Abstract
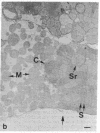
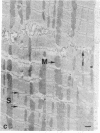
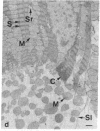
Brief perfusion of heart with calcium-free medium renders myocardial cells calcium-sensitive so that readmission of calcium results in uncontrolled Ca2+ entry and acute massive cell injury (calcium paradox). We investigated the hypothesis that polyamines may be involved in the mediation of abnormal Ca2+ influx and cell damage in the calcium paradox. The isolated perfused rat heart was used for these studies. Calcium-free perfusion promptly (less than 5 min) decreased the levels of polyamines and the activity of their rate-regulating synthetic enzyme, ornithine decarboxylase (ODC), and calcium reperfusion abruptly (less than 15-180 s) increased these components. alpha-Difluoromethylornithine (DFMO), a specific suicide inhibitor of ODC, suppressed the calcium reperfusion-induced increase in polyamines and the concomitant increase in myocardial cellular 45Ca influx, loss of contractility, release of cytosolic enzymes, myoglobin, and protein, and structural lesions. Putrescine, the product of ODC activity, nullified DFMO inhibition and restored the calcium reperfusion-induced increment in polyamines and the full expression of the calcium paradox. Putrescine itself enhanced the reperfusion-evoked release of myoglobin and protein in the absence of DFMO. Hypothermia blocked the changes in heart ODC and polyamines induced by calcium-free perfusion and calcium reperfusion and prevented the calcium paradox. These results indicate that rapid Ca2+-directed changes in ODC activity and polyamine levels are essential for triggering excessive transsarcolemmal transport of Ca2+ and explosive myocardial cell injury in the calcium paradox.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alto L. E., Dhalla N. S. Myocardial cation contents during induction of calcium paradox. Am J Physiol. 1979 Dec;237(6):H713–H719. doi: 10.1152/ajpheart.1979.237.6.H713. [DOI] [PubMed] [Google Scholar]
- Alto L. E., Dhalla N. S. Role of changes in microsomal calcium uptake in the effects of reperfusion of Ca2+-deprived rat hearts. Circ Res. 1981 Jan;48(1):17–24. doi: 10.1161/01.res.48.1.17. [DOI] [PubMed] [Google Scholar]
- Baker J. E., Bullock G. R., Hearse D. J. The temperature dependence of the calcium paradox: enzymatic, functional and morphological correlates of cellular injury. J Mol Cell Cardiol. 1983 Jun;15(6):393–411. doi: 10.1016/0022-2828(83)90323-1. [DOI] [PubMed] [Google Scholar]
- Chapman R. A. Control of cardiac contractility at the cellular level. Am J Physiol. 1983 Oct;245(4):H535–H552. doi: 10.1152/ajpheart.1983.245.4.H535. [DOI] [PubMed] [Google Scholar]
- Chapman R. A., Rodrigo G. C., Tunstall J., Yates R. J., Busselen P. Calcium paradox of the heart: a role for intracellular sodium ions. Am J Physiol. 1984 Nov;247(5 Pt 2):H874–H879. doi: 10.1152/ajpheart.1984.247.5.H874. [DOI] [PubMed] [Google Scholar]
- Costa M., Nye J. S. Calcium, asparagine and cAMP are required for ornithine decarboxylase activation in intact Chinese hamster ovary cells. Biochem Biophys Res Commun. 1978 Dec 14;85(3):1156–1164. doi: 10.1016/0006-291x(78)90663-0. [DOI] [PubMed] [Google Scholar]
- D'Amore P. A., Shepro D. Calcium flux and ornithine decarboxylase activity in cultured endothelial cells. Life Sci. 1978 Feb;22(7):571–576. doi: 10.1016/0024-3205(78)90335-1. [DOI] [PubMed] [Google Scholar]
- Dhalla N. S., Alto L. E., Singal P. K. Role of Na+-Ca2+ exchange in the development of cardiac abnormalities due to calcium paradox. Eur Heart J. 1983 Dec;4 (Suppl H):51–56. doi: 10.1093/eurheartj/4.suppl_h.51. [DOI] [PubMed] [Google Scholar]
- Djurhuus R. Ornithine decarboxylase (EC 4.1.1.17) assay based upon the retention of putrescine by a strong cation-exchange paper. Anal Biochem. 1981 May 15;113(2):352–355. doi: 10.1016/0003-2697(81)90088-9. [DOI] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Calcium and cardiac excitation-contraction coupling. Annu Rev Physiol. 1979;41:473–484. doi: 10.1146/annurev.ph.41.030179.002353. [DOI] [PubMed] [Google Scholar]
- Flamigni F., Rossoni C., Stefanelli C., Caldarera C. M. Polyamine metabolism and function in the heart. J Mol Cell Cardiol. 1986 Jan;18(1):3–11. doi: 10.1016/s0022-2828(86)80977-4. [DOI] [PubMed] [Google Scholar]
- Frank J. S., Rich T. L., Beydler S., Kreman M. Calcium depletion in rabbit myocardium. Ultrastructure of the sarcolemma and correlation with the calcium paradox. Circ Res. 1982 Aug;51(2):117–130. doi: 10.1161/01.res.51.2.117. [DOI] [PubMed] [Google Scholar]
- Ganote C. E., Nayler W. G. Contracture and the calcium paradox. J Mol Cell Cardiol. 1985 Aug;17(8):733–745. doi: 10.1016/s0022-2828(85)80035-3. [DOI] [PubMed] [Google Scholar]
- Gibbs J. B., Hsu C. Y., Terasaki W. L., Brooker G. Calcium and microtubule dependence for increased ornithine decarboxylase activity stimulated by beta-adrenergic agonists, dibutyryl cyclic AMP, or serum in a rat astrocytoma cell line. Proc Natl Acad Sci U S A. 1980 Feb;77(2):995–999. doi: 10.1073/pnas.77.2.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinwald P. M., Nayler W. G. Calcium entry in the calcium paradox. J Mol Cell Cardiol. 1981 Oct;13(10):867–880. doi: 10.1016/0022-2828(81)90286-8. [DOI] [PubMed] [Google Scholar]
- Gøtzsche O. Abnormal myocardial calcium uptake in streptozocin-diabetic rats. Evidence for a direct insulin effect on catecholamine sensitivity. Diabetes. 1985 Mar;34(3):287–290. doi: 10.2337/diab.34.3.287. [DOI] [PubMed] [Google Scholar]
- Gøtzsche O. Decreased myocardial calcium uptake after isoproterenol in streptozotocin-induced diabetic rats. Studies in the in vitro perfused heart. Lab Invest. 1983 Feb;48(2):156–161. [PubMed] [Google Scholar]
- Heby O. Role of polyamines in the control of cell proliferation and differentiation. Differentiation. 1981;19(1):1–20. doi: 10.1111/j.1432-0436.1981.tb01123.x. [DOI] [PubMed] [Google Scholar]
- Holland C. E., Jr, Olson R. E. Prevention by hypothermia of paradoxical calcium necrosis in cardiac muscle. J Mol Cell Cardiol. 1975 Dec;7(12):917–928. doi: 10.1016/0022-2828(75)90152-2. [DOI] [PubMed] [Google Scholar]
- Hunt W. G., Willis R. J. Calcium exposure required for full expression of injury in the calcium paradox. Biochem Biophys Res Commun. 1985 Jan 31;126(2):901–904. doi: 10.1016/0006-291x(85)90270-0. [DOI] [PubMed] [Google Scholar]
- Hunter D. R., Haworth R. A., Berkoff H. A. Cellular calcium turnover in the perfused rat heart: modulation by caffeine and procaine. Circ Res. 1982 Sep;51(3):363–370. doi: 10.1161/01.res.51.3.363. [DOI] [PubMed] [Google Scholar]
- Hunter D. R., Haworth R. A., Berkoff H. A. Measurement of rapidly exchangeable cellular calcium in the perfused beating rat heart. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5665–5668. doi: 10.1073/pnas.78.9.5665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal Z., Koenig H. Polyamines appear to be second messengers in mediating Ca2+ fluxes and neurotransmitter release in potassium-depolarized synaptosomes. Biochem Biophys Res Commun. 1985 Dec 17;133(2):563–573. doi: 10.1016/0006-291x(85)90943-x. [DOI] [PubMed] [Google Scholar]
- Koenig H., Goldstone A. D., Lu C. Y. Beta-adrenergic stimulation of Ca2+ fluxes, endocytosis, hexose transport, and amino acid transport in mouse kidney cortex is mediated by polyamine synthesis. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7210–7214. doi: 10.1073/pnas.80.23.7210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig H., Goldstone A., Lu C. Y. Polyamines regulate calcium fluxes in a rapid plasma membrane response. Nature. 1983 Oct 6;305(5934):530–534. doi: 10.1038/305530a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lamers J. M., Ruigrok T. J. Diminished Na+/K+ and Ca2+ pump activities in the Ca2+ depleted heart: possible role in the development of Ca2+ overload during the Ca2+ paradox. Eur Heart J. 1983 Dec;4 (Suppl H):73–79. doi: 10.1093/eurheartj/4.suppl_h.73. [DOI] [PubMed] [Google Scholar]
- Lamers J. M., Stinis J. T., Ruigrok T. J. Biochemical properties of membranes isolated from calcium-depleted rabbit hearts. Circ Res. 1984 Mar;54(3):217–226. doi: 10.1161/01.res.54.3.217. [DOI] [PubMed] [Google Scholar]
- Langdon R. C., Fleckman P., McGuire J. Calcium stimulates ornithine decarboxylase activity in cultured mammalian epithelial cells. J Cell Physiol. 1984 Jan;118(1):39–44. doi: 10.1002/jcp.1041180109. [DOI] [PubMed] [Google Scholar]
- Nawata H., Yamamoto R. S., Poirier L. A. Ornithine decarboxylase induction and polyamine levels in the kidney of estradiol-treated castrated male rats. Life Sci. 1980 Mar 3;26(9):689–698. doi: 10.1016/0024-3205(80)90258-1. [DOI] [PubMed] [Google Scholar]
- Nayler W. G., Elz J. S., Perry S. E., Daly M. J. The biochemistry of uncontrolled calcium entry. Eur Heart J. 1983 Dec;4 (Suppl H):29–41. doi: 10.1093/eurheartj/4.suppl_h.29. [DOI] [PubMed] [Google Scholar]
- Nayler W. G., Perry S. E., Elz J. S., Daly M. J. Calcium, sodium, and the calcium paradox. Circ Res. 1984 Aug;55(2):227–237. doi: 10.1161/01.res.55.2.227. [DOI] [PubMed] [Google Scholar]
- Pegg A. E. Recent advances in the biochemistry of polyamines in eukaryotes. Biochem J. 1986 Mar 1;234(2):249–262. doi: 10.1042/bj2340249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruaño-Arroyo G., Gerstenblith G., Lakatta E. G. 'Calcium paradox' in the heart is modulated by cell sodium during the calcium-free period. J Mol Cell Cardiol. 1984 Sep;16(9):783–793. doi: 10.1016/s0022-2828(84)80002-4. [DOI] [PubMed] [Google Scholar]
- Ruigrok T. J., Boink A. B., Spies F., Blok F. J., Maas A. H., Zimmerman A. N. Energy dependence of the calcium paradox. J Mol Cell Cardiol. 1978 Nov;10(11):991–1002. doi: 10.1016/0022-2828(78)90395-4. [DOI] [PubMed] [Google Scholar]
- Russell D. H. Ornithine decarboxylase: a key regulatory enzyme in normal and neoplastic growth. Drug Metab Rev. 1985;16(1-2):1–88. doi: 10.3109/03602538508991430. [DOI] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. Polyamines. Annu Rev Biochem. 1984;53:749–790. doi: 10.1146/annurev.bi.53.070184.003533. [DOI] [PubMed] [Google Scholar]
- Tunstall J., Busselen P., Rodrigo G. C., Chapman R. A. Pathways for the movements of ions during calcium-free perfusion and the induction of the 'calcium paradox'. J Mol Cell Cardiol. 1986 Mar;18(3):241–254. doi: 10.1016/s0022-2828(86)80406-0. [DOI] [PubMed] [Google Scholar]
- Zimmerman A. N., Hülsmann W. C. Paradoxical influence of calcium ions on the permeability of the cell membranes of the isolated rat heart. Nature. 1966 Aug 6;211(5049):646–647. doi: 10.1038/211646a0. [DOI] [PubMed] [Google Scholar]