Abstract
Chronic alcohol use leads to specific neurobiological alterations in the dopaminergic brain reward system, which probably are leading to a reward deficiency syndrome in alcohol dependence. The purpose of our study was to examine the effects of such hypothesized neurobiological alterations on the behavioral level, and more precisely on the implicit and explicit reward learning. Alcohol users were classified as dependent drinkers (using the DSM-IV criteria), binge drinkers (using criteria of the USA National Institute on Alcohol Abuse and Alcoholism) or low-risk drinkers (following recommendations of the Scientific board of trustees of the German Health Ministry). The final sample (n = 94) consisted of 36 low-risk alcohol users, 37 binge drinkers and 21 abstinent alcohol dependent patients. Participants were administered a probabilistic implicit reward learning task and an explicit reward- and punishment-based trial-and-error-learning task. Alcohol dependent patients showed a lower performance in implicit and explicit reward learning than low risk drinkers. Binge drinkers learned less than low-risk drinkers in the implicit learning task. The results support the assumption that binge drinking and alcohol dependence are related to a chronic reward deficit. Binge drinking accompanied by implicit reward learning deficits could increase the risk for the development of an alcohol dependence.
Keywords: Alcohol dependence, Binge drinking, Low risk alcohol use, Implicit and explicit reward learning
Highlights
-
•
Alcohol dependent patients were impaired in implicit and explicit reward learning.
-
•
Alcohol dependence may lead to implicit and explicit reward learning deficits.
-
•
Binge drinkers learned less than low-risk drinkers in the implicit learning task.
-
•
Binge drinking is related to implicit reward learning deficits.
1. Introduction
The social acceptance and availability of alcohol is reflected in an annual German per capita consumption of twelve liter pure alcohol. Within a time period of 30 days, alcohol is consumed by approximately 80% of German men and 70% of German women. Accordingly, there is a high number of people with alcohol use disorders, estimated 1.8 million in Germany (Bundesregierung, 2017; Heinz & Batra, 2003; John, Hanke, Freyer-Adam, Baumann, & Meyer, 2018; McCarty et al., 2004).
One way of risky alcohol use is binge drinking, which is defined as the consumption of five or more alcohol units (0.08 g/dl) for men and four or more alcohol units for women during a time episode of 2 h on at least one day in the past month (NIAAA, 2004; SAMHSA, 2016). Different longitudinal studies have demonstrated, that repeated excessive consumption of alcohol (e.g., binge drinking) leads to a higher risk of developing alcohol dependence and neuropsychological problems (Bourque et al., 2016; Jennison, 2004; Stolle, Sack, & Thomasius, 2009). Therefore, it is important to examine if binge drinking is, like alcohol dependence, accompanied by specific neuropsychological impairments, e.g., reward learning deficits.
1.1. Reward leaning in alcohol use disorders
One of the main causes for the addictive potential of alcohol is its stimulation of the brain reward system (BRS), particularly of the dopaminergic neurotransmission in the nucleus accumbens (NAc), which contributes to the subjectively experienced, rewarding effects of alcohol (Di Chiara, 1992; Di Chiara, 2002). The positive reinforcing effects include euphoria, pleasure, increased activity, and arousal (Koob, 2006). Stimuli which are associated with drug-taking behavior become themselves rewarding and attain high incentive salience (Robinson & Berridge, 1993). At the same time, the incentive salience of alternative reinforcers decreases (Garbusow et al., 2013; Robinson & Berridge, 1993, Volkow, Fowler, & Wang, 2003), and learning with non-drug reinforcing stimuli is impaired (Bühler et al., 2010). However, reward learning is not only relevant for alcohol dependence, but for sustained alcohol use in general. It is supposed, that already sustained risky alcohol consumption causes adaptive changes in mesolimbic regions, modulated by dopamine (DA). Postsynaptic DA-D2-receptor density is probably down-regulated and DA-release from VTA reduced (Volkow & Morales, 2015). Consequently, it was argued, that the resulting hypodopaminergic state might lead to a reduced sensitivity to natural reinforcers and other rewards (Hyman, Malenka, & Nestler, 2006; Volkow, Fowler, & Wang, 2002). Fitting to this, heavy social drinkers and alcohol dependent people reacted less to monetary reinforcers in behavioral impulsivity measures than light social drinkers and non-alcohol dependent people (Perry & Carrol, 2008).
The Incentive Sensitization Theory by Robinson and Berridge (2000) emphasizes the role of different associative learning processes in addiction. It focuses on drug-induced alterations in BRS circuitry and associated changes in motivational processes and associative learning (Everitt et al., 2008; Volkow & Morales, 2015). In their corresponding model of reward, Berridge, Robinson, and Aldridge (2009) distinguish three components of reward: liking, which is emotional and predominantly implicit; wanting, which is incentive motivational; and learning, which purveys the ability to predict reward, and hence forms the basis of wanting. Berridge et al. (2009) further distinguish between different associative learning processes that can be classified as explicit vs. implicit. Based on these models, we expect that implicit and explicit reward learning processes play distinct roles during development and maintenance of a dependence.
With regard to their neuropsychology, implicit and explicit reward learning involve distinct neural circuits (Frank & Claus, 2006). Frank and Claus (2006) proposed that the dopaminergic basal ganglia (BG) system mediates implicit, context-dependent response initiation based on the relative probability of positive or negative outcomes, hence implicit reward-dependent learning. The dopaminergic activity in the BG, particularly the ventral striatum, determines whether a response is executed or inhibited, according to the contingencies of the response (Nakanishi, Hikida, & Yawata, 2014). This is a slow, implicit associative learning process, where a positive outcome promotes a behavior and a negative outcome inhibits a behavior (Averbeck & Costa, 2017; Frank & Claus, 2006). Explicit response selection based on anticipated rewards, however, requires a top-down control of the dopaminergic activity in the BG by the orbitofrontal cortex (OFC), which provides estimates of reinforcement magnitudes activated in working memory. This explicit system is successful in estimating the true expected value of reward-related decisions and is fast in switching behavior when reinforcement contingencies change. Due to the alcohol-induced alterations in the BRS, including BG and OFC (Volkow, Fowler, Wang, & Goldstein, 2002; Volkow & Morales, 2015), implicit and explicit reward-related learning may be altered in chronic alcohol use. While neuropsychological deficits in reward learning and memory in alcohol dependence are well studied, less is known about impaired reward learning of alternative natural rewarding, non-alcohol related stimuli in repeated excessive alcohol use like binge drinking.
1.2. Previous research
To our knowledge, there are no behavioral studies that separately examined implicit and explicit reward learning in alcohol addiction or binge drinking using experimental tasks with non-drug-associated stimuli. Park et al. (2010) reported an impaired implicit reinforcement learning and an abnormal functional connectivity between DLPFC and striatum in people with alcohol dependence as compared to a control group. During the reinforcement learning task with probabilistic reward allocation, alcohol dependent patients needed more trials than the control group to meet a defined learning criterion. There are no behavioral studies yet on implicit reward learning in binge drinking, except in animal research. The existing studies only examined the impact of binge drinking on BRS on a neuronal level. But there are none investigating the behavioral level. Smith, Co, Mcintosh, and Cunningham (2008) found that repeated binge drinking causes a diminished DA-concentration in reward-related brain structures in rats. Similarly, Schulteis and Liu (2006) showed a temporary elevated reward-threshold as a result of repeated binge drinking in rodents.
Many studies demonstrated deficits in alcohol dependent patients in explicit reward related learning and reward related decision making (Galandra, Basso, Cappa, & Canessa, 2018; van Holst & Schilt, 2011; Yücel, Lubman, & Solowij, 2007), mainly tasks were applied with explicit instructions and choices such as Iowa Gambling Task (Bechara et al., 2001; Le Berre, Fama, & Sullivan, 2017; Rogers, Moeller, Swann, & Clark, 2010). Further, drug users, including smokers, alcoholics, cocaine users, and opiate addicts, performed more impulsively in such behavioral tasks (Vuchinich, Tucker, & Rudd, 1987; Bickel & Madden, 1999; Mitchell, 1999; Fillmore & Rush, 2002; Lejuez et al., 2003; Reynolds, Richards, Horn, & Karraker, 2004; Tanabe et al., 2009; Madden, Johnson, Brewer, Pinkston, & Fowler, 2010). However, evidence about the influences of binge drinking on explicit reward learning is inconsistent (Field, Wiers, Christiansen, Fillmore, & Verster, 2010; Lees et al., 2018). With the Iowa Gambling Task, Goudriaan, Grekin, and Sher (2007) found deficits in decision making in heavy binge drinkers only. Likewise, Rose and Grunsell (2008) found weak group-effects in a delay discounting task and no difference between binge drinkers and controls in a time estimation task. Lannoy, Maurage, D'Hondt, Billieux, and Dormal (2018) found no difference regarding inhibition abilities in a speeded Go/No-Go task between binge drinker and control participants.
1.3. Present research and predictions
Aim of the present study was to examine alterations in reward learning in alcohol use disorders. Besides distinguishing alcohol dependence along the DSM IV-criteria, repeated binge drinking and low risk alcohol use, we also considered two other relevant factors, namely explicit and implicit reward learning.
Impairments in explicit reward learning and reward based decision making are well studied in alcohol dependent patients. Studies are rare which investigate implicit reward learning of non-drug reinforcers. There is a study by Park et al. (2010), which has already shown deficits in a reinforcement learning task with probabilistic reward allocation in alcohol dependent patients. But regardless the probabilistic reward allocation in the task, the learning rule within each trial bloc was to be learned and therefore an explicit learning criterion had to be achieved. Accordingly, implicit reward learning in alcohol dependent patients has to be further investigated. The characteristic of implicit reward learning paradigms is, that the reward allocation rules are not recognized by participants, even though they steadily make better predictions (Knowlton, Squire, & Gluck, 1994). Therefore, it is important to investigate implicit and explicit reward learning in separate tasks. Furthermore, these separate functions are much less studied in binge drinkers. There are no studies about implicit reward learning and results in explicit reward based decision making tasks are inconsistent (Goudriaan et al., 2007; Bø, Aker, Billieux, & Landro, 2016; Lannoy et al., 2018; Lees et al., 2018; Rose & Grunsell, 2008). Therefore, we formulated hypothesis on the basis of neurobiological findings in alcohol use disorders.
Due to the neurobiological, empirical supported assumptions of a reduced number of dopaminergic D2 receptors, the generally dampened dopamine neurotransmission, and the reduced OFC regulated behavioral control, we expected impaired implicit and explicit reward learning in dependent and bingeing alcohol drinkers as compared to a control group of low-risk alcohol drinkers. We further hypothesized that the longer the duration of an alcohol use disorder, the lower the task performance.
2. Material and methods
2.1. Participants
The clinical sample consisted of n = 26 alcohol dependent patients from the German Salus-Clinic Lindow. The alcohol cessation treatment in Lindow usually follows the detoxification treatment in an acute hospital or a supervised or self-initiated withdrawal, before a patient is admitted into the clinic. Patients were only then asked to participate in the study, when the acute withdrawal was concluded. Accordingly, at the time of testing the patients in the alcohol dependent group were abstinent only some days or a few weeks. The n = 82 non-dependent participants were recruited with flyers, emails and in personal conversations in bars, cafés and at the university campus in Halle/Saale. All participants signed informed consent prior to the study. The study was accomplished in compliance with the declaration of Helsinki.
Four alcohol dependent patients with a comorbid depression and one with poly drug use were excluded from analysis. From the non-dependent subjects, three with a non-bingeing risky alcohol use, three with a chronic cannabis misuse, one with a phobic disorder, one with obsessive thoughts, and one with an anamnestic legasthenia were excluded from the analysis.
The final sample (n = 94) consisted of three groups: n = 36 low-risk alcohol drinkers, n = 37 binge drinkers, and n = 21 abstinent alcohol dependent patients fulfilling the DSM-IV criteria for alcohol dependence (Saß, Wittchen, & Zaudig, 1998). Binge drinking was defined according to the criteria of the US National Institute on Alcohol Abuse and Alcoholism (NIAAA, 2004; see also SAMHSA, 2016), that is four or more alcoholic drinks for women and five or more for men within 2 h, at least twice a month, and since the last six months or longer. These participants were visitors of alcohol selling bars, cafés and restaurants or students of the Martin-Luther-University Halle-Wittenberg and their relatives and acquaintances. Inclusion criteria for the binge drinking group were absence of an alcohol dependence according to the DSM IV criteria or another risky alcohol use beyond the recommendations of the German Health Ministry for a low-risk alcohol use (John et al., 2018; Keller, Maddock, Laforge, Velicer, & Basler, 2007). We defined low-risk alcohol use according to the recommendations of a maximal daily limit of alcohol use (no more than one daily alcoholic drink for women and no more than two for men on no more than on five days a week) of the German Health Ministry (John et al., 2018; Keller et al., 2007). Following Keller et al. (2007), we defined an alcoholic drink as one bottle of beer (0.33 l), one glass of wine or champagne (0.15 l), one glass of spirits or hard liquor (0.04 l), one bottle of alcopop (0.33 l), one cocktail or long drink.
Sex distribution did not differ between the groups. In the low-risk-group there were exactly the same number of women and men. In both of the other groups were five women less than men (Table 1). The groups differed regarding to age (Table 2). On average, the alcohol dependent patients were twice the age of binge drinkers and low-risk alcohol users.
Table 1.
Sex, education, and daily cigarette consumption for each group; last and second to the last columns depict results of group comparisons.
| (N = 108) | Low-risk drinker (n = 36) |
Binge drinker (n = 37) |
Dependent drinker (n = 21) |
Test statistics |
|
|---|---|---|---|---|---|
| Frequency | Frequency | Frequency | χ2 | p | |
| Gender | |||||
| Male | 18 | 21 | 13 | 0.81 | >.67 |
| Female | 18 | 16 | 8 | ||
| Highest educational outcome⁎⁎⁎ | |||||
| Occasional jobs | 1 | 0 | 1 | 32.31 | <.001 |
| Professional training | 7 | 4 | 14 | ||
| Middle leadership/students | 27 | 33 | 4 | ||
| Higher management/entrepreneur | 1 | 0 | 2 | ||
| Daily cigarette use⁎⁎⁎ | |||||
| None | 30 | 19 | 7 | 32.56 | <.001 |
| 1–10 | 5 | 13 | 3 | ||
| 11–20 | 1 | 4 | 6 | ||
| 21–30 | 0 | 0 | 3 | ||
| >31 | 0 | 1 | 2 | ||
p < .001.
Table 2.
Means, standard deviations and test statistics of age, crystallized, and fluid intelligence, neuropsychological variables, (attention, short-term- and working memory), mean depression score (BDI), and alcohol drinking variables separated for groups.
| (N = 108) | Low-risk drinker (n = 36) |
Binge drinker (n = 37) |
Dependent drinker (n = 21) |
Test statistics |
||||
|---|---|---|---|---|---|---|---|---|
| M | (SD) | M | (SD) | M | (SD) | F/t | p | |
| Age*** | 21.92 | (2.81) | 22.22 | (2.03) | 47.67 | (9.38) | 22.50 | <.001 |
| Vocabulary test | 30.36 | (4.07) | 29.89 | (2.27) | – | – | 0.37 | =.54 |
| Cryst I (LPS I) | 46.89 | (9.85) | 46.05 | (7.08) | – | – | 0.17 | =.68 |
| Fluid I (LPS II + III) | 31.25 | (6.33) | 29.46 | (4.94) | – | – | 1.82 | =.18 |
| STM (DSfw) | 8.25 | (1.81) | 8.00 | (1.55) | 8.81 | (1.63) | 1.58 | =.21 |
| WMn (DSbw) | 7.67 | (2.00) | 7.78 | (1.80) | 6.57 | (2.38) | 2.71 | =.07 |
| Executive attention (CWIT) | ||||||||
| °Reading*** | 26.61 | (3.29) | 26.93 | (3.96) | 32.10 | (4.21) | 16.29 | <.001 |
| °Naming*** | 39.35 | (5.41) | 39.99 | (5.79) | 47.34 | (5.88) | 15.05 | <.001 |
| °Interference*** | 64.88 | (9.51) | 67.40 | (10.47) | 91.71 | (17.47) | 37.06 | <.001 |
| BDI score*** | 3.17 | (3.03) | 4.59 | (3.37) | 13.33 | (8.78) | 29.86 | <.001 |
| Drinking variables | ||||||||
| °Alc drinks/m*** | 9.84 | (8.52) | 56.02 | (29.52) | – | – | −7.04 | <.001 |
| °Days alc use/m*** | 3.85 | (3.18) | 9.87 | (4.24) | – | – | −6.85 | <.001 |
| °Dur of alc use*** | 7.89 | (7.21) | 5.99 | (2.32) | 21.24 | (11.72) | 32.13 | <.001 |
| Dur of dependence | – | – | – | – | 13.33 | (2.52) | ||
| Cigarettes/day*** | 1.22 | (3.04) | 5.45 | (8.16) | – | – | −2.90 | =.005 |
Notes. np < .10, *p < .05; ***p < .001; alc drinks/m alcoholic drinks per mont; BDI Beck Depression Inventory; CWIT Colour-Word-Interference-Test; cryst cristallized; days alc use/m days with alcohol use per month; DSbw digit span backward; DSfw digit span forward; Dur duration (years); I intelligence; M mean; np = .10; LPS Leistungsprüfsystem; STM short term memory; SD standard deviation; WM working memory.
2.2. Psychological testing
Psychological testing was performed in the Department of Psychology (Martin-Luther-University Halle-Wittenberg) or in the Salus-Clinic Lindow. The examination lasted up to two and a half hours. After the assessment of biographical and clinical data, subjects performed the implicit and explicit reward learning tasks, and the Stroop task. After a short break, subjects were tested for short-term memory, working memory, attention, crystallized and fluid intelligence, followed by a semi-structured clinical interview and several clinical questionnaires (The Alcohol Use Disorders Identification Test AUDIT, Babor, Biddle-Higgins, Saunders, & Monteiro, 2001; The Health Behavior Survey HFS, Keller et al., 2007; Fagerstrøm Tolerance Questionnaire FTND, Heatherton, Kozlowski, Frecker, & Fagerstrom, 1991).
2.2.1. Implicit reward learning
To test implicit reward learning, we used the Ice-Cream-Seller-Task (IST, adapted after Shohamy et al., 2004), a probabilistic classification learning task (Knowlton et al., 1994) with two conditions: one implicit feedback condition and one explicit observation condition in a between-subjects-design. Subjects had to imagine they were an ice cream seller. They saw a puppet – the customer – in one of 14 configurations, resulting from the combination of four cues: a hat, glasses, a moustache and a bow tie. These cues were linked to the preference of the customer puppet for vanilla or chocolate ice cream. The task of the subjects was to predict the preferred ice cream. They received visual and monetary feedback after their verbal response. While in the beginning the subjects were only guessing, their predictions improved during the experiment. Subjects learned gradually which of the two outcomes (vanilla or chocolate ice-cream) would appear on each trial, given the distinct combination of cues (Knowlton, Mangels, & Squire, 1996). Following Shohamy and colleagues, each single cue was independently and probabilistically related to the outcome and the complex, probabilistic structure of the task prevented the verbalization or memorization of learning rules; that is, this task actually requires and thus, tests implicit learning (Shohamy et al., 2004).
A second, observational condition with an explicit learning instruction served to compare explicit and implicit learning (feedback condition). Here, the subjects observed only the puppet and its choice during the first 100 trials. They were explicitly instructed to learn which customer prefers which ice cream. During the second 100 trials, the subjects had to name the preferred ice cream without any feedback.
2.2.1.1. Stimuli
The cues were features (hat, glasses, moustache and/or a bow tie) of a plasticine puppet (Fig. 1) holding a vanilla or chocolate ice cream in its left hand. Stimuli were photographed using a digital camera and then combined into 14 configurations (A–N) for each of the possible outcomes (vanilla, chocolate ice-cream). All configurations were displayed on a beige background. The 14 different configurations per ice cream type were presented randomly in two blocks of 100 stimuli following a scheme modelled after Shohamy et al. (2004). The order was fixed for all subjects.
Fig. 1.
Plasticine puppet – the customer in the ice-cream parlor, created by the artist Thorsten Drössler – with four different cues and two possible outcomes (IST).
In total, both outcomes (vanilla and chocolate ice-cream) had the same frequency and a fixed, complementary probability per each cue: P(vanilla/hat present) = .80, P(vanilla/glasses present) = .60, P(vanilla/moustache present) = .40 and P(vanilla/bowtie present) = .20. The probability of the second outcome P(chocolate/cue present) amounted to 1 − P(vanilla/cue present). For example, cue 1 (hat present) was part of seven configurations and appeared in 100 trials; in 80 of these trials the outcome was vanilla ice cream and in 20 of these trials the outcome was chocolate ice cream.
2.2.1.2. Procedure
Subjects were seated in front of a computer screen at a comfortable viewing distance. They received a German translation of the experimental instruction taken from Shohamy et al. (2004). The configurations were presented on a 17-inch PC-notebook.
In the implicit feedback condition, an exemplification was presented after the instructions. Then, the subject could start the experiment by pressing the space bar. On each of the 200 trials the subject was asked “Which flavor do you think he wants?”. They had to respond by saying the German words for vanilla (“Vanille”) or chocolate (“Schokolade”). If the subject did not respond within 2 s, a reminder appeared “Please, answer now!” If the subject did not respond within the next 3 s, the correct answer was shown and this trial was rated as not solved correctly. After each correct answer the subjects received monetary reward of one Euro cent. The next trial started with a prompt “To proceed, press the space bar, please!”. The experimenter sat opposite to the subject, noted the responses and provided the reward.
The observation condition consisted of two phases, an observational and a testing phase. The subjects received a German translation of the experimental instruction taken from Shohamy et al. (2004). In each of the 100 trials of the observation phase, the customer puppet appeared for 5 s holding his favorite ice cream in the left hand. The order of observational trials was identical to that in the implicit feedback condition. After the last observational trial, the instruction for the 100-trial test phase appeared on the screen. The procedure of the test phase was similar to the second half (trial numbers 101–200) of the implicit feedback condition, except that no visual and monetary feedback was provided. The experimenter sat opposite of the subject and noted the responses.
We computed relative frequencies of optimal responses. A response was valued optimal if it matched the more probable outcome of the configuration (cf. Knowlton et al., 1994). An individual relative frequency was included if the participant responded above the guessing probability. To differentiate explicit and implicit learning, relative frequencies of the second half of the implicit feedback condition (100 trials) were compared to the 100 trials of the testing phase in the observation condition.
2.2.2. Explicit reward learning
To test explicit reward learning, we used a trial-and-error discrimination task with 80 playing cards with eight different, two-digit numbers (Card-Playing Task, CPT; Newman, Widom, & Nathan, 1985). All stimuli were presented ten times in random order. The participants received a German translation of the experimental instruction used by Newman et al. (1985). They had to learn, explicitly by trial and error, which of the eight numbers were target (S+) or non-target (S−) stimuli. Participants responded by tapping the targets and non-tapping the non-targets. The stimulus cards, one at a time, were placed in front of the subjects, and subjects had approximately 2 s to respond before the next card was presented. A response was recorded each time that a subject tapped a card with his or her finger (Newman et al., 1985).
There were two conditions. In the passive avoidance with loss of reward condition (PALR), subjects were rewarded with 1 Euro cent for each correct response (tapping S+) and punished by withdrawing 1 Euro cent for each wrong response (tapping S−). No rewards were won or lost when a subject did not respond. The reward for response inhibition condition (RRI) involved the same discrimination task. In contrast to the PALR condition, subjects were rewarded with 1 Euro cent for each inhibited response. Hence, subjects were rewarded for correct responses (tapping S+) and inhibited incorrect responses (not tapping S−); they were not punished for incorrect responses (tapping S−). To minimize practice, sequence, and interference effects we used eight new, randomly generated numbers for both conditions. We analyzed omission (OE) and commission (CE) errors. The number of omission errors reflected the tendency to avoid punishing stimuli and the number of commission errors the tendency to approach rewarding stimuli (Yechiam et al., 2006).
2.2.3. Neuropsychological assessment
To monitor the performance in explicit and implicit reward learning for common reported deficits of people with alcohol use disorders in memory and attention, and for differences in intelligence we used the following psychological tests.
2.2.3.1. Attention
Attention was measured using the three subtests reading, naming, and interference of the Stroop colour word interference test (FWIT, Bäumler, 1985).
2.2.3.2. Memory
Short-term- and working memory were measured with the forward and backward memory span of the Revised Wechsler Memory Scale (WMS-R, Markowitsch, Neufeld, Calabrese, Deisinger, & Kessler, 2000).
2.2.3.3. Intelligence
Premorbid intelligence was tested using a word recognition test (MWT-B Lehrl, 1995), which is functionally equivalent to the widely used NART test (Nelson & O'Connel, 1978). Crystallized and fluid intelligence were assessed with subtests 1 + 2, 3 from (LPS, Horn, 1983; Sturm, Willmes, & Horn, 1999), a German standard intelligence scale.
2.2.4. Addiction related measures
The daily nicotine consumption was measured with one question from the Fagerstrøm Tolerance Questionnaire (FTND, Heatherton et al., 1991). This variable was used as a covariate, because a prior study (Paelecke-Habermann, Paelecke, Reschke, Giegerich, & Kuebler, 2013) showed significant effects on implicit and explicit reward learning. Alcohol addiction and consumption related variables were measured with the following questionnaires and interviews. Data about alcohol use and abuse were collected with a custom-made structured interview. The dependence related questions were taken from the SCID (Wittchen, Wunderlich, Gruschwitz, & Zaudig, 1997), the CIDI (Composite International Diagnostic Interview, Wittchen & Semmler, 1990), and the AUDIT (The Alcohol Use Disorders Identification Test, Babor et al., 2001). All non-dependent participants filled in the Health Behavior Survey (HFS, Keller et al., 2007) which included questions regarding all substance related disorders.
2.2.5. Clinical psychological assessment
The presence of comorbid axis-1 disorders was assessed with the SCID (Wittchen et al., 1997). We asked for the number of consumed alcoholic drinks per month. Depressive symptoms were quantified with the German version of the Beck Depression Inventory (BDI, Hautzinger, Bailer, Worall, & Keller, 2000). This was necessary, because depressive symptoms could have an effect on implicit and explicit reward learning (Paelecke-Habermann, 2009; Whitton, Treadway, & Pizzagalli, 2015).
2.3. Statistical analysis
All analyses were corrected for age, BDI and daily cigarette consumption, when there was a significant influence in the analysis. For the ice cream seller task (IST) we calculated an analysis of covariance (ANOVA/ANCOVA) with task condition (feedback vs. observation) and group (low risk drinkers vs. binge drinkers and alcohol dependent patients) as between subject factors. To examine the reward dependent learning progress, we calculated a repeated measures ANOVA/ANCOVA for the first and second half of the feedback condition with group as between subject factors.
For the explicit learning task, we conducted 2 × 3 ANOVA/ANCOVAs with task (PALR, RRI) as within and group as between subject factors for each of the three dependent variables (reaction time, omission errors, commission errors) and the relevant covariates (age, depressive symptoms via BDI, daily nicotine consumption). A priori planned contrasts (one-tailed t-tests) were calculated to test the directional hypotheses (low risk drinkers vs. binge drinkers and alcohol dependent patients, PALR vs. RRI, omission vs. commission errors).
We examined comparability of the groups in biographical, neuropsychological, substance related, and clinical variables using ANOVAs or student's t-tests. Parametric correlations were used to check for the relationship between dependent and control variables (age, BDI, premorbid, crystallized and fluid intelligence, interference, short-term- and working memory) and substance related variables (duration of alcohol use, duration of dependence). The levels of statistical significance of correlation coefficients were adjusted for multiple comparisons (ten for each dependent variable) by the Bonferroni-Holm procedure (Holm, 1979).
Where an a priori hypothesis was established, test for significance were one-tailed, otherwise two-tailed. The significance level was set to alpha = 0.05. The effect size parameters d (0.20 < d < 0.50 = small, 0.50 < d < 0.80 = medium, 0.80 < d = large), r (0.20 < r < 0.50 = small, 0.50 < r < 0.80 = medium, 0.80 < r = large), and η2 (0.01 < η2 < 0.06 = small, 0.06 < η2 < 0.14 = medium, 0.14 < η2 = large) are reported for the univariate and multivariate tests (Cohen, 1988).
3. Results
3.1. Clinical and control variables
Distribution of education and daily cigarette consumption differed between groups (Table 1). Mean scores and standard deviations of age, intelligence, neuropsychological variables and clinical features in the three groups are listed in Table 2. The three groups differed with regard to age, short-term-memory, working memory, attention (FWIT, Bäumler, 1985), depression (BDI, Hautzinger et al., 2000), daily cigarette consumption, and the duration since first alcohol use. Overall groups the duration of use was positively correlated with age (r = 0.64; p ≤ .001). The single group analysis revealed a significant correlation only for the binge drinking group (r = 0.79; p ≤ .001).
Binge drinkers and low-risk drinkers performed comparably in a German vocabulary test (MWTB, Lehrl, 1995) and a crystallized and fluid intelligence test (LPS, Horn, 1983; subtests 1 + 2/3), but differed with respect to the number of alcoholic drinks per month and the number of days with alcohol use per month.
3.2. Implicit reward learning
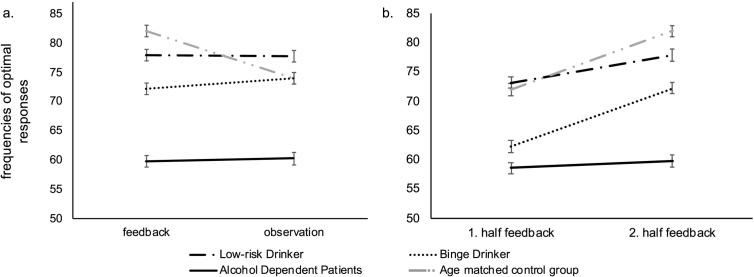
Means, standard deviations, and standard errors of the mean for frequencies of optimal responses, are listed in Table 3 for groups and conditions of the IST. Fig. 2a/b depicts means and standard errors of the mean frequencies of optimal responses for the three groups and both conditions.
Table 3.
Means and standard deviations of frequencies of optimal responses in the IST and correct responses and error rates in the CPT.
| (N = 94) | Low-risk drinker (n = 36) |
Binge drinker (n = 37) |
Dependent drinker (n = 21) |
|||
|---|---|---|---|---|---|---|
| M | (SD) | M | (SD) | M | (SD) | |
| Implicit reward learning (IST) | ||||||
| Fre FB 1.H | 73.16 | (11.20) | 62.24 | (13.48) | 57.70 | (6.04) |
| Fre FB 2.H | 77.89 | (10.98) | 72.24 | (7.56) | 59.78 | (5.82) |
| Fre OB | 77.77 | (10.20) | 74.00 | (10.37) | 60.25 | (9.91) |
| Explicit reward learning (CPT) | ||||||
| CR PALR | 58.75 | (9.03) | 58.05 | (9.19) | 50.43 | (9.24) |
| OE PALR | 14.31 | (8.93) | 14.49 | (8.93) | 17.86 | (9.99) |
| CE PALR | 6.92 | (4.64) | 7.46 | (4.64) | 11.71 | (7.87) |
| CR RRI | 62.00 | (9.76) | 62.22 | (8.62) | 49.14 | (9.29) |
| OE RRI | 7.25 | (5.05) | 8.95 | (5.05) | 14.86 | (6.61) |
| CE RRI | 10.72 | (6.94) | 8.84 | (6.94) | 16.00 | (5.20) |
Notes. CE commission error; CR correct reactions; FB feedback condition [IST]; Fre relative frequency; H test half; IST Ice cream-Seller Task; M mean; PALR Passive avoidance with loss of reward; OB observation condition; OE omission error; RRI Reward for response inhibition; SD standard deviation.
Fig. 2.
a/b. Corrected means and standard errors of the mean frequencies of optimal responses in the IST for the three groups (black: Low-risk Drinker, Binge Drinker, Alcohol Dependent Patients). The light grey lines show means and standard errors of the mean frequencies of a healthy group of middle-aged participants from another study (unpublished data, Michel, Paelecke-Habermann, & Leplow, 2008). These data were used for discussion of the results only. a: feedback vs. observation condition b: first vs. second half of the feedback condition.
The 2 (condition) × 3 (group) ANCOVA yielded no interaction of condition and group and no main effect of condition, but a significant main effect of group (F (2, 83) = 20.49; p = .000; η2 = 0.33; 1 – β = 1.00; Fig. 2a). Alcohol dependent patients and binge drinkers learned less than low-risk drinkers in both conditions (contrast 1 0–1: F (2, 83) = 17.82; p = .000; η2 = 0.33; 1 − β = 1.00; contrast 0 1–1: F (2, 83) = 4.71; p = .04; η2 = 0.05; 1 − β = 0.54). Furthermore, alcohol dependent patients learned less than binge drinkers (contrast 0 1–1: f (2, 83) = 4.39; p < .001; η2 = 0.21; 1 − β = 0.97).
The repeated measures ANOVA proved a significant effect of learning (F (1, 47) = 13.15; p = .001; η2 = 0.34; 1 – β = 1.00; Fig. 2b), group (F (1, 47) = 12.37; p = .000; η2 = 0.35; 1 − β = 0.99) and a significant interaction between learning and group (F (1, 47) = 3.47; p = .04; η2 = 0.35; 1 – β = 0.62). None of the covariates were significant. The duration of alcohol abuse (binge drinker and alcohol dependents) significantly correlated with the mean frequency of optimal responses in the feedback condition (r = −0.54; p = .001). There were no significant correlations between control and dependent variables after Bonferroni-Holm correction.
3.3. Explicit reward learning
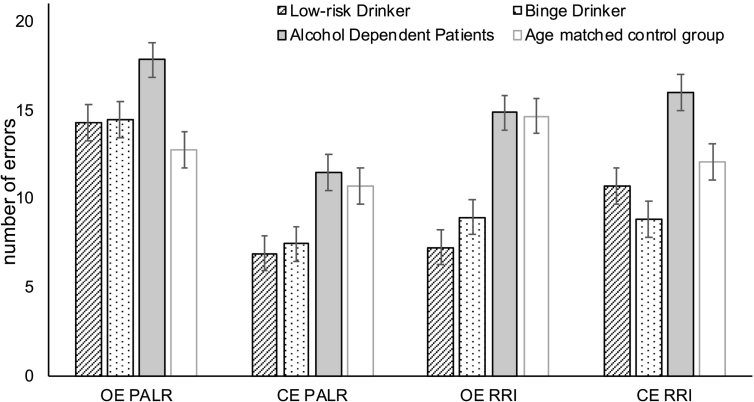
Means and standard deviations of the mean for correct reactions and omission and commission errors, for each group and both conditions of the CPT are listed in Table 3. Fig. 3 depicts means and standard errors of the error rates for groups and conditions.
Fig. 3.
Corrected means and standard errors of the error rates for the groups (black: Low-risk Drinker, Binge Drinker, Alcohol Dependent Patients) and both conditions of the CPT. The light grey bars show means and standard errors of the mean error rates of a healthy group of middle-aged participants from another study (unpublished data, Michel et al., 2008). These data were used for discussion of the results only. CE = commission error; CPT Card Playing Task; OE = omission error; PALR = passive avoidance with loss of reward; RRI = reward for response inhibition.
3.3.1. Correct reactions
The 2 (condition) × 3 (group) repeated measures ANCOVA yielded a significant effect of group (F (1, 90) = 8.86; p = .000; η2 = 0.16; 1 − β = 0.97) but no main effect of condition and no interaction effect. Alcohol dependent patients showed less correct reactions than low risk and binge drinkers (contrast 1 0–1: F (1, 90) = 12.54; p = .001; η2 = 0.12; 1 − β = 0.94; contrast 0 1–1: F (1, 90) = 16.70; p = .000; η2 = 0.16; 1 − β = 0.98). Binge drinker and low risk drinker did not differ significantly. Cigarette consumption influenced the number of correct reactions such that the higher the number of cigarettes per day, the lower was the frequency of optimal responses (F (1, 90) = 3.25; p = .08; η2 = 0.04; 1 − β = 0.43). The duration of the alcohol use disorder (binge drinker and alcohol dependents) significantly correlated with the number of correct responses in the RRI condition (r = −0.42; p = .001). There were no significant correlations between control and dependent variables after Bonferroni-Holm correction.
3.3.2. Omission errors
The 2 (condition) × 3 (group) repeated measures ANCOVA yielded a main effect of condition (F (1, 89) = 18.13; p = .000; η2 = 0.17; 1 − β = 0.98) and group (F (1, 89) = 18.13; p = .000; η2 = 0.17; 1 − β = 0.98), but no interaction effect. Alcohol dependent patients showed more omission errors than low risk and binge drinkers (contrast 1 0–1: F (1, 91) = 8.87; p = .004; η2 = 0.09; 1 − β = 0.84; contrast 0 1–1: F (1, 90) = 8.82; p = .004; η2 = 0.09; 1 − β = 0.84). Binge drinker and low risk drinker did not differ significantly. Cigarette consumption and the BDI score influenced the number of omission errors such that the higher the number of cigarettes per day and the BDI score, the higher was the number of omission errors (Cigarettes: F (1, 89) = 6.26; p = .01; η2 = 0.07; 1 − β = 0.70; BDI: F (1, 89) = 6.01; p = .02; η2 = 0.06; 1 − β = 0.68). Alcohol dependent patients tendentially made more omission errors in the PALR condition than in the RRI condition (t(21) = 1.39; p = .09; difference: M = 3.00, SE = 2.16). The duration of alcohol abuse (binge drinker and alcohol dependents) significantly correlated with the number of omission errors in the RRI condition (r = −0.36; p = .003). There were no significant correlations between control and dependent variables after Bonferroni-Holm correction.
3.3.3. Commission errors
The 2 (condition) × 3 (group) repeated measures ANCOVA yielded significant main effects of condition (F (1, 91) = 19.87; p = .000; η2 = 0.18; 1 − β = 0.99) and group (F (1, 91) = 10.73; p = .000; η2 = 0.19; 1 − β = 0.99), but no interaction effects. Alcohol dependent patients showed more omission errors than low risk drinkers (contrast 1 0–1: F (1, 91) = 15.05; p = .000; η2 = 0.14; 1 − β = 0.97; contrast 0 1–1: F (1, 90) = 19.52; p = .000; η2 = 0.18; 1 − β = 0.99). Binge drinker and low risk drinker did not differ significantly. Alcohol dependent patients made significantly more commission errors in the RRI condition than in the PALR condition (t(21) = 2.47; p = .02; difference: M = 4.29, SE = 1.74). The duration of alcohol abuse (binge drinker and alcohol dependents) significantly correlated with the number of omission errors in the RRI condition (RRI: r = 0.34; p = .004). There were no significant correlations between control and dependent variables after Bonferroni-Holm correction.
4. Discussion
4.1. Summary of results
Our investigation aimed at testing for reward learning deficits in people with alcohol use disorders. We compared alcohol dependent patients, binge drinkers, and low risk drinkers with regard to reward related learning and we were able to differentiate between implicit and explicit reward learning, which recruit different brain structures (Frank & Claus, 2006). We found deficits for both kinds of reward learning in alcohol dependent patients and an impaired implicit reward learning even in binge drinking.
Alcohol dependent patients and binge drinking participants performed worse in the implicit reward learning task as compared to low risk drinking participants. Furthermore, we found a negative correlation of implicit learning performance with the duration of alcohol use in binge drinking and dependent participants. This result supports the assumption of impaired implicit reward learning in alcohol use disorders.
Likewise, alcohol dependent patients performed worse in the explicit reward learning task than binge and low risk drinking participants. They made more omission and commission errors than the other groups in both conditions of the explicit reward learning task. Compared to the other groups, alcohol dependent patients generally reacted more disinhibited, i.e., more commission errors, and even more so when commission errors were not punished as in the reward for response inhibition condition. The duration of alcohol use in binge drinking and dependent participants was correlated with the number of omission and commission errors. These results support the assumption of an impairment in explicit reward learning in alcohol use disorders.
We found no difference in explicit reward learning between the binge drinking and low-risk drinking group, yet negative correlations of the explicit reward learning performance with the duration of alcohol use in the binge drinking and alcohol dependent alcohol use disorders. Possibly, explicit reward learning in people with alcohol use disorders decreases more slowly over the years and, thus, not only implicit but also explicit reward learning processes are affected. This is an important question, which has to be examined in a future longitudinal study.
4.2. Implicit vs. explicit reward learning in dependent and binge drinking participants
Our study expands on previous findings: Firstly, we were able to differentiate between implicit and explicit reward learning, which both recruit different brain structures (Frank & Claus, 2006). We found deficits for both kinds of reward learning in alcohol dependent patients. Secondly, only few studies to date compared reward related functions in alcohol dependent patients and binge drinking people to low risk drinking participants. Thirdly, our results point to an impaired implicit reward learning even in binge drinking. This supports the assumption, that binge drinking people are at a higher risk of becoming alcohol dependent than low risk drinking people (Grüsser, Mörsen, & Flor, 2006; Jennison, 2004; McCarty et al., 2004; Stolle et al., 2009). But only a longitudinal study with young binge drinking persons could give more information about the effects of repeated binge drinking on reward learning over time (Bourque et al., 2016).
Our study complements previously reported results on reward learning deficits in alcohol dependent patients (Beck et al., 2009; Lane, Yechiam, & Busemeyer, 2006; Loeber & Duka, 2009; Mackillop et al., 2010; Park et al., 2010; Richards, Zhang, Mitchell, & de Wit, 1999; Wrase et al., 2007). Chronic alcohol use affects the tonic, and phasic DA signals in the BRS (Di Chiara, 1999; Volkow et al., 2007). In line with our results, Makris et al. (2008) reported a decreased total reward-network volume in alcoholic subjects. Phasic DA signals indicate the expectation or the unexpected attainment of a reward (Schultz, 2002). If this phasic DA signaling is disturbed, then implicit reward learning is possibly also impaired. Sevy et al. (2006) found implicit reward learning deficits caused by a blockade of DA-transmission, which can be compared with the reduced DA-activity in alcohol dependents. Our findings are in line with these neurobiological findings of dopaminergic changes in chronic alcohol consumption and repeated bingeing. It seems that a reduced ability to learn implicit from non-drug reinforcers is a relevant factor for the development of an addiction. But the question if an impaired reward learning is a risk factor or a consequence for misusing alcohol remains unclear.
Alcohol dependent subjects showed deficits in explicit learning of information about reward and punishment. Accordingly, we found a decreased behavioral inhibition in both conditions of the explicit reward learning task. These results are in line with the well-confirmed assumption of a reduced frontal control in addiction (Jentsch & Taylor, 1999). This assumption was supported by many neurobiological (Di Chiara, 1999; Volkow & Fowler, 2000; Volkow, Fowler, & Wang, 2002; Volkow, Fowler, Wang, & Goldstein, 2002) and behavioral studies. Deficits were reported in dependent (Bechara et al., 2001; Goldstein, Volkow, Wang, Fowler, & Rajaram, 2001; Goudriaan, Oosterlaan, de Beurs, & van den Brink, 2006a; Lane et al., 2006) and binge drinkers (Goudriaan et al., 2007) and in heavy compared to light drinkers (Nederkoorn, Baltus, Guerrieri, & Wiers, 2009). Studies using the Iowa Gambling Task (Bechara et al., 2001) reported a lower performance of dependent patients, which was interpreted as a behavioral disinhibition and a higher preference for faster, but more disadvantageous decisions in contrast to delayed, but more advantageous rewards. In our study, we distinguished between two experimental conditions, reward with (PALR) and without (RRI) punishment. The higher number of commission errors in both conditions of the explicit reward learning task in the dependent group suggests disinhibited behavior which could be a result of incomplete processing of stimuli indicating reward and punishment. Accordingly, alcohol dependent patients report often about automated, non-conscious drug taking behavior, which cannot be controlled or only with great effort (Stacy & Wiers, 2010).
We found no major differences between binge and low-risk drinkers in explicit reward learning. This is in line with the fact, that these users can control their drug-taking behavior in daily life. Most of them reported an intention to alcohol bingeing in specific situations mostly together with peers (Oei & Morawska, 2004). In a study of Bø et al. (2016) the binge score could not predict inhibition performance in a stop signal-task, but an impairment in response adjustment after errors in this task. Rose and Grunsell (2008) and Lannoy et al. (2018) also found no association of binge drinking with impulsivity task performance. Using the Iowa Gambling Task (IGT) Goudriaan et al. (2007) only found deficits in decision making in heavy binge drinkers. However, the group of heavy binge drinkers was characterized by a higher number of other mental disorders. This limitation does not allow for an appropriate inference of binge drinking effects on reward-related decision making (Goudriaan et al., 2007). Although most studies suggest no meaningful deficits in explicit learning in binge drinkers, we found a correlation between both, the error rates (positive) and correct responses (negative) with the duration of alcohol use in non-dependent drinkers including binge drinkers and low risk drinkers. Thus, we argue that deficits in explicit reward learning evolve gradually with consumption (Koob, 2003). Our results are in line with the reward model of Berridge and Robinson (2003). The model assumes that addictive behavior is learned implicitly, but in the beginning, drug-taking behavior underlies deliberate frontal control of behavior. With continuous high risk alcohol consumption such as seen in binge drinkers frontal control is continuously reduced.
4.3. Neurobiological implications for binge drinking
The present paper showed an impaired implicit reward learning in people who are binge drink. This could be interpreted as an evidence for a decreased sensitivity and implicit processing of substance-unrelated reinforcers (Volkow, Fowler, & Wang, 2002; Volkow, Fowler, Wang, & Goldstein, 2002; Blum, Gardner, Oscar-Berman, & Gold, 2012). This result points to behavioral deficits, which are probably related to neuroadaptive processes within the dopaminergic BRS. The impairment in implicit learning during the presentation of substance-unrelated reinforcers might be related to a down-regulation of dopaminergic receptors (Koob, 2006; Volkow & Morales, 2015) and a diminished phasic DA-neurotransmission (Schultz, Tremblay, & Hollerman, 2000; Keiflin & Janak, 2015).
With regard to explicit reward learning, our results indicate an intact executive control of reward-dependent behavior in people who are binge drink. Their explicit response selection, based on anticipated non-drug rewards, which requires a regulation of the dopaminergic activity in the BG by the OFC (Frank & Claus, 2006; Pauli, Hazy, & O'Reilly, 2012), may be still intact. Possibly, the controlling function of OFC still ensures adaptive and flexible decision making as well as goal-directed behavior in non-alcohol associated situation in non-alcohol associated situations in binge drinking alcohol users (Frank & Claus, 2006). Thus, we were able to demonstrate differential effects of binge drinking on explicit and implicit reward learning.
4.4. Limitations
As the three groups differed in age, we controlled all analyses for age. Age showed no significant influence within the analysis. The control group was matched to the binge drinking group but not to alcohol dependent patients. Yet, the performance of alcohol dependent patients is in line with previous research (Galandra et al., 2018). Nonetheless, aging goes along with a decline in the structural integrity of the prefrontal cortex and significant losses of dopamine receptors and transporters, which could compromise the dopaminergic modulation (Dreher, Meyer-Lindenberg, Kohn, & Berman, 2008). Particularly since we found a correlation of the duration of alcohol use with our dependent variables in alcohol dependents and binge drinking people. Therefore, it can be assumed that aging might have also an influence on cortical reward processing, as seen in suboptimal financial decision making (Samanez-Larkin, Kuhnen, Yoo, & Knutson, 2010). On the other hand, a correlation between age and the duration of alcohol use was only found in binge drinkers but not in alcohol dependent patients. Furthermore, for an illustrating effect we used the data of a healthy middle-aged group from a study examining age effects on implicit and explicit reward learning. In the graphical presentation of the results (light grey lines/bars in Fig. 2, Fig. 3) it is shown, that the performance scores of this healthy middle-aged group in implicit reward learning are much higher than these of the alcohol dependent patients in most of the conditions. This would be an argument that age is not the only explaining factor for the group differences in our results, and that indeed alcohol dependence and binge drinking did have a significant effect on the reward learning performance. Nevertheless, it was another study with another purpose and therefore we cannot make valid conclusions from these data. A second one to one age-matched control group would be necessary to disentangle the influence of age from that of alcohol dependence on reward learning. Nonetheless, it would be not possible to find an age matched group of binge drinking participants, because binge drinking is most common in the young-adulthood between age of 21–25 years and after that age gradually falls of Winograd and Sher (2015). Compared to this, alcohol dependent patients who are in psychotherapeutic treatment, are inevitably older. However, our conclusions regarding to binge and low-risk drinkers are not affected hereby, because both groups were comparable in age.
We found a significant difference in daily cigarette use between the groups. Dependent and binge drinkers were more often smokers and smoked more cigarettes per day than low-risk drinkers. Nicotine is a highly addictive substance and also stimulates the BRS (Di Chiara, 1992; Di Chiara, 2002; Mao & Mcgehee, 2010). Thus, our findings may be not only due the alcohol but also due to the nicotine consumption. Accordingly, Paelecke-Habermann et al. (2013) found a decreased performance in implicit and explicit reward learning with the same tasks in dependent and occasional smokers. However, we controlled all analyses for daily cigarette use and found no effects or correlations.
We also found a significant group difference in the amount of depressive symptoms. Depressive symptoms are common withdrawal signs and are linked to a reduced DA transporter availability during withdrawal (Laine, Ahonen, Räsänen, & Tiihonen, 1999). This effect might decrease reward processes by itself (Martin-Soelch, 2009), therefore we controlled all analyses for depressive symptoms (BDI), but we found no effects or correlations.
It was shown, that probabilistic reward learning is influenced by sustained attention (Markou et al., 2013), working memory and speed of processing (Bismark et al., 2018). We found no differences in short-term and working memory between the three groups and no correlations with the dependent variables. The three groups differed in their reaction times in the Colour-Word-Interference-Test in all three conditions (reading, naming, and interference). But, we found no correlations of the reactions times with the frequencies in implicit reward learning and the error rates in explicit reward learning within groups and over all groups. So, we assume that reward learning in our reward learning tasks was not associated with attention, processing speed, and working memory. This is in line with the study of Lewandowski and colleagues, which found no association between reward learning and neurocognitive performance (Lewandowski et al., 2016).
Unfortunately, we had no information about the medication of alcohol dependent patients. The common goals of psychopharmacological therapies for alcohol dependent patients are the treatment of intoxication and minimizing physical withdrawal symptoms (Wilcox & Bogenschutz, 2013). Yet, the patients who participated in our study were asked to participate only after the acute withdrawal was finished.
It is also possible that some of the patients received a medical cessation treatment after the acute withdrawal (e.g., naltrexone, disulfiram, or acamprosate; Tretter, 2017). Performance of the patient group in explicit reward learning may have been affected by a possible medication with one of the three medications named above, as they have a high potential to improve conditioned learning and decision making (Salamone et al., 2018). Any medication effects would therefore counteract our hypothesis.
Concerning implicit reward learning, we are not aware of any studies on the effects of such medication in alcohol dependent patients. However, we found performance deficits in our patient group as well as in our binge drinking participants that received no medication. This supports our view that the performance deficits of the patient group in implicit reward learning could not be explained by medical treatment effects.
5. Conclusion
We combined three groups of drinking behavior in one experimental design with implicit and explicit reward measures. Deficits in explicit and implicit reward learning of dependent drinkers support the reward deficiency syndrome hypothesis, that dependent patients suffer from a generally reduced reward reactivity (Blum et al., 2000). In particular, binge drinkers were impaired in implicit reward learning. Possibly, this is indicative for binge drinking as a high risk factor for the development of alcohol dependence. Explicit and implicit learning deficits appear to evolve gradually with the amount and duration of alcohol consumption.
To further elucidate whether impaired explicit and implicit reward learning is not only a marker of alcohol and nicotine addiction (Martin-Soelch et al., 2009; Martin-Soelch, Missimer, Leenders, & Schultz, 2003; Paelecke-Habermann et al., 2013) but for addiction in general, studies should be extended to other drugs (e.g., opiates, cannabis, cocaine, Martin-Soelch et al., 2001; Martin-Soelch et al., 2009) and behavioral addictions, such as addictive shopping, gambling, or binge eating (Goudriaan, Oosterlaan, de Beurs, & van den Brink, 2006b; Sobottka, 2007). To determine causal relationships between deficient reward learning an addiction longitudinal epidemiological studies are required.
Role of funding source
This publication was funded by the German Research Foundation (DFG) and the University of Würzburg in the funding programme Open Access Publishing.
Contributors
Author Yvonne Paelecke-Habermann designed the study, wrote the protocol, did the statistical analysis, prepared and wrote the manuscript. Author Marko Paelecke co-wrote the manuscript. Authors Juliane Mauth and Juliane Tschisgale co-wrote the manuscript, performed the experiment and collected the data. Authors Andrea Kübler and Johannes Lindenmeyer supervised data collection, co-wrote and approved the manuscript. All authors contributed to and approved the final manuscript.
Conflict of interest
No conflict declared.
Acknowledgments
We thank Sophia Neugebauer for testing alcohol dependent patients in the Salus-Clinic Lindow. We thank Thorsten Drössler (MA) for providing images of the ice cream seller-puppet.
References
- Averbeck B.B., Costa V.D. Motivational neural circuits underlying reinforcement learning. Nature Neuroscience. 2017;20(4):505–512. doi: 10.1038/nn.4506. [DOI] [PubMed] [Google Scholar]
- Babor T.F., Biddle-Higgins J.C., Saunders J.B., Monteiro M.G. World Health Organization; Geneva, Switzerland: 2001. AUDIT: The alcohol use disorders identification test: Guidelines for use in primary health care. [Google Scholar]
- Bäumler G. Hogrefe-Verlag; Göttingen: 1985. Farbe-Wort-Interferenztest nach R. J. Stroop (FWIT). [Colour word interference test adapted from R. J. Stroop] [Google Scholar]
- Bechara A., Dolan S., Denburg N., Hindes A., Anderson S., Nathan P. Decision-making deficits, linked to a dysfunctional ventromedial prefrontal cortex, revealed in alcohol and stimulant abusers. Neuropsychologia. 2001;39(4):376–389. doi: 10.1016/s0028-3932(00)00136-6. [DOI] [PubMed] [Google Scholar]
- Beck A.T., Schlagenhauf F., Wüstenberg T., Hein J., Kienast T., Kahnt T.…Wrase J. Ventral striatal activation during reward anticipation correlates with impulsivity in alcoholics. Biological Psychiatry. 2009;66(8):734–742. doi: 10.1016/j.biopsych.2009.04.035. [DOI] [PubMed] [Google Scholar]
- Berridge K., Robinson T. Parsing reward. Trends in Neurosciences. 2003;26(9):507–513. doi: 10.1016/S0166-2236(03)00233-9. [DOI] [PubMed] [Google Scholar]
- Berridge K.C., Robinson T.E., Aldridge J.W. Dissecting components of reward: ‘Liking’, ‘wanting’, and learning. Current Opinion in Pharmacology. 2009;9(1):65–73. doi: 10.1016/j.coph.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel W., Madden G. A comparison of measures of relative reinforcing efficacy and behavioral economics: Cigarettes and money in smokers. Behavioural Pharmacology. 1999;10(6–7):627–637. doi: 10.1097/00008877-199911000-00009. [DOI] [PubMed] [Google Scholar]
- Bismark A.W., Thomas M.L., Tarasenko M., Shiluck A.L., Rackelmann S.Y., Young J.W., Light G.A. Relationship between effortful motivation and neurocognition in schizophrenia. Schizophrenia Research. 2018;193:69–76. doi: 10.1016/j.schres.2017.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum K., Braverman E., Holder J., Lubar J., Monastra V., Miller D.…Comings D. Reward deficiency syndrome: A biogenetic model for the diagnosis and treatment of impulsive, addictive, and compulsive behaviors. Journal of Psychoactive Drugs. 2000;32(S I-IV):1–112. doi: 10.1080/02791072.2000.10736099. [DOI] [PubMed] [Google Scholar]
- Blum K., Gardner E., Oscar-Berman M., Gold M. “Liking” and ‘wanting’ linked to reward deficiency syndrome (RDS): Hypothesizing differential responsivity in brain reward circuitry. Current Pharmaceutical Design. 2012;18(1):113–118. doi: 10.2174/138161212798919110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bø R., Aker M., Billieux J., Landro N.I. Binge drinkers are fast, able to stop - But they fail to adjust. Journal of the International Neuropsychological Society. 2016;22(1):38–46. doi: 10.1017/S1355617715001204. [DOI] [PubMed] [Google Scholar]
- Bourque J., Baker T.E., Dagher A., Evans A., Garavan H., Leyton M.…Conrod P. Effects of delaying binge drinking on adolescent brain development: A longitudinal neuroimaging study. BMC Psychiatry. 2016;16 doi: 10.1186/s12888-016-1148-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bühler M., Vollstädt-Klein S., Kobiella A., Budde H., Reed L.J., Braus D.F.…Smolka M.N. Nicotine dependence is characterized by disordered reward processing in a network driving motivation. Biological Psychiatry. 2010;67:745–752. doi: 10.1016/j.biopsych.2009.10.029. [DOI] [PubMed] [Google Scholar]
- Bundesregierung D.D.d. Bundesgesundheitsministerium für Gesundheit und soziale Sicherung; Berlin: 2017. Drogen- und Suchtbericht. [Drug and addiction report] [Google Scholar]
- Cohen J. Lawrence Erlbaum Associates; Hillsdale: 1988. Statistical power analysis for the behavioral sciences. [Google Scholar]
- Di Chiara G. Mesolimbic dopamine and incentive properties of nicotine. Clinical Neuropharmacology. 1992;15(1):564A–565A. doi: 10.1097/00002826-199201001-00293. [DOI] [PubMed] [Google Scholar]
- Di Chiara G. Drug addiction as dopamine-dependent associative learning disorder. European Journal of Pharmacology. 1999;375(1–3):13–30. doi: 10.1016/s0014-2999(99)00372-6. [DOI] [PubMed] [Google Scholar]
- Di Chiara G. Nucleus accumbens shell and core dopamine: Differential role in behavior and addiction. Behavior Brain Research. 2002;137(1–2):75–114. doi: 10.1016/s0166-4328(02)00286-3. [DOI] [PubMed] [Google Scholar]
- Dreher J., Meyer-Lindenberg A., Kohn P., Berman K.F. From the cover: Age-related changes in midbrain dopaminergic regulation of the human reward system. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(39) doi: 10.1073/pnas.0802127105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt B., Belin D., Economidou D., Pelloux Y., Dalley J., Robbins T. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philosophical Transactions of the Royal Society B. 2008;363:3125–3135. doi: 10.1098/rstb.2008.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field M., Wiers R., Christiansen P., Fillmore M.T., Verster J.C. Acute alcohol effects on inhibitory control and implicit cognition: Implications for loss of control over drinking. Alcoholism: Clinical and Experimental Research. 2010;34(8):1346–1352. doi: 10.1111/j.1530-0277.2010.01218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillmore M., Rush C. Impaired inhibitory control of behavior in chronic cocaine users. Drug and Alcohol Dependence. 2002;66(3):265–273. doi: 10.1016/s0376-8716(01)00206-x. [DOI] [PubMed] [Google Scholar]
- Frank M., Claus E. Anatomy of a decision: Striato-orbitofrontal interactions in reinforcement learning, decision making, and reversal. Psychological Review. 2006;113(2):300–326. doi: 10.1037/0033-295X.113.2.300. [DOI] [PubMed] [Google Scholar]
- Galandra C., Basso G., Cappa S., Canessa N. The alcoholic brain: Neural bases of impaired reward-based decision-making in alcohol use disorders. Neurological Sciences. 2018;39(3):423–435. doi: 10.1007/s10072-017-3205-1. [DOI] [PubMed] [Google Scholar]
- Garbusow M., Friedel E., Sebold M., Beck A., Heinz A., Smolka M.N. Pathways leading to addiction: Study design for the assessment of risk factors for developing an alcohol addiction. Sucht. 2013;59(4):187–199. [Google Scholar]
- Goldstein R., Volkow N., Wang G., Fowler J., Rajaram S. Addiction changes orbitofrontal gyrus function: Involvement in response inhibition. Neuroreport. 2001;12(11):2595–2599. doi: 10.1097/00001756-200108080-00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudriaan A., Oosterlaan J., de Beurs E., van den Brink W. Neurocognitive functions in pathological gambling: A comparison with alcohol dependence, tourette syndrome and normal controls. Addiction. 2006;101(4):534–547. doi: 10.1111/j.1360-0443.2006.01380.x. [DOI] [PubMed] [Google Scholar]
- Goudriaan A., Oosterlaan J., de Beurs E., van den Brink W. Psychophysiological determinants and concomitants of deficient decision making in pathological gamblers. Drug and Alcohol Dependence. 2006;84(3):231–239. doi: 10.1016/j.drugalcdep.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Goudriaan A.E., Grekin E.R., Sher K.J. Decision making and binge drinking: A longitudinal study. Alcoholism: Clinical and Experimental Research. 2007;31(6):928. doi: 10.1111/j.1530-0277.2007.00378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grüsser S.M., Mörsen C.P., Flor H. Alcohol craving in problem and occasional alcohol drinkers. Alcohol. 2006;41(4):421–425. doi: 10.1093/alcalc/agl035. [DOI] [PubMed] [Google Scholar]
- Hautzinger M., Bailer M., Worall H., Keller F. Hogrefe-Verlag; Göttingen: 2000. Beck-Depressions-Inventar (BDI). [Beck depression inventory] [Google Scholar]
- Heatherton T., Kozlowski L., Frecker R., Fagerstrom K. The fagerstrom test for nicotine dependence: A revision of the fagerstrom tolerance questionnaire. British Journal of Addiction. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Heinz A., Batra A. Kohlhammer; Stuttgart: 2003. Neurobiologie der Alkohol- und Nikotinabhängigkeit. [Neurobiology of alcohol and nicotine dependence.] [Google Scholar]
- Holm A. A simple sequentially rejective multiple test procedure. Scandinavian Journal of Statistics. 1979;6:65–70. S. [Google Scholar]
- Horn W. Hogrefe-Verlag; Goettingen: 1983. Leistungspruefsystem (LPS) (Performance examination test) [Google Scholar]
- Hyman S., Malenka R., Nestler E. Neural mechanisms of addiction: The role of reward-related learning and memory. Annual Review of Neuroscience. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Jennison K. The short-term effects and unintended long-term consequences of binge drinking in college: A 10-year follow-up study. American Journal of Drug and Alcohol Abuse. 2004;30(3):659–684. doi: 10.1081/ada-200032331. [DOI] [PubMed] [Google Scholar]
- Jentsch J., Taylor J. Impulsivity resulting from frontostriatal dysfunction in drug abuse: Implications for the control of behavior by reward-related stimuli. Psychopharmacology. 1999;146(4):373–390. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- John U., Hanke M., Freyer-Adam J., Baumann S., Meyer C. Alkohol. In: Deutsche Hauptstelle für Suchtfragen, editor. Jahrbuch Sucht 2018. Pabst; Lengerich: 2018. [Google Scholar]
- Keiflin R., Janak P.H. Dopamine prediction errors in reward learning and addiction: From theory to neural circuitry. Neuron. 2015;88(2):247–263. doi: 10.1016/j.neuron.2015.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller S., Maddock J.E., Laforge R.G., Velicer W.F., Basler H.D. Binge drinking and health behavior in medical students. Addictive Behaviour. 2007;32(3):505–515. doi: 10.1016/j.addbeh.2006.05.017. [DOI] [PubMed] [Google Scholar]
- Knowlton B., Mangels J., Squire L. A neostriatal habit learning system in humans. Science. 1996;273:1399–1402. doi: 10.1126/science.273.5280.1399. [DOI] [PubMed] [Google Scholar]
- Knowlton B., Squire L., Gluck M. Probabilistic classification learning in amnesia. Learning and Memory. 1994;1(2):106–120. [PubMed] [Google Scholar]
- Koob G. Alcoholism: Allostasis and beyond. Alcoholism: Clinical and Experimental Research. 2003;27(2):232–243. doi: 10.1097/01.ALC.0000057122.36127.C2. [DOI] [PubMed] [Google Scholar]
- Koob G. The neurobiology of addiction: A neuroadaptational view relevant for diagnosis. Addiction. 2006;101(S1):23–30. doi: 10.1111/j.1360-0443.2006.01586.x. [DOI] [PubMed] [Google Scholar]
- Laine T., Ahonen A., Räsänen P., Tiihonen J. Dopamine transporter availability and depressive symptoms during alcohol withdrawal. Psychiatry Research: Neuroimaging. 1999;90(3):153–157. doi: 10.1016/s0925-4927(99)00019-0. [DOI] [PubMed] [Google Scholar]
- Lane S., Yechiam E., Busemeyer J. Application of a computational decision model to examine acute drug effects on human risk taking. Experimental and Clinical Psychopharmacology. 2006;14(2):254–264. doi: 10.1037/1064-1297.14.2.254. [DOI] [PubMed] [Google Scholar]
- Lannoy S., Maurage P., D'Hondt F., Billieux J., Dormal V. Executive impairments in binge drinking: Evidence for a specific performance-monitoring difficulty during alcohol-related processing. European Addiction Research. 2018;24(3):118–127. doi: 10.1159/000490492. [DOI] [PubMed] [Google Scholar]
- Le Berre A.-P., Fama R., Sullivan E.V. Executive functions, memory, and social cognitive deficits and recovery in chronic alcoholism: A critical review to inform future research. Alcoholism: Clinical and Experimental Research. 2017;41(8):1432–1443. doi: 10.1111/acer.13431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees B., Mewton L., Stapinski L., Squeglia L.M., Rae C., Teesson M. Binge drinking in young people: Protocol for a systematic review of neuropsychological, neurophysiological and neuroimaging studies. BMJ Open. 2018;8(7) doi: 10.1136/bmjopen-2018-023629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrl S. [Multiple choice vocabulary intelligence test]. Perimed-Spitta; Balingen: 1995. Mehrfachwahl-Wortschatz-Intelligenztest (MWT-B) [Google Scholar]
- Lejuez C., Aklin W., Jones H., Richards J., Strong D., Kahler C., Read J. The balloon analogue risk task (bart) differentiates smokers and nonsmokers. Experimental and Clinical Psychopharmacology. 2003;11(1):26. doi: 10.1037//1064-1297.11.1.26. [DOI] [PubMed] [Google Scholar]
- Lewandowski K.E., Whitton A.E., Pizzagalli D.A., Norris L.A., Ongur D., Hall M.-H. Reward learning, neurocognition, social cognition, and symptomatology in psychosis. Frontiers in Psychiatry. 2016;7 doi: 10.3389/fpsyt.2016.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeber S., Duka T. Acute alcohol impairs conditioning of a behavioural reward-seeking response and inhibitory control processes implications for addictive disorders. Addiction. 2009;104(12):2013–2022. doi: 10.1111/j.1360-0443.2009.02718.x. [DOI] [PubMed] [Google Scholar]
- Mackillop J., Miranda R., Monti P., Ray L.A., Murphy J., Rohsenow D.…Gwaltney C.J. Alcohol demand, delayed reward discounting, and craving in relation to drinking and alcohol use disorders. Journal of Abnormal Psychology. 2010;119(1):106–114. doi: 10.1037/a0017513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden G., Johnson P., Brewer A., Pinkston J., Fowler S. Effects of pramipexole on impulsive choice in male wistar rats. Experimental and Clinical Psychopharmacology. 2010;18(3):267–276. doi: 10.1037/a0019244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris N., Oscarberman M., Jaffin S., Hodge S., Kennedy D., Caviness V.…Harris G. Decreased volume of the brain reward system in alcoholism. Biological Psychiatry. 2008;64(3):192–202. doi: 10.1016/j.biopsych.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao D., Mcgehee D. Nicotine and behavioral sensitization. Journal of Molecular Neuroscience. 2010;40(1–2):154–163. doi: 10.1007/s12031-009-9230-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markou A., Salamone J.D., Bussey T.J., Mar A.C., Brunner D., Gilmour G., Balsam P. Measuring reinforcement learning and motivation constructs in experimental animals: Relevance to the negative symptoms of schizophrenia. Neuroscience and Biobehavioral Reviews. 2013;37(9):2149–2165. doi: 10.1016/j.neubiorev.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowitsch H.J., Neufeld R., Calabrese P., Deisinger W., Kessler J. Verlag Hans Huber; Bern: 2000. Wechsler-Gedächtnistest - revidierte Fassung: WMS-R. Manual deutsche Adaptation der revidierten Fassung der Wechsler Memory Scale. [Wechsler Memory Scale-Revised] [Google Scholar]
- Martin-Soelch C. Is depression associated with dysfunction of the central reward system? Biochemical Society Transactions. 2009;37:313–317. doi: 10.1042/BST0370313. [DOI] [PubMed] [Google Scholar]
- Martin-Soelch C., Chevalley A., Kunig G., Missimer J., Magyar S., Mino A.…Leenders K. Changes in reward-induced brain activation in opiate addicts. European Journal of Neuroscience. 2001;14(8):1360–1368. doi: 10.1046/j.0953-816x.2001.01753.x. [DOI] [PubMed] [Google Scholar]
- Martin-Soelch C., Kobel M., Stoecklin M., Michael T., Weber S., Krebs B., Opwis K. Reduced response to reward in smokers and cannabis users. Neuropsychobiology. 2009;60(2):94–103. doi: 10.1159/000239685. [DOI] [PubMed] [Google Scholar]
- Martin-Soelch C., Missimer J., Leenders K., Schultz W. Neural activity related to the processing of increasing monetary reward in smokers and nonsmokers. European Journal of Neuroscience. 2003;18(3):680–688. doi: 10.1046/j.1460-9568.2003.02791.x. [DOI] [PubMed] [Google Scholar]
- McCarty C., Ebel B., Garrison M., DiGiuseppe D., Christakis D., Rivara F. Continuity of binge and harmful drinking from late adolescence to early adulthood. Pediatrics. 2004;114(3):714. doi: 10.1542/peds.2003-0864-L. [DOI] [PubMed] [Google Scholar]
- Michel T., Paelecke-Habermann Y., Leplow B. University of Halle-Wittenberg; Halle/Saale: 2008. Die Wirkung von Alter und Geschlecht auf die Belohnungsreaktivität und spezifische motorische Fähigkeiten. (Unpublished Diploma Thesis) (Effects of age and gender on reward reactivity and specific motor abilities) [Google Scholar]
- Mitchell S. Measures of impulsivity in cigarette smokers and non-smokers. Psychopharmacology. 1999;146(4):455–464. doi: 10.1007/pl00005491. [DOI] [PubMed] [Google Scholar]
- Nakanishi S., Hikida T., Yawata S. Distinct dopaminergic control of the direct and indirect pathways in reward-based and avoidance learning behaviors. Neuroscience. 2014;282:49–59. doi: 10.1016/j.neuroscience.2014.04.026. [DOI] [PubMed] [Google Scholar]
- Nederkoorn C., Baltus M., Guerrieri R., Wiers R.W. Heavy drinking is associated with deficient response inhibition in women but not in men. Pharmacology, Biochemistry and Behavior. 2009;93(3):331–336. doi: 10.1016/j.pbb.2009.04.015. [DOI] [PubMed] [Google Scholar]
- Nelson H.-E., O'Connel A. Dementia: The estimation of premorbid intelligence levels using a new adult reading test. Cortex. 1978;14:234–244. doi: 10.1016/s0010-9452(78)80049-5. [DOI] [PubMed] [Google Scholar]
- Newman J., Widom C., Nathan S. Passive avoidance in syndromes of disinhibition: Psychopathy and extraversion. Journal of Personality and Social Psychology. 1985;48(5):1316–1327. doi: 10.1037//0022-3514.48.5.1316. [DOI] [PubMed] [Google Scholar]
- NIAAA - National Institute on Alcohol Abuse and Alcoholism . NIAAA Newsletter; 2004, Winter. NIAAA council approves definition of binge drinking.http://pubs.niaaa.nih.gov/publications/Newsletter/winter2004/Newsletter_Number3.pdf Available at: (No. 3) [Google Scholar]
- Oei T., Morawska A. A cognitive model of binge drinking: The influence of alcohol expectancies and drinking refusal self-efficacy. Addictive Behaviors. 2004;29(1):159–179. doi: 10.1016/s0306-4603(03)00076-5. [DOI] [PubMed] [Google Scholar]
- Paelecke-Habermann Y. Martin-Luther-University of Halle-Wittenberg; Halle/Saale: 2009. Belohnungslernen bei Sucht und Depression.http://digital.bibliothek.uni-halle.de/hs/content/titleinfo/233922 (Doctoral Dissertation) [Google Scholar]
- Paelecke-Habermann Y., Paelecke M., Reschke K., Giegerich K., Kuebler A. Implicit and explicit reward processing in chronic nicotine use. Drug and Alcohol Dependence. 2013;129(1–2):8–17. doi: 10.1016/j.drugalcdep.2012.09.004. [DOI] [PubMed] [Google Scholar]
- Park S., Kahnt T., Beck A.T., Cohen M., Dolan R., Wrase J., Heinz A. Prefrontal cortex fails to learn from reward prediction errors in alcohol dependence. Journal of Neuroscience. 2010;30(22):7749–7753. doi: 10.1523/JNEUROSCI.5587-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauli W.M., Hazy T.E., O'Reilly R.C. Expectancy, ambiguity, and behavioral flexibility: Separable and complementary roles of the orbital frontal cortex and amygdala in processing reward expectancies. Journal of Cognitive Neuroscience. 2012;24(2):351–366. doi: 10.1162/jocn_a_00155. [DOI] [PubMed] [Google Scholar]
- Perry J., Carrol M.E. The role of impulsive behavior in drug abuse. Psychopharmacology. 2008;200(1):1–26. doi: 10.1007/s00213-008-1173-0. [DOI] [PubMed] [Google Scholar]
- Reynolds B., Richards J., Horn K., Karraker K. Delay discounting and probability discounting as related to cigarette smoking status in adults. Behavioural Processes. 2004;65(1):35–42. doi: 10.1016/s0376-6357(03)00109-8. [DOI] [PubMed] [Google Scholar]
- Richards J., Zhang L., Mitchell S., de Wit H. Delay or probability discounting in a model of impulsive behavior: Effect of alcohol. Journal of the Experimental Analysis of Behavior. 1999;71(2):121–143. doi: 10.1901/jeab.1999.71-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson T., Berridge K. The neural basis of drug craving: An incentive-sensitization theory of addiction. Brain Research Review. 1993;18(3):247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Robinson T., Berridge K. The psychology and neurobiology of addiction: An incentive-sensitization view. Addiction. 2000;95(S2):S91–117. doi: 10.1080/09652140050111681. [DOI] [PubMed] [Google Scholar]
- Rogers R., Moeller F.G., Swann A.C., Clark L. Recent research on impulsivity in individuals with drug use and mental health disorders: Implications for alcoholism. Alcoholism: Clinical and Experimental Research. 2010;34(8):1319–1333. doi: 10.1111/j.1530-0277.2010.01216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose A.K., Grunsell L. The subjective, rather than the disinhibiting, effects of alcohol are related to binge drinking. Alcoholism: Clinical and Experimental Research. 2008;32(6):1096–1104. doi: 10.1111/j.1530-0277.2008.00672.x. [DOI] [PubMed] [Google Scholar]
- Salamone J.D., Correa M., Ferrigno S., Yang J.-H., Rotolo R.A., Presby R.E., Dantzer R. The psychopharmacology of effort-related decision making: Dopamine, adenosine, and insights into the neurochemistry of motivation. Pharmacological Reviews. 2018;70(4):747–762. doi: 10.1124/pr.117.015107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanez-Larkin G., Kuhnen C., Yoo D., Knutson B. Variability in nucleus accumbens activity mediates age-related suboptimal financial risk taking. Journal of Neuroscience. 2010;30(4):1426. doi: 10.1523/JNEUROSCI.4902-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAMHSA - Substance Abuse and Mental Health Services Administration Binge drinking: Terminology and patterns of use. 2016. https://www.samhsa.gov/capt/tools-learning-resources/binge-drinking-terminology-patterns Available at.
- Saß H., Wittchen H.-U., Zaudig M. [Diagnostic and statistical manual of mental disorders]. Hogrefe; Göttingen: 1998. Diagnostisches und statistisches Manual psychischer Störungen (DSM-IV) [Google Scholar]
- Schulteis G., Liu J. Brain reward deficits accompany withdrawal (hangover) from acute ethanol in rats. Alcohol. 2006;39(1):21–28. doi: 10.1016/j.alcohol.2006.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Getting formal with dopamine and reward. Neuron. 2002;36(2):241–263. doi: 10.1016/s0896-6273(02)00967-4. [DOI] [PubMed] [Google Scholar]
- Schultz W., Tremblay L., Hollerman J. Reward processing in primate orbitofrontal cortex and basal ganglia. Cerebral Cortex. 2000;10(3):272–284. doi: 10.1093/cercor/10.3.272. [DOI] [PubMed] [Google Scholar]
- Sevy S., Hassoun Y., Bechara A., Yechiam E., Napolitano B., Burdick K.…Malhotra A. Emotion-based decision-making in healthy subjects: Short-term effects of reducing dopamine levels. Psychopharmacology. 2006;188(2):228–235. doi: 10.1007/s00213-006-0450-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shohamy D., Myers C., Grossman S., Sage J., Gluck M., Poldrack R. Cortico-striatal contributions to feedback-based learning: Converging data from neuroimaging and neuropsychology. Brain. 2004;127(4):851–859. doi: 10.1093/brain/awh100. [DOI] [PubMed] [Google Scholar]
- Smith J., Co C., Mcintosh S., Cunningham C. Chronic binge-like moderate ethanol drinking in rats results in widespread decreases in brain serotonin, dopamine, and norepinephrine turnover rates reversed by ethanol intake. Journal of Neurochemistry. 2008;105(6):2134–2155. doi: 10.1111/j.1471-4159.2008.05296.x. [DOI] [PubMed] [Google Scholar]
- Sobottka B. Pabst Science Publishers; 2007. Entscheidungsverhalten bei pathologischen Glücksspielern. [Decision making in pathological gambling] [Google Scholar]
- Stacy A.W., Wiers R.W. Implicit cognition and addiction: A tool for explaining paradoxical behavior. Annual Review of Clinical Psychology. 2010;6(6):551–575. doi: 10.1146/annurev.clinpsy.121208.131444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolle M., Sack P., Thomasius R. Binge drinking in childhood and adolescence: Epidemiology, consequences, and interventions. Deutsches Ärzteblatt International. 2009;106(19):323. doi: 10.3238/arztebl.2009.0323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm W., Willmes K., Horn W. [Performance examination test for 50-year-old people]. Hogrefe-Verlag; Goettingen: 1999. Leistungspruefsystem fuer ueber 50-jaehrige (LPS 50+) [Google Scholar]
- Tanabe J., Tregellas J., Dalwani M., Thompson L., Owens E., Crowley T., Banich M. Medial orbitofrontal cortex gray matter is reduced in abstinent substance-dependent individuals. Biological Psychiatry. 2009;65(2):160–164. doi: 10.1016/j.biopsych.2008.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tretter F. [Addiction medicine compact. Addictions in clinic and practice]. Schattauer; Stuttgart: 2017. Suchtmedizin kompakt. Suchtkrankheiten in Klinik und Praxis. [Google Scholar]
- van Holst R.J., Schilt T. Drug-related decrease in neuropsychological functions of abstinent drug users. Current Drug Abuse Reviews. 2011;4:42–56. doi: 10.2174/1874473711104010042. [DOI] [PubMed] [Google Scholar]
- Volkow N., Fowler J. Addiction, a disease of compulsion and drive: Involvement of the orbitofrontal cortex. Cerebral Cortex. 2000;10(3):318–325. doi: 10.1093/cercor/10.3.318. [DOI] [PubMed] [Google Scholar]
- Volkow N., Fowler J., Wang G. Role of dopamine in drug reinforcement and addiction in humans: Results from imaging studies. Behavioural Pharmacology. 2002;13(5–6):355–366. doi: 10.1097/00008877-200209000-00008. [DOI] [PubMed] [Google Scholar]
- Volkow N., Fowler J., Wang G. Positron emission tomography and single-photon emission computed tomography in substance abuse research. Seminars in Nuclear Medicine. 2003;33(2):114–128. doi: 10.1053/snuc.2003.127300. [DOI] [PubMed] [Google Scholar]
- Volkow N., Fowler J., Wang G., Goldstein R. Role of dopamine, the frontal cortex and memory circuits in drug addiction: Insight from imaging studies. Neurobiology of Learning and Memory. 2002;78(3):610–624. doi: 10.1006/nlme.2002.4099. [DOI] [PubMed] [Google Scholar]
- Volkow N., Wang G., Newcorn J., Telang F., Solanto M., Fowler J.…Swanson J. Depressed dopamine activity in caudate and preliminary evidence of limbic involvement in adults with attention-deficit/hyperactivity disorder. Archives of General Psychiatry. 2007;64(8):932–940. doi: 10.1001/archpsyc.64.8.932. [DOI] [PubMed] [Google Scholar]
- Volkow N.D., Morales M. The brain on drugs: From reward to addiction. Cell. 2015;162(4):712–725. doi: 10.1016/j.cell.2015.07.046. [DOI] [PubMed] [Google Scholar]
- Vuchinich R.E., Tucker J.A., Rudd E.J. Preference for alcohol consumption as a function of amount and delay of alternative reward. Journal of Abnormal Psychology. 1987;96(3):181–195. doi: 10.1037//0021-843x.96.3.259. [DOI] [PubMed] [Google Scholar]
- Whitton A.E., Treadway M.T., Pizzagalli D.A. Reward processing dysfunction in major depression, bipolar disorder and schizophrenia. Current Opinion in Psychiatry. 2015;28(1):7–12. doi: 10.1097/YCO.0000000000000122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox C., Bogenschutz M. Pharmacotherapies for alcohol and drug use disorders. In: Maccrady B.S., Epstein E.E., editors. Addictions. A comprehensive guidebook. Oxford University Press; New York: 2013. [Google Scholar]
- Winograd R., Sher K.J. Hogrefe Publishing; 2015. Binge drinking and alcohol misuse among college students and young adults. [Google Scholar]
- Wittchen H.-U., Semmler G. Beltz-Test; Weinheim: 1990. Composite international diagnostic interview (CIDI) [Google Scholar]
- Wittchen H.-U., Wunderlich U., Gruschwitz S., Zaudig M. [Structured Clinical Interview for DSM-IV. Axis i: Mental disorders]. Hogrefe; Göttingen: 1997. Strukturiertes klinisches interview für DSM-IV. Achse I: Psychische Störungen (SKID) [Google Scholar]
- Wrase J., Schlagenhauf F., Kienast T., Wustenberg T., Bermpohl F., Kahnt T.…Heinz A. Dysfunction of reward processing correlates with alcohol craving in detoxified alcoholics. Neuroimage. 2007;35(2):787–794. doi: 10.1016/j.neuroimage.2006.11.043. [DOI] [PubMed] [Google Scholar]
- Yechiam E., Goodnight J., Bates J., Busemeyer J., Dodge K., Pettit G., Newman J. A formal cognitive model of the go/no-go discrimination task: Evaluation and implications. Psychological Assessment. 2006;18(3):239–249. doi: 10.1037/1040-3590.18.3.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yücel M., Lubman D., Solowij N. Understanding drug addiction: A neuropsychological perspective. Australian and New Zealand Journal of Psychiatry. 2007;41:957–968. doi: 10.1080/00048670701689444. [DOI] [PubMed] [Google Scholar]