-lactam antibiotics are ineffective against it. The absence of a cell wall also confers some protection from innate immunity, due to the absence of cell wall-derived stimulators such as lipopolysaccharide and peptidoglycan. Therefore, patients with M. pneumoniae infection classically demonstrate fewer physical signs than symptoms would suggest, absence of leukocytosis and patchy infiltrates rather than lobar consolidation on chest radiography.
M. pneumoniae most commonly causes a mild, self-limiting respiratory illness, including pharyngitis and acute bronchitis. Typical presenting symptoms are headache, fever and malaise, followed by an intractable dry cough. Coryzal symptoms and wheeze may occur in younger patients. Pneumonia is less common, affecting approximately 10% of patients with respiratory symptoms [1]. Extrapulmonary, ‘atypical’ manifestations of M. pneumoniae infection can include central nervous system (CNS) involvement, cold agglutinin haemolysis, skin eruptions, cardiac involvement and arthritis. CNS complications occur more frequently in children and are associated with significant morbidity and mortality. The pathogenesis is likely immune-mediated inflammation. Antibodies to M. pneumoniae may cross-react with myelin glycolipids [2]. Encephalitis (most common), aseptic meningitis, transverse myelitis, peripheral neuropathy, cerebellar ataxia, cranial nerve palsies and acute disseminated encephalomyelitis can occur. In one study of 61 patients with neurologic disease attributed to M. pneumoniae, 23% had severe sequelae and 8% died [3].
When available, nucleic acid amplification testing is now considered the diagnostic test of choice for M. pneumoniae infection, facilitating early diagnosis. Multiplex PCR assays using nasopharyngeal samples have been well-validated for M. pneumoniae [4]. Serologic testing can be used in addition or as an alternative to PCR testing. The gold standard for serologic diagnosis of M. pneumoniae is a fourfold rise in IgG antibody titres over time. However, this is generally impractical as it requires collection of acute and convalescent samples (approximately 4 weeks after illness onset). Alternatively, a single high titre of IgM antibodies can be diagnostic, as IgM antibodies rise earlier than IgG. IgM titres begin to rise 7–10 days after infection and peak after 3–4 weeks. One study of PCR using throat samples demonstrated a sensitivity of 82% and specificity of 99% among children with M. pneumoniae pneumonia, with IgG serology used as the reference standard [5]. We recommend a combination of PCR and IgM testing as the optimal approach for early diagnosis of M. pneumoniae infection.
Directed treatment for M. pneumoniae infection includes macrolides or doxycycline. In vitro studies have found azithromycin to be the most active drug [6]. Azithromycin is therefore recommended for confirmed M. pneumoniae infection, with intravenous administration indicated for severe disease. A 5-day course is adequate in most cases, as the organism can invade intracellularly and azithromycin has a long intracellular half-life. A 7- to 14-day course is usually necessary if using doxycycline or clarithromycin. However, treatment duration should be tailored to clinical response. Doxycycline is contraindicated in children under 8 years of age due to dental and bony side effects.
In conclusion, this case report aims to prompt clinicians to consider extrapulmonary M. pneumoniae infection in the differential diagnosis for patients presenting with acute neurologic and respiratory symptoms. We also propose that the routine addition of M. pneumoniae PCR to hospital rapid respiratory PCR panels may promote early directed therapy and antimicrobial stewardship.
Conflicts of interest
None to declare.
Acknowledgements
We thank all Top End Health Service clinical and laboratory staff involved in the patient's care. We particularly thank Prof Bart Currie, Dr Matthew O'Brien and A/Prof Robert Baird for their expertise and clinical input.
References
- 1.Mansel J.K., Rosenow E.C., 3rd, Smith T.F., Martin J.W., Jr. Mycoplasma pneumoniae pneumonia. Chest. 1989;95(3):639. doi: 10.1378/chest.95.3.639. [DOI] [PubMed] [Google Scholar]; Mansel JK, Rosenow EC 3rd, Smith TF, Martin JW Jr. Mycoplasma pneumoniae pneumonia. Chest. 1989;95(3):639. [DOI] [PubMed]
- 2.Meyer Sauteur P.M., Jacobs B.C., Spuesens E.B. Antibody responses to Mycoplasma pneumoniae: role in pathogenesis and diagnosis of encephalitis? PLoS Pathog. 2014;10(6) doi: 10.1371/journal.ppat.1003983. [DOI] [PMC free article] [PubMed] [Google Scholar]; Meyer Sauteur PM, Jacobs BC, Spuesens EB et al. Antibody responses to Mycoplasma pneumoniae: role in pathogenesis and diagnosis of encephalitis? PloS Pathog. 2014; 10(6):e1003983. [DOI] [PMC free article] [PubMed]
- 3.Koskiniemi M. CNS manifestations associated with Mycoplasma pneumoniae infections: summary of cases at the University of Helsinki and review. Clin. Infect. Dis. 1993;17(Suppl1):S52. doi: 10.1093/clinids/17.Supplement_1.S52. [DOI] [PMC free article] [PubMed] [Google Scholar]; Koskiniemi M. CNS manifestations associated with Mycoplasma pneumoniae infections: summary of cases at the University of Helsinki and review. Clin Infect Dis. 1993;17 Suppl1:S52. [DOI] [PMC free article] [PubMed]
- 4.Loens K., Ieven M. Mycoplasma pneumoniae: current knowledge on nucleic acid amplification techniques and serological diagnosis. Front. Microbiol. 2016;7:448. doi: 10.3389/fmicb.2016.00448. [DOI] [PMC free article] [PubMed] [Google Scholar]; Loens K & Ieven M. Mycoplasma pneumoniae: Current Knowledge on Nucleic Acid Amplification Techniques and Serological Diagnosis. Front. Microbiol. 2016;7:448 [DOI] [PMC free article] [PubMed]
- 5.Medjo B., Atanaskovic-Markovic M., Radic S. Mycoplasma pneumoniae as a causative agent of community-acquired pneumonia in children: clinical features and laboratory diagnosis. Ital. J. Pediatr. 2014;40:104. doi: 10.1186/s13052-014-0104-4. [DOI] [PMC free article] [PubMed] [Google Scholar]; Medjo B, Atanaskovic-Markovic M, Radic S et al. Mycoplasma pneumoniae as a causative agent of community-acquired pneumonia in children: clinical features and laboratory diagnosis. Ital. J. Pediatr. 2014;40:104. [DOI] [PMC free article] [PubMed]
- 6.Critchley I.A., Jones M.E., Heinze P.D. In vitro activity of levofloxacin against contemporary clinical isolates of Legionella pneumophila, Mycoplasma pneumoniae and Chlamydia pneumoniae from North America and Europe. Clin. Microbiol. Infect. 2002;8(4):214. doi: 10.1046/j.1469-0691.2002.00392.x. [DOI] [PubMed] [Google Scholar]; Critchley IA, Jones ME, Heinze PD et al. In vitro activity of levofloxacin against contemporary clinical isolates of Legionella pneumophila, Mycoplasma pneumoniae and Chlamydia pneumoniae from North America and Europe. Clin Microbiol Infect. 2002;8(4):214. [DOI] [PubMed]