Summary
Bacterial blight caused by the infection of Xanthomonas oryzae pv. oryzae (Xoo) is a devastating disease that severely challenges the yield of rice. Here, we report the identification of a “SAPK10-WRKY72-AOS1” module, through which Xoo infection stimulates the suppression of jasmonic acid (JA) biosynthesis to cause Xoo susceptibility. WRKY72 directly binds to the W-box in the promoter of JA biosynthesis gene AOS1 and represses its transcription by inducing DNA hypermethylation on the target site, which finally led to lower endogenous JA level and higher Xoo susceptibility. Abscisic acid (ABA)-inducible SnRK2-type kinase SAPK10 phosphorylates WRKY72 at Thr 129. The SAPK10-mediated phosphorylation impairs the DNA-binding ability of WRKY72 and releases its suppression on AOS1 and JA biosynthesis. Our work highlights a module of how pathogen stimuli lead to plant susceptibility, as well as a potential pathway for ABA-JA interplay with post-translational modification and epigenetic regulation mechanism involved.
Subject Areas: Biochemistry, Cell Biology, Genetics, Microbiology, Molecular Biology, Plant Biology
Graphical Abstract
Highlights
-
•
WRKY72 negatively regulates rice resistance to Xoo infection and JA synthesis
-
•
SAPK10 phosphorylates WRKY72 at Thr 129 to impair its DNA binding on AOS1
-
•
WRKY72 directly represses AOS1 transcription to attenuate JA synthesis
-
•
WRKY72 recruits hyper DNA methylation on AOS1 promoter
Biochemistry; Cell Biology; Genetics; Microbiology; Molecular Biology; Plant Biology
Introduction
The plant innate immune system is considered to contain two interconnected layers termed PTI (pathogen-associated molecular patterns-triggered immunity) and ETI (effector-triggered immunity) (Jones and Dangl, 2006). Once plant intercepts pathogen-associated molecular patterns (PAMPs) such as chitin and flagellin, it activates downstream defense signaling to provide the first layer (Jones and Dangl, 2006, Saijo et al., 2018). Some virulent pathogens secrete effector proteins to suppress PTI. To fight back, plant resistance (R) proteins trigger ETI that provokes highly efficient defense responses upon effectors (Jones and Dangl, 2006, Peng et al., 2017). PTI and ETI usually result in massive transcriptional reprogramming of defense genes, which indicates the existence of a complex regulatory circuitry composed of transcriptional activators and repressors (Agarwal and Chikara, 2011, Madhunita and Ralf, 2014).
The WRKY family proteins are plant special transcription factors. The WRKY domain contains a conserved WRKYGQK sequence followed by a Cys2His2 or Cys2HisCys zinc-binding motif (Eulgem et al., 2000). WRKY proteins recognize the W-box (T)TGAC(C/T) or W-like box cis-regulatory elements, which are often found in many defense gene promoters. In addition to the W-box, it can bind other cis elements, such as sugar-responsive element (AA/TAA) in barley and pathogen response element (TACTGCGCTTAGT) in rice (Cai et al., 2008, Cheng et al., 2015, Sun et al., 2003).
WRKY proteins have been reported to play broad and pivotal roles in plant-pathogen interactions and act in a complex signaling network as both positive and negative regulators of disease resistance (Chen and Chen, 2002, Eulgem et al., 2000, Li et al., 2004, Zheng et al., 2006). Till date, a total of 125 WRKY gene family members have been identified and uniquely named by the rice WRKY working group to avoid confusions in nomenclature (hereafter, we follow the nomenclature of rice WRKY working group) (Rice WRKY Working Group, 2012, Ross et al., 2007). Ryu et al. (2006) demonstrated that one-third of the 45 tested WRKY genes in rice were remarkably responsive to the inoculation of bacterial pathogen Xanthomonas oryzae pv. oryzae (Xoo) and the fungal pathogen Magnaporthe grisea, which indicated that WRKY genes are extensively involved in plant defense to pathogens (Ryu et al., 2006). Till date, at least 12 WRKY genes have been characterized to be involved in rice disease resistance through diverse mechanisms (Cheng et al., 2015). WRKY12 (originally named as WRKY03), WRKY13, WRKY22, WRKY30, WRKY55 (originally named as WRKY31), WRKY53, WRKY71, and WRKY104 (originally named as WRKY89) are positive regulators of rice resistance to pathogens. WRKY12, WRKY55, WRKY53, WRKY71, and WRKY104 could enhance rice resistance to M. grisea by up-regulating pathogenesis-related (PR) genes such as NPR1, ZB8, POX22.3, PR1b, PBZ1, and Sci2 (Chujo et al., 2014, Liu et al., 2005, Liu et al., 2007, Zhang et al., 2008). Some of the WRKY-positive regulators were implicated in the biosynthesis and signaling of phytohormones such as salicylic acid (SA) and jasmonic acid (JA). For example, WRKY30 activated SA signaling genes in response to M. grisea infection, whereas WRKY13 activated a series of SA synthesis and signaling genes and simultaneously suppressed JA synthesis and responsive genes to enhance rice resistance to M. grisea and Xoo (Cheng et al., 2015, Qiu et al., 2007, Qiu et al., 2008). Reported negative regulators include WRKY28, WRKY42, WRKY62, and WRKY76. Most of them increase rice susceptibility to pathogens by suppressing the transcription of defense-related genes, phytoalexin synthesis-related genes, or resistance (R) genes (Cheng et al., 2015, Chujo et al., 2013, Peng et al., 2008, Yokotani et al., 2013). Notably, WRKY45 positively regulates rice resistance to M. grisea, but it plays dual roles in rice resistance to bacteria via an alternative splicing model (Shimono et al., 2007, Shimono et al., 2012, Tao et al., 2009). Knockout of the variant WRKY45-1 showed increased resistance to Xoo and Xanthomonas oryzae pv oryzicola (Xoc), which was accompanied by a higher level of SA and JA. On the contrary, the variant WRKY45-2 was found to promote accumulation of JA, but not SA, and eventually play positive functions in response against Xoo and Xoc. The opposite roles of the two variants in rice-Xoo interaction are possibly attributed to their mediation of different defense signaling pathways.
Despite the fact that tremendous progresses on WRKYs have been achieved, it is believed that more WRKY members are involved in rice immunity, given the extensive involvement of WRKYs and complication of rice-pathogen interplays. The current study revealed that Xoo-inducible WRKY72 negatively regulates rice resistance against bacterial blight. WRKY72 directly binds to the promoter of a JA biosynthesis enzyme gene AOS1 and suppresses its transcription by recruiting DNA methylation on it. SAPK10-mediated phosphorylation on Thr129 of WRKY72 weakens its DNA-binding ability to AOS1, promotes the endogenous JA level, and finally enhances Xoo resistance.
Results
Transcription of WRKY72 Is Induced by Xoo Infection and Exogenous JA
To find out the rice WRKYs involved in defense against bacterial blight, we performed qRT-PCR analysis of various WRKYs in a time line after Xoo inoculation and found that WRKY72 (LOC_Os11g29870) is highly induced. The 3,736-bp-long gene encodes a 243-amino acid protein. In its only intron, we identified a SINE (short interspersed nuclear elements)-type transposon (2,812–3,024 bp) (http://www.repeatmasker.org/cgi-bin/WEBRepeatMasker). WRKY72 displayed significantly elevated transcriptional level since 12 HAI (hours after inoculation) and reached highest level (over eight times up-regulation) at 72 HAI, when compared with the 0-HAI samples (Figure 1A). In addition, WRKY72 also responds to treatment of exogenous phytohormones JA and ABA. WRKY72 was immediately suppressed by ABA treatment at 4 and 8 HAI, and then increased to a higher level at 12 HAI (Figure 1B). JA treatment showed a similar induction pattern as ABA (Figure 1C). WRKY72 is constitutively transcribed in various tissues, including leaf, root, panicle, callus, stem, and developing seeds (Figure 1D). To figure out the subcellular localization of WRKY72, we constructed a pro35S:WRKY72-GFP vector and co-transformed it with a marker nuclear protein pro35S:D53-mKate into rice protoplast. As expected, WRKY72 co-localized with D53 in the nucleus, which supported its functional annotation as a transcription factor (Figure 1E).
Figure 1.
The Temporal-Spatial and Stress Expression Profiles of WRKY72
(A–C) The time course expression level of WRKY72 at Xoo inoculation and ABA and JA treatment. (A) Xoo and (B) ABA and (C) JA concentrations were ∼1 × 108 colony-forming unit/mL and 100 μM, respectively.
(D) The expression analysis of WRKY72 in various tissues and stages. The expression level of leaves was set as 1. DAP: day after pollination.
(E) Subcellular localization of WRKY72. 35S:WRKY72-GFP was co-transformed with a nuclear marker 35S:D53-mKate into protoplast. The fluorescent protein signals from left to right: 35S:WRKY72-GFP, 35S:D53-mKate, bright field, and merged. Scale bars, 10 μm. All data are shown as means ± SD of at least three biological replicates.
WRKY72 Suppresses Rice Resistance to Xoo
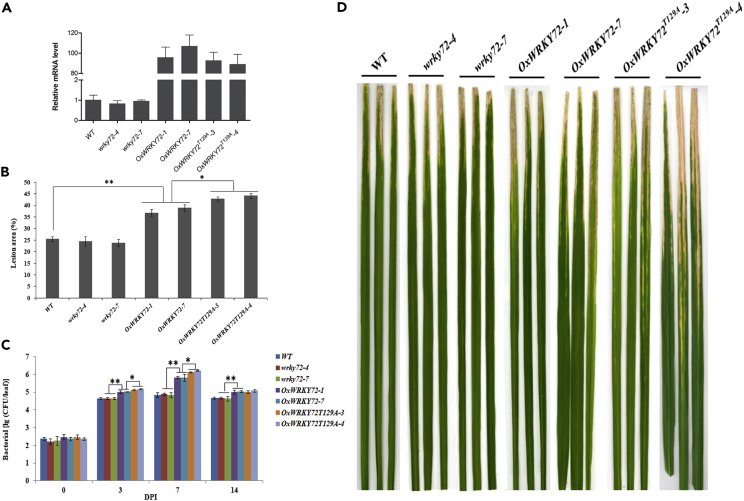
To dissect the biological roles of WRKY72 in rice bacterial blight resistance, we generated both CRISPR/Cas9-mediated mutant lines and over-expression lines of the gene. In T1 generation, two representative homozygous mutant lines wrky72-4 and wrky72-7 and two highly over-expressed lines OxWRKY72-1 and OxWRKY72-7 were used for phenotyping. wrky72-4 and wrky72-7 harbored a G and a T nucleotide insertion in the coding sequence, respectively, which should have disrupted the WRKY72 translation by shifting the open reading frame, although the transcription of the mutated genes were at the same level as native WRKY72 (Figures 2A and S1). Both OxWRKY72-1 and OxWRKY72-7 showed over 80-fold increase of expression level. The major agronomic traits of the plants were largely the same, except that OxWRKY72s had lower yield per plant (Table S1). Upon artificial inoculation of Xoo, OxWRKY72-1 and OxWRKY72-7 became more susceptible than the wild-type (WT), as the lesion area on OxWRKY72-1 and OxWRKY72-7 were 37% and 39%, respectively, whereas 25% of the leaf area of WT displayed necrosis (Figures 2B and 2D). It was also revealed that the Xoo growth rates in OxWRKY72s were significantly higher than those in the WT at 3, 7, and 14 DPI (days post inoculation) (p < 0.05) (Figure 2C). All these disease index data demonstrated that WRKY72 negatively regulated rice resistance to Xoo. However, wrky72-4 and wrky72-7 mutant lines displayed almost identical Xoo susceptibility as the WT did, which may be attributed to the functional redundancy of other sibling WRKY genes.
Figure 2.
Phenotypical Characterization of wrky72s, OxWRKY72s, and WT against Xoo
(A) The expression analysis of WRKY72 in wrky72, OxWRKY72, and WT plant lines.
(B–D) (B) The lesion area (%), (C) bacterial growth rate, and (D) necrosis lesion symposium in wrky72, OxWRKY72, and WT plant lines. wrky72-4 and wrky72-7: WRKY72 CRISPR/Cas9 lines; OxWRKY72-1 and OxWRKY72-7: WRKY72 over-expressing lines; OxWRKY72T129A-3 and OxWRKY72T129A-4: WRKY72 over-expressing lines with Thr129 substitution; WT: wild-type. Data are shown as means ± SD of at least three biological replicates. *p ≤ 0.05, **p ≤ 0.01 by the Student's t test.
WRKY72 Is Phosphorylated by SAPK10 at Thr129
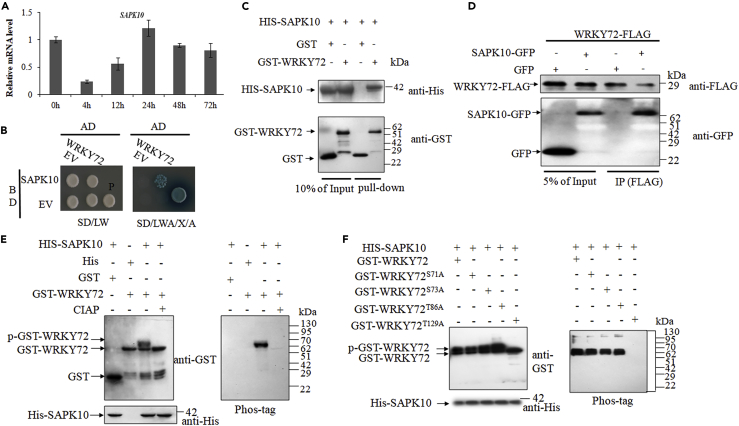
We screened over 1 million colonies from a cDNA library derived from rice young seedlings by using yeast two-hybrid method and detected an interactive kinase SAPK10 (LOC_Os03g41460). SAPK10 may be a negative regulator in response to Xoo infection, as its transcription was significantly suppressed by Xoo at the first 12 h after inoculation (Figure 3A). We repeated the yeast two-hybrid assay by a reciprocal hybridization and confirmed the SAPK10-WRKY72 interaction in yeast (Figure 3B). The interaction was further verified by pull-down in vitro and co-immunoprecipitation in vivo assays. The in vitro pull-down assay demonstrated that GST-WRKY72 protein could be pulled down by HIS-SAPK10 protein (Figure 3C). Meanwhile, SAPK10-GFP protein, but not the GFP tag control, was specifically co-immunoprecipitated with the WRKY72-FLAG in tobacco (Figure 3D). Thus, we proved the SAPK10-WRKY72 interaction in yeast, in vitro and in vivo. Subsequently, an E. coli kinase assay was performed to examine the kinase-substrate relationship between SAPK10 and WRKY72. When co-transformed with HIS-SAPK10, GST-WRKY72 showed a lagged band as detected by the GST antibody, indicating a phosphorylation has occurred on it (Figure 3E). The phosphorylation was further confirmed by the Phos-tag detection (Figure 3E). Therefore, we concluded that SAPK10 phosphorylates WRKY72 in vitro. To specify the SAPK10-dependent phosphosites on WRKY72, we generated the truncated forms of WRKY72 for the kinase assay. The phosphosite was gradually narrowed down to the fragment 1–135 with four potential sites (Figure S2), and finally localized at Thr129 (129th threonine), which is out of, but very close to, the annotated WRKY domain (138–193 amino acid) (Figure 3F).
Figure 3.
Protein-Protein Interaction and Phosphorylation Site Analysis of WRKY72 and SAPK10
(A) A time course expression level of SAPK10 at Xoo inoculation. Data are shown as means ± SD of at least biological triplicates.
(B) Yeast two-hybrid assays. BD: pGBKT7; AD: pGADT7; EV: empty vector; SD/LW: -Leu-Trp; SD/LWA/X/A: -Leu-Trp-Ade with the addition of X-α-Gal and aureobasidin A; P: positive control, pGADT7-T/pGBKT7-53.
(C) In vitro pull-down of SAPK10 and WRKY72. His-SAPK10 and GST-WRKY72 and GST were expressed and purified in E. coli and subjected to GST pull-down assays, then detected by immunoblotting using anti-GST and anti-His antibodies, respectively.
(D) In vivo co-immunoprecipitation (IP) assay of SAPK10 and WRKY72. GFP, SAPK10-GFP, and WRKY72-FLAG extracted from infiltrated Nicotiana benthamiana leaves were used in a coIP assay. Precipitates were immunoblotted with GFP and FLAG antibodies, respectively.
(E and F) WRKY72 is phosphorylated by SAPK10 at Thr129. The recombinant protein GST-WRKY72 (E) and GST-WRKY72 with potential phosphosites substituted (F) were co-expressed with His-SAPK10 in E. coli, respectively. Equal amounts of the GST purified recombinant proteins were detected by immunoblotting using indicated antibodies. CIAP: Calf Intestine Alkaline Phosphatase; p-GST-WRKY72: phosphorylated GST-WRKY72; Phos-tag: biotinylated Phos-tag zinc BTL111 complex.
To test the effect of SAPK10-mediated phosphorylation on the function of WRKY72, we further generated OxWRKY72T129A lines, in which the only phosphosite Thr129 was mutated into an alanine (Ala) to block the phosphorylation on it. Two OxWRKY72T129A lines with comparable levels of WRKY72 expression as the OxWRKY72s were chosen for Xoo susceptibility assay (Figure 2A). Interestingly, OxWRKY72T129A lines exhibited higher susceptibility to Xoo than the OxWRKY72 lines (Figure 2D), although the other major agronomic traits remained largely the same (Table S1), indicating that the phosphorylation on Thr129 might be a key switch to turn off the WRKY72 negative function in disease resistance.
WRKY72 Regulates Defense-Related Genes and JA Biosynthesis Genes
To further elucidate the regulation network, we first performed RT-qCR to examine the transcriptional levels of a couple of JA synthesis genes in OxWRKY72, OxWRKY72T129A, and WT lines. The results suggested that the all the tested genes including AOC (Allene Oxide Cyclase, LOC_Os03g32314), AOS1 (Allene Oxide Synthase 1, LOC_Os03g55800), AOS2 (Allene Oxide Synthase 2, LOC_Os03g12500), LOX1 (Lipoxygenase 1, LOC_Os03g49380), and LOX2 (Lipoxygenase 2, LOC_Os03g08220) were significantly down-regulated in OxWRKY72, with the only exception of OPR7 (12-Oxophytodienoate reductase, LOC_Os08g35740), which showed an opposite tendency (Figure 4A). More interestingly, the levels of AOC, AOS1, and AOS2 were further reduced in OxWRKY72T129A when compared with OxWRKY72 (p < 0.05), which is in accordance with the observed higher susceptibility of OxWRKY72T129A. We also investigated the levels of PR protein genes PR1a, PR1b, PR5, and PR10. PR1b, and PR10 may contribute to the elevated susceptibility of OxWRKY72 and OxWRKY72T129A lines, as both genes were significantly down-regulated (Figure 4B).
Figure 4.
The Transcriptional Abundances of JA Biosynthesis and Pathogenesis-Related Genes and Endogenous JA Level Analysis in WT, OxWRKY72, and OxWRKY72T129A
(A and B) The transcriptional level of JA biosynthesis and pathogenesis-related genes in WT, OxWRKY72, and OxWRKY72T129A at 72 h after Xoo infection.
(C) The time course endogenous JA level in WT, OxWRKY72, and OxWRKY72T129A. WT: wild-type; OxWRKY72: WRKY72 over-expression line 1 and line7 sample mixed; OxWRKY72T129A: WRKY72 with Thr129 substitution form's over-expressing line 3 and 4 sample mixed. FW: fresh weight.
Data are shown as means ± SD of three biological replicates. * And ** represent significant difference in the comparison at p ≤ 0.05 and p ≤ 0.01, respectively, as determined by the Student's t test.
Given the obvious down-regulation of the key enzyme genes for JA biosynthesis, we examined the endogenous JA level in OxWRKY72, OxWRKY72T129A, and WT (Figure 4C). Before the Xoo infection, the three types of plants were in a similar and relatively low endogenous JA level, although OxWRKY72 had a slightly higher level (p < 0.05). In the WT, Xoo infection significantly stimulated the accumulation of JA to about 16 times higher level than that in the pre-infection condition. However, the Xoo infection appeared to have triggered the suppression function of WRKY72 on JA synthesis, as we found that the JA level was significantly reduced in OxWRKY72 at 72 HAI. Moreover, OxWRKY72T129A displayed even lower JA level than OxWRKY72 at 72 HAI, which is consistent with the observation that OxWRKY72T129A lines were more susceptible to Xoo than OxWRKY72s. The results above suggested that WRKY72 negatively regulates Xoo resistance by suppressing JA biosynthesis.
WRKY72 Directly Suppresses AOS1 Transcription to Attenuate JA Biosynthesis
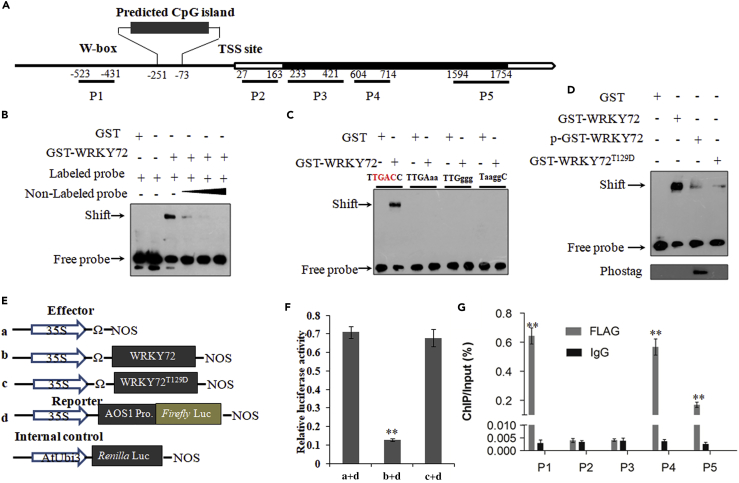
The reduced level of endogenous JA and JA biosynthesis rate-limiting genes in OxWRKY72 lines intrigued us to speculate that WRKY72 directly suppresses the transcription of these genes. To test this hypothesis, we checked the in vitro binding of WRKY72 on AOC, AOS1, and LOX1 promoter regions by EMSA (electrophoretic mobility shift assay). The results showed that WRKY72 only bound to the promoter of AOS1, where a conserved W-box cis element exists (Figures 5A, 5B, and S3). The shifted bands were substantially weakened when non-labeled competitive probes were applied, suggesting a highly specific binding of WRKY72 to the promoter of AOS1 in vitro (Figure 5B). Meanwhile, the binding was completely impaired when the conserved W-box was mutated; hence this 6-nucleotide sequence (TTGACC) might be the core cis element for the binding of WRKY72 (Figure 5C). Moreover, we performed EMSA to investigate the DNA-binding ability of WRKY72 in different phosphorylation status. As shown in Figure 5D, p-GST-WRKY72 (Thr129 site phosphorylated) and WRKY72T129D, in which Thr129 was mutated to mimic constitutive phosphorylation status, both had substantially reduced signal of shifted bands when compared with the non-phosphorylated WRKY72. This observation suggested that SAPK10-mediated phosphorylation on Thr129 turns down WRKY72 function by weakening its DNA-binding ability. Subsequently, we generated proUbi:WRKY72-FLAG lines and used the leaves at 72 HAI for chromatin immunoprecipitation-qPCR assay. In total, five fragments representing the promoter, UTR, and coding sequence regions were examined, and we found that WRKY72 was significantly enriched in the W-box region of the AOS1 promoter when compared with the mock, which proved the binding of WRKY72 to AOS1 promoter in vivo (Figure 5G). Finally, we conducted a dual luciferase (LUC) transient transcriptional activity assay to test the effect of WRKY72 on AOS1 transcription. When compared with the empty effector, pro35S:WRKY72:tNOS dramatically reduced the firefly LUC reporter level (Figures 5E and 5F), which is in agreement with the observed down-regulation of AOS1 in OxWRKY72 lines, indicating that WRKY72 directly suppresses AOS1 transcription. In support to the observed lower DNA-binding ability of WRKY72T129D in EMSA, we also found that the suppression on AOS1 was significantly reduced when WRKY72T129D was used as the effector (Figures 5E and 5F).
Figure 5.
WRKY72 Directly Binds to the W-Box of AOS1 Promoter and Suppresses Its Transcription, and Phosphorylation of WRKY72 can Reduce its Binding and Suppression Activity
(A) Schematic presentation of the AOS1 gene structures. Black boxes: exons; blank box: untranslated region; line: promoter. Transcription starting site (TSS) was set as 0. Numbers indicate the distances (bps) to the TSS.
(B) EMSA assay showing WRKY72 could directly bind to the promoter of AOS1. The 5-, 10-, and 30-fold excess non-labeled probes were used for competition.
(C) EMSA showing TTGACC is required by WRKY-72 binding to the promoter of AOS1. Probe sequence (60 bp) containing W-box (TTGACC). W-box region is shown in (A). TTGACC was substituted by TaaggC, TTGggg, and TTGAaa in the mutant probe, and the substitution nucleotide acids were marked with lowercases.
(D) EMSA assay showing the binding between WRKY72 and AOS1 is suppressed by phosphorylation of Thr129 in WRKY72 protein. GST-WRKY72, purified protein; p-GST-WRKY72: phosphorylated proteins; GST-WRKY72T129D: mimic phosphorylated proteins. The phosphorylated proteins were chemiluminescence detected by biotinylated Phos-tag zinc complex.
(E) Scheme of the constructs used in LUC transient transcriptional activity assay.
(F) LUC transient transcriptional activity assay in rice protoplast. Reporter: proAOS1:LUC; effectors: pro35S:WRKY72:tNOS and WRKY72T129D. The fLUC/rLUC ratio represents the relative activity of 35S promoter.
(G) Validation of the direct binding of WRKY72 to the promoter and coding sequence (CDS) of AOS1 by chromatin immunoprecipitation (ChIP)-qPCR. P1–P5 indicates the regions detected by ChIP-qPCR shown in (A).The enrichment values were normalized to Input. IgG-immunoprecipitated DNA was used as a control check (CK).
All values are mean ± SD with biological triplicates. **p < 0.01 by the Student's t test.
To figure out the biological functions of AOS1 in rice disease resistance, we further over-expressed AOS1 in Nipponbare background. The OxAOS1 lines had substantially elevated transcription level of AOS1 and became dwarf, which is a typical phenotype of plants with high endogenous JA levels, given that JA generally represses plant growth (Figures S4A–S4C). After the artificial inoculation of Xoo, OxAOS1 lines exhibited less lesion areas in the leaf and lower bacterial growth rate, suggesting a positive role of AOS1 in response to Xoo infection (Figures 6A–6C).
Figure 6.
Phenotypical Characterization of OxAOS1s and WT against Xoo
(A–C) Necrosis lesion symposium (A), the lesion area (%) (B), and bacterial growth rate (C) in OxAOS1 and WT plant lines. OxAOS1-2 and OxAOS1-3: AOS1 over-expressing lines; WT: wild-type. Data are shown as means ± SD of at least three biological replicates. *p ≤ 0.05, **p ≤ 0.01 by the Student's t test.
WRKY72 Induces DNA Hypermethylation on AOS1 Promoter
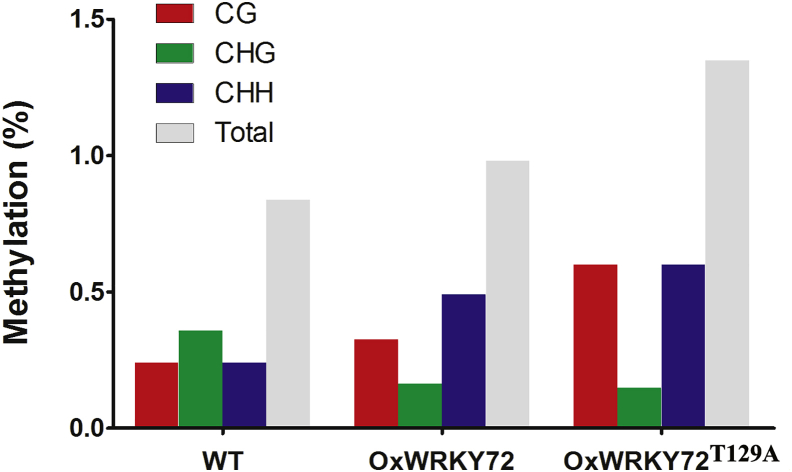
DNA methylation has been revealed as a profound epigenetic mechanism involved in gene repression. To address the question that how is AOS1 suppressed by WRKY72, we checked the DNA methylation pattern and level on the AOS1 promoter region (minus 251 – minus 73), which is the only potential CpG island as predicted by MethPrimer (http://www.urogene.org/cgi-bin/methprimer/methprimer.cgi) (Figure 5A). When compared with the WT, the CG and CHH levels of OxWRKY72 were significantly increased, which led to a significant increase of the total DNA methylation level, although the CHG level was decreased (Figure 7). WRKY72T129A exhibited hypermethylation on this region, when compared with WT and OxWRKY72 (Figure 7). The correlation between the DNA methylation and AOS1 transcription levels suggested that WRKY72 may induce DNA hypermethylation on AOS1 promoter to suppress its transcription.
Figure 7.
DNA Methylation Patterns and Level in WT, OxWRKY72, and OxWRKY72T129A
CG, CHG and CHH: three methylation patterns; WT: wild-type; OxWRKY72: WRKY72 over-expression line 1 and line7 sample mixed; OxWRKY72T129A: WRKY72 with Thr129 substitution form's over-expressing line 3 and 4 sample mixed. DNA methylation region detected is shown in Figure 5A.
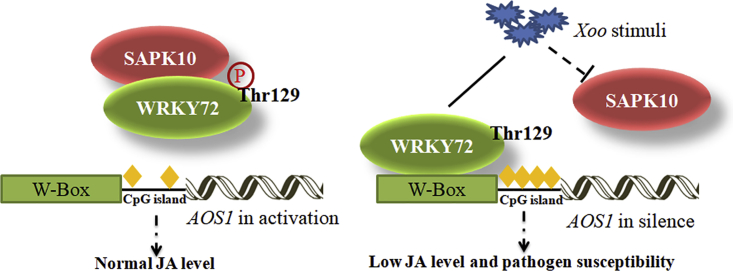
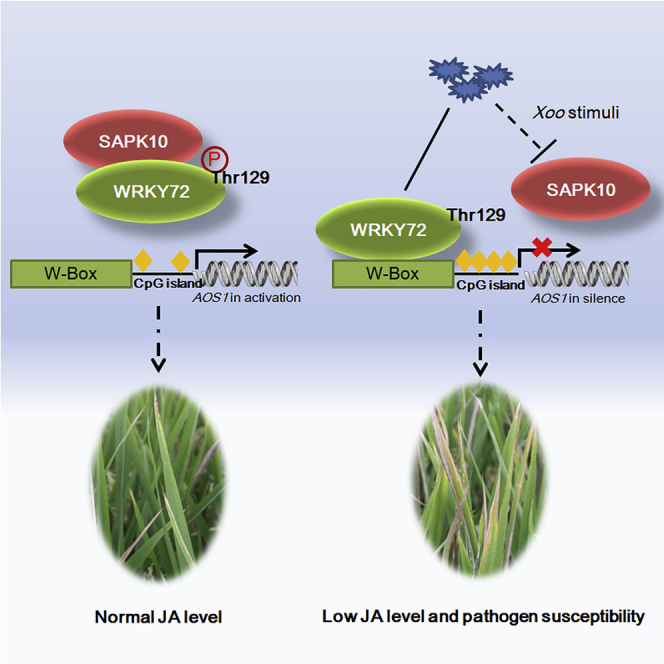
From the results above, a working model of WRKY72 was proposed (Figure 8). Under normal growth conditions, WRKY72 is phosphorylated by SAPK10 at Thr 129, which releases the suppression of WRKY72 to AOS1 by impairing WRKY72 DNA-binding ability and lowering the DNA methylation level on AOS1 promoter, thus maintaining a normal endogenous JA level for growth. Under Xoo infection, the stimuli represses SAPK10 transcription and makes the WRKY72 in a non-phosphorylated status, which facilitates the binding of WRKY72 to the W-box cis element of AOS1 promoter to suppress AOS1 transcription by recruiting hyper DNA methylation on it, and eventually contributes to plant susceptibility by suppressing the endogenous JA biosynthesis (Figure 8). It should be noted that, although our working model proposed a suppressing pathway of JA biosynthesis induced by Xoo infection, we did observe that the final endogenous JA level in Nipponbare plants was drastically increased by Xoo stimuli at 72 HAI (Figure 4C). Such a phenomenon suggested the existence of a feedback loop comprising suppressing pathways such as SAPK10-WRKY72-AOS1 as well as some unknown activating pathways of JA biosynthesis, whose counterbalance finally decides the endogenous level in rice. In the time point for JA quantification assay, possibly due to the weak level of WRKY72 in WT plants, the WRKY72-mediated suppression might be overcounted by the JA biosynthesis activation pathways, which eventually gave rise to the elevated JA level in the WT plant. Nevertheless, in the OxWRKY72 lines with magnified WRKY72 effects, the suppression pathway overrode the activating pathways and finally led to lower JA level and pathogen susceptibility after Xoo infection, which perfectly matched the JA quantification assay result in Figure 4C.
Figure 8.
Working Model of WRKY72-Mediated Regulation against Xoo Infection in Rice
Line and dotted line indicate positive and negative regulation in transcription, respectively; ⓟ indicates the phosphorylation status of the protein; and yellow rhombus indicates DNA methylation on the region.
Discussion
WRKY72 Suppresses Endogenous JA Level in Defense
It has been well known that JA, as an activating signal molecule, triggers immunity to confer broad-spectrum resistance for plants (Okada et al., 2015). Pathogen infection or other forms of biotic attack stimulate rapid biosynthesis of JA and its derivatives, which would promote the expression of defense-related proteins and secondary metabolites such as alkaloids, terpenoids, and PR proteins (Campos et al., 2014). Meanwhile, genetically knocking out or knocking down JA biosynthesis or signaling genes led to higher susceptibility of the plants to various pathogens. In Arabidopsis, disruption of JA receptor gene Coi1 or JA-lle synthesis gene JAR1 makes the plants susceptible to necrotrophic pathogens or soil fungus, respectively (Staswick et al., 2002, Staswick et al., 2010). Likewise, rice jasmonate-deficient plants cpm2 and hebiba were found to lose their resistance to an originally incompatible avirulent strain of M. grease, whereas ectopic expression of AOS2 encoding a JA production enzyme enhanced the plant resistance to pathogenic fungi (Mei et al., 2006, Riemann et al., 2013). JA may also promote plant resistance to hemi-necrotrophic Xoo. For example, JA signaling genes JAZ8 and MYC2 both are involved in rice resistance to bacterial blight (Uji et al., 2016, Yamada et al., 2012).
WRKYs are very important transcription factors in plants. The majority of over 100 members in rice are found to be involved in plant defense response in either a negative or a positive manner. The negative regulator members include WRKY28, WRKY42, WRKY62, and WRKY76, but their regulatory mechanism may vary from each other. WRKY62 and WRKY76 cause pathogen susceptibility by regulating a list of defense-related genes or interacting with the intracellular kinase domain of Xa21 to affect its protein cleavage and nuclear localization (Park and Ronald, 2012, Peng et al., 2008, Yokotani et al., 2013). Recently, emerging evidences linked the WRKY-regulated pathogen response to JA accumulation or signaling. For example, WRKY42-knockdown and WRKY42-over-expressing plants showed increased resistance and susceptibility to M. oryzae, which are accompanied by increased and reduced JA content, respectively (Cheng et al., 2015). In this research, we found that WRKY72 negatively regulates rice response to Xoo infection by suppressing JA biosynthesis, as the WRKY72 over-expression lines became more susceptible upon Xoo inoculation. A couple of JA biosynthesis genes were significantly down-regulated in OxWRKY72 lines. Moreover, we provided several layers of in vivo and in vitro evidences to show that WRKY72 directly binds to the conserved W-box cis element of JA biosynthesis rate-limiting enzyme gene AOS1 and suppresses AOS1 transcription, which eventually reduced endogenous JA level and rice resistance to bacterial blight. AOS enzymes catalyze the conversion of 13-HPOT (13-hydroperoxy-9,11,15-octadecatrienoic acid) to 12,13-EOT ((9Z,11E,15Z,13S,12R)-12,13-epoxy-9,11,15-octadecatrienoic), which is the first step toward JA biosynthesis (Schaller, 2001). Among the four AOS genes in rice (AOS1-AOS4), AOS2 has been characterized as a pathogen-inducible gene, and its over-expression lines had higher levels of JA and stronger resistance to M. grisea (Mei et al., 2006). Likewise, the current study revealed that over-expression of AOS1 also enhanced plant resistance to Xoo infection, when compared with the WT. In another study, AOS1 was isolated by positional cloning as Pre (precocious) controlling juvenile-to-adult phase transition in rice. Pre exhibited long leaf with precociously acquired adult features in midrib formation, shoot meristem size and plastochron, and more importantly, lower endogenous JA level (Hibara et al., 2016). Although the authors did not explore the potential roles of AOS1 in disease resistance, it is rational to expect a higher susceptibility of aos1 to Xoo infection.
SAPK10-Mediated Phosphorylation Turns Down WRKY72 Function as a Repressor
Post-translational modifications, particularly protein phosphorylation, have been long recognized as a significant regulatory mechanism controlling transcription factor activity (Meng et al., 2013, Yang et al., 2017). In plant defense, MAPK (mitogen-activated protein kinase) is a major type of kinase that can phosphorylate disease resistance-related transcription factors such as WRKYs. Phosphorylation within the SP cluster of WRKY proteins by MAPKs is thought to exert a booster function in the expression of downstream genes (Asai et al., 2002, Ishihama and Yoshioka, 2012, Pitzschke et al., 2009). In Arabidopsis, AtMPK3 and AtMPK6 directly phosphorylated AtWRKY33 to enhance the production of phytoalexin camalexin and phytohormone ethylene, whereas non-phosphorylated AtWRKY33 was not able to fully rescue wrky33 mutant, implying that MAPK-dependent phosphorylation activates AtWRKY33 function (Li et al., 2012, Mao et al., 2011, Wang et al., 2018). Similarly, Chujo et al. (2014) found that WRKY53-mediated resistance to rice blast fungus strain Ina86-137 relied on the phosphorylation on its serine-proline residues by MPK3/MPK6. Over-expressing a phosphomimic mutated version of WRKY53 (WRKY53SD) rice plants elevated the expression level of defense-related genes and enhanced disease resistance to M. oryzae compared with native WRKY53-over-expressing rice plants (Chujo et al., 2014). It was suggested that the positive effect of MAPK-dependent phosphorylation on WRKYs might be achieved by increasing its DNA-binding activities on target genes (Ishihama and Yoshioka, 2012, Koo et al., 2009, Menke et al., 2005).
In this study, we demonstrated that SAPK10 kinase cloud physically binds to and phosphorylates WRKY72 at Thr129. In contrast to the above-mentioned cases that phosphorylation activated WRKYs, the phosphorylation on WRKY72 weakened its DNA-binding ability to AOS1 promoter, to thus release the inhibition on JA accumulation. In support of this finding, the OxWRKY72T129A lines, which had blocked SAPK10 target phosphosite, showed drastically reduced transcription of downstream JA biosynthesis genes, endogenous JA level, and resistance to Xoo infection than the native OxWRKY72 lines. Hence, the indication is that the SAPK10-dependent phosphorylation on WRKY72 turns down its function as a transcription repressor in plant defense, representing a diverse mechanism to the previously reported MAPK-WRKY module. SAPK10 is an ABA-inducible SnRK2-type kinase involved in ABA signaling (Kobayashi et al., 2010). So far, the effects of ABA on plant disease resistance remain elusive. ABA likely plays negative roles in plant defense; however, the interplay of ABA with other phytohormones often produces complicated network and possibly promotes defense in plants (Ton et al., 2009). Notably, a couple of rice SnRK2s have been implicated in response to Xoo infection (Xu et al., 2013). Our results hint a pathway “SAPK10-WRKY72-AOS1” in the cross talk of ABA-JA as well as in the ABA-mediated plant defense response, which will be further explored in our future study.
WRKY72 Recruits DNA Hypermethylation to Repress AOS1
DNA cytosine methylation is usually connected with transcriptional silence of the target genes in numerous biological processes, including plant defense (Chen and Zhou, 2013, Wang et al., 2018). RdDM (RNA-directed DNA methylation) is a major mechanism to recruit DNA methyltransferases to the target site to execute DNA methylation. In such a case, DNA methylation is guided by a series of 21- to 24-nt small interfering RNA (siRNAs) with high homology with the target sites. The siRNAs could be derived from viral replication intermediates, products of endogenous RNA-directed RNA polymerase, transcribed inverted repeats, or TEs (transposable elements) (Wassenegger et al., 1994). One of the well-documented RdDM cases in rice defense is TE-siR815 (Zhang et al., 2016). TE-siR815 is an siRNA that originates from a MITE (miniature inverted repeat transposable elements). TE existed in the first intron of WRKY45, whose two variants WRKY45-1 and WRKY45-2 play opposite roles in response to Xoo and Xoc infection (Tao et al., 2009). Only the negative player WRKY45-1 produces TE-siR815, which imposes DNA hypermethylation on ST1 via an RdDM mechanism to abolish WRKY45-mediated pathogen resistance (Zhang et al., 2016). In our study, the total DNA cytosine methylation level on AOS1 promoter was negatively correlated with the AOS1 transcription level and endogenous JA level in WT, OxWRKY72, and OxWRKY72T129A lines, implying that the repressor role of WRKY72 may be achieved by inducing DNA methylations on the promoter region of its direct target AOS1. Nevertheless, the question that how was the DNA methyltransferase recruited to the target sites remain to be addressed. The identified SINE TE in WRKY72 intron might be a good clue that WRKY72 suppresses target genes through an RdDM mechanism, given the reported example of its homolog WRKY45 and many SINE-directed DNA methylation cases in mouse and human beings (Estécio et al., 2012, Yates et al., 2003).
Limitations of the Study
In this study, we revealed a “SAPK10-WRKY72-AOS1” module, through which Xoo infection suppresses JA biosynthesis to cause Xoo susceptibility. However, as we have discussed in the article, the final endogenous JA level in plant is determined by the counterbalance of both activation and suppression pathways. Therefore, figuring out the Xoo-activated JA biosynthesis regulatory pathways would be of great interest for us to elucidate the comprehensive reaction of rice in response to Xoo infection. In addition, although we provided clues that WRKY72 represses AOS1 transcription via RdDM, the detailed mechanism needs to be explored in future studies.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
The authors are grateful to Prof. Zhongchao Yin, Zhaohui Chu, Xinhua Ding, Meng Yuan, and Dr. Babatunde K. Bello for reviewing the manuscript. This work was supported by National Natural Science Foundation of China (grant number: 31601288, 31701395, and 31871229), Key R&D Project of Zhejiang Province (2019C02018), Agricultural Sciences and Technologies Innovation Program of Chinese Academy of Agricultural Sciences to Rice Reproductive Developmental Biology Group, and Chinese High-yielding Rice Transgenic Program (grant No. 2016ZX08001004-001).
Author Contributions
J.Z. and S.H. planned and designed the research; Y.H., Y.W., L.T., X.T., L.W., and L.L. performed experiments; Y.H., Y.W., and J.Z. analyzed data; Y.H. and J.Z. wrote the manuscript. Y.H. and Y.W. contributed equally.
Declaration of Interests
All the authors declare no conflicts of interests in this paper.
Published: June 28, 2019
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2019.06.009.
Contributor Information
Shiwen Huang, Email: huangshiwen@caas.cn.
Jian Zhang, Email: zhangjian@caas.cn.
Supplemental Information
References
- Agarwal P., Chikara J. WRKY: its structure, evolutionary relationship, DNA-binding selectivity, role in stress tolerance and development of plants. Mol. Biol. Rep. 2011;38:3883–3896. doi: 10.1007/s11033-010-0504-5. [DOI] [PubMed] [Google Scholar]; Agarwal, P., and Chikara, J.. (2011). WRKY: its structure, evolutionary relationship, DNA-binding selectivity, role in stress tolerance and development of plants. Mol. Biol. Rep. 38, 3883-3896. [DOI] [PubMed]
- Asai T., Tena G., Plotnikova J., Willmann M., Chiu W.-L., Gomez-Gomez L., Boller T., Ausubel F.M., Sheen J. MAP kinase signalling cascade in Arabidopsis innate immunity. Nature. 2002;415:977–983. doi: 10.1038/415977a. [DOI] [PubMed] [Google Scholar]; Asai, T., Tena, G., Plotnikova, J., Willmann, M., Chiu, W.-L., Gomez-Gomez, L., Boller, T., Ausubel, F.M., and Sheen, J.. (2002). MAP kinase signalling cascade in Arabidopsis innate immunity. Nature 415, 977-983. [DOI] [PubMed]
- Cai M., Qiu D., Yuan T., Ding X., Li H., Duan L., Xu C., Li X., Wang S. Identification of novel pathogen-responsive cis-elements and their binding proteins in the promoter of OsWRKY13, a gene regulating rice disease resistance. Plant Cell Environ. 2008;31:86–96. doi: 10.1111/j.1365-3040.2007.01739.x. [DOI] [PubMed] [Google Scholar]; Cai, M., Qiu, D., Yuan, T., Ding, X., Li, H., Duan, L., Xu, C., Li, X., and Wang, S.. (2008). Identification of novel pathogen-responsive cis-elements and their binding proteins in the promoter of OsWRKY13, a gene regulating rice disease resistance. Plant Cell Environ. 31, 86-96. [DOI] [PubMed]
- Campos M.L., Kang J.H., Howe G.A. Jasmonate-triggered plant immunity. J. Chem. Ecol. 2014;40:657–675. doi: 10.1007/s10886-014-0468-3. [DOI] [PMC free article] [PubMed] [Google Scholar]; Campos, M.L., Kang, J.H., and Howe, G.A.. (2014). Jasmonate-triggered plant immunity. J. Chem. Ecol. 40, 657-675. [DOI] [PMC free article] [PubMed]
- Chen C., Chen Z. Potentiation of developmentally regulated plant defense response by AtWRKY18, a pathogen-induced Arabidopsis transcription factor. Plant Physiol. 2002;129:706–716. doi: 10.1104/pp.001057. [DOI] [PMC free article] [PubMed] [Google Scholar]; Chen, C., and Chen, Z.. (2002). Potentiation of developmentally regulated plant defense response by AtWRKY18, a pathogen-induced Arabidopsis transcription factor. Plant Physiol. 129, 706-716. [DOI] [PMC free article] [PubMed]
- Chen X., Zhou D.X. Rice epigenomics and epigenetics: challenges and opportunities. Curr. Opin. Plant Biol. 2013;16:164–169. doi: 10.1016/j.pbi.2013.03.004. [DOI] [PubMed] [Google Scholar]; Chen, X., and Zhou, D.X.. (2013). Rice epigenomics and epigenetics: challenges and opportunities. Curr. Opin. Plant Biol. 16, 164-169. [DOI] [PubMed]
- Cheng H., Liu H., Deng Y., Xiao J., Li X., Wang S. The WRKY45-2 WRKY13 WRKY42 transcriptional regulatory cascade is required for rice resistance to fungal pathogen. Plant Physiol. 2015;167:1087–1099. doi: 10.1104/pp.114.256016. [DOI] [PMC free article] [PubMed] [Google Scholar]; Cheng, H., Liu, H., Deng, Y., Xiao, J., Li, X., and Wang, S.. (2015). The WRKY45-2 WRKY13 WRKY42 transcriptional regulatory cascade is required for rice resistance to fungal pathogen. Plant Physiol. 167, 1087-1099. [DOI] [PMC free article] [PubMed]
- Chujo T., Miyamoto K., Ogawa S., Masuda Y., Shimizu T., Kishi-Kaboshi M., Takahashi A., Nishizawa Y., Minami E., Nojiri H. Overexpression of phosphomimic mutated OsWRKY53 leads to enhanced blast resistance in rice. PLoS One. 2014;9:e98737. doi: 10.1371/journal.pone.0098737. [DOI] [PMC free article] [PubMed] [Google Scholar]; Chujo, T., Miyamoto, K., Ogawa, S., Masuda, Y., Shimizu, T., Kishi-Kaboshi, M., Takahashi, A., Nishizawa, Y., Minami, E., Nojiri, H., et al. (2014). Overexpression of phosphomimic mutated OsWRKY53 leads to enhanced blast resistance in rice. PLoS One 9, e98737. [DOI] [PMC free article] [PubMed]
- Chujo T., Miyamoto K., Shimogawa T., Shimizu T., Otake Y., Yokotani N., Nishizawa Y., Shibuya N., Nojiri H., Yamane H. OsWRKY28, a PAMP-responsive transrepressor, negatively regulates innate immune responses in rice against rice blast fungus. Plant Mol. Biol. 2013;82:23–37. doi: 10.1007/s11103-013-0032-5. [DOI] [PubMed] [Google Scholar]; Chujo, T., Miyamoto, K., Shimogawa, T., Shimizu, T., Otake, Y., Yokotani, N., Nishizawa, Y., Shibuya, N., Nojiri, H., Yamane, H., et al. (2013). OsWRKY28, a PAMP-responsive transrepressor, negatively regulates innate immune responses in rice against rice blast fungus. Plant Mol. Biol. 82, 23-37. [DOI] [PubMed]
- Estécio M.R.H., Gallegos J., Dekmezian M., Yue L., Liang S., Issa J.P.J. SINE retrotransposons cause epigenetic reprogramming of adjacent gene promoters. Mol. Cancer Res. 2012;10:1332. doi: 10.1158/1541-7786.MCR-12-0351. [DOI] [PMC free article] [PubMed] [Google Scholar]; Estecio, M.R.H., Gallegos, J., Dekmezian, M., Yue, L., Liang, S., and Issa, J.P.J.. (2012). SINE retrotransposons cause epigenetic reprogramming of adjacent gene promoters. Mol. Cancer Res. 10, 1332. [DOI] [PMC free article] [PubMed]
- Eulgem T., Rushton P.J., Robatzek S., Somssich I.E. The WRKY superfamily of plant transcription factors. Trends Plant Sci. 2000;5:199–206. doi: 10.1016/s1360-1385(00)01600-9. [DOI] [PubMed] [Google Scholar]; Eulgem, T., Rushton, P.J., Robatzek, S., and Somssich, I.E.. (2000). The WRKY superfamily of plant transcription factors. Trends Plant Sci. 5, 199-206. [DOI] [PubMed]
- Hibara K.I., Isono M., Mimura M., Sentoku N., Kojima M., Sakakibara H., Kitomi Y., Yoshikawa T., Itoh J.I., Nagato Y. Jasmonate regulates juvenile-adult phase transition in rice. Development. 2016;143:3407. doi: 10.1242/dev.138602. [DOI] [PubMed] [Google Scholar]; Hibara, K.I., Isono, M., Mimura, M., Sentoku, N., Kojima, M., Sakakibara, H., Kitomi, Y., Yoshikawa, T., Itoh, J.I., and Nagato, Y.. (2016). Jasmonate regulates juvenile-adult phase transition in rice. Development 143, 3407. [DOI] [PubMed]
- Ishihama N., Yoshioka H. Post-translational regulation of WRKY transcription factors in plant immunity. Curr. Opin. Plant Biol. 2012;15:431–437. doi: 10.1016/j.pbi.2012.02.003. [DOI] [PubMed] [Google Scholar]; Ishihama, N., and Yoshioka, H.. (2012). Post-translational regulation of WRKY transcription factors in plant immunity. Curr. Opin. Plant Biol. 15, 431-437. [DOI] [PubMed]
- Jones J.D.G., Dangl J.L. The plant immune system. Nature. 2006;444:323–329. doi: 10.1038/nature05286. [DOI] [PubMed] [Google Scholar]; Jones, J.D.G., and Dangl, J.L.. (2006). The plant immune system. Nature 444, 323-329. [DOI] [PubMed]
- Kobayashi Y., Murata M., Minami H., Yamamoto S., Kagaya Y., Hobo T., Yamamoto A., Hattori T. Abscisic acid-activated SNRK2 protein kinases function in the gene-regulation pathway of ABA signal transduction by phosphorylating ABA response element-binding factors. Plant J. 2010;44:939–949. doi: 10.1111/j.1365-313X.2005.02583.x. [DOI] [PubMed] [Google Scholar]; Kobayashi, Y., Murata, M., Minami, H., Yamamoto, S., Kagaya, Y., Hobo, T., Yamamoto, A., and Hattori, T.. (2010). Abscisic acid-activated SNRK2 protein kinases function in the gene-regulation pathway of ABA signal transduction by phosphorylating ABA response element-binding factors. Plant J. 44, 939-949. [DOI] [PubMed]
- Koo S.C., Moon B.C., Kim J.K., Kim C.Y., Sung S.J., Kim M.C., Cho M.J., Cheong Y.H. OsBWMK1 mediates SA-dependent defense responses by activating the transcription factor OsWRKY33. Biochem. Biophys. Res. Commun. 2009;387:365–370. doi: 10.1016/j.bbrc.2009.07.026. [DOI] [PubMed] [Google Scholar]; Koo, S.C., Moon, B.C., Kim, J.K., Kim, C.Y., Sung, S.J., Kim, M.C., Cho, M.J., and Cheong, Y.H.. (2009). OsBWMK1 mediates SA-dependent defense responses by activating the transcription factor OsWRKY33. Biochem. Biophys. Res. Commun. 387, 365-370. [DOI] [PubMed]
- Li G., Meng X., Wang R., Mao G., Han L., Liu Y., Zhang S. Dual-level regulation of ACC synthase activity by MPK3/MPK6 cascade and its downstream WRKY transcription factor during ethylene induction in Arabidopsis. PLoS Genet. 2012;8:e1002767. doi: 10.1371/journal.pgen.1002767. [DOI] [PMC free article] [PubMed] [Google Scholar]; Li, G., Meng, X., Wang, R., Mao, G., Han, L., Liu, Y., and Zhang, S.. (2012). Dual-level regulation of ACC synthase activity by MPK3/MPK6 cascade and its downstream WRKY transcription factor during ethylene induction in Arabidopsis. PLoS Genet. 8, e1002767. [DOI] [PMC free article] [PubMed]
- Li J., Brader G., Palva E.T. The WRKY70 transcription factor: a node of convergence for jasmonate-mediated and salicylate-mediated signals in plant defense. Plant Cell. 2004;16:319–331. doi: 10.1105/tpc.016980. [DOI] [PMC free article] [PubMed] [Google Scholar]; Li, J., Brader, G., and Palva, E.T.. (2004). The WRKY70 transcription factor: a node of convergence for jasmonate-mediated and salicylate-mediated signals in plant defense. Plant Cell 16, 319-331. [DOI] [PMC free article] [PubMed]
- Liu X., Bai X., Wang X., Chu C. OsWRKY71, a rice transcription factor, is involved in rice defense response. J. Plant Physiol. 2007;164:969–979. doi: 10.1016/j.jplph.2006.07.006. [DOI] [PubMed] [Google Scholar]; Liu, X., Bai, X., Wang, X., and Chu, C.. (2007). OsWRKY71, a rice transcription factor, is involved in rice defense response. J. Plant Physiol. 164, 969-979. [DOI] [PubMed]
- Liu X.Q., Bai X.Q., Qian Q., Wang X.J., Chen M.S., Chu C.C. OsWRKY03, a rice transcriptional activator that functions in defense signaling pathway upstream of OsNPR1. Cell Res. 2005;15:593–603. doi: 10.1038/sj.cr.7290329. [DOI] [PubMed] [Google Scholar]; Liu, X.Q., Bai, X.Q., Qian, Q., Wang, X.J., Chen, M.S., and Chu, C.C.. (2005). OsWRKY03, a rice transcriptional activator that functions in defense signaling pathway upstream of OsNPR1. Cell Res. 15, 593-603. [DOI] [PubMed]
- Madhunita B., Ralf O. WRKY transcription factors: jack of many trades in plants. Plant Signal. Behav. 2014;9:e27700. doi: 10.4161/psb.27700. [DOI] [PMC free article] [PubMed] [Google Scholar]; Madhunita, B., and Ralf, O.. (2014). WRKY transcription factors: jack of many trades in plants. Plant Signal. Behav. 9, e27700. [DOI] [PMC free article] [PubMed]
- Mao G., Meng X., Liu Y., Zheng Z., Chen Z., Zhang S. Phosphorylation of a WRKY transcription factor by two pathogen-responsive MAPKs drives phytoalexin biosynthesis in Arabidopsis. Plant Cell. 2011;23:1639–1653. doi: 10.1105/tpc.111.084996. [DOI] [PMC free article] [PubMed] [Google Scholar]; Mao, G., Meng, X., Liu, Y., Zheng, Z., Chen, Z., and Zhang, S.. (2011). Phosphorylation of a WRKY transcription factor by two pathogen-responsive MAPKs drives phytoalexin biosynthesis in Arabidopsis. Plant Cell 23, 1639-1653. [DOI] [PMC free article] [PubMed]
- Mei C., Qi M., Sheng G., Yang Y. Inducible overexpression of a rice allene oxide synthase gene increases the endogenous jasmonic acid level, PR gene expression, and host resistance to fungal infection. Mol. Plant Microbe Interact. 2006;19:1127–1137. doi: 10.1094/MPMI-19-1127. [DOI] [PubMed] [Google Scholar]; Mei, C., Qi, M., Sheng, G., and Yang, Y.. (2006). Inducible overexpression of a rice allene oxide synthase gene increases the endogenous jasmonic acid level, PR gene expression, and host resistance to fungal infection. Mol. Plant Microbe Interact. 19, 1127-1137. [DOI] [PubMed]
- Meng X., Xu J., He Y., Yang K.Y., Mordorski B., Liu Y., Zhang S. Phosphorylation of an ERF transcription factor by Arabidopsis MPK3/MPK6 regulates plant defense gene induction and fungal resistance. Plant Cell. 2013;25:1126–1142. doi: 10.1105/tpc.112.109074. [DOI] [PMC free article] [PubMed] [Google Scholar]; Meng, X., Xu, J., He, Y., Yang, K.Y., Mordorski, B., Liu, Y., and Zhang, S.. (2013). Phosphorylation of an ERF transcription factor by Arabidopsis MPK3/MPK6 regulates plant defense gene induction and fungal resistance. Plant Cell 25, 1126-1142. [DOI] [PMC free article] [PubMed]
- Menke F.L., Kang H.Z., Park J.M., Kumar D., Klessig D.F. Tobacco transcription factor WRKY1 is phosphorylated by the MAP kinase SIPK and mediates HR-like cell death in tobacco. Mol. Plant Microbe Interact. 2005;18:1027–1034. doi: 10.1094/MPMI-18-1027. [DOI] [PubMed] [Google Scholar]; Menke, F.L., Kang, H.Z., Park, J.M., Kumar, D., and Klessig, D.F.. (2005). Tobacco transcription factor WRKY1 is phosphorylated by the MAP kinase SIPK and mediates HR-like cell death in tobacco. Mol. Plant Microbe Interact. 18, 1027-1034. [DOI] [PubMed]
- Okada K., Abe H., Arimura G. Jasmonates induce both defense responses and communication in monocotyledonous and dicotyledonous plants. Plant Cell Physiol. 2015;56:16–27. doi: 10.1093/pcp/pcu158. [DOI] [PubMed] [Google Scholar]; Okada, K., Abe, H., and Arimura, G.. (2015). Jasmonates induce both defense responses and communication in monocotyledonous and dicotyledonous plants. Plant Cell Physiol. 56, 16-27. [DOI] [PubMed]
- Park C.J., Ronald P.C. Cleavage and nuclear localization of the rice XA21 immune receptor. Nat. Commun. 2012;3:920. doi: 10.1038/ncomms1932. [DOI] [PMC free article] [PubMed] [Google Scholar]; Park, C.J., and Ronald, P.C.. (2012). Cleavage and nuclear localization of the rice XA21 immune receptor. Nat. Commun. 3, 920. [DOI] [PMC free article] [PubMed]
- Peng Y., Bartley L.E., Chen X., Dardick C., Chern M., Ruan R., Canlas P.E., Ronald P.C. OsWRKY62 is a negative regulator of basal and Xa21-mediated defense against Xanthomonas oryzae pv. oryzae in rice. Mol. Plant. 2008;1:446–458. doi: 10.1093/mp/ssn024. [DOI] [PubMed] [Google Scholar]; Peng, Y., Bartley, L.E., Chen, X., Dardick, C., Chern, M., Ruan, R., Canlas, P.E., and Ronald, P.C.. (2008). OsWRKY62 is a negative regulator of basal and Xa21-mediated defense against Xanthomonas oryzae pv. oryzae in rice. Mol. Plant 1, 446-458. [DOI] [PubMed]
- Peng Y., Wersch R.V., Zhang Y. Convergent and divergent signaling in PAMP-triggered immunity and Effector-triggered immunity. Mol. Plant Microbe Interact. 2017;31:403–409. doi: 10.1094/MPMI-06-17-0145-CR. [DOI] [PubMed] [Google Scholar]; Peng, Y., Wersch, R.V., and Zhang, Y.. (2017). Convergent and divergent signaling in PAMP-triggered immunity and Effector-triggered immunity. Mol. Plant Microbe Interact. 31, 403-409. [DOI] [PubMed]
- Pitzschke A., Schikora A., Hirt H. MAPK cascade signalling networks in plant defence. Curr. Opin. Plant Biol. 2009;12:421–426. doi: 10.1016/j.pbi.2009.06.008. [DOI] [PubMed] [Google Scholar]; Pitzschke, A., Schikora, A., and Hirt, H.. (2009). MAPK cascade signalling networks in plant defence. Curr. Opin. Plant Biol. 12, 421-426. [DOI] [PubMed]
- Qiu D., Xiao J., Ding X., Xiong M., Cai M., Cao Y., Li X., Xu C., Wang S. OsWRKY13 mediates rice disease resistance by regulating defense-related genes in salicylate- and jasmonate-dependent signaling. Mol. Plant Microbe Interact. 2007;20:492–499. doi: 10.1094/MPMI-20-5-0492. [DOI] [PubMed] [Google Scholar]; Qiu, D., Xiao, J., Ding, X., Xiong, M., Cai, M., Cao, Y., Li, X., Xu, C., and Wang, S.. (2007). OsWRKY13 mediates rice disease resistance by regulating defense-related genes in salicylate- and jasmonate-dependent signaling. Mol. Plant Microbe Interact. 20, 492-499. [DOI] [PubMed]
- Qiu D., Xiao J., Xie W., Liu H., Li X., Xiong L., Wang S. Rice gene network inferred from expression profiling of plants overexpressing OsWRKY13, a positive regulator of disease resistance. Mol. Plant. 2008;1:538–551. doi: 10.1093/mp/ssn012. [DOI] [PubMed] [Google Scholar]; Qiu, D., Xiao, J., Xie, W., Liu, H., Li, X., Xiong, L., and Wang, S.. (2008). Rice gene network inferred from expression profiling of plants overexpressing OsWRKY13, a positive regulator of disease resistance. Mol. Plant 1, 538-551. [DOI] [PubMed]
- Rice WRKY Working Group Nomenclature report on rice WRKY's - conflict regarding gene names and its solution. Rice. 2012;5:3. doi: 10.1186/1939-8433-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]; Rice WRKY Working Group (2012). Nomenclature report on rice WRKY's - conflict regarding gene names and its solution. Rice 5, 3. [DOI] [PMC free article] [PubMed]
- Riemann M., Haga K., Shimizu T., Okada K., Ando S., Mochizuki S., Nishizawa Y., Yamanouchi U., Nick P., Yano M. Identification of rice Allene Oxide Cyclase mutants and the function of jasmonate for defence against Magnaporthe oryzae. Plant J. 2013;74:226–238. doi: 10.1111/tpj.12115. [DOI] [PubMed] [Google Scholar]; Riemann, M., Haga, K., Shimizu, T., Okada, K., Ando, S., Mochizuki, S., Nishizawa, Y., Yamanouchi, U., Nick, P., and Yano, M.. (2013). Identification of rice Allene Oxide Cyclase mutants and the function of jasmonate for defence against Magnaporthe oryzae. Plant J. 74, 226-238. [DOI] [PubMed]
- Ross C., Liu Y., Shen Q. The WRKY gene family in rice (Oryza sativa) J. Integr. Plant Biol. 2007;49:827–842. [Google Scholar]; Ross, C., Liu, Y., and Shen, Q.. (2007). The WRKY gene family in rice (Oryza sativa). J. Integr. Plant Biol. 49, 827-842.
- Ryu H.S., Han M., Lee S.K., Cho J.I., Ryoo N., Heu S., Lee Y.H., Bhoo S.H., Wang G.L., Hahn T.R. A comprehensive expression analysis of the WRKY gene superfamily in rice plants during defense response. Plant Cell Rep. 2006;25:836–847. doi: 10.1007/s00299-006-0138-1. [DOI] [PubMed] [Google Scholar]; Ryu, H.S., Han, M., Lee, S.K., Cho, J.I., Ryoo, N., Heu, S., Lee, Y.H., Bhoo, S.H., Wang, G.L., Hahn, T.R., et al. (2006). A comprehensive expression analysis of the WRKY gene superfamily in rice plants during defense response. Plant Cell Rep. 25, 836-847. [DOI] [PubMed]
- Saijo Y., Loo E.P., Yasuda S. Pattern recognition receptors and signaling in plant-microbe interactions. Plant J. 2018;93:592–613. doi: 10.1111/tpj.13808. [DOI] [PubMed] [Google Scholar]; Saijo, Y., Loo, E.P., and Yasuda, S.. (2017). Pattern recognition receptors and signaling in plant-microbe interactions. Plant J. 93.592-613 [DOI] [PubMed]
- Schaller F. Enzymes of the biosynthesis of octadecanoid-derived signalling molecules. J. Exp. Bot. 2001;52:11–23. [PubMed] [Google Scholar]; Schaller, F.. (2001). Enzymes of the biosynthesis of octadecanoid-derived signalling molecules. J. Exp. Bot. 52, 11-23. [PubMed]
- Shimono M., Koga H., Akagi A., Hayashi N., Goto S., Sawada M., Kurihara T., Matsushita A., Sugano S., Jiang C.J. Rice WRKY45 plays important roles in fungal and bacterial disease resistance. Mol. Plant Pathol. 2012;13:83–94. doi: 10.1111/j.1364-3703.2011.00732.x. [DOI] [PMC free article] [PubMed] [Google Scholar]; Shimono, M., Koga, H., Akagi, A., Hayashi, N., Goto, S., Sawada, M., Kurihara, T., Matsushita, A., Sugano, S., and Jiang, C.J.. (2012). Rice WRKY45 plays important roles in fungal and bacterial disease resistance. Mol. Plant Pathol. 13, 83-94. [DOI] [PMC free article] [PubMed]
- Shimono M., Sugano S., Nakayama A., Jiang C.J., Ono K., Toki S., Takatsuji H. Rice WRKY45 plays a crucial role in Benzothiadiazole-inducible blast resistance. Plant Cell. 2007;19:2064–2076. doi: 10.1105/tpc.106.046250. [DOI] [PMC free article] [PubMed] [Google Scholar]; Shimono, M., Sugano, S., Nakayama, A., Jiang, C.J., Ono, K., Toki, S., and Takatsuji, H.. (2007). Rice WRKY45 plays a crucial role in Benzothiadiazole-inducible blast resistance. Plant Cell 19, 2064-2076. [DOI] [PMC free article] [PubMed]
- Staswick P.E., Tiryaki I., Rowe M.L. Jasmonate response locus JAR1 and several related Arabidopsis genes encode enzymes of the firefly luciferase superfamily that show activity on jasmonic, salicylic, and indole-3-acetic acids in an assay for adenylation. Plant Cell. 2002;14:1405–1415. doi: 10.1105/tpc.000885. [DOI] [PMC free article] [PubMed] [Google Scholar]; Staswick, P.E., Tiryaki, I., and Rowe, M.L.. (2002). Jasmonate response locus JAR1 and several related Arabidopsis genes encode enzymes of the firefly luciferase superfamily that show activity on jasmonic, salicylic, and indole-3-acetic acids in an assay for adenylation. Plant Cell 14, 1405-1415. [DOI] [PMC free article] [PubMed]
- Staswick P.E., Yuen G.Y., Lehman C.C. Jasmonate signaling mutants of Arabidopsis are susceptible to the soil fungus Pythium irregulare. Plant J. 2010;15:747–754. doi: 10.1046/j.1365-313x.1998.00265.x. [DOI] [PubMed] [Google Scholar]; Staswick, P.E., Yuen, G.Y., and Lehman, C.C.. (2010). Jasmonate signaling mutants of Arabidopsis are susceptible to the soil fungus Pythium irregulare. Plant J. 15, 747-754. [DOI] [PubMed]
- Sun C., Palmqvist S., Olsson H., Borén M., Ahlandsberg S., Jansson C. A Novel WRKY transcription factor, SUSIBA2, participates in sugar signaling in barley by binding to the sugar-responsive elements of the iso1 promoter. Plant Cell. 2003;15:2076. doi: 10.1105/tpc.014597. [DOI] [PMC free article] [PubMed] [Google Scholar]; Sun, C., Palmqvist, S., Olsson, H., Boren, M., Ahlandsberg, S., and Jansson, C.. (2003). A Novel WRKY transcription factor, SUSIBA2, participates in sugar signaling in barley by binding to the sugar-responsive elements of the iso1 promoter. Plant Cell 15, 2076. [DOI] [PMC free article] [PubMed]
- Tao Z., Liu H., Qiu D., Zhou Y., Li X., Xu C., Wang S. A pair of allelic WRKY genes play opposite roles in rice-bacteria interactions. Plant Physiol. 2009;151:936–948. doi: 10.1104/pp.109.145623. [DOI] [PMC free article] [PubMed] [Google Scholar]; Tao, Z., Liu, H., Qiu, D., Zhou, Y., Li, X., Xu, C., and Wang, S.. (2009). A pair of allelic WRKY genes play opposite roles in rice-bacteria interactions. Plant Physiol. 151, 936-948. [DOI] [PMC free article] [PubMed]
- Ton J., Flors V., Mauchmani B. The multifaceted role of ABA in disease resistance. Trends Plant Sci. 2009;14:310–317. doi: 10.1016/j.tplants.2009.03.006. [DOI] [PubMed] [Google Scholar]; Ton, J., Flors, V., and Mauchmani, B.. (2009). The multifaceted role of ABA in disease resistance. Trends Plant Sci. 14, 310-317. [DOI] [PubMed]
- Uji Y., Taniguchi S., Tamaoki D., Shishido H., Akimitsu K., Gomi K. Overexpression of OsMYC2 results in the up-regulation of early JA-rresponsive genes and bacterial blight resistance in rice. Plant Cell Physiol. 2016;57:1814–1827. doi: 10.1093/pcp/pcw101. [DOI] [PubMed] [Google Scholar]; Uji, Y., Taniguchi, S., Tamaoki, D., Shishido, H., Akimitsu, K., and Gomi, K.. (2016). Overexpression of OsMYC2 results in the up-regulation of early JA-rresponsive genes and bacterial blight resistance in rice. Plant Cell Physiol. 57, 1814-1827. [DOI] [PubMed]
- Wang Y., Schuck S., Wu J., Yang P., Doering A.C., Zeier J., Tsuda K. A MPK3/6-WRKY33-ALD1-pipecolic acid regulatory loop contributes to systemic acquired resistance. Plant Cell. 2018;30:2480–2494. doi: 10.1105/tpc.18.00547. [DOI] [PMC free article] [PubMed] [Google Scholar]; Wang, Y., Schuck, S., Wu, J., Yang, P., Doering, A.C., Zeier, J., and Tsuda, K.. (2018). A MPK3/6-WRKY33-ALD1-pipecolic acid regulatory loop contributes to systemic acquired resistance. Plant Cell..30, 2480-2494. [DOI] [PMC free article] [PubMed]
- Wassenegger M., Heimes S., Riedel L., Sänger H.L. RNA-directed de novo methylation of genomic sequences in plants. Cell. 1994;76:567–576. doi: 10.1016/0092-8674(94)90119-8. [DOI] [PubMed] [Google Scholar]; Wassenegger, M., Heimes, S., Riedel, L., and Sanger, H.L.. (1994). RNA-directed de novo methylation of genomic sequences in plants. Cell 76, 567-576. [DOI] [PubMed]
- Xu M., Huang L., Zhang F., Zhu L., Zhou Y., Li Z. Genome-wide phylogenetic analysis of stress-activated protein kinase genes in rice (OsSAPKs) and expression profiling in response to xanthomonas oryzae pv. oryzicola infection. Plant Mol. Biol. Rep. 2013;31:877–885. [Google Scholar]; Xu, M., Huang, L., Zhang, F., Zhu, L., Zhou, Y., and Li, Z.. (2013). Genome-wide phylogenetic analysis of stress-activated protein kinase genes in rice (OsSAPKs) and expression profiling in response to xanthomonas oryzae pv. oryzicola infection. Plant Mol. Biol. Rep. 31, 877-885.
- Yamada S., Kano A., Tamaoki D., Miyamoto A., Shishido H., Miyoshi S., Taniguchi S., Akimitsu K., Gomi K. Involvement of OsJAZ8 in jasmonate-induced resistance to bacterial blight in rice. Plant Cell Physiol. 2012;53:2060–2072. doi: 10.1093/pcp/pcs145. [DOI] [PubMed] [Google Scholar]; Yamada, S., Kano, A., Tamaoki, D., Miyamoto, A., Shishido, H., Miyoshi, S., Taniguchi, S., Akimitsu, K., and Gomi, K.. (2012). Involvement of OsJAZ8 in jasmonate-induced resistance to bacterial blight in rice. Plant Cell Physiol. 53, 2060-2072. [DOI] [PubMed]
- Yang W., Zhang W., Wang X. Post-translational control of ABA signalling: the roles of protein phosphorylation and ubiquitination. Plant Biotechnol. J. 2017;15:4–14. doi: 10.1111/pbi.12652. [DOI] [PMC free article] [PubMed] [Google Scholar]; Yang, W., Zhang, W., and Wang, X.. (2017). Post-translational control of ABA signalling: the roles of protein phosphorylation and ubiquitination. Plant Biotechnol. J. 15, 4-14. [DOI] [PMC free article] [PubMed]
- Yates P.A., Burman R., Simpson J., Ponomoreva O.N., Thayer M.J., Turker M.S. Silencing of mouse Aprt is a gradual process in differentiated cells. Mol. Cell. Biol. 2003;23:4461–4470. doi: 10.1128/MCB.23.13.4461-4470.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]; Yates, P.A., Burman, R., Simpson, J., Ponomoreva, O.N., Thayer, M.J., and Turker, M.S.. (2003). Silencing of mouse Aprt is a gradual process in differentiated cells. Mol. Cell. Biol. 23, 4461-4470. [DOI] [PMC free article] [PubMed]
- Yokotani N., Sato Y., Tanabe S., Chujo T., Shimizu T., Okada K., Yamane H., Shimono M., Sugano S., Takatsuji H. WRKY76 is a rice transcriptional repressor playing opposite roles in blast disease resistance and cold stress tolerance. J. Exp. Bot. 2013;64:5085–5097. doi: 10.1093/jxb/ert298. [DOI] [PMC free article] [PubMed] [Google Scholar]; Yokotani, N., Sato, Y., Tanabe, S., Chujo, T., Shimizu, T., Okada, K., Yamane, H., Shimono, M., Sugano, S., Takatsuji, H., et al. (2013). WRKY76 is a rice transcriptional repressor playing opposite roles in blast disease resistance and cold stress tolerance. J. Exp. Bot. 64, 5085-5097. [DOI] [PMC free article] [PubMed]
- Zhang H., Zeng T., Hong H., Chen Z., Wu C., Li X., Xiao J., Wang S. Transposon-derived small RNA is responsible for modified function of WRKY45 locus. Nat. Plants. 2016;2:16016. doi: 10.1038/nplants.2016.16. [DOI] [PubMed] [Google Scholar]; Zhang, H., Zeng, T., Hong, H., Chen, Z., Wu, C., Li, X., Xiao, J., and Wang, S.. (2016). Transposon-derived small RNA is responsible for modified function of WRKY45 locus. Nat. Plants 2, 16016. [DOI] [PubMed]
- Zhang J., Peng Y., Guo Z. Constitutive expression of pathogen-inducible OsWRKY31 enhances disease resistance and affects root growth and auxin response in transgenic rice plants. Cell Res. 2008;18:508–521. doi: 10.1038/cr.2007.104. [DOI] [PubMed] [Google Scholar]; Zhang, J., Peng, Y., and Guo, Z.. (2008). Constitutive expression of pathogen-inducible OsWRKY31 enhances disease resistance and affects root growth and auxin response in transgenic rice plants. Cell Res. 18, 508-521. [DOI] [PubMed]
- Zheng Z., Qamar S.A., Chen Z., Mengiste T. Arabidopsis WRKY33 transcription factor is required for resistance to necrotrophic fungal pathogens. Plant J. 2006;48:592–605. doi: 10.1111/j.1365-313X.2006.02901.x. [DOI] [PubMed] [Google Scholar]; Zheng, Z., Qamar, S.A., Chen, Z., and Mengiste, T.. (2006). Arabidopsis WRKY33 transcription factor is required for resistance to necrotrophic fungal pathogens. Plant J. 48, 592-605. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.