Abstract
Introduction:
This report presents uterine smooth muscle tumors of uncertain malignant potential course with an unpredictable clinical behavior and late metastases. Metastases have been described to the humerus, lung, and peritoneum.
Case Presentation:
Hereby we present the case of a 71-year-old woman with a past surgical history of hysterectomy and bilateral adnexectomy due to a smooth muscle tumor of unknown malignant potential, who was evaluated 6 years later after the appearance of a mass in the proximal third of the right lower limb. The mass was diagnosed as a G1 epithelioid leiomyosarcoma and was surgically removed with immediate reconstruction with a tendinous transfer to the tibialis posterior muscle to maintain foot dorsiflexion.
Conclusion:
Patients diagnosed with smooth uterine muscle tumors of uncertain malignant potential should be closely followed up given the possibility of recurrence and late metastases, bearing in mind uncommon locations as well, such as the lower limb.
Keywords: smooth muscle tumors of unknown malignant potential, leiomyosarcoma, metastases, lower extremity
Introduction
Smooth uterine muscle tumors of uncertain malignant potential (STUMPs) comprise a group of mesenchymal tumors that cannot be clearly categorized as benign or malignant lesions.1 They present an unpredictable clinical course, usually presenting with clinical benign features but late recurrence, and unfrequently, with distant dissemination.2 We present the case of a patient diagnosed with a leiomyosarcoma of the lower limb 6 years after a hysterectomy due to a smooth muscle tumors of unknown malignant potential.
Case Report
Hereby we present the case of a 71-year-old woman with a past surgical history of a hysterectomy and bilateral adnexectomy in December 2011 as a consequence of a smooth muscle tumors of unknown malignant potential of 16 × 11 cm2. Histologically, this uterine tumor was conformed by a highly cellular proliferation of mesenchymal cells disposed in a fascicular growth pattern. The cells were fusiform with zones of epithelioid morphology (20% of the cellularity). The mitotic count per 10 high power fields was of 5%. There were zones with moderate atypia and coagulative necrosis. There was not infiltrative border or vascular invasion. In 2017, the patient was evaluated for a 3-month evolution mass in the proximal third of the right lower limb, and the patient remained asymptomatic. On physical examination, a soft, nonpainful, fixed to deep structures nodule was observed.
Ultrasonography studies showed a solid and heterogeneous (with cystic features) lesion of 110 × 40 × 40 mm3. The lesion had well-defined borders and was located in the anterior intramuscular compartment of the right lower limb. It showed increased venous and arterial vascularization. On magnetic resonance imaging (MRI), malignant features were detected, with a vast infiltrative component of the adjacent neurovascular structures, suggestive of sarcoma of undetermined characteristics (Figure 1).
Figure 1.

STIR FSE coronal MRI showing a soft tissue tumor in the proximal third of the right leg with fusiform morphology. FSE indicates fast spin echo; STIR, Short-TI Inversion Recovery; MRI, magnetic resonance imaging.
Surgical removal of the lesion was accomplished in March 2017 through an anterior approach, resecting the muscles from the aforementioned compartment, and with immediate reconstruction with a tendinous transfer of the tibialis posterior muscle, so the patient would be able to retain foot dorsiflexion (Figure 2). The pathology report described a 13 × 5 × 5 cm3 pearl-colored mass with multiple cystic areas that exhibited elastic characteristics. These areas were not in contact with the surgical margins (Figure 3).
Figure 2.
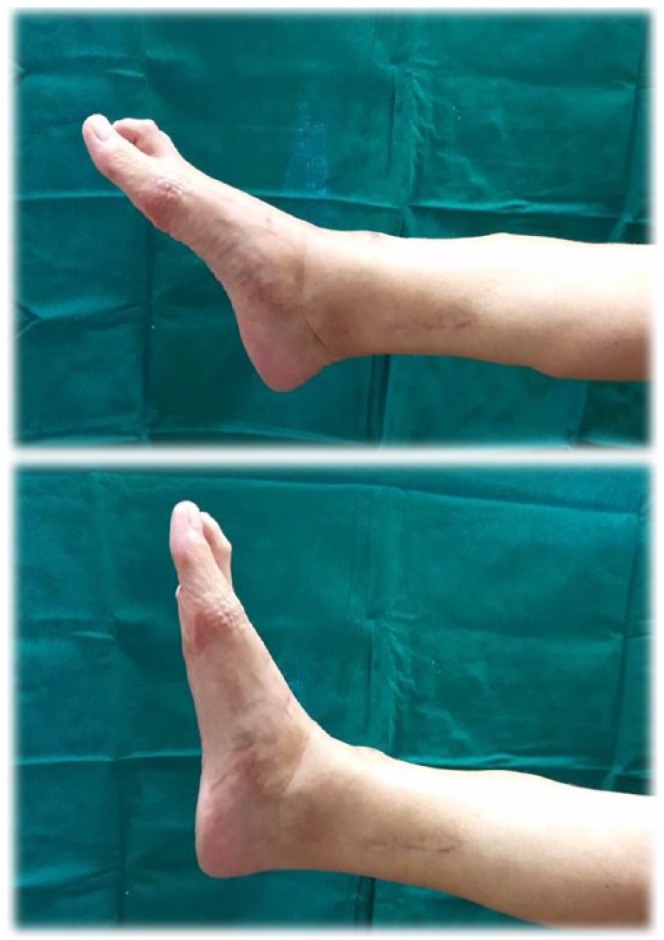
Foot dorsiflexion motion after resection and tendinous transfer surgery.
Figure 3.

Macroscopic image corresponding to the bloc, free-border resection of the tumor located in the lower limb. Tumor size 13 × 5 × 5 cm3. Pearl-colored lesion, with focal yellowish areas, that represents necrosis areas and minor read-colored areas that correspond with hemorrhage foci.
Histologically, the mass showed a hypercellular atypical proliferation of mesenchymal cells, disposed in a fascicular and storiform pattern and displayed an epithelioid morphology with zones of fusocellular morphology (Figure 4). Immunohistochemistry was positive for vimentin, cytokeratin AE1-AE3, desmin, calponin, caldesmon and weak for estrogens receptors (Figure 5). Proliferation rate measured by Ki67 was of 5%, counting 5 mitosis in 10 fields under high magnification and a 15% necrosis rate. With all these characteristics, the lesion was classified as an epithelioid leiomyosarcoma G1. The patient did not receive adjuvant treatment and remains asymptomatic, ambulating and without the need of an anti-equinus splint.
Figure 4.

Hematoxylin-eosin (10×): atypical cellular proliferation of mesenchymal cells of epithelioid and fusiform morphology, disposed in a fascicular and storiform pattern.
Figure 5.
Immunohistochemistry: caldesmon, actin, calponin, and desmin diffuse positive, which confirms smooth muscle origin and the diagnosis of leiomyosarcoma. Nuclear positivity to estrogen receptor suggests a metastatic gynecological origin; in this case, a STUMP. STUMP indicates smooth uterine muscle tumors of uncertain malignant potential.
Discussion
Uterine mesenchymal smooth muscle tumors comprise the most common gynecological neoplasms. These can be divided into benign, malignant, and uterine mesenchymal smooth muscle tumors of uncertain malignancy.
It is crucial to correctly diagnose this type of tumors given its prognosis and clinical course. High-grade leiomyosarcomas frequently present with an aggressive evolution, early recurrence, and metastases. On the contrary, STUMPs exhibit a slower growth rate and its recurrence is often delayed from its initial diagnosis.3
Smooth uterine muscle tumors of uncertain malignant potential are uncommon, and the true incidence of these tumors remains unknown. To aid with diagnosis, the Stanford Criteria resumed 3 main factors, which consist of the presence of cytologic atypia, mitotic index, and tumor necrosis.4
Recurrence rate of STUMPs has been estimated between 8.7% and 11%, with the potential to present as a leiomyosarcoma. Survival rate is higher than that in low recurrence rate of leiomyosarcomas. Till date, no consensus has been established as to what histological features, demographic variables, or serum markers will predict recurrence rate or the clinical course of these lesions.3 The importance of translational research to improve the understanding of the biology of this disease must be underlined.5
Humerus, liver, and peritoneum have been described as the most common metastatic sites of STUMPs.6,7 The singularity of the case presented is that it affected musculature of the lower limb as opposed to the most commonly described distant locations.
Serrano et al8 describe a pulmonary metastasis in the form of leiomyosarcoma with immunohistochemical positivity for estrogens and progesterone receptors following and STUMP also with positivity for estrogens and progesterone receptors, low mitotic rate, the absences of necrosis, and focal atypia. Our case shows a similar morphology of the STUMP and also shows positivity for estrogens receptor, but the metastasis in our case was in the lower limb.
Treatment for distant lesions consists of surgical excision that can be further aided with radiotherapy, chemotherapy, or hormone therapy8; however, as in the case presented, surgery as the sole treatment has been reported with favorable outcomes. Nevertheless, close monitoring of the patient is imperative.
Conclusions
Patients diagnosed with smooth muscle tumors of uncertain malignant potential should be closely followed-up given the possibility of recurrence and late metastases, bearing in mind uncommon locations as well, such as the lower limb.
Footnotes
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: Acquisition of data: García-Sánchez JM, Bauza M.
Analysis and interpretation of data: García-Sánchez JM, Bauza M.
Critical revision: Pérez García A, Mayordomo Aranda E.
Drafting of manuscript: García-Sánchez JM, Bauza M, Ruiz Valls A.
Final approval of the version to be published: Pérez García A, Mayordomo Aranda E.
Study conception and design: García-Sánchez JM, Bauza M, Ruiz Valls A, Pérez García A, Mayordomo Aranda E.
Patient Consent: Patient consent was obtained for publication.
References
- 1. Artola Pérez de Azanza M, Navarro Echeverría L, Tejerina González E, Cristóbal García I. Tumoraciones mesenquimales de músculo liso uterino de potencial incierto: revisión anatomopatológica y pronóstica de un caso clínico. Prog Obstet Ginecol. 2013;56:418–423. [Google Scholar]
- 2. Ip PPC, Tse KY, Tam KF. Uterine smooth muscle tumors other than the ordinary leiomyomas and leiomyosarcomas: a review of selected variants with emphasis on recent advances and unusual morphology that may cause concern for malignancy. Adv Anat Pathol. 2010;17:91–112. [DOI] [PubMed] [Google Scholar]
- 3. Dall’Asta A, Gizzo S, Musaro A, et al. Uterine smooth muscle tumors of uncertain malignant potential (STUMP): pathology, follow-up and recurrence. Int J Clin Exp Pathol. 2014;7:8136–8142. [PMC free article] [PubMed] [Google Scholar]
- 4. Mowers EL, Skinner B, McLean K, Reynolds RK. Effects of morcellation of uterine smooth muscle tumor of uncertain malignant potential and endometrial stromal sarcoma: case series and recommendations for clinical practice. J Minim Invasive Gynecol. 2015;22:601–606. [DOI] [PubMed] [Google Scholar]
- 5. De Vita A, Mercatali L, Miserocchi G, et al. Establishment of a primary culture of patient-derived soft tissue sarcoma. J Vis Exp. 2018;2018:e56767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Atkins KA, Arronte N, Darus CJ, Rice LW. The use of p16 in enhancing the histologic classification of uterine smooth muscle tumors. Am J Surg Pathol. 2008;32:98–102. [DOI] [PubMed] [Google Scholar]
- 7. Shapiro A, Ferenczy A, Turcotte R, Bruchim I, Gotlieb WH. Uterine smooth-muscle tumor of uncertain malignant potential metastasizing to the humerus as a high-grade leiomyosarcoma. Gynecol Oncol. 2004;94:818–820. [DOI] [PubMed] [Google Scholar]
- 8. Serrano C, Nucci MR, Tirumani SH, Raut CP, George S. Hormone dependency in metastatic low-grade leiomyosarcoma following uterine smooth muscle tumour of uncertain malignant potential. BMJ Case Rep. 2014;2014:bcr2013202107. [DOI] [PMC free article] [PubMed] [Google Scholar]



