Abstract
Objective
Recently, questions have been raised concerning the potential endocrine disrupting effects of bisphenol A (BPA). This substance is a constituent in many different products which we frequently come into contact with, such as food containers and receipts. Resin-based dental filling materials are another source of exposure, although according to previous studies the amount and potential risks are not clear. Thus, the aims of the present study were (1) to identify if direct dental filling materials are liable to leak BPA and (2) to investigate if this leakage could lead to any adverse effects on health.
Materials and methods
A literature search was made with PubMed as the primary source, subsequently complemented with reference tracking.
Results
A total of 26 articles were included, 24 of which were used for the first aim (leakage) and 2 for the second aim (health risks). The majority of studies, including all in vivo studies, showed leakage of BPA from dental materials in various amounts and during different time intervals. The findings showed a contradiction in results regarding the connection between dental materials and adverse health effects.
Conclusions
There is leakage of BPA from some dental materials, but critical levels are not evident. Bis-DMA contents might convert to BPA in the oral cavity. There is a contradiction between in vitro and in vivo studies concerning BPA leakage and finally, there is a lack of studies investigating the association between BPA exposure and its adverse effects on human health.
Keywords: Public health, Endocrinology, Materials chemistry, Dentistry
1. Introduction
Among many endocrine-disrupting chemicals, bisphenol A (BPA) is currently at the forefront of discussion. The substance was synthesized approximately 100 years ago and in the 1930s it was identified as one of the first synthetic estrogens [1, 2, 3, 4]. Since the 1950s, BPA has been used commercially in the manufacture of polycarbonates and epoxy resins, which are used in a variety of commonly used products. Polycarbonates are rigid plastics used in toys, water bottles, eyeglass lenses and compact discs (CDs). Epoxy resins are used in protective linings in cans, as strong adhesives and in dental sealants [4, 5]. BPA is also a constituent in thermal paper, such as receipts and fax machine paper.
The primary cause of exposure to BPA is canned food due to leakage from the protective lining, followed by the second largest source which is thermal paper [6].
Due to its wide range of uses, people are exposed to this substance in their daily lives, possibly without being aware of it. More than 90% of the US population revealed detectable BPA in their urine samples based on the report and updated tables report by the Centre for Disease Control and Prevention [7, 8], which demonstrated frequent and persisting exposure to BPA. In the early 1990s, scientists discovered BPA leakage from polycarbonates, which provided the starting point for further research regarding the possible adverse effects this might have [4]. In 1996, Olea et al. [9] reported a significant leakage of BPA from dental sealants into the saliva of patients which resulted in increased concerns about the potential estrogenicity of dental materials [3, 10]. Another study followed by Fung et al. [11] showed that BPA released orally from a dental sealant may not be absorbed or may be present in nondetectable amounts in systemic circulation. Due to its structure, pure BPA is an estrogen-mimicking compound (xenoestrogen) which can bind to nuclear receptors such as estrogen receptors (ER) and also to androgen receptor (AR), estrogen-related receptors (ERRs) and have negative effect on thyroid hormone receptor (THR) and further to membrane receptors and potentially exert endocrine-disrupting effects [12].
In a review by Nadal et al. [13] it was stipulated that in the last years increasing number of studies have demonstrated that BPA can elicit estrogen-like effects with the same potency as E2 (17ß-estradiol), challenging the concept of BPA as a weak estrogen with no effects at low doses. The review also points out several other important effects by PBA on pancreatic b-cells affecting insulin production and play a role in diabetes mellitus type 2, as well as targeting intracellular Ca2+ promoting arythmia in female heart.
Exposure to xenoestrogens can affect not only humans but also wildlife by disturbing their natural processes, such as reproductive cycles and development and the presence and and bioaccumulation of BPA in wildlife is being reported from almost the whole world [14].
The monomer bisphenol A glycidyl dimethacrylate (Bis-GMA) is the most commonly used BPA derivative in epoxy-resin-based dental composites [2]. Bis-GMA is a monomer with methyl methacrylate groups bound to hydroxyl groups of BPA. Another BPA derivative used in dental materials is bisphenol A dimethacrylate (Bis-DMA) which, in contrast to bis-GMA, hydrolyses into BPA by salivary esterase due to structural differences between these monomers [2, 15]. Bis-DMA is rarely used in dental materials [16]. BPA itself does not have a functional role in dental composites, as it exists as an impurity due to the incomplete manufacturing process or as a degradation product as per the process described above [17]. Suppliers often list limited information about the specific composition of dental materials in their safety data sheets [18]. The European Parliament's regulations state that the producers of dental materials are only obliged to declare toxic chemicals in concentrations of 0.1%–1%. This concentration is dependent on the compound's level of toxicity [16]. Dental materials can be marketed as “private labels” which means that retailers put their own brand on a product manufactured by another company. This makes it difficult for a user (dentist) to track the manufacturing process and the actual content of the material [19]. For example, the manufacturer of a commonly used resin-based root canal sealer does list BPA as a component in its safety data sheet, but the amount is not specified [20]. Therefore, it is unclear to the reader whether the level of concentration is high enough to cause any adverse effects, or whether a safe level even exists.
Although pure BPA is not a component of dental composite resins the derivatives of BPA are and in a survey by Dursun E et al [21] a list was presented of 18 composite resins, out of 130 identified, that contain no BPA derivative, but no manufacturer reported pure PBA. As BPA is an endocrine-disrupting chemical, its use is regulated by several organizations around the world, for example the European Food Safety Authority (EFSA) and the U.S. Food and Drug Administration (FDA). These organizations set restrictions for the use of BPA based on the results of scientific studies by introducing a tolerable daily intake (TDI) dose of BPA [22]. In 1988, the Environmental Protection Agency (EPA) set the first safety standard at 50 μg/kg bodyweight (bw)/day [4]. EFSA revised this TDI in January 2015 to a considerably lower and temporary level of 4 μg/kg bw/day, indicating a major uncertainty regarding the adverse health effects of BPA. EFSA estimates that the combined dietary and non-dietary exposure for adults is 1.449 μg/kg bw/day, which is approximately 3 times lower than the current temporary TDI [6]. The aim of the present study was (1) to investigate resin-based dental materials regarding their BPA content and possible leakage of the substance after insertion, and (2) to see if adverse health effects correlated to those materials have been reported in the literature.
2. Materials and methods
2.1. Search strategy
A review of current literature was carried out using the PubMed database. The literature search was conducted on 12th February, 2015. “MeSH terms” and “free-text words” were used as search terms. The keywords used were: bisphenol A, bisphenol A epoxy resin, diglycidyl bisphenol A ether, endocrine disruptors, estrogens, resin composites, pit and fissure. Besides database searches, reference tracking was also used to search for studies.
An update of the literature search was carried out on 20th September, 2017, using the same database and search terms.
2.2. Inclusion and exclusion criteria
No publication date limit was used for the primary literature search or the following reference tracking. Further inclusion criteria were studies which only analyzed leakage (or synonyms thereof) of BPA from an oral environment or in vitro, as well as health effects caused by this leakage. Exclusion criteria were reviews and studies on animals. The direct dental filling materials included in this review are resin-based composites and pit and fissure sealants. Other resin-based dental materials such as root canal sealer and orthodontic adhesives were excluded.
2.3. Other
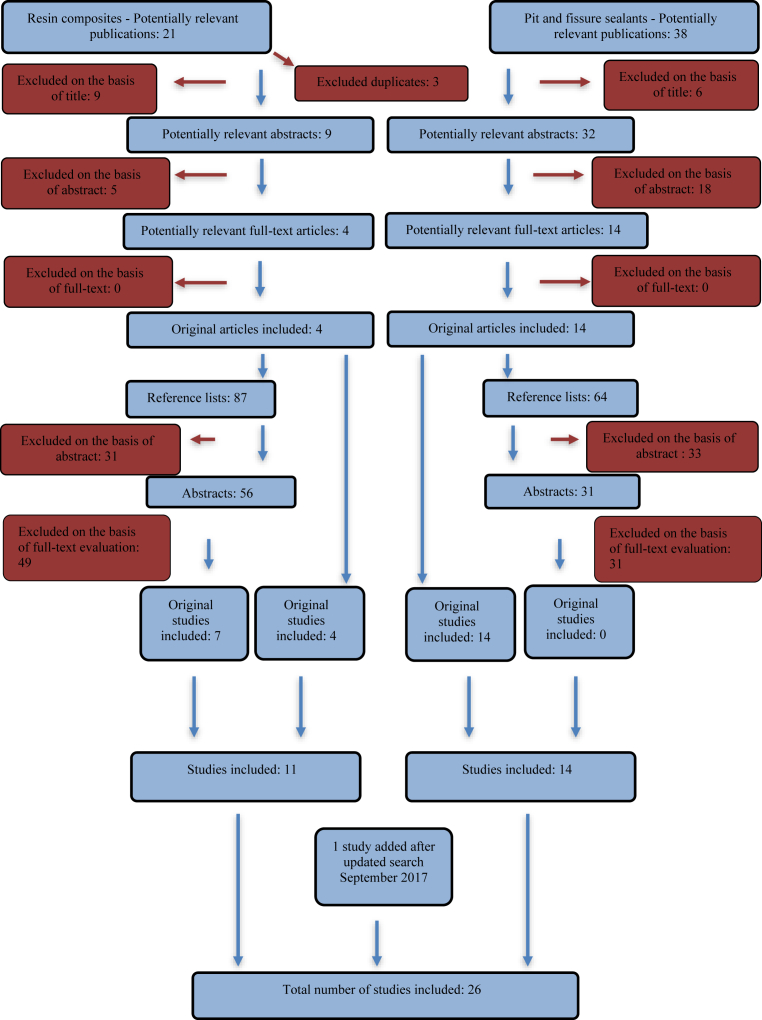
A report by the Swedish National Board of Health and Welfare (Socialstyrelsen) was used as support for detailed information in Tables 1, 2, and 3 [23] (see Fig. 1).
Table 1.
In vitro studies showing no leakage of BPA.
| Study | Method | Solvent | Time | DL QL |
Material | Detectable amount of BPA |
|---|---|---|---|---|---|---|
| A study of component release from resin pit and fissure sealants in vitro, Hamid et al., 1997 | HPLC | water | 24 h (several time intervals were tested) | DL:0.07–0.09 μg/ml | Concise™, Ultraseal®, Pit and Fissure Sealant, Prisma Shield Compules®, Helioseal®F, Delton® (Ash Dent GER), Delton® (Johnson & Johnson) | ND |
| In vitro elution of leachable components from dental sealants, Nathanson et al., 1997 | HPLC-UV/VIS |
ethanol | 4 minutes | – | Delton®, Concise™, Helioseal®, Prisma®:Shield, Seal-Rite™ I (BIS-GMA), Seal-Rite™ II (UDMA), Defender™ | ND |
| Variability of cytotoxicity and leaching of substances from four light-curing pit and fissure sealants, Geurtsen et al., 1999 | GC/MS | water | 24 h | - | Fissurit®F, Helioseal®, Visioseal™, Delton® Plus | ND |
| Analysis of the degradation of a model dental composite, Koin et al., 2008 | HPLC, LC/MS | water | 2 weeks | - | Bis-GMA | ND |
| Water sorption and solubility of dental composites and identification of monomers released in an aqueous environment, Örtengren et al., 2001 | HPLC | water | 4 h | - | Alcaglass, C&B™Cement, Sono-Cem, Targis®, TPH Spectrum®, Vario-link® II | ND |
| 24 h | ND | |||||
| 7 days | ND | |||||
| 60 days | ND | |||||
| 180 days | ND |
ND: Not detected, DL: Detection limit, QL: Quantification limit, h: hour.
Table 2.
In vivo studies showing leakage of BPA.
| Study | Method | Biologic fluid | Time |
DL QL |
Material | Detectable amount of BPA |
|---|---|---|---|---|---|---|
| Dental composite fillings and bisphenol A among children: a survey in South Korea, Chung et al., 2012 | HPLC-ESI MS⁄MS | urine | - | - | Fissure sealant material Composite filling material and fissure sealant material |
Highest mean conc.: 9.13 μg/g creatinine Highest mean conc.: 2.68 μg/g creatinine |
| Time-related bisphenol-A content and estrogenic activity in saliva samples collected in relation to placement of fissure sealants, Arenholt-Bindslev et al., 1999 | HPLC | saliva | 0 h 1 h 24 h 0 h 1 h 24 h |
DL: 0.1 ppm QL: 0.3 ppm |
Delton®LC Visio™Seal |
0.3–2.8 ppm ND ND ND ND ND |
| Pharmacokinetics of bisphenol A released from a dental sealant Fung et al., 2000 | HPLC | saliva | baseline 1 h 3 h 1day 3days 5days |
DL: 5ppb (0.005ppm) | Delton® LC | ND 5.8–105.6 ppb 5.8–105.6 ppb ND ND ND |
| Salivary bisphenol-A levels detected by ELISA after restoration with composite resin, Sasaki et al., 2005 | ELISA | saliva | pre-treatment/after treatment | - | Z100™, Progress, Palfique®Toughwell, Metafil Flo, Unifil S, Beautifil, Xeno®CFII, Prodigy™, Clearfil™ST |
Mean detected: 32.1 ng/ml Highest detected: 60.1 ng/ml |
| Salivary bisphenol-A levels due to dental sealant/resin: a case-control study in Korean children, Han et al., 2012 | ELISA | saliva | - | - | existing dental sealant or resin | 0.002–8.305 μg/L |
| Salivary bisphenol A levels and their association with composite resin restoration, Lee et al., 2016 | ELISA | saliva | immediately before 5minutes 7days |
- | Filtek™ Z350 XT | Average amount: 0.15 μg/liter 3.64 μg/liter 0.59 μg/liter |
| Exposure to bisphenol A from bis-glycidyl dimethacrylate-based dental sealants, Joskow et al., 2006 | Sensitive isotope-dilution mass spectrometry | saliva urine |
pre-treatment immediately after 1 h pre-treatment 1 h 24 h |
- | Delton® LC, Helioseal® F |
Mean value: 0.30 ng/ml 26.5 ng/ml 5.12 ng/ml 0.64 μg/g cr* 8.70 μg/g cr* 1.68 μg/g cr* |
| Bisphenol A blood and saliva levels prior to and after dental sealant placement in adults Zimmerman-Downs et al., 2010 |
ELISA | saliva | baseline 1–3 h 24 h |
DL: 0.05 μg/L | Delton® Pit and fissure sealant-Light Cure Opaque | 0.07–6.00 ng/ml low-dose group: 3.98 ng/ml high-dose group: 9.08 ng/ml Return to baseline |
| Estrogenicity of resin-based composites and sealants used in dentistry, Olea et al., 1996 | HPLC, GC/MS | saliva | 1 h pre-treatment 1 h post-treatment |
- | Delton® Pit and fissure sealant | ND-2123 ng/ml 3300–30000 ng/ml |
ND: Not detected, DL: Detection limit, QL: Quantification limit, Baseline = Pre-treatment: Before start of experiment, 1ppm: 1000 ppb, *cr = creatinine, h: hour.
Table 3.
In vitro studies showing lekage of BPA.
| Study | Method | Solvent | Time |
DL QL |
Material | Detectable amount of BPA |
|---|---|---|---|---|---|---|
| Effect of hydrogen peroxide on the three-dimensional polymer network in composites, Durner et al., 2011 | GC/MS | methanol | 24 h |
- | Tetric® Flow Tetric® Ceram Filtek™Supreme XT |
PF 15% without/with bleaching: 2.09 μmol/l/14.49 μmol/(4.78 μmol/l/5.07 μmol/l*) without/with bleaching: 1.82 μmol/l/11.45 μmol/l (3.24 μmol/l/7.66 μmol/l *) without/with bleaching: 1.70 μmol/l/12.41 μmol/l (2.43 μmol/l/4.62 μmol/l*) |
| Effect of bleaching on the elution of monomers from modern dental composite materials, Polydorou et al., 2009 | LC-MS/MS | saliva ethanol |
24 h 7 days 28 days 24 h 7 days 28 days |
QL: 0.5 μg/ml | Filtek™Supreme X Ceram X® |
0.614 μg/ml 1.837 μg/ml 0.580 μg/ml 3.145 μg/ml 7.149 μg/ml 3.898 μg/ml |
| Stability of bisphenol A, triethylene-glycol dimethacrylate, and bisphenol A dimethacrylate in whole saliva, Atkinson et al., 2002 | GC/MS, HPLC | whole saliva water |
up to 4 months | QL for BPA: 1 ng/ml QL for BisDMA: 10 ng/ml QL for TEGDMA: 500 ng/ml |
BPA BisDMA TEGDMA |
See text below |
| Elution of bisphenol A from composite resin: a model experiment Imai et al., 2000 | HPLC | water methanol |
up to 7 days (in water) up to 28 days (in methanol) |
- | Z100™ | See text below |
| Detection of bisphenol-A in dental materials by gas chromatography-mass spectrometry, Manabe et al., 2000 | GC/MS | phosphate-buffered saline | 24h | - | Silux Plus™ Concise™ Teethmate™ |
Cured material 91.4 ng/g material 19.8 ng/g material 55.5 ng/g material |
| Release of monomers from different core build-up materials Polydorou et al., 2009 | LC-MS/MS | ethanol | 24 h 7 days 28 days 24 h 7 days 28 days 24 h 7 days 28 days |
QL: 0.5 μg/ml | Clearfil™ Photo Core Clearfil™ DC Core Automix Clearfil™Core |
Mean values ND 1.92 μg/ml 6 μg/ml 5.19 μg/ml 3.98 μg/ml 6.14 μg/ml ND ND ND |
| Long-term release of monomers from modern dental-composite materials, Polydorou et al., 2009 | LC/MS | ethanol | 24 h to 1year | QL: 0.5 μg/ml | Ceram X® Filtek™ Supreme XT Clearfil™ Core |
5.25 μg/ml ND ND |
| Determination of bisphenol A and related aromatic compounds released from bis-GMA-based composites and sealants by high performance liquid chromatography. Pulgar et al., 2000 | GC/MS, HPLC | water | 24 h | DL: 0.20 ppm (0.20 μg/ml), QL: 0.23 ppm (0.23 μg/ml) | Charisma® Pekalux Polofil Silux-Plus™ Z-100™ Tetric® Brillant™ Delton® |
pH 7, cured and uncured 1.4 μg/ml 0.6 μg/ml 2.8 μg/ml 16.5 μg/ml 0.3 μg/ml 12.9 μg/ml ND 42.8 μg/ml |
| Bisphenol-A content of resin monomers and related degradation products, Schmalz et al., 1999 | HPLC | saliva | 0.3 h-24 h 0.3 h 1 h 2 h 24 h |
DL: 1–104 ppm | Bis-GMA Bis-DMA |
Conversion rate: <0.2% for all times 44.7% 73.4% 70.2% 81.4% |
ND: Not detected, DL: Detection limit, QL: Quantification limit, PF = Potassium nitrate and fluoride, * = Detected amount after 7 days, h: hour.
Fig. 1.
Search strategy and results, search table modified after Papia et al [66].
3. Results
A total of 24 studies analyzing the elution of BPA from resin composites and pit and fissure sealant materials were selected for this review [9, 11, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45].
3.1. Studies showing no leakage of BPA
Using high performance liquid chromatography (HPLC), Hamid et al. [25] analyzed seven light-cured pit and fissure sealants to identify the components released. Fissure-sealed extracted molars incubated in water were used in the study. No detectable amount of BPA was found in any of the eluates. The authors also stated that the present data does not prove the absence of BPA leakage, but if there was a release, it was below the detection level.
Seven light-cured pit and fissure sealants were also analyzed by Nathanson et al. [43] with regard to the elution of BPA in vitro. Each sample was light-cured, placed in ethanol for 4 minutes and thereafter removed. The solutions were analyzed and no detectable amount of BPA was found. Geurtsen et al. [26] investigated four types of light-cured pit and fissure sealants in order to determine their composition and their cytotoxicity. The results showed that different components of the material, such as co-monomers and initiating substances, leaked into water. However, no BPA, which is easy to detect by means of gas chromatography-mass spectrometry (GC/MS), was found. In their report, Koin et al. [27] studied the degradation of dental composites in vitro. They stored a Bis-GMA-coated surface in distilled water for two weeks. HPLC did not find any detectable amounts of BPA but did find intact Bis-GMA and other BPA derivatives. It was stated by the authors that, due to the BPA content in these degradation products, health concerns might arise. The water sorption and solubility of six resin composites were investigated by Örtengren et al. [46]. The test was carried out using HPLC to analyze the eluted monomers after storage times ranging from 4 hours to 180 days. No detectable quantities of BPA were found during the test period.
3.2. Studies showing leakage of BPA
In an in vivo study, Chung et al. [29] investigated the relationship between urinary BPA concentrations and the presence of composite restorations or sealants in 496 South Korean children. Urine samples were collected and analyzed to measure the BPA concentration. They concluded that having many dental composite filling surfaces may increase the urinary BPA concentration compared to few or no filled surfaces. Arenholt-Bindslev et al. [30] placed clinically appropriate amounts of either Delton® LC or Visio-Seal™ pit and fissure sealant in eight healthy male volunteers and measured BPA in saliva samples collected from the subjects at various intervals. The authors only found BPA in samples collected immediately after placement of Delton® LC. Fung et al. [11] applied the dental sealant Delton® LC to the teeth of 40 adults with no history of fissure sealants or composite resin fillings. The results showed that BPA was only detectable in some of the saliva samples collected at 1 and 3 hours after insertion. No detectable amounts of BPA in blood samples suggest no or undetectable quantities of systemic circulation absorption. Sasaki et al. [31] analyzed changes in the salivary BPA concentration linked to the placement of a composite filling in 21 subjects. Saliva samples were collected before and immediately after placement, as well as after gargling with tepid water. All samples showed a higher concentration of BPA immediately after placement of the filling compared to baseline but the concentrations declined significantly after gargling. The authors concluded that efficient gargling may reduce the risk of BPA exposure. In a case control study, Han et al. [32] investigated the relationship between the number of tooth surfaces with sealant/resin fillings and the amount of BPA in saliva. They collected saliva samples from 124 children, 50 % of whom formed the control group with no dental fillings and the remaining 50 % of whom had more than four surfaces filled. Due to the positive correlation that was discovered, the authors suggested that there might be a connection between dental sealant/resin fillings and salivary BPA levels. Lee et al. [42] measured changes in the BPA level in saliva associated with composite resin restoration using ELISA (enzyme-linked immunosorbent assay). The participants were 30 volunteers who received one or several composite restorations. Saliva was collected before and after the filling procedure. The results showed an increased average level of BPA after 5 minutes compared to baseline. After 7 days, the amount of BPA detected had decreased. A higher number of treated surfaces resulted in higher levels of BPA. Joskow et al. [33] measured the BPA concentration in saliva and urine after the placement of fissure sealants in 14 men. The fissure sealant materials used were Delton®LC and Helioseal™ F, the former showing a low level of BPA exposure and the latter showing negligible exposure. The authors recorded that the collection of saliva samples probably reduced the systemic absorption and consequently the measured concentrations of BPA in urine. The effects of pit and fissure sealant material on BPA levels in blood and saliva over time were analyzed by Zimmerman–Downs et al. [34]. Saliva and serum samples from 30 randomly chosen adults, with no previous fillings, were collected at various time intervals and divided into two groups: low-dose and high-dose samples. The subjects were treated with either one occlusal sealant or four occlusal sealants respectively. The results showed that BPA was detected in the saliva of all the participants at baseline. The concentration of BPA, however, increased in both groups after applying the sealants and the level peaked within three hours. In the high-dose group, the peak level of BPA was more than twice the amount measured in the low-dose group. No BPA was found in any serum samples. Olea et al. [9] also investigated the leakage of BPA and estrogenicity of dental sealants and composites both in vivo and in vitro. Eighteen adults were selected and 12 dental fissure sealants were applied to their molars. Saliva samples were collected one hour before and one hour after treatment. BPA was identified in the post-treatment samples in varying amounts. The components of uncured resin composites (Tetric®, Charisma® and Pekalux™) and fissure sealants (Delton®) were analyzed in vitro at different pH levels. All of the materials showed a leakage of BPA at pH 7.
Durner et al. [35] investigated how the leakage of BPA and other monomers from three dental composites is affected by bleaching with Opalescence® tooth whitening system which is based on potassium nitrate and fluoride (PF) 15% or PF 35%. Polymerized composite specimens were bleached and thereafter stored in methanol for 24 hours or 7 days. The results showed that bleaching with hydrogen peroxide increased the elution of the monomers. Specimens bleached with PF 15% and thereafter stored for 24 hours showed the highest leakage of BPA.
The effect of bleaching on the elution of monomers from dental composite materials in vitro was further analyzed by Polydorou et al. [45]. Two different resin composites were polymerized and thereafter bleached with one of two different bleaching products (Opalescence® Xtra® Boost™ or Opalescence® PF™ 15%). BPA was detected in all samples before and after bleaching. The amount of leached BPA from Ceram X®was significantly lower in the bleached samples compared to the control. There was no significant change in the elution of BPA from Filtek™Supreme XT after bleaching. The amount of leached BPA from Filtek™Supreme XT was, however, lower than the amount detected in the Ceram X® samples. The following two studies analyzed how leakage of BPA was affected by pH and came to the same conclusion. Atkinson et al. [36] analyzed the conversion rate of BPA, Bis-DMA and TEGDMA in whole saliva stored at -20 °C or -70 °C. Solutions of the substances were added to saliva samples collected from subjects with no previous dental sealants or composite restorations and the mixtures were stored for various times. The authors also investigated the stability of Bis-DMA mixed with whole saliva or stored in water at 37 °C. BPA was stable at all times and at all temperatures, Bis-DMA, on the other hand, was highly unstable. After incubation for four months at -20 °C, almost all Bis-DMA was converted to BPA. All samples were stable at -70 °C and only a slight decrease of Bis-DMA was detected in the water samples. Bis-DMA samples stored at 37 °C demonstrated a rapid and significant conversion to BPA, the Bis-DMA concentration decreased from 200 ng/ml to 21.8 ng/ml after 24 hours while the BPA concentration, which was undetectable at baseline, increased to 100 ng/ml. The results also showed that a lower salivary pH might decrease the leakage of BPA.
Pulgar et al. [41] analyzed BPA release from dental composites and dental sealants at different pH [1, 7, 10, 16]. The dental materials were used both in polymerized and non-polymerized form. The authors found BPA leakage from all samples which increased with a more alkaline pH. Non-polymerized Charisma® composite showed the highest leakage amount of BPA (1.8 μg/mg). Imai et al. [37] analyzed leakage of BPA from a composite resin material in water and methanol at 37 °C. Each specimen was placed in water and observed at different time intervals from 5 minutes up to 7 days. They were then transferred to methanol and observed again for various time intervals, up to 28 days. The result showed that BPA elutes more rapidly in both water and methanol solvents within three hours. The amount of eluted BPA in water became constant after seven days, but when the material was transferred into methanol, the leakage increased.
In their in vitro study, Manabe et al. [38] demonstrated that GC/MS is a reliable method for detecting BPA in dental materials. This method was used to detect the elution of BPA from uncured as well as cured dental bonding agents, dental sealants and dental composites. Three pieces of polymerized dental sealants or composites were placed in phosphate-buffered saline for 24 hours and thereafter analyzed using GC/MS. The authors concluded that these three materials all leached BPA but in quantities less than 1/1000 of the reported dose (2 μg/kg body weight/day) required for xenoestrogenisity in vivo. Polydorou et al. [39] investigated the elution of BPA and other monomers from three dental composites: one chemically-cured, one photo-cured and one dual-cured. The cured samples were stored in ethanol and the eluates were analyzed after 24 hours, 7 days, 28 days and 1 year. No BPA leached from the chemically-cured material, a small amount of BPA leached from the other samples, mainly from the dual-cured composite. The results also showed a slight increase of the eluted BPA with time. In another in vitro study, leakage of BPA was investigated both from light-cured composite materials (Ceram X® and Filtek™ Supreme XT) and from chemically-cured ones (Clearfil™ Core). Polydorou et al. [40] compared the elution of BPA based on different curing and incubation time. Only one of the light-cured composite materials (Ceram X®) showed leakage of BPA and that occurred between day 1 and day 28 but not after 1 year. The curing time did not have a significant effect on the amount of leaked BPA. G. Schmalz et al. [24] analyzed the BPA content of different monomers in fissure sealant materials and their leakage of BPA under different hydrolytic conditions using HPLC. They found no BPA release from Bis-GMA under any of the conditions used. They did find, however, that the main proportion of Bis-DMA converted to BPA under the same conditions, leading to the conclusion that BPA release is attributed to the Bis-DMA content of the fissure sealant tested. The conversion was time-related with a continuous increase in conversion rate from Bis-DMA to BPA during a 24-hour period.
3.3. Retrospective cross-sectional study
The association between dental sealants and BPA level in urine was examined by McKinney et al. [44]. Measurements of urinary BPA and oral examination data were analyzed using data from the 2003–2004 National Health and Nutrition Examination Survey (NHANES). The study categorized 6-19-year-old children whose BPA measurements were available. The results showed no statistically significant association between the number of dental sealants or restorations and urinary BPA concentrations.
3.4. Studies investigating bisphenol A from dental materials and health
Only two of the studies reviewed analyzed the association between BPA release from dental materials and its effect on health [1, 47]. Maserejian et al. [1] compared physical development in children with amalgam or composite fillings over 5 years. The subjects were randomly selected to receive filling treatment with either one of these two materials. The authors measured changes in body mass index (BMI), body fat percentage and growth rate. Their conclusion was that there were no significant correlations between composite or amalgam fillings and physical development. Maserejian et al. [47] also analyzed the association between Bis-GMA-based composite fillings and psychosocial functions in 534 children. Some of the eligibility criteria were no psychosocial diagnosis, no amalgam fillings and ≥2 posterior teeth with occlusal caries in need of treatment. The participants were randomly selected to be treated with either amalgam, composite or compomer. After five years, the children's psychosocial function was followed-up. The authors concluded that children with higher exposure to Bis-GMA-based composites showed impaired psychosocial functions, for example anxiety and depression. There was no correlation between these functions and the exposure to amalgam or compomer.
4. Discussion
Leakage of BPA from dental filling materials has been analyzed in different studies using different methods, with the majority showing a leakage of BPA in varying amounts. The studies that showed no leakage were all performed in vitro [25, 26, 27, 46] and no in vivo studies could confirm this result. This suggests that BPA does leak in an oral environment, which may be due to salivary enzymatic processes or other environmental conditions that could not be mimicked in vitro. The findings are in accordance with other literature reviews [48].
The use of different investigation methods as well as varying detection limitations and quantification limits complicates the comparison process. Only 10 studies have provided information about the detection and quantification limits used, and there is no consensus in this respect [11, 24, 25, 30, 34, 36, 39, 40, 41, 45]. Some of the reviewed studies measured time-dependent elution of BPA. They show a higher elution immediately after placement of a filling which decreased with time [11,30, 33, 34, 37]. Meanwhile, the conversion rate from Bis-DMA to BPA increased continuously [24, 36]. Based on these findings, it is important to take measures to reduce the initial elution by thorough polishing of the filling and rinsing the mouth immediately after treatment. The “no touch technique” should be applied to minimize the clinician's contact with uncured dental materials in order to avoid possible allergic reactions. This technique may also indirectly reduce the exposure risk to BPA in the clinical situation. The “no touch technique” is an established clinical recommendation [17]. Two of the studies included investigated the relationship between BPA from dental materials and adverse health effects. A study by Maserejian et al. was the only study reviewed which showed a significant positive correlation between BPA from dental composite restorations and adverse effects on health [47]. The other study did not find any correlation in this respect. Two studies are not enough evidence to conclude the effect of BPA leakage on health. Thus, EFSA claims that there is a need for further studies on BPA and its effect on health [6]. EFSA published its first report on BPA and its risk characterization from dietary exposure in 2006 [6]. This report gave rise to establishing the TDI for BPA at 50 μg/kg bw/day, which was based on several rodent studies showing adverse effects at considerably higher doses. In 2010, EFSA concluded that there are uncertainties regarding the possible toxicological effects of BPA due to a lack of scientific evidence. In late 2011, EFSA published a statement [49] on the French Agency for Food, Environmental and Occupational Health and Safety (ANSES) report on BPA [50] due to a discrepancy between this and the 2010 EFSA report [51]. According to this statement, the main reason for variance is that ANSES included non-dietary sources in addition to dietary sources as opposed to EFSA which only included dietary sources. EFSA did state, however, that there is need for further reviews of new studies in order to arrive at an accurate conclusion. In EFSA's report from 2015 [6], the sources for external non-dietary exposure were: thermal paper, air (inhalation), dust, toys (which may come in contact with the mouth) and cosmetics. Dental materials were not included as a potential source of BPA in this report and therefore the results do not provide an accurate picture of the existing sources.
Due to all the uncertainty regarding the TDI of BPA, the EU restricted the use of BPA in infant feeding bottles in 2011 as a precautionary measure [6]. Infants, children and women of childbearing age are all considered as vulnerable groups for BPA exposure. One of the factors for this is that infants generally consume a larger amount of food and drink per body weight than adults, which in turn leads to a higher concentration of BPA. Some studies analyzed how BPA can affect the fetus through placental exchange and could cause adverse effects [52, 53]. This is a reason for women of childbearing age, including pregnant women, to be considered as vulnerable to this exposure.As previously mentioned, people are exposed to BPA from different sources in daily life. It can be anything from receipts to canned food as well as dental materials. EFSA's current opinion is that BPA poses no health risks as the exposure levels are far below the TDI. The studies that these conclusions are based on, however, do not consider dental materials as a source of BPA exposure. There may be other unknown sources of BPA which have not been included in these studies. Based on this, one cannot be sure that our daily exposure is significantly lower than the TDI. Hypothetically, BPA in a lower dose may also be part of a cocktail effect in combination with other endocrine-disrupting chemicals to which we are exposed. This includes a wide range of substances such as dichloro-diphenyl-trichloroethane (DDT) synthetic insecticide, pharmaceuticals, dioxin and dioxin-like compounds, which might act synergistically and lead to unpredictable adverse effects [54]. The current TDI level of BPA set by EFSA, 4 μg/kg bw/day, is temporary and until January 2015 it was 50 μg/kg bw/day. The new TDI is not final due to EFSA awaiting results from an ongoing long-term rodent study. This indicates the level of uncertainty regarding the subject. Due to this fact, it is important to limit exposure to BPA sources, specifically for the vulnerable groups, such as children and pregnant women [6]. As previously discussed, the European Union (EU) has already taken measures to restrict the usage of BPA-containing materials in infant feeding bottles. Meanwhile, EFSA's official statement [6] is that the daily intake of BPA is far below the TDI. In conclusion, limiting a child's exposure to BPA is an important precaution. Fissure sealants, which are frequently used nowadays in a preventative way to reduce the risk of caries among children, may increase the exposure amount of BPA. Even though studies indicate a low risk of BPA leakage from sealants, it should still be a matter of concern, as they are frequently used in children who are considered a risk group. Based on EFSA's reasoning behind restricting the use of BPA in feeding bottles, it may be argued that the use of resin-based sealants should also be discussed. Due to all the uncertainties regarding the exposure level, it has been suggested as a legitimate measure to exclude pregnant women from getting composite restorations unless it is urgent [2]. Considering that the main reason for placing composite fillings is caries, the best way to decrease the possible BPA exposure from composites is to prevent the disease in the first place. In light of the current debate on the daily usage of fluoride from water and toothpaste etc., it is important not to forget its major preventative effects on caries disease. Many studies show the positive effects of fluoride in caries prevention [55], so it could be argued that the recommended fluoride usage indirectly decreases the exposure to BPA due to less need for composite fillings.At present, many of the studies regarding the endocrine-disrupting properties of BPA are conducted on animals. Some of the studies showed that BPA exposure correlated with adverse effects on reproductive as well as non-reproductive systems, including neurobehavioral development and the endocrine system [56, 57, 58, 59,60, 61, 62]. However, it is important to consider that the source of BPA exposure in those studies was not dental materials but pure BPA solvent, which was administered subcutaneously, intragastrically or orally.
Fenichel et al stated that BPA has been found to leach out from food and beverage, as well as from dental sealants, and that exposure to environmental nanomolar concentrations of BPA is ubiquitous and continuous, via different routes: oral absorption, air, skin. Further; In humans, free active unconjugated BPA is metabolized by rapid glucurono- orsulfono-conjugation and eliminated via renal clearance and that BBA is present in the urine and in most bloods, maternal milk or amniotic fluid [63].
According to the findings by Taylor et al., the route of administration has no significant effect on plasma BPA levels [64]. Thus, the route of administration should not be a reason to dismiss the results from studies using a non-oral route.
In a later study, however, by Gayrard it was showed that BPA can be efficiently and very rapidly absorbed through the oral mucosa after sublingual exposure in dogs. This efficient systemic entry route of BPA may lead to far higher BPA internal exposures than known for BPA absorption from the gastrointestinal tract [65].
Several factors, including metabolism and exposure per kilogram bodyweight, need to be taken into consideration before drawing conclusions regarding human health based on results from animal studies. Based on one study [57], the pharmacokinetics in women, female monkeys and mice are similar; however, the plasma concentration of BPA in rodents in these studies is substantially higher than the equivalent concentration in humans. Based on the results of the studies reviewed, it can be determined whether the dental materials analyzed contain and/or leak BPA. It would be valuable for the clinician to obtain this information by reading the safety data sheet. This is not the case, however, since the current regulation policy set by the European Parliament does not require that companies fully declare the content of their products [16]. There might be a reason to re-evaluate the regulations in order to allow caregivers to consider the true content of materials versus possible risks when treating their patients.
5. Conclusions
-
•
There is a leakage of BPA from some resin-based dental materials. However, what constitutes critical levels is not evident.
-
•
Bis-DMA contents in resin-based dental materials might convert to BPA under different conditions in the oral cavity.
-
•
There is a contradiction between in vitro and in vivo studies concerning BPA leakage from resin-based dental materials. This may be due to difficulties in mimicking the oral environmental conditions in vitro.
-
•
There is a lack of studies analyzing the association between BPA exposure from dental materials and its adverse effects on human health, and consequently there is a need for further studies on this matter.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgments
The authors would like to express their gratitude to associate professor Lars Ehrnford for providing inspiration and expertise.
References
- 1.Maserejian N.N., Hauser R., Tavares M., Trachtenberg F.L., Shrader P., McKinlay S. Dental composites and amalgam and physical development in children. J. Dent. Res. 2012;91:1019–1025. doi: 10.1177/0022034512458691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fleisch A.F., Sheffield P.E., Chinn C., Edelstein B.L., Landrigan P.J. Bisphenol A and related compounds in dental materials. Pediatrics. 2010;126:760–768. doi: 10.1542/peds.2009-2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anusavice K.J. twelfth ed. Elsevier/Saunders; St. Louis, Mo: 2013. Phillips' Science of Dental Materials. [Google Scholar]
- 4.Vogel S.A. The politics of plastics: the making and unmaking of bisphenol a "safety. Am. J. Public Health. 2009;99(Suppl 3):559–566. doi: 10.2105/AJPH.2008.159228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kamrin M.A. Bisphenol A: a scientific evaluation. MedGenMed. 2004;6:7. [PMC free article] [PubMed] [Google Scholar]
- 6.European Food Safety Authority . first ed. European Food Safety Authority; Parma: 2015. Scientific Opinion on the Risks to Public Health Related to the Presence of Bisphenol A (BPA) in Foodstuffs: Executive Summary. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Department of Health and Human Services Centers for Disease Control and Prevention, National Center for Environmental Health . 2009. Fourth National Report on Human Exposure to Environmental Chemicals. [Google Scholar]
- 8.Department of Health and Human Services Centers for Disease Control and Prevention, National Center for Environmental Health . 2018. Fourth National Report on Human Exposure to Environmental Chemicals Updated Tables, March 2018, Volume One. [Google Scholar]
- 9.Olea N., Pulgar R., Perez P., Olea-Serrano F., Rivas A., Novillo-Fertrell A. Estrogenicity of resin-based composites and sealants used in dentistry. Environ. Health Perspect. 1996;104:298–305. doi: 10.1289/ehp.96104298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schafer T.E., Lapp C.A., Hanes C.M., Lewis J.B., Wataha J.C., Schuster G.S. Estrogenicity of bisphenol A and bisphenol A dimethacrylate in vitro. J. Biomed. Mater. Res. 1999;45:192–197. doi: 10.1002/(sici)1097-4636(19990605)45:3<192::aid-jbm5>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 11.Fung E.Y., Ewoldsen N.O., St Germain H.A., Jr., Marx D.B., Miaw C.L., Siew C. Pharmacokinetics of bisphenol A released from a dental sealant. J. Am. Dent. Assoc. 2000;131:51–58. doi: 10.14219/jada.archive.2000.0019. [DOI] [PubMed] [Google Scholar]
- 12.Acconcia F., Pallottini V., Marino M. Molecular mechanisms of action of BPA. Dose Response. 2015 Oct 7;13(4) doi: 10.1177/1559325815610582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nadal A., Fuentes E., Ripoll C., Villar-Pazos S., Castellano-Muñoz M., Soriano S., Martinez-Pinna J., Quesada I., Alonso-Magdalena P. Extranuclear-initiated estrogenic actions of endocrine disrupting chemicals: is there toxicology beyond paracelsus? J. Steroid Biochem. Mol. Biol. 2018 Feb;176:16–22. doi: 10.1016/j.jsbmb.2017.01.014. Epub 2017 Jan 31. [DOI] [PubMed] [Google Scholar]
- 14.Corrales J., Kristofco L.A., Steele W.B., Yates B.S., Breed C.S., Williams E.S., Brooks B.W. Global assessment of bisphenol a in the environment: review and analysis of its occurrence and bioaccumulation. Dose Response. 2015 Jul 29;13(3) doi: 10.1177/1559325815598308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soderholm K.J., Mariotti A. BIS-GMA-based resins in dentistry: are they safe? J. Am. Dent. Assoc. 1999;130:201–209. doi: 10.14219/jada.archive.1999.0169. [DOI] [PubMed] [Google Scholar]
- 16.Socialstyrelsen . 2012. Bisfenol A I Dentala Material. (in Swedish) [Google Scholar]
- 17.Ehrnford L. Lars E.M. Ehrnford AB; Malmö: 2014. De Vita Materialen I Praktiken 2014-2015. (in Swedish) [Google Scholar]
- 18.Ehrnford L. 2015. Vad är det egentligen vi stoppar i munnen på våra patienter?http://dental24.se/dentalkanalen/vad-ar-det-egentligen-vi-stoppar-i-munnen-pa-vara-patienter/ Available at: (in Swedish) [Google Scholar]
- 19.Ehrnford L. 2013. Private labels. Focus Tann Vår. [Google Scholar]
- 20.Dentsply DeTrey GmbH . 2005. AH plus root canal sealer.http://www.dentsply.de/bausteine.net/f/7299/SCAHPlus050419rMV[Germanmarket].pdf?fd=2 Available at: [Google Scholar]
- 21.E1 Dursun. Fron-chabouis H2, attal JP2, Raskin A3. Bisphenol a release: survey of the composition of dental composite resins. Open Dent. J. 2016 Aug 31;10:446–453. doi: 10.2174/1874210601610010446. eCollection 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.News Desk . 2015. EFSA Risk Assessment on Bisphenol A Finds No Consumer Health Risks.http://www.foodsafetynews.com/2015/01/bpa-exposure-so-low-europe-further-reduces-daily-tolerable-intake-level/#.VmFmQ96FPIW Available at: [Google Scholar]
- 23.Socialstyrelsen . 2015. Bisfenol A I Dentala Material - En Systematisk Kartläggning Av Vetenskapliga Studier. (in Swedish) [Google Scholar]
- 24.Schmalz G., Preiss A., Arenholt-Bindslev D. Bisphenol-A content of resin monomers and related degradation products. Clin. Oral Investig. 1999 Sep;3(3):114–119. doi: 10.1007/s007840050088. [DOI] [PubMed] [Google Scholar]
- 25.Hamid A., Hume W.R. A study of component release from resin pit and fissure sealants in vitro. Dent. Mater. 1997;13:98–102. doi: 10.1016/s0109-5641(97)80018-8. [DOI] [PubMed] [Google Scholar]
- 26.Geurtsen W., Spahl W., Leyhausen G. Variability of cytotoxicity and leaching of substances from four light-curing pit and fissure sealants. J. Biomed. Mater. Res. 1999;44(1):73–77. doi: 10.1002/(sici)1097-4636(199901)44:1<73::aid-jbm8>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 27.Koin P.J., Kilislioglu A., Zhou M., Drummond J.L., Hanley L. Analysis of the degradation of a model dental composite. J. Dent. Res. 2008;87:661–665. doi: 10.1177/154405910808700712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ortengren U. On composite resin materials. Degradation, erosion and possible adverse effects in dentists. Swed. Dent. J. Suppl. 2000;(141):1–61. [PubMed] [Google Scholar]
- 29.Chung S.Y., Kwon H., Choi Y.H., Karmaus W., Merchant A.T., Song K.B. Dental composite fillings and bisphenol A among children: a survey in South Korea. Int. Dent. J. 2012;62:65–69. doi: 10.1111/j.1875-595X.2011.00089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arenholt-Bindslev D., Breinholt V., Preiss A., Schmalz G. Time-related bisphenol-A content and estrogenic activity in saliva samples collected in relation to placement of fissure sealants. Clin. Oral Investig. 1999;3:120–125. doi: 10.1007/s007840050089. [DOI] [PubMed] [Google Scholar]
- 31.Sasaki N., Okuda K., Kato T., Kakishima H., Okuma H., Abe K. Salivary bisphenol-A levels detected by ELISA after restoration with composite resin. J. Mater. Sci. Mater. Med. 2005;16:297–300. doi: 10.1007/s10856-005-0627-8. [DOI] [PubMed] [Google Scholar]
- 32.Han D.H., Kim M.J., Jun E.J., Kim J.B. Salivary bisphenol-A levels due to dental sealant/resin: a case-control study in Korean children. J. Korean Med. Sci. 2012;27:1098–1104. doi: 10.3346/jkms.2012.27.9.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Joskow R., Barr D.B., Barr J.R., Calafat A.M., Needham L.L., Rubin C. Exposure to bisphenol A from bis-glycidyl dimethacrylate-based dental sealants. J. Am. Dent. Assoc. 2006;137:353–362. doi: 10.14219/jada.archive.2006.0185. [DOI] [PubMed] [Google Scholar]
- 34.Zimmerman-Downs J.M., Shuman D., Stull S.C., Ratzlaff R.E. Bisphenol A blood and saliva levels prior to and after dental sealant placement in adults. J. Dent. Hyg. 2010;84:145–150. [PubMed] [Google Scholar]
- 35.Durner J., Stojanovic M., Urcan E., Spahl W., Haertel U., Hickel R. Effect of hydrogen peroxide on the three-dimensional polymer network in composites. Dent. Mater. 2011;27:573–580. doi: 10.1016/j.dental.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 36.Atkinson J.C., Diamond F., Eichmiller F., Selwitz R., Jones G. Stability of bisphenol A, triethylene-glycol dimethacrylate, and bisphenol A dimethacrylate in whole saliva. Dent. Mater. 2002;18:128–135. doi: 10.1016/s0109-5641(01)00031-8. [DOI] [PubMed] [Google Scholar]
- 37.Imai Y., Komabayashi T. Elution of bisphenol A from composite resin: a model experiment. Dent. Mater. J. 2000;19:133–138. doi: 10.4012/dmj.19.133. [DOI] [PubMed] [Google Scholar]
- 38.Manabe A., Kaneko S., Numazawa S., Itoh K., Inoue M., Hisamitsu H. Detection of bisphenol-A in dental materials by gas chromatography-mass spectrometry. Dent. Mater. J. 2000;19:75–86. doi: 10.4012/dmj.19.75. [DOI] [PubMed] [Google Scholar]
- 39.Polydorou O., Hammad M., Konig A., Hellwig E., Kummerer K. Release of monomers from different core build-up materials. Dent. Mater. 2009;25:1090–1095. doi: 10.1016/j.dental.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 40.Polydorou O., Konig A., Hellwig E., Kummerer K. Long-term release of monomers from modern dental-composite materials. Eur. J. Oral Sci. 2009;117:68–75. doi: 10.1111/j.1600-0722.2008.00594.x. [DOI] [PubMed] [Google Scholar]
- 41.Pulgar R., Olea-Serrano M.F., Novillo-Fertrell A., Rivas A., Pazos P., Pedraza V. Determination of bisphenol A and related aromatic compounds released from bis-GMA-based composites and sealants by high performance liquid chromatography. Environ. Health Perspect. 2000;108:21–27. doi: 10.1289/ehp.0010821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee J.H., Yi S.K., Kim S.Y., Kim J.S., Son S.A., Jeong S.H. Salivary bisphenol A levels and their association with composite resin restoration. Chemosphere. 2017;172:46–51. doi: 10.1016/j.chemosphere.2016.12.123. [DOI] [PubMed] [Google Scholar]
- 43.Nathanson D., Lertpitayakun P., Lamkin M.S., Edalatpour M., Chou L.L. In vitro elution of leachable components from dental sealants. J. Am. Dent. Assoc. 1997;128:1517–1523. doi: 10.14219/jada.archive.1997.0091. [DOI] [PubMed] [Google Scholar]
- 44.McKinney C., Rue T., Sathyanarayana S., Martin M., Seminario A.L., DeRouen T. Dental sealants and restorations and urinary bisphenol A concentrations in children in the 2003-2004 National health and Nutrition examination survey. J. Am. Dent. Assoc. 2014;145:745–750. doi: 10.14219/jada.2014.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Polydorou O., Beiter J., Konig A., Hellwig E., Kummerer K. Effect of bleaching on the elution of monomers from modern dental composite materials. Dent. Mater. 2009;25:254–260. doi: 10.1016/j.dental.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 46.Ortengren U., Wellendorf H., Karlsson S., Ruyter I.E. Water sorption and solubility of dental composites and identification of monomers released in an aqueous environment. J. Oral Rehabil. 2001;28:1106–1115. doi: 10.1046/j.1365-2842.2001.00802.x. [DOI] [PubMed] [Google Scholar]
- 47.Maserejian N.N., Trachtenberg F.L., Hauser R., McKinlay S., Shrader P., Tavares M. Dental composite restorations and psychosocial function in children. Pediatrics. 2012;130:e328–e338. doi: 10.1542/peds.2011-3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kloukos D.1, Pandis N., Eliades T. Vivo bisphenol-a release from dental pit and fissure sealants: a systematic review. J. Dent. 2013 Aug;41(8):659–667. doi: 10.1016/j.jdent.2013.04.012. Epub 2013 May 1. [DOI] [PubMed] [Google Scholar]
- 49.European Food Safety Authority . 2011. SCIENTIFIC OPINION, Statement on the ANSES Reports on Bisphenol A - EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids. [Google Scholar]
- 50.ANSES (Agence Nationale de Sécurité sanitaire, de l’alimentation, de l’environement et du travail) 2011. Effets sanitaires du bisphénol A. Rapport d’expertise collective. [Google Scholar]
- 51.European Food Safety Authority . 2010. Scientific Opinion on Bisphenol A: Evaluation of a Study Investigating its Neurodevelopmental Toxicity, Review of Recent Scientific Literature on its Toxicity and Advice on the Danish Risk Assessment of Bisphenol A. [Google Scholar]
- 52.Morck T.J., Sorda G., Bechi N., Rasmussen B.S., Nielsen J.B., Ietta F. Placental transport and in vitro effects of Bisphenol A. Reprod. Toxicol. 2010;30:131–137. doi: 10.1016/j.reprotox.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 53.Sugiura-Ogasawara M., Ozaki Y., Sonta S., Makino T., Suzumori K. Exposure to bisphenol A is associated with recurrent miscarriage. Hum. Reprod. 2005;20:2325–2329. doi: 10.1093/humrep/deh888. [DOI] [PubMed] [Google Scholar]
- 54.National Institute of Environmental Health Sciences . 2016. Endocrine disruptors.http://www.niehs.nih.gov/health/topics/agents/endocrine/ Available at: [Google Scholar]
- 55.Toumba J. Fluoride. Eur. Arch. Paediatr. Dent. 2009;10:127. doi: 10.1007/BF03262672. [DOI] [PubMed] [Google Scholar]
- 56.Xu X., Tian D., Hong X., Chen L., Xie L. Sex-specific influence of exposure to bisphenol-A between adolescence and young adulthood on mouse behaviors. Neuropharmacology. 2011;61:565–573. doi: 10.1016/j.neuropharm.2011.04.027. [DOI] [PubMed] [Google Scholar]
- 57.Al-Hiyasat A.S., Darmani H., Elbetieha A.M. Leached components from dental composites and their effects on fertility of female mice. Eur. J. Oral Sci. 2004;112:267–272. doi: 10.1111/j.1600-0722.2004.00136.x. [DOI] [PubMed] [Google Scholar]
- 58.Nah W.H., Park M.J., Gye M.C. Effects of early prepubertal exposure to bisphenol A on the onset of puberty, ovarian weights, and estrous cycle in female mice. Clin Exp Reprod Med. 2011;38:75–81. doi: 10.5653/cerm.2011.38.2.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Palanza P., Gioiosa L., vom Saal F.S., Parmigiani S. Effects of developmental exposure to bisphenol A on brain and behavior in mice. Environ. Res. 2008;108:150–157. doi: 10.1016/j.envres.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 60.Tian Y.H., Baek J.H., Lee S.Y., Jang C.G. Prenatal and postnatal exposure to bisphenol a induces anxiolytic behaviors and cognitive deficits in mice. Synapse. 2010;64:432–439. doi: 10.1002/syn.20746. [DOI] [PubMed] [Google Scholar]
- 61.Yu C., Tai F., Song Z., Wu R., Zhang X., He F. Pubertal exposure to bisphenol A disrupts behavior in adult C57BL/6J mice. Environ. Toxicol. Pharmacol. 2011;31:88–99. doi: 10.1016/j.etap.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 62.Rubin B.S., Murray M.K., Damassa D.A., King J.C., Soto A.M. Perinatal exposure to low doses of bisphenol A affects body weight, patterns of estrous cyclicity, and plasma LH levels. Environ. Health Perspect. 2001;109:675–680. doi: 10.1289/ehp.01109675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fenichel P., Chevalier N., Brucker-Davis F. Bisphenol A: an endocrine and metabolic disruptor. Ann. Endocrinol. 2013 Jul;74(3):211–220. doi: 10.1016/j.ando.2013.04.002. Epub 2013 Jun 21. [DOI] [PubMed] [Google Scholar]
- 64.Taylor J.A., Welshons W.V., Vom Saal F.S. No effect of route of exposure (oral; subcutaneous injection) on plasma bisphenol A throughout 24h after administration in neonatal female mice. Reprod. Toxicol. 2008;25:169–176. doi: 10.1016/j.reprotox.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gayrard V., Lacroix M.Z., Collet S.H., Viguié C., Bousquet-Melou A., Toutain P.L., Picard-Hagen N. High bioavailability of bisphenol A from sublingual exposure. Environ. Health Perspect. 2013 Aug;121(8):951–956. doi: 10.1289/ehp.1206339. Epub 2013 Jun 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Papia E., Larsson C., du Toit M., Vult von Steyern P. Bonding between oxide ceramics and adhesive cement systems: a systematic review. J. Biomed. Mater. Res. B Appl. Biomater. 2014;102:395–413. doi: 10.1002/jbm.b.33013. [DOI] [PubMed] [Google Scholar]