Abstract
Current groundwater treatment facilities, mostly relying on aeration-filtration configurations, aim at the removal of iron (Fe), ammonia (NH4+) and manganese (Mn). However, recently water companies expressed the ambition to also reduce arsenic (As) concentrations in these rapid sand filters. The aim of this study was to investigate the effect of the Fe oxidation state entering a biological filter bed on As removal. By varying supernatant water level, either Fe(II) or Fe(III) in the form of hydrous ferric oxides (HFO) could be stimulated to enter the filter bed at alkaline groundwater pH (7.6). The experimental pilot column filters showed that once the As(III) oxidation stabilised in the top layer of the filter sand, As removal reached its maximum (±75% at 120 cm supernatant level and 1.5 m/h filtration velocity). The increase in supernatant level from 5 to 120 cm resulted in additional HFO production prior to rapid filtration (1.5, 5 and 10 m/h), i.e. homogeneous Fe(II) oxidation and flocculation, and subsequently, HFO ending up deeper into the filter bed (120 cm filter depth). At a low supernatant water level of 5 cm, Fe(II) oxidised heterogeneously and was removed within the top 20 cm of the filter bed. Consequently, filters with high supernatant levels removed As to lower levels (by 20%) than in filters with low supernatant water levels. The benefits of Fe(II) oxidation prior to filtration for As removal was confirmed by comparing Fe(III) to Fe(II) additions in the supernatant water or in the filter bed. Overall it is concluded that in biological groundwater filters, the combination of a higher supernatant level and/or Fe(III) addition with biological As(III) oxidation in the top of the filter bed promotes As removal.
Keywords: Arsenic removal, Supernatant water level, Fe(II)/Fe(III) additions, Rapid sand filtration
Graphical abstract

Highlights
-
•
As(III) oxidation in the top of a biological rapid sand filter contributed to effective As removal.
-
•
Fe(II) oxidation before rapid sand filtration improved As(III) removal.
-
•
FeCl2 or FeCl3 added in the supernatant water removed As more than Fe added in the filter bed.
1. Introduction
Current groundwater treatment facilities, mostly relying on aeration-filtration configurations, aim at the removal of iron (Fe), ammonia (NH4+) and manganese (Mn). However, recently water companies have expressed the ambition to reduce arsenic (As) concentrations to below 1 μg/L, to exclude potential undesired health effects (Middleton et al., 2016; van Halem et al., 2009; WHO, 2011). In Belgium and the Netherlands As levels in groundwater are generally low (<10 μg/L), though at some pumping stations concentrations as high as ±70 μg/L are observed. As a consequence, the existing drinking water treatment infrastructure, frequently in good condition, needs upgrading for the removal of several μg As/L. For water companies, optimisation of existing treatment processes for As removal is preferred over implementation of new treatment steps (e.g., adsorbents, membrane filtration) or introduction of invasive chemicals (e.g., strong oxidants).
In groundwater filters, rapid oxidation of As(III) to As(V) is crucial for effective As removal. Previous research has found As(III) oxidation to occur in the top of the filter bed (Bissen and Frimmel, 2003; Gude et al., 2016), facilitated by bacteria (Gude et al., 2018b; Katsoyiannis et al., 2004; Kumari and Jagadevan, 2016). The oxidised As(V) is subsequently removed by adsorption onto hydrous ferric oxides (HFO) that originate from oxidised and subsequently flocculated or adsorbed Fe(II) (Dixit and Hering, 2003; Gude et al., 2017; Sharma et al., 2001; van Beek et al., 2015). HFO is either produced in the supernatant water or in the filter bed which is determined to a large extent by the supernatant water level (SWL), but also on water quality (e.g., pH), filter design (e.g., filter material) and operational conditions (e.g., flow rate) (Stumm and Lee, 1961; Vries et al., 2017). Therefore, the aim of this study was to investigate the effect of the Fe oxidation state, entering the biological filter bed, on As removal. The Fe oxidation state, entering the filter bed, can be controlled by adjusting the SWL. The supernatant water can be regarded as a completely stirred tank reactor (Vries et al., 2017), therefore the major difference between high and low SWL is residence time of the aerated groundwater. Mn(II), NH4+ and As(III) do not homogeneously oxidise in the timespan of 1 h (Diem and Stumm, 1984; Kim and Nriagu, 2000; Tatari et al., 2016), which can be considered the practical maximum residence time, however, depending on O2 concentrations and pH part of the Fe(II) will homogeneously oxidise and form HFO flocs (de Ridder and van Halem, 2017; Sharma et al., 2001; Stumm and Lee, 1961). The produced HFO typically adsorbs about 20–40% As(III) depending on, among other parameters, As/Fe ratio and residence time (Dixit and Hering, 2003; Gude et al., 2017, 2016; Qiao et al., 2012). The Fe(II) that reaches the filter bed can heterogeneously (or at pH < 7.5 partly biologically (de Vet et al., 2011)) oxidise on the filter grains. Kinetics of heterogeneous Fe(II) oxidation are faster than homogenous (Tamura et al., 1980), the HFO formed is less voluminous and causes less filter resistance than HFO floc-filtration (Sharma et al., 2001) and could have a lower sorption site density, as was observed by Dixit and Hering (2003) for different Fe oxides minerals (HFO, Goethite and Magnetite), where the site density on the mineral surface increased as the density of the mineral decreased. Also confirmed by Senn et al. (2018) where aging HFO at 40 °C caused a release of As(V).
Impact of SWL on the oxidation can only be expected at alkaline groundwater which guarantee a sufficiently high homogeneous Fe(II) oxidation rate (see Fig. 1). To test the effect of SWL, and thus the Fe oxidation state, on the removal of As during rapid sand filtration, two pilot scale sand columns with a filter bed height of 1.4 m were operated for a period of nine months. The filter columns were fed with aerated, natural alkaline (±pH 7.6) groundwater, containing 13 μg/L As(III), 2 mg/L Fe(II), 0.6 mg/L NH4+ and 20 μg/L Mn(II). One column was operated with high (>1 m) SWL while the other was operated with low SWL (<0.05 m). In addition, to have a direct comparison on the influence of either Fe(III) or Fe(II) entering the filter bed on the removal of As(III), an experiment was included by dosing 1 mg/L Fe(II) or Fe(III) at equal SWL. To observe As(III) oxidation and Fe/HFO mobility in the filter bed, water samples were measured over the height of the filter bed, during start-up of the sand filters (i.e. ripening) as well as during stable operation.
Fig. 1.
Groundwater treatment: abstraction, followed by aeration and rapid filtration. Increasing the supernatant level, increases the amount of HFO entering the filter bed by lengthening residence time in the SWL.
2. Materials and methods
2.1. Groundwater quality
The pilot experiments were performed at drinking water production plant Hoogstraten in Belgium (Pidpa). The groundwater quality is depicted in Table 1.
Table 1.
Groundwater quality of WTP Hoogstraten and feed water of the column experiment.
| Water quality parameters | Units | avr. | Min. | Max. |
|---|---|---|---|---|
| Temperature | ˚C | 11.7 | 10.5 | 12.8 |
| O2 | mg/L | <0.01 | <0.01 | <0.01 |
| ORP | mV | −129 | −188 | −61 |
| EC | μS/cm | 406 | 386 | 432 |
| pH | [−] | 7.56 | 7.45 | 7.70 |
| HCO3 | mg/L | 225 | 214 | 229 |
| As | μg/L | 12.5 | 8.3 | 15.0 |
| Fe | mg/L | 1.97 | 0.89 | 2.27 |
| Mn | μg/L | 17.7 | 11.9 | 25.9 |
| Ca | mg/L | 69.3 | 65.6 | 75.3 |
| Mg | mg/L | 5.2 | 4.6 | 5.8 |
| NH4+ | mg/L | 0.68 | 0.62 | 0.74 |
| PO43- | μg/L P | 257 | 248 | 283 |
| SiO2 | mg/L | 23 | 22.1 | 23.9 |
2.2. Experimental column set-up
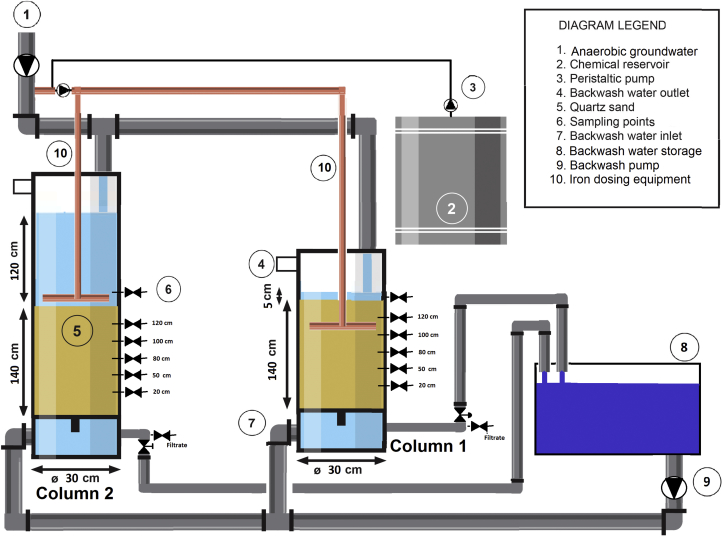
The experimental set-up consisted of two columns with a diameter of 300 mm (Fig. 2). Both columns were filled with sand to 140 cm bed height, where one column was 2 m in height and one column was 3 m in height to allow for a high SWL. For equal flow distribution and spray aeration, raw groundwater was increased in pressure with a centrifugal pump at the intake and manually set (reduced in pressure) to the desired flow just before the columns. Aeration was achieved by spraying in the columns with a fall height of 30 cm into the supernatant water, resulting in an average dissolved oxygen (DO) concentration of 6.6 mg/L with a standard deviation of 1.1 mg/L. The calculated DO demand of the Fe(II), Mn(II), NH4+ and As(III) combined is 2.78 mg/L O2. The additional 1 mg/L Fe(II) adds 0.14 mg/L, making the total DO demand 2.92 mg/L O2. The applied aeration sufficed to ensure oxic conditions in the filter bed. The SWL was maintained constant throughout the runtime with a control valve compensating for the increased pressure drop due to clogging of the filter bed. The columns were filled with 1.4 m quartz filter sand of size 0.70–1.25 mm. Before starting the experiment the columns were intensively backwashed with tap water to remove all fines. Over the course of the experiment the filtrate of the columns was collected and used as backwash water. Throughout the seven months experiment, the columns were continuously fed with aerated groundwater and were situated in a location deprived of (sun)light.
Fig. 2.
Schematic overview of the filter column set-up including sample points over the height of the filter bed. Fe(II)/Fe(III) dosing equipment was installed only for specific experiments.
2.3. Experimental conditions
The experiment started with virgin sand, which was ripened with aerated groundwater for nine weeks at a velocity of 1.5 m/h with 5 cm SWL and 120 cm SWL for column 1 and 2, respectively. Once the biological processes sufficiently progressed (only Mn was not completely removed), the filtration velocity was increased. The first three weeks to 5 m/h and the subsequent four weeks it was increased to 10 m/h. Afterwards the filtration velocity was set back to 5 m/h and subsequently reduced to 1.5 m/h. For a complete overview of the experimental conditions is referred to Table 2. In week 22, the SWL was set to 50 cm in both columns for a period of three weeks to stabilise conditions in both columns. At this point two identical PVC Fe(II)/Fe(III) dosing unit were installed. The outlet of the first was installed in the top of the filter bed of column 1, at a depth of 30 cm (Fig. 2) and the second in the SWL of column 2. 10% of the anaerobic groundwater was pumped through the dosing units, right before the centrifugal pump the FeCl was dosed with a peristaltic pump (Watson marlow U120) to ensure proper mixing. Successively, 1 mg/L Fe(II) was dosed in the filter bed in column 1 and 1 mg/L Fe(II) in the SWL of column 2. Samples throughout the complete installation were taken at 3 and 7 days of chemical injection and were averaged. The same experiment was repeated for Fe(III) dosing. Finally the columns were disinfected to confirm the biological nature of the processes by pumping chlorinated water of 1.5 times the reactor volume into the columns and allowed a reaction time of 24 h. After 24 h, the columns were backwashed and operated as before.
Table 2.
Experimental overview.
| Period |
Filtration velocity |
SWL column 1 |
SWL column 2 |
Experiment |
|---|---|---|---|---|
| [week] | [m/h] | [m] | [m] | |
| 1–9 | 1.5 | 0.05 | 1.20 | Biological ripening |
| 10–13 | 5 | 0.05 | 1.20 | Flowrate increase to 5 m/h |
| 14–19 | 10 | 0.05 | 1.20 | Flowrate increase to 10 m/h |
| 20 | 5 | 0.05 | 1.20 | Flowrate return to 5 m/h |
| 21 | 1.5 | 0.05 | 1.20 | Flowrate reduction to 1.5 m/h |
| 22 | 5 | 0.5 | 0.5 | Stable operation at equal SWL |
| 26 | 5 | 0.5 | 0.5 | 1 mg/L Fe(II) addition in filter bed (column 1) and supernatant (column 2) |
| 27 | 5 | 0.5 | 0.5 | 1 mg/L Fe(III) addition in filter bed (column 1) and supernatant (column 2) |
| 30 | 5 | 0.5 | 0.5 | Disinfection with Cl2 |
2.4. Chemicals, sampling and analytical methods
Fe was obtained from Sigma Aldrich: FeCl2·4H2O, mw 198.81 g/mol (99.99%) and FeCl3·6H2O mw 270.30 g/mol (99%). 15% stock solution NaOCl was diluted to 150 mg/L with drinking water. 10% of the anoxic main flow was diverted and injected with the undiluted FeCl2/FeCl3. The chemicals were continuously pumped into a PVC dosing unit placed just above the filter bed in the supernatant water and at 30 cm depth (from the top) in the sand bed. The dosing unit consisted of a cross with 5 small holes per arm to enhance the mixing of the Fe. The default sample frequency was once per week and while sampling the sample-water flow remained always below 10% of the main flow to prevent large changes in filtration velocity. Samples were processed via three methods: (1) direct, (2) filtered over 0.45 μm, and (3) filtered over 0.45 μm and anionic resin for the purpose of As speciation.
As speciation was done according the method proposed by Karori et al., (2006). Here, 150 mL sample is passed through an anionic resin (80 mL Amberlite® IRA-400 chlorite form resin in a 100-mL syringe) that retains only the charged As(V) species. The filtrate from the resin is considered to be As(III). As(V) is then calculated by subtracting As(III) from the measured total As concentration. The first 50 mL was always discarded, the remaining 100 mL was collected and analysed using Inductively Coupled Plasma Mass Spectrometry (ICP-MS). This is considered to be a robust method, however, at neutral pH the resin unavoidably retains 14% of As(III) (min = 7%, max = 23%; n = 24) (Gude et al., 2018b), which was not compensated for in the Figures. pH, electrical conductivity (EC) and O2 were measured with WTW electrodes (SenTix 940, TerraCon 925 and FDO925).
Determination of total iron concentration was performed spectrophotometrically by the phenanthroline method (American Public Health Association, 1985). As, Mn and P were analysed with ICP-MS (type XSeries2 van Thermo Scientific), while NH4+, NO2-, NO3+ were analysed by a discrete analyser spectrophotometry (Aquakem 250, company: Thermo Scientific).
3. Results and discussion
3.1. Biological ripening of As(III), Mn(II) and NH4+
In Fig. 3, the speciation of the dissolved As in the two filter columns with different SWL is depicted in the supernatant water and over the height of the filter bed at day 1, 8, 15 and 36.
Fig. 3.
As(III) and As(V) speciation of dissolved As over the filter bed height for 5 cm SWL (left) and 120 cm SWL (right) with filtration velocity of 1.5 [m/h] for day 1, 8 15, 36.
The gradually increased As(V) concentrations over the filter bed profile illustrates the onset of biological As(III) oxidation. After 36 days, before NH4+ and Mn started (Fig. 4), As in the filtrate was completely in the As(V) form, both in the 5 and 120 cm SWL columns.
Fig. 4.
NH4+ (left) and Mn (right) concentrations over the height of the filter bed after 36, 49 and 63 days, for 5 cm (open) and 120 cm (closed) SWL.
Over the first 5 weeks, the As(III) conversion gradually moved upwards in the filter bed, as a result of filter ripening. This process only slowed down until the major part was oxidised in the top 20 cm of the filter bed. While As(III) oxidation occurred higher in the filter bed, total As removal efficiencies increased in the filtrate. It was observed that even though within 2–3 weeks complete oxidation was achieved in the filtrate, the removal efficiency in the 120 cm SWL column gradually increased until week 5–6. Apparently, the biological As(III) oxidation occurring in the top of the filter bed contributes to increased As removal.
Apart from As(III) conversion, other biological processes also started in the ripening stage of groundwater filters. The NH4+ and Mn concentration profile over the filter bed are depicted in Fig. 4 during the first 63 days of operation.
NH4+ oxidation commenced and was (almost) complete in the filtrate at day 49 and, like As(III), climbed to the top of the filter bed at least until day 63. Although NH4+ oxidation started at day 36 for both 5 and 120 cm SWL, by day 49 and 63 more was oxidised in the 120 cm SWL column, and oxidation occurred higher in the filter bed. Mn(II) removal did not fully develop during the ripening stage, as only 29% and 45% was removed in the 5 and 120 cm SWL columns, respectively. However, similar to both NH4+ and As(III), the 120 cm SWL column removed more Mn than the 5 cm SWL column at day 63, which suggests that biological processes benefit from Fe(II) oxidation prior to filtration. The development of biomass for As(III) conversion was fastest, followed by NH4+ and subsequently Mn, which is in-line with results from ripening experiments with other natural groundwaters containing, As(III), Mn(II) and NH4+ (Gude et al., 2018a, 2018b). The results in Fig. 4 suggest that specific As(III) oxidising bacteria were accumulated in sand filters since As(III) oxidation developed prior to NH4+ and Mn(II) oxidation. This is in line with observations in Gude et al. (2018b) which showed that As(III) oxidising bacteria accumulated rapidly on As(III)-substrate in an environment of NH4+ without NO3- and vise versa, in both these systems no MnO2 minerals were present on the filter sand.
The results of increasing the filter loading (e.g. filtration velocity) are depicted in Fig. 5. Here As(III) and As(V) concentration profiles are shown after 1 week of increasing the flow from 1.5 m/h to 5 m/h.
Fig. 5.
Loading increase and measurements after 1 week 1.5–5 m/h As(III) and As(V) profile over the height of the filter bed at 5 cm SWL (left) and 120 cm SWL (right).
As(III) concentrations throughout both columns increased as a result of an increased loading. Apparently the biological oxidation was in equilibrium with the loading of 1.5 m/h and the columns could not directly cope with the, more than tripling, of As(III) loading at 5 m/h. The elevated As(III) concentrations were accompanied by a decrease in As removal efficiency. To confirm that As(III) conversion was a biological process in the columns, the columns were disinfected with Cl2 at the end of seven months of experiments. Fig. 6 depicts the arsenic speciation in the columns after returning to regular operational mode (at 5 m/h) for a period of 24 h.
Fig. 6.
As(III) and As(V) profile through the filter bed after disinfection with Cl2.
After disinfection, the As(III)/As(V) profile over the filter bed closely resembled the profile of the first measurement at day 1. Hence, As(III) oxidation stopped almost completely after disinfection. This confirms that the As(III) oxidation during these experiments was of biological nature. Although not measured over the height of the filter bed, NH4+ concentrations in the filtrate (0.60 mg/L) remained identical to the influent concentrations (0.61 mg/L), indicating that the disinfection inactivated the nitrifiers as well, also observed by Gagnon et al. (2005). On the other hand, Fe removal was just as effective after chlorination as before chlorination, with Fe concentrations in both columns below 0.01 mg/L. Therefore, although biological Fe(II) oxidation in the filter bed could have occurred (de Vet et al., 2011; van Beek et al., 2015), it is demonstrated that for these columns with alkaline groundwater homogeneous and heterogeneous Fe(II) oxidation sufficed for Fe removal. However, it is clear that the nature of As(III) oxidation was predominantly biological, as after disinfection the majority of the As in the filtrate was in the As(III) form. Minerals, present on the filter sand, potentially potent for As(III) oxidation, like MnO2 (Gude et al., 2017; Jones et al., 2012), apparently did not oxidise As(III) while the bacteria were inactivated.
3.2. SWL and As removal
Extending residence times in the supernatant water results in increased homogeneous HFO production, and reduces the contribution of heterogeneous Fe(II) oxidation in the filter bed. Fe removal over the filter bed height, both total Fe and 0.45 μm filtered Fe, are depicted in Fig. 7 together with dissolved and adsorbed As.
Fig. 7.
Dissolved As and adsorbed As to mobile HFO (yet to be retained by the sand filter) in μg/L. On secondary axis Fe removal both over the filter bed depth (FB) at 5 cm SWL (top) and 120 cm SWL (bottom). Filtration velocity 1.5 m/h. Data shown are averaged values of week 6 and 7 of operation.
The profiles of the columns with 5 and 120 cm SWL were distinctly different for As and Fe. At 5 cm SWL, Fe removal was rapid and efficient since 98.6% was removed in the first measuring interval at 20 cm from the top of the filter bed. In this system, the majority of the Fe enters the filter bed as Fe(II) (82% was not retained by a 0.45 μm filter) and apparently this was efficiently removed. The rapid Fe removal resulted in a low level of As adsorption throughout the filter bed. 5 μg/L As (total) was removed in the first 20 cm of the filter bed, whereas in the rest of the 120 cm sand filter only 1 μg/L As was removed.
In the 120 cm SWL column, the Fe(II) was allowed to be homogenously oxidised for 45 min at 5 mg/L O2 and pH 7.6. The retention time caused 53% of the 1.93 mg/L Fe(II) to be retained by a 0.45 μm filter, apparently being oxidised and flocculated into HFO flocs. These flocs were observed throughout the filter bed and were only completely removed after 120 cm of sand filtration (deep-bed filtration). Apparently homogeneously formed HFO in the supernatant water was more mobile in sand filters than dissolved Fe(II) as was also observed by Sharma et al. (2001). This deep-bed filtration of HFO changed the As profile compared to the profile in the 5 cm SWL column. In terms of As removal the 120 cm SWL column outperformed the 5 cm SWL column. In the first 20 cm of the filter bed, an additional decrease of 2 μg/L dissolved As and an additional 2.5 μg/L adsorbed As was observed. The adsorbed As to the (mobile) HFO was subsequently removed in the filter bed. This observation is in-line with an As removal profile in a full-scale rapid filter which was operated with a high supernatant level and a NaOH injection (Gude et al., 2016).
An additional observation is that after 20 cm of the sand filter, the dissolved As remained steady for the rest of the filter bed. Probably, the HFO deeper in the filter bed had already reached equilibrium sorption and/or further flocculation of the HFO prevented additional adsorption of the produced As(V). Overall, increased SWL, at a pH high enough for homogeneous Fe(II) oxidation to occur, resulted in increased HFO production prior to filtration which was beneficial to As adsorption onto HFO and subsequent removal in the filter bed.
3.3. Supernatant level and filtration velocity
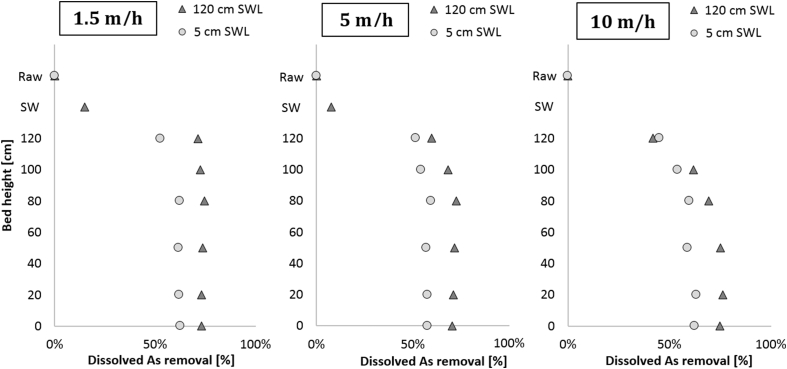
Increasing the filtration velocity reduces the homogeneous oxidation time in the supernatant water and therefore the HFO production. To observe whether the positive effect of a high SWL is maintained at higher filtration velocities, the results of the As removal at different velocities are depicted in Fig. 8 (1.5, 5 and 10 m/h).
Fig. 8.
Dissolved As removal from raw water to supernatant and throughout the filter bed at 1.5 m/h left (day 120), 5 m/h middle (day 148) and 10 m/h right (day 155).
Independent of the filtration velocity, 120 cm SWL resulted in an increased As removal compared to 5 cm SWL throughout the filter bed. The increase in As removal in the filtrate was 10%, 12% and 12% respectively for 1.5, 5 and 10 m/h. The minor differences are more likely to be explained by the variations in water quality than in process conditions.
At an increased filtration velocity, As removal moved deeper into the filter bed. At 1.5 m/h, As removal occurred in the upper layer of the filter bed and no additional removal was observed further in the filter bed, while at 5 m/h and even more pronounced at 10 m/h As removal was occurring deeper in the filter bed. Apparently the increased filter loading and/or the decreased contact time caused the process to acquire additional surface area at higher filtration rates. In this experiment however, although the removal occurred deeper in the filter bed, no effect on the total As removal in the complete filter bed was observed.
3.4. Fe(II)/Fe(III) addition in supernatant water
To have a direct comparison of the influence of either Fe(III) or Fe(II) entering the filter bed on the removal of As, an experiment was included adding 1 mg/L Fe(II) or Fe(III). Fig. 9 depicts either Fe(II) or Fe(III) additions, as well as the natural groundwater composition with process conditions of 50 cm SWL and 5 m/h filtration velocity.
Fig. 9.
Dissolved As concentrations over the bed filter bed height for addition of 1 mg/L Fe(II) or Fe(III) to supernatant water with 50 cm SWL. Native Fe concentration was 2 mg/L (as Fe(II)) and filtration velocity 5 m/h.
Both Fe(II) and Fe(III) additions resulted in a more effective removal of As than was the case for natural groundwater without addition, indicating that additional formation of HFO resulted in increased As removal, as also previously observed by Qiao et al. (2012) where As(V) removal percentages increased at lower As(V)/Fe(III) ratios. Chiew et al. (2009) observed more As removal in filters where more Fe was present. However, Fe(III) addition in the supernatant water was more effective in decreasing As compared to Fe(II) addition. Evidently, Fe(III) entering the filter bed is more effective at removing As than Fe(II), which is in-line with the SWL experiments presented earlier. The ±1 μg/L increase in removal was obtained in the upper layer of the filter bed and remained more or less stable further throughout the filter bed. The mechanism involved here is that the HFO flocs formed by Fe(III) are not accumulating on the surface of the top of the filter bed where the As(III) is oxidised (observed at 120 cm SWL in Fig. 7 and by Sharma et al. (2001)), but in majority passing them in the pore volume of the filter bed and subsequently absorbed the produced As(V) more effectively.
3.5. Fe(II)/Fe(III) addition in filter bed
To obtain HFO in the zone of the filter bed where As(V) is present, in order to better adsorb As in rapid filters, also Fe(II) and Fe(III) were dosed in the filter bed at 30 cm under the top of the filter bed and compared to an equal Fe addition in the supernatant water. The results for dissolved As and Fe are depicted in Fig. 10.
Fig. 10.
Fe(III) (left) and Fe(II) (right) addition of 1 mg/L in supernatant water and filter bed at 110 cm bed height. All experiments were performed with 50 cm SWL and filtration velocity of 5 m/h.
As removal as a result of Fe addition in the supernatant causes the majority of the As removal to take place in the upper layer of the filter bed. This in contrast to the Fe addition at 30 cm in the filter bed. Here the removal was achieved over a larger filter bed height and remained constant after 60 cm of sand filtration. The Fe addition in the supernatant water caused the overall As removal to be more effective than Fe addition in the filter bed. For Fe(III) addition in the supernatant, 1.2 μg/L additional As was removed. In the filter bed where the Fe was dosed a drop of 1.0 μg/L is observed. However, this did not result in the desired effect since the dissolved As concentration remained higher than in the same sampling point while dosing in the supernatant water. For Fe(II), a similar trend is observed. Fe(II) dosed in the filter bed caused some additional removal compared to highest sampling point in the filter bed. However it did not result in increased removal compared to the Fe(II) added in the supernatant water.
The Fe profile as a result of Fe(III) additions was clearly different for dosing in the supernatant and dosing in the filter bed. Additions in the supernatant water effectively caused (a maximum ±1 mg/L) increased Fe concentrations over the first 90 cm of the filter bed. On the other hand, while adding the same concentration Fe in the filter bed almost no additional Fe was observed in the filter bed. Apparently adding in the filter bed, the flocs were more effectively locally retained due to improper mixing and direct availability of sand surfaces, potentially explaining the reduced As removal compared to the well-mixed dosing in the supernatant water similar to observations of Sharma et al. (2001), here Fe(III) entering the filter bed resulted in 2.5 times high filter resistance (rapidly clogging a filter bed by voluminous flocs). The Fe profile for Fe(II) addition in the filter bed and in the supernatant was similar, apart from 2 outliers; which was, in both experiments, dominated by rapid heterogeneous oxidation (Hiemstra and van Riemsdijk, 2007; Tamura et al., 1980) and thus, similarly to previous experiments, not resulting in better As removal than when adding Fe(III).
Conclusions
The research aimed at establishing an As(III) oxidising biomass in the top of the filter bed and investigating the role of Fe oxidation state prior to rapid filtration. For this purpose the SWL was varied and Fe(II) and Fe(III) additions were compared in a pilot plant using alkaline groundwater (pH 7.6) with naturally containing As(III). From the experiments it may be concluded that As(III) oxidation gradually moved upward in the filter columns during ripening of biological sand filters, similar to NH4+ but more rapidly. Once the biomass established itself in the top of the filter bed, As removal efficiency stabilised at its maximum. Disinfection by chlorine caused the As(III)/As(V) profile to return to its initial values, confirming the biological nature of this process. As removal greatly benefitted from increasing the SWL from 5 cm to 120 cm, for all tested filtration velocities (1.5, 5 and 10 m/h). A closer look in the filter bed revealed that HFO penetrated the filter bed further and seemed to be more efficiently used as an adsorbent at a higher SWL. The beneficial properties of HFO formed prior to rapid filtration were confirmed by the increased As adsorption by Fe(III) addition in the supernatant water compared to Fe(II) addition and/or Fe injection into the filter bed.
Declaration of interests
None.
Acknowledgements
This research is supported by a Dutch Technology Foundation NWO-TTW grant, part of the Netherlands Organisation for Scientific Research. Project code: 13343 (FixAs). The authors want to thank David Geysen, Martine Cuypers and Wendy Engelen for their valuable assistance in planning, operating and installing the pilot plant. In addition, the authors are grateful for the hosting of Pidpa for the on-site experiments at Water Treatment Plant Hoogstraten.
References
- American Public Health Association . 16th ed. 1985. Standard Method for the Examination of Water and Waste Water. Washington DC. [Google Scholar]
- Bissen M., Frimmel F.H. Arsenic— a review. Part II: oxidation of arsenic and its removal in water treatment. Acta Hydrochim. Hydrobiol. 2003;31:97–107. [Google Scholar]
- Chiew H., Sampson M.L., Huch S., Ken S., Bostick B.C. Effect of groundwater iron and phosphate on the efficacy of arsenic removal by iron-amended BioSand filters. Environ. Sci. Technol. 2009;43:6295–6300. doi: 10.1021/es803444t. [DOI] [PubMed] [Google Scholar]
- de Ridder D.J., van Halem D. Influence of particle properties on iron flocculation. Water Sci. Technol. Water Supply. 2017;3 ws2017216. [Google Scholar]
- de Vet W.W.J.M., Dinkla I.J.T., Rietveld L.C., van Loosdrecht M.C.M. Biological iron oxidation by Gallionella spp. in drinking water production under fully aerated conditions. Water Res. 2011;45:5389–5398. doi: 10.1016/j.watres.2011.07.028. [DOI] [PubMed] [Google Scholar]
- Diem D., Stumm W. Is dissolved Mn2+ being oxidized by O2 in absence of Mn-bacteria or surface catalysts? Geochem. Cosmochim. Acta. 1984;48:1571–1573. [Google Scholar]
- Dixit S., Hering J.G. Comparison of arsenic(V) and arsenic(III) sorption onto iron oxide minerals: implications for arsenic mobility. Environ. Sci. Technol. 2003;37:4182–4189. doi: 10.1021/es030309t. [DOI] [PubMed] [Google Scholar]
- Gagnon G.A., Rand J.L., O'Leary K.C., Rygel A.C., Chauret C., Andrews R.C. Disinfectant efficacy of chlorite and chlorine dioxide in drinking water biofilms. Water Res. 2005;39:1809–1817. doi: 10.1016/j.watres.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Gude J.C.J., Rietveld L.C., van Halem D. Biological As(III) oxidation in rapid sand filters. J. Water Process Eng. 2018;21:107–115. [Google Scholar]
- Gude J.C.J., Rietveld L.C., van Halem D. 2018. As(III) Removal in Rapid Filters: Effect of pH, Fe(II)/Fe(III), Filtration Velocity and Media Size. [DOI] [PubMed] [Google Scholar]
- Gude J.C.J., Rietveld L.C., van Halem D. As(III) oxidation by MnO2 during groundwater treatment. Water Res. 2017;111:41–51. doi: 10.1016/j.watres.2016.12.041. [DOI] [PubMed] [Google Scholar]
- Gude J.C.J., Rietveld L.C., van Halem D. Fate of low arsenic concentrations during full-scale aeration and rapid filtration. Water Res. 2016 doi: 10.1016/j.watres.2015.10.034. [DOI] [PubMed] [Google Scholar]
- Hiemstra T., van Riemsdijk W.H. Adsorption and surface oxidation of Fe(II) on metal (hydr)oxides. Geochem. Cosmochim. Acta. 2007;71:5913–5933. [Google Scholar]
- Jones L.C., Lafferty B.J., Sparks D.L. Additive and competitive effects of bacteria and Mn oxides on Arsenite oxidation kinetics. Environ. Sci. Technol. 2012;46:6548–6555. doi: 10.1021/es204252f. [DOI] [PubMed] [Google Scholar]
- Karori S., Clifford D., Ghurye G., Gautam S. Development of a field speciation method for inorganic arsenic species in groundwater. J. Am. Water Works Assoc. 2006:128–141. [Google Scholar]
- Katsoyiannis I.A., Zouboulis A.I., Jekel M. Kinetics of bacterial As(III) oxidation and subsequent As(V) removal by sorption onto biogenic manganese oxides during groundwater treatment. Ind. Eng. Chem. Res. 2004;43:486–493. [Google Scholar]
- Kim M.J., Nriagu J. Oxidation of arsenite in groundwater using ozone and oxygen. Sci. Total Environ. 2000;247:71–79. doi: 10.1016/s0048-9697(99)00470-2. [DOI] [PubMed] [Google Scholar]
- Kumari N., Jagadevan S. Genetic identification of arsenate reductase and arsenite oxidase in redox transformations carried out by arsenic metabolising prokaryotes – a comprehensive review. Chemosphere. 2016;163:400–412. doi: 10.1016/j.chemosphere.2016.08.044. [DOI] [PubMed] [Google Scholar]
- Middleton D.R.S., Watts M.J., Hamilton E.M., Ander E.L., Close R.M., Exley K.S., Crabbe H., Leonardi G.S., Fletcher T., Polya D.A. Urinary arsenic profiles reveal exposures to inorganic arsenic from private drinking water supplies in Cornwall, UK. Sci. Rep. 2016;6:25656. doi: 10.1038/srep25656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao J., Jiang Z., Sun B., Sun Y., Wang Q., Guan X. Arsenate and arsenite removal by FeCl3: effects of pH, As/Fe ratio, initial As concentration and co-existing solutes. Separ. Purif. Technol. 2012;92:106–114. [Google Scholar]
- Senn A.C., Hug S.J., Kaegi R., Hering J.G., Voegelin A. Arsenate co-precipitation with Fe(II) oxidation products and retention or release during precipitate aging. Water Res. 2018;131:334–345. doi: 10.1016/j.watres.2017.12.038. [DOI] [PubMed] [Google Scholar]
- Sharma S.K., Kappelhof J., Groenendijk M., Schippers J.C. Comparison of physicochemical iron removal mechanisms in filters. J. Water Supply Res. Technol. 2001;1:187–198. [Google Scholar]
- Stumm W., Lee G.F. Oxygenation of ferrous iron. Ind. Eng. Chem. 1961;53:143–146. [Google Scholar]
- Tamura H., Kawamura S., Hagayama M. Acceleration of the oxidation of Fe2+ ions by Fe(III)-oxyhydroxides. Corrosion Sci. 1980;20:963–971. [Google Scholar]
- Tatari K., Smets B.F., Albrechtsen H.J. Depth investigation of rapid sand filters for drinking water production reveals strong stratification in nitrification biokinetic behavior. Water Res. 2016;101 doi: 10.1016/j.watres.2016.04.073. [DOI] [PubMed] [Google Scholar]
- van Beek C.G.E.M., Dusseldorp J., Joris K., Huysman K., Leijssen H., Schoonenberg Kegel F., de Vet W.W.J.M., van de Wetering S., Hofs B. Contributions of homogeneous, heterogeneous and biological iron(II) oxidation in aeration and rapid sand filtration (RSF) in field sites. J. Water Supply Res. Technol. - Aqua. 2015;65:1–13. [Google Scholar]
- van Halem D., Bakker S. a., Amy G.L., van Dijk J.C. Arsenic in drinking water: a worldwide water quality concern for water supply companies. Drink. Water Eng. Sci. 2009;2:29–34. [Google Scholar]
- Vries D., Bertelkamp C., Schoonenberg Kegel F., Hofs B., Dusseldorp J., Bruins J.H., de Vet W., van den Akker B. Iron and manganese removal: recent advances in modelling treatment efficiency by rapid sand filtration. Water Res. 2017;109:35–45. doi: 10.1016/j.watres.2016.11.032. [DOI] [PubMed] [Google Scholar]
- WHO Arsenic in drinking-water. Backgr. Doc. Dev. WHO Guidel. Drink. Qual. 2011 [Google Scholar]