Abstract
Background
Aging plays an important role in endothelial dysfunction. Fluid shear stress (FSS) can activate endothelial cells (ECs). Herein, we tested the hypothesis that this endothelial impairment could be improved by elevated FSS (EFSS) in aged rats.
Material/Methods
EFSS was created through ligation of the unilateral common iliac artery in 20-−month-old rats, evaluated by measuring blood flow velocity with Doppler spectrum. The effect of FSS on aged ECs was examined by senescence-associated β-galactosidase (SA-β-Gal) staining, ultrastructural observation, and immunostaining and qPCR analysis of eNOS and SIRT1 expression on both the mRNA and protein levels.
Results
(1) FSS was significantly increased in the right common iliac artery (RCIA) in rats with the ligation of the left common iliac artery (LCIA). (2) SA-β-Gal staining was significantly attenuated by EFSS in the RCIA of aged rats. (3) Ultrastructural observation showed that ECs in the RCIA of normal aged rats became irregular and enlarged, with increasingly polypoid nuclei and fewer mitochondria, whereas ECs in the RCIA of aged rats with LCIA ligation became more prominent and contained more mitochondria. (4) eNOS and SIRT1 expression in the RCIA of aged rats with LCIA ligation was significantly upregulated compared with that in control group rats.
Conclusions
The present study for the first time shows that EFSS has the ability to improve age-related impairment of endothelial structure and functions.
MeSH Keywords: Aged, Endothelial Cells, Nitric Oxide Synthase Type III, Sirtuin 1
Background
Endothelial cells (ECs) form the inner lining of the vasculature and regulate vascular functions such as exchange of fluid and molecules between the blood and surrounding tissues, maintenance of cardiovascular homeostasis and vascular tone, creation of new vascular network, and participation in and facilitation of the immune response through production and release of a broad spectrum of biologically active molecules, including nitric oxide (NO) and prostacyclin [1–5].
Arterial endothelial dysfunction refers to the decrease or loss of regulatory function in the normal ECs, and displays pro-oxidant, vasoconstrictor, pro-inflammatory, and pro-thrombotic properties. Increasing evidence indicates that vascular endothelial dysfunction develops with aging. One hallmark of vascular endothelial dysfunction is impaired endothelial-dependent dilation (EDD). Celermajer et al. demonstrated that EDD begins to decline in the early 40s for men and early 50s for women [6]. Egashira et al. reported lower EDD of coronary arteries evoked by acetylcholine with aging in humans [7]. Trott et al. showed that aging impairs PI3 K/Akt signaling and NO-mediated dilation in soleus muscle feed arteries [8]. It has been proposed that age-related endothelial dysfunction contributes to reduced perfusion of oxidative skeletal muscle [1], decreased exercise tolerance [9], enhanced permeability, infiltration of inflammatory cells, and thrombotic events [10], and impaired arteriogenesis [11]. Because NO produced by ECs plays critical roles in regulating vascular tone, inhibiting vascular inflammation, thrombotic events, and aberrant cell proliferation, endothelial dysfunction is largely attributed to decreased production and bioavailability of NO [12].
Multiple molecular and cellular alterations take place in aged ECs. Cavallaro et al. demonstrated that aged ECs became larger and irregular in shape [13]. Similar morphological features have also been observed in aged ECs in vitro [14,15]. Aged ECs were also characterized by a decline in mitochondrial mass [16] and reduced expression of SIRT1, a member of the sirtuin family of enzymes associated with lifespan extension and other anti-aging effects [17].
Recently, several lines of experimental evidence have indicated that age-related endothelial dysfunction and adverse arterial remodeling could be reversed or prevented by exercise training and overexpression of SIRT1 [18–20]. Furthermore, fluid shear stress (FSS) has been reported to have the ability to activate ECs and regulate SIRT1 expression [21]. However, it is unclear whether age-related impairment of endothelial structure and functions can be improved by elevated FSS (EFSS). To clarify this issue, we used the unilateral common iliac artery ligature model to study the effects of EFSS on endothelial function and structural remodeling in aged rats by use of histochemistry, ultrastructural observation, and immunostaining with specific antibodies against SIRT1 and endothelial nitric oxide synthase (eNOS). We found that elevated FSS improved age-related endothelial dysfunction and reversed adverse endothelial remodeling in aged rats.
Material and Methods
Animal model
The present study was performed according to the Guide for the Care and Use of Laboratory Animals by China’s Ministry of Science and Technology, and was approved by the Animal Care and Use Committee of Central South University (Ethics approval number: LLSC(LA)2017-006). Aged (20 months old) Sprague-Dawley (SD) rats obtained from the Animal Experimental Center of the Third Xiangya Hospital, Central South University) underwent unilateral (left) common iliac artery (LCIA) ligation. Anesthesia was performed using 10% chloral hydrate (3.5 ml/kg, intraperitoneally). A midline incision was made in the abdomen and the LCIA was separated from the accompanying vein and ligated with 5-0 silk, which led to an increase of blood flow in the contralateral (right) common iliac artery (RCIA). Then, the abdomen was closed in 2 layers. The animals had free access to food and water during perioperative recovery. The RCIA from both the aged and adult (12 months old) rats without ligation of the LCIA were used as controls. A total of 30 SD rats were used for the experiment.
Preparation of the tissue
The rats were sacrificed under deep anesthesia 1 week after the operation. We chose a 1-week experimental duration because our collateral research showed that endothelial cell activation is achieved by 7-day FSS stimulation [22,23]. The RCIA was isolated from each group of animals, and extreme care was taken at the time of isolation to minimize tissue damage. The tissue was cleaned with phosphate-buffered saline (PBS) containing 20 unit/ml heparin to remove any unwanted blood. For SA-β-gal staining and immunofluorescence, after quick-freezing in liquid nitrogen, the RCIA tissues were mounted with Tissue Tek (Tissue Tek O.C.T, SAKURA, CA, USA) and kept at −80°C until use. For RNA analysis, theRCIA tissues were stored in liquid nitrogen until RNA isolation.
Senescence-associated β-Galactosidase staining (SA-β-gal staining)
The SA-β-gal staining kit (Cell Signaling Technology, Beverly, MA) was used to confirm vascular cellular senescence according to the manufacturer’s instructions. Briefly, frozen tissue sections were initially fixed for 15 min with 4% paraformaldehyde. After washing 3 times with PBS, frozen tissue sections were then incubated with the SA-β-gal staining solution overnight at 37°C in a CO2-free atmosphere. The stained senescent cells (green color) were detected by conventional microscopy.
Immunofluorescence
Cryosections with a thickness of 5 μm were prepared and fixed with freshly prepared 4% paraformaldehyde. To block nonspecific staining, the sections were incubated with BSA (Aurion Co.) at a dilution of 1: 500. Then, the sections were incubated with primary antibodies (against SIRT1 and eNOS, purchased from Vector Lab, CA, USA, Cell Signaling Technology and BD Transduction Laboratories, respectively), secondary antibodies (anti-mouse or anti-rabbit IgG Vector lab, CA, USA), Cy3-conjugated Streptavidin (BioTrend, Germany), and DAPI (Invitrogen, Life Technologies, OR, USA). Finally, the sections were sealed with coverslips and examined under a confocal microscope (Nikon, Japan).
To exclude nonspecific binding of the secondary antibody, omission of the first antibody served as a negative control.
Doppler spectrum measurement
Blood flow velocity and lumen diameter of the RCIA were measured by Doppler spectrum (model Aplio 500, TOSHIBA, Japan) equipped with a linear probe (14L5) immediately before and 7 days after ligature of the LCIA in aged rats. During the measurements, the settings were 9 MHz probe frequency and 1-mm sampling volume. The rats were anaesthetized and placed in supine position. Images were obtained and transferred to a computer.
Real-time PCR
Total RNA was extracted from the RCIA by using TRIzol (Invitrogen, Life Technologies, CA, USA) according to the manufacturer’s protocol. Total RNA was isolated using TRIzol (Invitrogen, Life Technologies, CA, USA) according to the manufacturer’s protocol. Then, 1 μg total RNA was transcribed to cDNA (Fermentas, Thermo Scientific, Lithuania) and real-time PCR was performed on a Mastercycler Nexus instrument (Eppendorf, Germany). The expression of RNA 18s served as a loading control. The primer sequences for each gene were:
SIRT1, 5′-ATAGATACCTTGGAGCAGGTTGC-3′ (forward),
5′-CACGAACAGCTTCACAATCAACT-3′ (reverse);
eNOS, 5′-ACAAGGCAGCAGTGGAAATTAAC-3′ (forward),
5′-CTGCTCATTTTCCAAGTGCTTCA-3′ (reverse).
TEM
Freshly dissected samples were immediately immersion-fixed in a mixture of 2% paraformaldehyde and 1% glutaraldehyde in 0.1 M sodium cacodylate buffer followed by postfixation in 2% osmium tetroxide and embedding in Epon. Ultrathin sections were stained with uranyl acetate and lead citrate, viewed, and photographically recorded using a Philips CM10 electron microscope.
Hematoxylin–eosin staining
Cryosections with a thickness of 5 μm were prepared and fixed with freshly prepared 4% paraformaldehyde. HE staining was performed according to routine protocols.
Quantification of fluorescence intensity
The quantification of fluorescence intensity of eNOS and SIRT1 was conducted with representative images obtained from a confocal microscope (Nikon, Japan), using EZ-C1 3.70 software. During measurements, 0-pixel to 255-pixel intensity level (a full range of gray values from black to peak white) was set. The fluorescence intensity was presented as arbitrary units AU/μm2.
Statistical analysis
All values are expressed as mean ±SEM. The difference between normal adult and aged groups and between ligated and unligated groups was compared by t test. A P value <0.05 was considered statistically significant.
Results
Effect of ligation of the LCIA on blood flow velocity and diameter of RCIA in aged rats
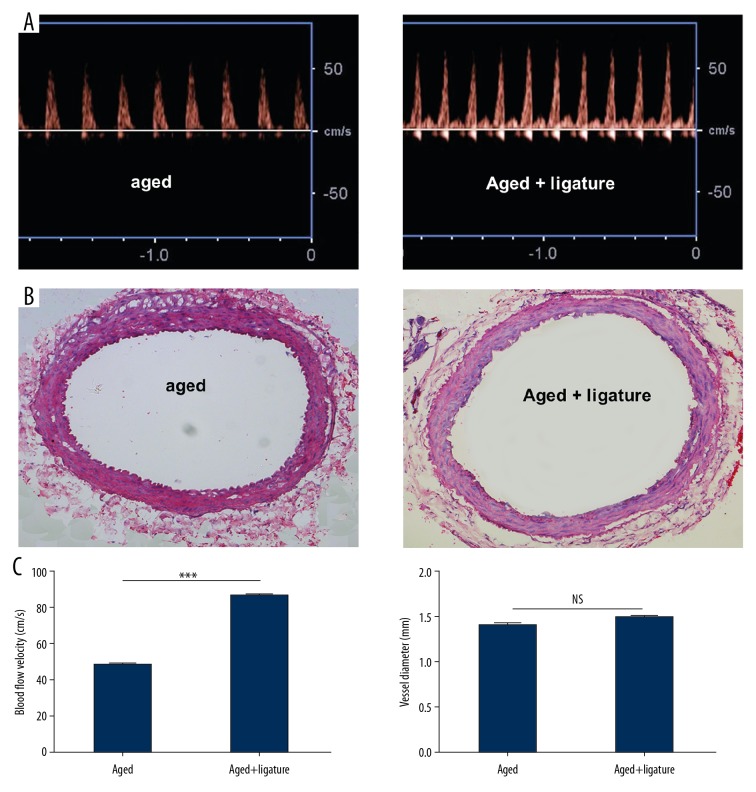
To determine the effect of ligation of LCIA on fluid shear stress alteration in RCIA in aged rats, the blood flow velocity of RCIA before and after ligation of LCIA in rats was measured by Doppler ultrasound, and the lumen diameter of the RCIA was measured on tissue sections after HE staining using the Image J system. Our data showed that the blood flow velocity of RCIA after ligation of LCIA (85.9±2.1 cm/s)was significantly increased (P<0.001) as compared to before surgery (49.6±1.3 cm/s) (Figure 1). In contrast, there was no significant variation in the lumen diameter of RCIA after ligation (Figure 1).
Figure 1.
Blood flow velocity and diameter of RCIA in aged rats. (A) Blood flow velocity of RCIA before and after ligation of LCIA in aged rats. (B) HE staining of RCIA before and after ligation of LCIA in aged rats. (C) Quantitative analysis of blood flow velocity and lumen diameter of RCIA before and after ligation of LCIA in aged rats. *** p<0.001; NS – not significant. n=10 per group.
FSS depends on blood viscosity (μ), vessel radius (r), and blood flow velocity. In a laminar state, FSS can be estimated by the formula: τ=4 μQ/πr3 and blood flux Q is determined by the blood flow velocity and the cross-sectional area. In our case, the blood viscosity and radius were not significantly different after the operation, so the changes in FSS originated mainly from the variation in blood flow velocity, and shear stress is proportional to blood flow velocity. Our experimental results indicated that blood flow velocity of the RICA was increased in rats via ligation of the LCIA; therefore, we concluded that increased FSS was induced in this model.
SA-β-gal staining was significantly attenuated by elevated FSS in the RCIA after ligation of LCIA in aged rats
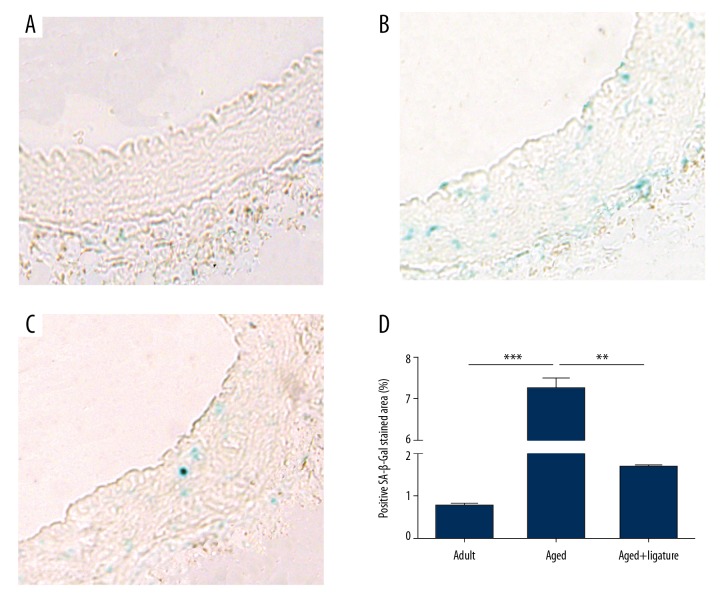
Positive SA-β-gal staining is a common feature of the senescent phenotype, which is largely independent of cell type. Therefore, we used SA-β-gal staining to determine cellular senescence in RCIA of aged rats. We found that positive SA-β-gal staining was rarely detected in the RCIA of normal adult rats; in contrast, it was apparent in the RCIA of normal aged rats and could be observed in all 3 layers of the wall (Figure 2). Compared with the normal aged rats, SA-β-gal staining was significantly attenuated in the RCIA of the aged rats with ligation of the LCIA (Figure 2).
Figure 2.
SA-β-gal staining in RCIA in rats. (A) RCIA of normal adult rats; (B) RCIA of normal aged rats; (C) RCIA of aged rats with ligation of LCIA; (D) quantitative analysis of SA-β-gal positive staining in 3 groups. ** p<0.01, *** p<0.001. n=3 per group.
The effect of elevated FSS on the ultrastructural changes of the endothelium in the RCIA in aged rats
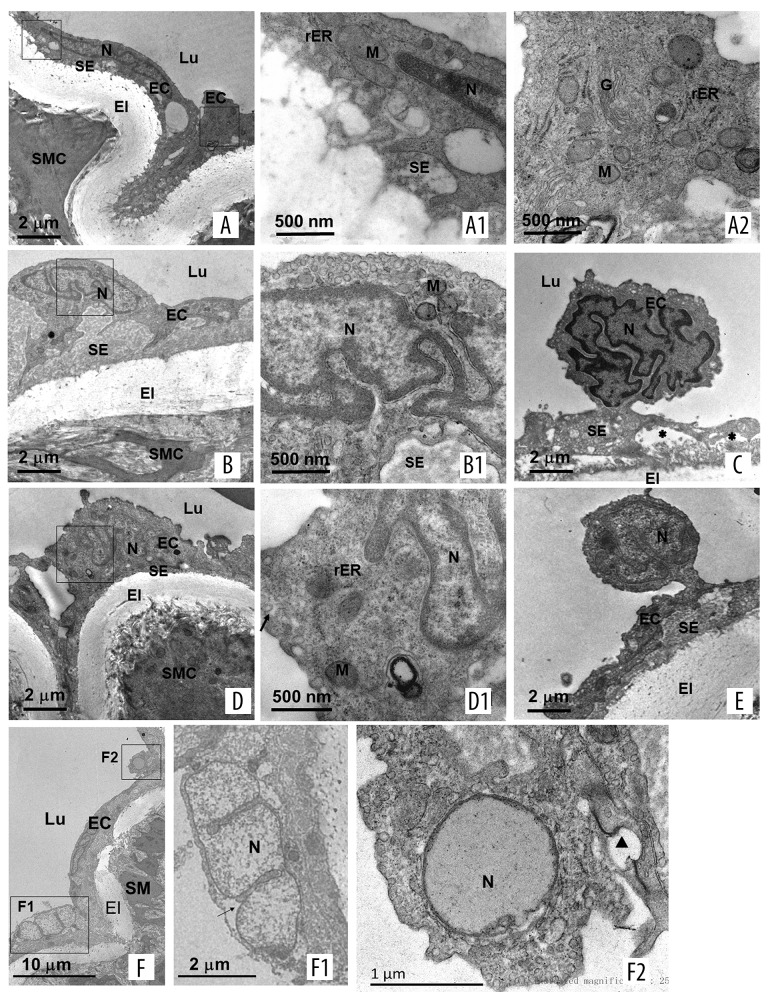
The effect of elevated FSS induced by ligation of LCIA on ultrastructural changes in RCIA of aged rats was investigated by TEM. In RCIA of normal adult rats, ECs was thin and flat, with an elongated nucleus. Numerous mitochondria were visible in the cytoplasm. A few small projections at the abluminal side extended into the subendothelial matrix. The basement membrane was not observed in the endothelium, and the subendothelial matrix was very thin (Figure 3). In the RCIA of normal aged rats, ECs became irregular in shape and enlarged in size and had an increasingly polypoid nucleus. There was evident transition to the lipofuscin bodies of damaged mitochondria. A part of the enlarged cell bodies and their long projections at the abluminal side were embedded in the subendothelial matrix (Figure 3). The thickness of the subendothelial matrix was increased, but without infiltration of SMCs. Apoptotic-like senescent ECs were observed (Figure 3). We found occasional dying senescent ECs whose nuclei were degraded and dead senescent ECs with disintegrating nuclei. In the RCIA of aged rats with ligation of the LCIA, ECs became prominent, but the nuclei were still polypoid. Numerous mitochondria were visible in the cytoplasm. The cell bodies and their projections of ECs at the abluminal side withdrew from the subendothelial matrix (Figure 3).
Figure 3.
TEM image showing ultrastructural of the endothelium in RCIA in rats. (A, A1, A2): RCIA of normal adult rats; (B, B1, F, F1, F2): RCIA of normal aged rats; (C, D, D1, E): RCIA of aged rats with ligation of LCIA. EC – endothelial cells; N – nuclei; SMC – smooth muscle cells; EI – elastica interna; M – mitochondria; rER – rough endoplasmic reticulum; Lu – lumen; SE – subendothelial layer; G – Golgi complex. n=3 per group.
Elevated FSS significantly increased protein and mRNA levels of eNOS and SIRT1 in the RCIA after ligation of LCIA in aged rats
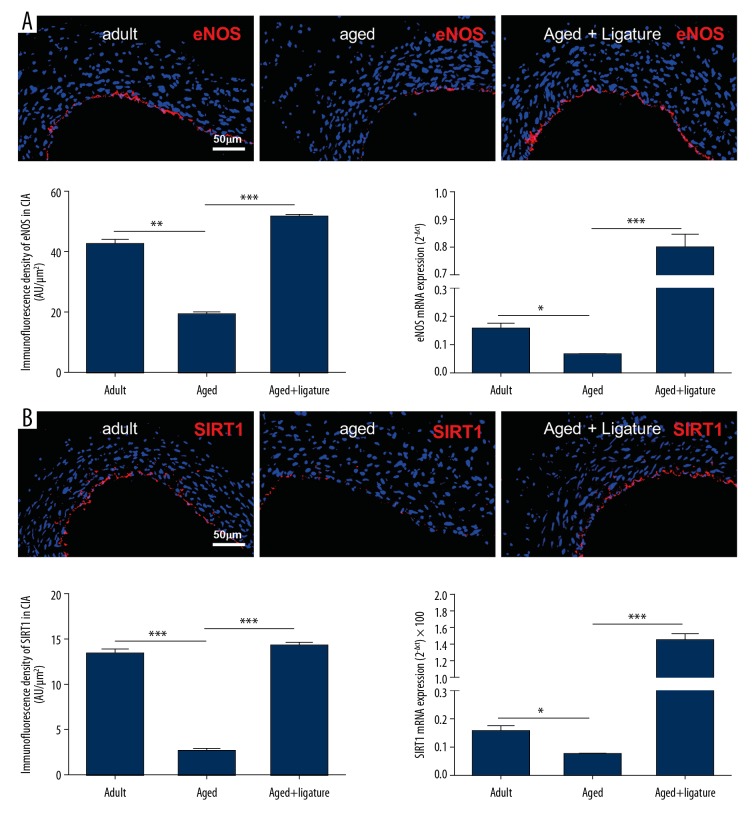
The effect of elevated FSS induced by ligation of LCIA on the expression of eNOS and SIRT1 in the RCIA of aged rats was investigated by immunofluorescence and q-PCR. We found that in the sham-operated aged rats, the contents at the protein and mRNA levels of eNOS in the RCIA were significantly lower compared with that in normal adult rats. After ligation of the LCIA, eNOS contents at the protein and mRNA levels in the RCIA were significantly higher than in sham-operated aged rats (Figure 4). Immunostaining of SIRT1 showed that this protein could be detected in the nucleus and cytoplasm of ECs. Similar to the eNOS expression pattern, expression of SIRT1 at the protein and mRNA levels in the endothelium of the RCIA of aged rats was significantly lower than in normal adult rats. These decreased levels in the RCIA of aged rats were reversed by elevated FSS induced by ligation of the LCIA (Figure 4).
Figure 4.
The expression of eNOS and SIRT1 on protein and mRNA levels in RCIA. (A) The expression of eNOS protein and mRNA in RCIA; blue: DAPI; red: eNOS; (B) The expression of SIRT1 protein and mRNA in RCIA; blue: DAPI; red: SIRT1. * p<0.05; ** p<0.01; *** p<0.001. For immunofluorescence: n=3 per group; for RNA analysis: n=4 per group.
Discussion
The main results of our study are: (1) in the RCIA of aged rats, aging led to impairment of endothelial structures, and this impairment was attenuated by the elevated FSS induced by ligation of the LCIA; (2) in the RCIA of aged rats, aging led to decreased expression of eNOS and SIRT1, and this decrease was reversed by the elevated FSS induced by ligation of the LCIA.
SA-β-gal staining is widely used to identify the senescent phenotype. In the present study, we found positive SA-β-gal staining in all 3 layers of the wall of the RCIA of normal aged rats, but staining was rare in the RCIA of normal adult rats, indicating that the presence of senescent vascular cells in aging RCIA vasculature. This is consistent with the results reported by Fenton et al., who first demonstrated senescent endothelial and vascular smooth muscle cells in balloon-injured vasculature [24] by SA-β-gal staining. Our data supports that SA-β-gal is one of the best currently available markers of senescence [25]. In RCIA of aged rats with ligation of the LCIA, there was significantly less SA-β-gal staining than in normal aged rats, indicating that elevated FSS induced by ligation of LCIA can reverse age-related vascular senescence.
Based on our SA-β-gal staining results, we investigated age-related ultrastructural changes in the endothelium of the RCIA and the effect of elevated FSS induced through ligation of LCIA on this change. We focused on the endothelium because it plays an important role in regulating vascular functions [1–5]. We found clear evidence of age-related impairment of endothelial structures, as shown by the observation that in the endothelium of the RCIA of adult rats, ECs were thin and flat with an elongated nucleus and contained numerous mitochondria, whereas in aged individuals, ECs became irregular and enlarged, with an increasingly polypoid nucleus, and had transitioned to the lipofuscin bodies of damaged mitochondria. Similar morphological features have also been observed in several previous studies [14,16,26,27]. In addition to the above-mentioned changes, the present study reveals a new morphological feature of aged ECs by showing that in the endothelium of the RCIA of aged rats, some of the enlarged cell bodies and their long projections at the abluminal side were embedded in the subendothelial matrix. To the best of our knowledge, no similar observation has been previously reported. We speculate that this might be a self-protective adaptative mechanism by which these ECs prevent themselves from being detached from the vascular wall through increasing contacts with the subendothelial matrix. To clarify this issue, further investigations are required. In our study, the basement membrane (BM) was not observed in the endothelium of the RCIA, and ECs contacted directly with subendothelial matrix. Subendothelial thickness increased with increasing age, but contained no smooth muscle cells (SMCs). We were not surprised by the absence of BM in the endothelium of the RCIA since endothelial BM mainly exists in arteriolar vessels and capillaries, whereas the RCIA is a kind of transitional vessel between large and muscle arteries. Similarly, under normal circumstances, intima SMCs are mainly observed in large arterial vessels, and they are rare in coronary arteriolar vessels, with the exception of muscle arteries. Thus, we suggest that in the RCIA of aged rats, the increased subendothelial matrix is produced by ECs, not SMCs. Recently, several reports showed that apoptotic cell death in ECs was increased in aging vessels, including peripheral arteries and capillaries [28,29]. In those studies, apoptosis was detected by terminal deoxynucleotidyl transferase-mediated dUTP nick-end labelling assay. In our study, apoptotic-like senescent ECs that were detached from the subendothelial matrix were also observed under TEM. This result is similar to that of Lakatta et al., who found that senescent ECs were detached from the basement membrane [10]. In addition to apoptotic-like cell death, dying and dead senescent ECs with degraded and disintegrating nuclei, respectively, were also observed. To the best of our knowledge, this type of cell death in senescent ECs has not been previously reported. The mechanism responsible for this cell death and its importance requires further investigations. The FSS-mediated EC response has attracted intense research interest for several decades. The dramatic effects of FSS on EC shape and structure have been well established. Kim et al. showed that exercise-mediated wall shear stress increases mitochondrial biogenesis in vascular endothelium [30]. Nerem et al. reported that the high and unidirectional FSS induces elongated EC shape and aligned cytoskeletal components, whereas low and oscillatory FSS induces cobblestone-like EC shape and randomly oriented cytoskeletal components [31]. Furthermore, others found that FSS induced-remodeling of the cytoskeleton, as well as focal adhesion and extracellular matrix assembly, is correlated with EC shape changes [32,33]. In line with the above-mentioned literature, the present study demonstrated that elevated FSS also induced aged endothelial shape and structural remodeling, showing that in the RCIA of aged rats with ligation of LCIA, ECs contain numerous mitochondria, and their bodies and projections at the abluminal side withdrew from the subendothelial matrix and became protruded into the lumen. These findings suggest that aged ECs are activated by elevated FSS, which is supported by data from collateral vessel research in which activated ECs were found to be morphologically prominent [34]. To the best of our knowledge, this is the first study showing that age-related impairment of EC structure can be reversed by elevated FSS.
Following morphological observation, further research was carried out on age-related functional change in the endothelium of the RCIA and the effect of elevated FSS on this change by examining the expression of both SIRT1 and eNOS. We found that in aged rats, eNOS expression at protein and mRNA levels in the RCIA was significantly lower than in normal adult rats. This result is in agreement with previously published studies showing that the production of eNOS in the aorta decreases with aging, and aged HUVECs showed significantly reduced eNOS expression and a decrease in the overall S-NO content [35,36]. eNOS is the key enzyme for NO production, and decreased NO bioavailability and NO deficiency are considered hallmarks of endothelial dysfunction and vascular aging [12]; therefore, the finding of deceased expression of eNOS in the aged RCIA indicates the presence of age-related impairment of endothelial function in aged rats. As expected, there was increased eNOS expression in the RCIA caused by by elevated FSS induced through ligation of the LCIA. On the one hand, the level of FSS, the most important factor regulating the production of eNOS, is reduced with aging [37]. On the other hand, aged ECs become less responsive to FSS [12]. Therefore, the observation that elevated FSS was accompanied by increased eNOS production in aged RCIA is reasonable. These data suggest that aged-related impairment of endothelial function could be reversed or attenuated by elevated FSS. In support of this, several previously published studies demonstrated that NO-mediated dilation was impaired with age and improved with exercise training or following exposure to increased intraluminal pressure or shear stress [38,39].
As with eNOS expression, we found that SIRT1 expression was also significantly decreased in aged RCIA. This is consistent with a previous report that SIRT1 expression is reduced in ECs obtained from arteries of older human adults compared to those from younger adults [40]. SIRT1, an NAD+-dependent deacetylase, is considered to be an anti-aging molecule. In ECs, downregulation of SIRT1 evokes cellular senescence [41]. Thus, the reduced SIRT1 in aged RCIA also indicates the presence of endothelial dysfunction. As mentioned earlier, FSS is the most important factor for activation of Ecs [21]. Consistent with this notion, in vitro experiments demonstrated shear stress upregulates SIRT1 expression in Ecs [21,42]. The present study is the first to show that elevated FSS can upregulate SIRT1 expression, likely initiating an anti-aging process.
In the present study, expression of eNOS and SIRT1 shared the same pattern, showing both were decreased in aged ECs and were upregulated by elevated FSS, suggesting that there is cross-talk between these 2 molecules. Indeed, Nisoli et al. demonstrated that SIRT1 expression is induced by increased NO and is blunted in eNOS-deficient mice [43], whereas Mattagajasingh et al. reported that SIRT1 promotes NO production by targeting eNOS for deacetylation [44]. Based on the literature and our data, we suggest that Sirt1 and eNOS interact with each other in regulating ECs function.
Conclusions
We demonstrated that aging leads to impairment of the structure and function (reduced expression of eNOS and SIRT1) of the endothelium in the RCIA of aged rats. We for the first time show that this age-related impairment of the endothelium in the aged RCIA can be reversed through ligation of the LCIA. Our data highlight the importance of FSS for the reactivation of aged ECs.
Footnotes
Source of support: This work was supported by a grant from the National Natural Science Foundation of China (No. 81370248)
Conflict of interest
None.
References
- 1.Bonetti PO, Lerman LO, Lerman A. Endothelial dysfunction: A marker of atherosclerotic risk. Arterioscler Thromb Vasc Biol. 2003;23:168–75. doi: 10.1161/01.atv.0000051384.43104.fc. [DOI] [PubMed] [Google Scholar]
- 2.Widlansky ME, Gokce N, Keaney JF, Vita JA. The clinical implications of endothelial dysfunction. J Am Coll Cardiol. 2003;42:1149–60. doi: 10.1016/s0735-1097(03)00994-x. [DOI] [PubMed] [Google Scholar]
- 3.Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–76. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 4.Busse R, Fleming I. Vascular endothelium and blood flow. Handb Exp Pharmacol. 2006;(176 Pt 2):43–78. doi: 10.1007/3-540-36028-x_2. [DOI] [PubMed] [Google Scholar]
- 5.Pober JS, Min W, Bradley JR. Mechanisms of endothelial dysfunction, injury, and death. Annu Rev Pathol. 2009;4:71–95. doi: 10.1146/annurev.pathol.4.110807.092155. [DOI] [PubMed] [Google Scholar]
- 6.Celermajer DS, Sorensen KE, Spiegelhalter DJ, et al. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J Am Coll Cardiol. 1994;24:471–76. doi: 10.1016/0735-1097(94)90305-0. [DOI] [PubMed] [Google Scholar]
- 7.Egashira K, Inou T, Hirooka Y, et al. Effects of age on endothelium-dependent vasodilation of resistance coronary artery by acetylcholine in humans. Circulation. 1993;88:77–81. doi: 10.1161/01.cir.88.1.77. [DOI] [PubMed] [Google Scholar]
- 8.Trott DW, Luttrell MJ, Seawright JW, Woodman CR. Aging impairs PI3K/Akt signaling and NO-mediated dilation in soleus muscle feed arteries. Eur J Appl Physiol. 2013;113(8):2039–46. doi: 10.1007/s00421-013-2639-2. [DOI] [PubMed] [Google Scholar]
- 9.Musch TI, Eklund KE, Hageman KS, Poole DC. Altered regional blood flow responses to submaximal exercise in older rats. J Appl Physiol (1985) 2004;96:81–88. doi: 10.1152/japplphysiol.00729.2003. [DOI] [PubMed] [Google Scholar]
- 10.Lakatta EG. The reality of aging viewed from the arterial wall. Artery Res. 2013;7(2):73–80. doi: 10.1016/j.artres.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang J, Peng X, Lassance-Soares RM, et al. Aging-induced collateral dysfunction: Impaired responsiveness of collaterals and susceptibility to apoptosis via dysfunctional eNOS signaling. J Cardiovasc Transl Res. 2011;4:779–89. doi: 10.1007/s12265-011-9280-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Collins C, Tzima E. Hemodynamic forces in endothelial dysfunction and vascular aging. Exp Gerontol. 2011;46:185–88. doi: 10.1016/j.exger.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cavallaro U, Castelli V, Del MU, Soria MR. Phenotypic alterations in senescent large-vessel and microvascular endothelial cells. Mol Cell Biol Res Commun. 2000;4:117–21. doi: 10.1006/mcbr.2000.0263. [DOI] [PubMed] [Google Scholar]
- 14.van der Loo B, Fenton MJ, Erusalimsky JD. Cytochemical detection of a senescence-associated beta-galactosidase in endothelial and smooth muscle cells from human and rabbit blood vessels. Exp Cell Res. 1998;241:309–15. doi: 10.1006/excr.1998.4035. [DOI] [PubMed] [Google Scholar]
- 15.Hohensinner PJ, Kaun C, Buchberger E, et al. Age intrinsic loss of telomere protection via TRF1 reduction in endothelial cells. Biochim Biophys Acta. 2016;1863:360–67. doi: 10.1016/j.bbamcr.2015.11.034. [DOI] [PubMed] [Google Scholar]
- 16.Ungvari Z, Labinskyy N, Gupte S, et al. Dysregulation of mitochondrial biogenesis in vascular endothelial and smooth muscle cells of aged rats. Am J Physiol Heart Circ Physiol. 2008;294:H2121–28. doi: 10.1152/ajpheart.00012.2008. [DOI] [PubMed] [Google Scholar]
- 17.Ungvari Z, Parrado-Fernandez C, Csiszar A, de Cabo R. Mechanisms underlying caloric restriction and lifespan regulation: Implications for vascular aging. Circ Res. 2008;102:519–28. doi: 10.1161/CIRCRESAHA.107.168369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trott DW, Gunduz F, Laughlin MH, Woodman CR. Exercise training reverses age-related decrements in endothelium-dependent dilation in skeletal muscle feed arteries. J Appl Physiol (1985) 2009;106:1925–34. doi: 10.1152/japplphysiol.91232.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hanna MA, Taylor CR, Chen B, et al. Structural remodeling of coronary resistance arteries: Effects of age and exercise training. J Appl Physiol (1985) 2014;117:616–23. doi: 10.1152/japplphysiol.01296.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guo Y, Xu A, Wang Y. SIRT1 in endothelial cells as a novel target for the prevention of early vascular aging. J Cardiovasc Pharmacol. 2016;67:465–73. doi: 10.1097/FJC.0000000000000344. [DOI] [PubMed] [Google Scholar]
- 21.Chen Z, Peng IC, Cui X, et al. Shear stress, SIRT1, and vascular homeostasis. Proc Natl Acad Sci USA. 2010;107:10268–73. doi: 10.1073/pnas.1003833107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang BL, Wu S, Wu X, et al. Effect of shunting of collateral flow into the venous system on arteriogenesis and angiogenesis in rabbit hind limb. Acta Histochem Cytochem. 2013;46:1–10. doi: 10.1267/ahc.12025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luo MY, Yang BL, Ye F, et al. Collateral vessel growth induced by femoral artery ligature is impaired by denervation. Mol Cell Biochem. 2011;354:219–29. doi: 10.1007/s11010-011-0821-6. [DOI] [PubMed] [Google Scholar]
- 24.Fenton M, Barker S, Kurz DJ, Erusalimsky JD. Cellular senescence after single and repeated balloon catheter denudations of rabbit carotid arteries. Arterioscler Thromb Vasc Biol. 2001;21:220–26. doi: 10.1161/01.atv.21.2.220. [DOI] [PubMed] [Google Scholar]
- 25.Erusalimsky JD, Kurz DJ. Cellular senescence in vivo: Its relevance in ageing and cardiovascular disease. Exp Gerontol. 2005;40:634–42. doi: 10.1016/j.exger.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 26.Repin VS, Dolgov VV, Zaikina OE, et al. Heterogeneity of endothelium in human aorta. A quantitative analysis by scanning electron microscopy. Atherosclerosis. 1984;50:35–52. doi: 10.1016/0021-9150(84)90006-6. [DOI] [PubMed] [Google Scholar]
- 27.Bürrig KF. The endothelium of advanced arteriosclerotic plaques in humans. Arterioscler Thromb. 1991;11:1678–89. [PubMed] [Google Scholar]
- 28.Asai K, Kudej RK, Shen YT, et al. Peripheral vascular endothelial dysfunction and apoptosis in old monkeys. Arterioscler Thromb Vasc Biol. 2000;20:1493–99. doi: 10.1161/01.atv.20.6.1493. [DOI] [PubMed] [Google Scholar]
- 29.Wang H, Listrat A, Meunier B, et al. Apoptosis in capillary endothelial cells in ageing skeletal muscle. Aging Cell. 2014;13:254–62. doi: 10.1111/acel.12169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim B, Lee H, Kawata K, Park JY. Exercise-mediated wall shear stress increases mitochondrial biogenesis in vascular endothelium. PLoS One. 2014;9:e111409. doi: 10.1371/journal.pone.0111409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nerem RM, Levesque MJ, Cornhill JF. Vascular endothelial morphology as an indicator of the pattern of blood flow. J Biomech Eng. 1981;103:172–76. doi: 10.1115/1.3138275. [DOI] [PubMed] [Google Scholar]
- 32.Osborn EA, Rabodzey A, Dewey CF, Hartwig JH. Endothelial actin cytoskeleton remodeling during mechanostimulation with fluid shear stress. Am J Physiol Cell Physiol. 2006;290:C444–52. doi: 10.1152/ajpcell.00218.2005. [DOI] [PubMed] [Google Scholar]
- 33.Mott RE, Helmke BP. Mapping the dynamics of shear stress-induced structural changes in endothelial cells. Am J Physiol Cell Physiol. 2007;293:C1616–26. doi: 10.1152/ajpcell.00457.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schapers W. Arteriogenesis. 1st ed. Massachusetts: Kluwer Academic Publisher; 2004. [Google Scholar]
- 35.Tanabe T, Maeda S, Miyauchi T, et al. Exercise training improves ageing-induced decrease in eNOS expression of the aorta. Acta Physiol Scand. 2003;178:3–10. doi: 10.1046/j.1365-201X.2003.01100.x. [DOI] [PubMed] [Google Scholar]
- 36.Hoffmann J, Haendeler J, Aicher A, et al. Aging enhances the sensitivity of endothelial cells toward apoptotic stimuli: Important role of nitric oxide. Circ Res. 2001;89:709–15. doi: 10.1161/hh2001.097796. [DOI] [PubMed] [Google Scholar]
- 37.Carallo C, Tripolino C, De Franceschi MS, et al. Carotid endothelial shear stress reduction with aging is associated with plaque development in twelve years. Atherosclerosis. 2016;251:63–69. doi: 10.1016/j.atherosclerosis.2016.05.048. [DOI] [PubMed] [Google Scholar]
- 38.Woodman CR, Price EM, Laughlin MH. Shear stress induces eNOS mRNA expression and improves endothelium-dependent dilation in senescent soleus muscle feed arteries. J Appl Physiol (1985) 2005;98:940–46. doi: 10.1152/japplphysiol.00408.2004. [DOI] [PubMed] [Google Scholar]
- 39.Seawright JW, Luttrell M, Trache A, Woodman CR. Short-term increases in pressure and shear stress attenuate age-related declines in endothelial function in skeletal muscle feed arteries. Eur J Appl Physiol. 2016;116:1305–11. doi: 10.1007/s00421-016-3388-9. [DOI] [PubMed] [Google Scholar]
- 40.Donato AJ, Magerko KA, Lawson BR, et al. SIRT-1 and vascular endothelial dysfunction with ageing in mice and humans. J Physiol. 2011;589:4545–54. doi: 10.1113/jphysiol.2011.211219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bai B, Vanhoutte PM, Wang Y. Loss-of-SIRT1 function during vascular ageing: hyperphosphorylation mediated by cyclin-dependent kinase 5. Trends Cardiovasc Med. 2014;24:81–84. doi: 10.1016/j.tcm.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 42.Liu J, Bi X, Chen T, et al. Shear stress regulates endothelial cell autophagy via redox regulation and Sirt1 expression. Cell Death Dis. 2015;6:e1827. doi: 10.1038/cddis.2015.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nisoli E, Tonello C, Cardile A, et al. Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science. 2005;310:314–17. doi: 10.1126/science.1117728. [DOI] [PubMed] [Google Scholar]
- 44.Mattagajasingh I, Kim CS, Naqvi A, et al. SIRT1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. Proc Natl Acad Sci USA. 2007;104:14855–60. doi: 10.1073/pnas.0704329104. [DOI] [PMC free article] [PubMed] [Google Scholar]