Abstract
Native right coronary artery (RCA) spasm is a less frequent early complication of perioperative coronary artery bypass grafting. Late presentation at 6 days postoperation is scarce and its relationship with an anomalous coronary artery is unknown. The optimal management and prevention remains controversial. In the case presented, the patient’s anomalous left coronary artery originating from the right coronary cusp underwent ligation at its proximal segment at the time of bypass grafting. This ligation was preformed to prevent competitive flow. Six days postoperation, a refractory spasm of dominant native RCA occurred. The spasm resulted in right ventricular failure. Administration of intracoronary verapamil had a longer sustained vasodilatory effect and resolution of coronary spasm when compared with intracoronary nitroglycerine injection. An intra-aortic balloon pump, inotropic agents and low-dose nitroglycerine were used to maintain adequate haemodynamic support. Right ventricular systolic function recovery was noted within 2 days postintervention.
Keywords: cardiothoracic surgery, interventional cardiology, heart failure, ischaemic heart disease, cardiovascular system
Background
Whether the coronary artery spasm (CAS) is a manifestation of postoperative pathology or related to concurrent presentation of an anomalous left coronary artery (ALCA) anatomy is unknown. Several hypotheses are proposed to explain the mechanism of ischaemia and sudden cardiac death (SCD) in patients with ALCA intra-arterial course. Previously proposed mechanisms include compression of ALCA between aorta and pulmonary trunk, slit-like orifice, acute angle take off, small arteries, and spontaneous vasospasm.1 This vasospasm was thought to be associated with the culprit coronary anomaly. The presented case rather involves a potentially fatal CAS that is not associated with the anomalous artery.
Case presentation
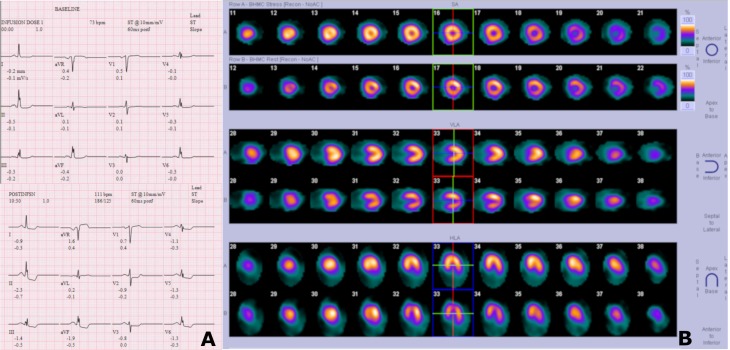
A 61-year-old black woman presented with a complaint of recurrent, intermittent, and non-exertional chest pain with radiation to the left arm for several months. She reported a history of percutaneous coronary intervention with a stent to the circumflex coronary artery (Cx) in 2016. Transthoracic echocardiography (TTE) showed a preserved left ventricular systolic function with no evidence of significant valvulopathy or diastolic dysfunction. Regadenoson cardiac stress test, 1 month prior, was positive for inferior and lateral ST segment depression with associated chest pain that resolved with nitroglycerine administration (figure 1A). Interestingly, the nuclear medicine images did not show any stress-induced reversible myocardial ischaemia or fixed defects (figure 1B). Cardiac enzymes were negative. Baseline ECG did not show any evidence of ischaemia. Regardless, the result of cardiac stress test prompted further investigation with coronary artery cardiac catheterization at our institution.
Figure 1.
Regadenoson cardiac stress test with ECG (A) and nuclear imaging (B).
Cardiac catheterization showed a Cx originating from the left coronary cusp with total occlusion at its take-off where the previous stent was placed. ALCA was visualised to originate from the right coronary cusp with its ostium located next to the ostium of right coronary artery (RCA). The ALCA extending as the left anterior descending artery (LAD) has a medium-sized septal perforator and three diagonal branches. First diagonal branch (D1) has 70% stenosis at its proximal segment. The RCA is a large-sized vessel, dominant, and supplies right ventricular branch (figure 2A,B).
Figure 2.
Preoperative cardiac catheterization, showing right coronary artery free of significant disease with no evidence of spasm (A); selective angiography of anomalous LAD with its septal perforators and diagonal branches (B).
ALCA was evaluated with coronary CT angiography (CCTA). The imaging indicates that the anomalous origin of LAD is from the right coronary cusp with an intra-arterial (between the aorta and the pulmonary artery) course (figure 3A). There is no evidence of slit-like orifice or intramural origin. The RCA, free of significant stenosis, originates from the right aortic cusp and does not share its origin with the anomalous LAD (figure 3B). The Cx originates from the left coronary cusp and has in-stent stenosis at its proximal section that was consistent with chronic total occlusion (figures 3C and 4A,B).
Figure 3.
Axial view of coronary CT angiography showing anomalous left coronary artery with intra-arterial course (A); dominant RCA without any evidence of stenosis (B); Cx with in-stent stenosis and delayed filling (C). RCA, right coronary artery.
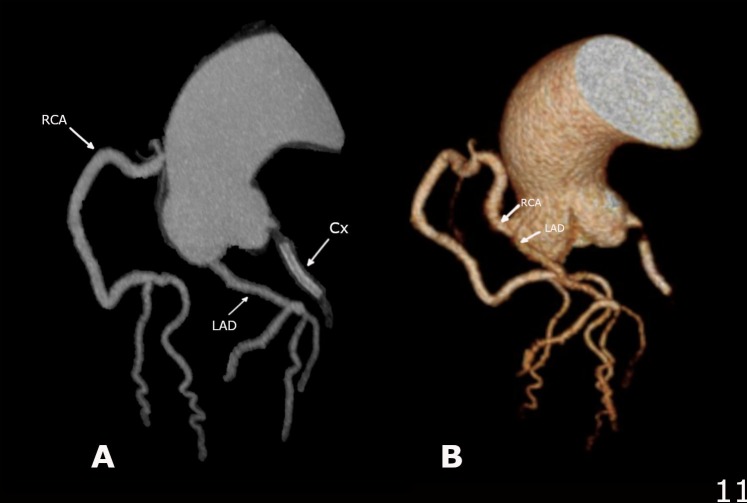
Figure 4.
Three-dimensional reconstruction CT with 45° rotational view between (A) and (B) to appreciate RCA and anomalous left coronary artery anatomy with separate but neighbouring ostia and their branches. RCA, right coronary artery.
Coronary artery bypass grafting (CABG) was performed using the left internal mammary artery to mid-LAD and reverse saphenous vein graft to D1. Patient tolerated the procedure well without any immediate postoperative complication. Six days after CABG, patient developed gradual hypotension with symptoms of orthostatic dizziness, lightheadedness, and fatigue. At this time, the patient denied any chest pain and remained comfortable in bed. A 12-lead ECG showed ST segment elevation in inferior leads. Troponin-I level was initially elevated at 63.01 ng/mL and peaked to 129.29 ng/mL. C reactive protein (CRP) was elevated at 15.42 mg/dL (0–0.80). Erythrocyte sedimentation rate (ESR) levels, which were within normal limits on admission, increased to 40 mm/h (0–30).
Differential diagnosis
The onset of mild to moderate hypotension and lightheadedness are the only sign and symptom that was presented to us as a change in patient’s status. Medication and vagal-related hypotension were our initial consideration for aetiology of patient’s presentation. Patient was placed into Trendelenburg position and 1 litre bolus of normal saline was administered. While the lightheadedness resolved, the blood pressure’s improvement was only transient.
Persistent hypotension led to further investigation with a 12-lead ECG. We considered several differential diagnoses once ST-segment elevation in inferior leads was noted. A bypass graft closure was contemplated but did not correlate to the localisation on inferior lead ST-segment elevation. Patient did not have any grafts to the large dominant native RCA. Thus, new plaque rupture to RCA was entertained. Although such event is expected to present with more pronounced symptoms, including chest pain, cardiac catheterization was indicated for further investigation.
Treatment
Emergent cardiac catheterization was preformed and showed patent grafts while the distal RCA had severe spasm with Thrombolysis in Myocardial Infarction (TIMI) grade 2 distal flow that resolved transiently with administration of intracoronary nitroglycerine (figure 5A–C). TTE during cardiac catheterization showed severely reduced right ventricular systolic function with severe dilatation, dilated inferior vena cava, and turbulent flow across the tricuspid valve (figure 5D,E). Meanwhile, the left ventricular systolic function remained intact. Intra-aortic balloon pump was inserted, and dopamine infusion at 10 µg/kg/min was initiated. The patient developed third-degree atrioventricular block, requiring a transvenous pacemaker. During the insertion of transvenous pacer, the patient went into pulseless ventricular tachycardia. Return of spontaneous circulation was achieved. After the arrest, additional imaging of the RCA revealed severe refractory spasm. Intracoronary verapamil was then administered resulting in more sustained patency of the vessel. Additional positive inotropic and vasopressor support was added due to refractory hypotension. Intravenous nitroglycerine infusion was initiated at 20 µg/kg/min to help alleviate CAS.
Figure 5.
Cardiac catheterization showing severe right coronary artery (RCA) spasm at distal segment with TIMI-2 flow (A); moderate improvement in spasm with intracoronary administration of nitroglycerine (B); marked improvement and sustained patency with intracoronary administration of verapamil (C). Transthoracic echocardiogram performed during active RCA spam showing severe dilation of right atrium and ventricle with basal right ventricular diameter of 4.20 cm (D); elevated right-sided pressure illustrated with dilated and non-collapsible inferior vena cava with diameter 2.40 cm (E).
Outcome and follow-up
On the second day of monitoring in the Cardiac Care Unit, repeat TTE showed recovery of the right ventricular systolic function and right ventricular dilatation. Swan Ganz haemodynamic monitoring showed improvement in the cardiac index from 1.6 to 3.5 (L/min/m2).
Discussion
This case highlights multiple topics that include rare presentation of late onset CAS after CABG leading to right ventricular failure, ALCA with an intra-arterial course, as well as its surgical treatment in the setting of existing coronary artery disease (CAD). Individual topics are discussed below.
Coronary artery anomalies are associated with myocardial ischaemia, SCD, and life-threatening arrhythmias. ALCA with intra-arterial course is at higher risk for SCD than other subtypes.2 This correlation has been suggested in those patients under the age of 30. Most studies have excluded patient populations above the age of 30.3 Therefore, there is a knowledge gap in our understanding of the presentation and risks of ALCA in later years of life. Patients with ALCA that present with chest pain later in life may have concurrent CAD similar to our patient. Presenting symptoms are thought to be secondary to either existing obstructive CAD or ALCA anatomy. The challenge of recognising the aetiology of symptoms may expose the patient to unnecessary percutaneous coronary intervention of non-culprit coronary obstructive lesion.
Cardiac stress testing modalities have been inconsistent in evaluating coronary ischaemia and risk of SCD in this subset of population.4 ST-segment changes are often transient and may yield a negative cardiac stress test.5 CAS, in general, may present in a similar fashion. Transient ST-segment changes on ECG and negative cardiac stress imaging in patients with ALCA have previously been illustrated in the literature which is similar to our patient.1
With the presence of concurrent obstructive coronary disease, CABG with ligation of proximal LACA was utilised to alleviate concerns for competitive flow. This technique remains controversial for concerns of reliance on the graft patency and risk of necessity for repeat revascularisation.6 Ligation of proximal ALCA (left main) was possible as the vessel did not share the ostium with native RCA. Given the absence of ALCA intramural segment, other methods such as unroofing and coronary translocation were not necessary.7
Several publications have presented both native coronary artery and graft artery spasm in the early perioperative phase of CABG. Some of these CAS have occurred intraoperative and up to few hours postoperative CABG.5 8 Our search of medical literature indicates that late onset of native CAS after CABG is scarce. Interestingly, a normal dominant RCA has often been the commonly reported spastic artery perioperative to CABG.9 In the case presented, the RCA spasm occurred very late after CABG and intervention of ALCA. Therefore, the aetiology of spasm remains uncertain.
There are multiple gaps existing in current knowledge of anomalous aortic origin of a coronary artery and its ALCA subtype presented in this case report. The CAS may persist in adulthood and persist after CABG. This CAS may occur within a different artery from the anomalous-related anatomy. Additionally, rising CRP and ESR levels may indicate an underlying slow progression of an inflammatory process perioperatively to incite this CAS as an incidental event.
This case is unique, as a collection of rare topics presented in one patient. In conclusion, regardless of aetiology of CAS, related myocardial ischaemia could present without typical chest pain symptoms and with ST-segment elevation at rest.10 Unexplained hypotension may be the only presenting sign. Refractory CAS may respond to combination of intracoronary use of nitroglycerine and verapamil with longer sustained vasodilatory effect. Vasopressors and positive inotropic agents were utilised in combination with nitroglycerine infusion.
Learning points.
The association of an existing anomalous coronary artery anatomy with onset of spontaneous native coronary artery spasm (CAS) postcoronary artery bypass grafting is unknown.
Late perioperative CAS after bypass graft is unusual and presents with atypical features.
Sudden unexplained hypotension may be the only early sign of CAS which can lead to severe myocardial infarction and death. Early recognition is crucial for a better prognosis.
Footnotes
Contributors: HZ conceptualised the design of the manuscript. HZ and RKB both drafted the manuscript and revised the work for important intellectual content. HZ, RKB, and VAM participated in clinical work presented in this manuscript. HZ, RKB, and VAM approved the final version of the paper that has been submitted and are in agreement to be accountable for all aspects of the work.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial, or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Next of kin consent obtained.
References
- 1. Nakazato J, Hirata K, Wake M. Coronary spasm as the cause of myocardial ischaemia in a patient with anomalous origin of the left anterior descending artery from the proximal right coronary artery. Case Reports 2014;2014:bcr2014204408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cheezum MK, Liberthson RR, Shah NR, et al. Anomalous Aortic Origin of a Coronary Artery From the Inappropriate Sinus of Valsalva. J Am Coll Cardiol 2017;69:1592–608. 10.1016/j.jacc.2017.01.031 [DOI] [PubMed] [Google Scholar]
- 3. Poynter JA, Williams WG, McIntyre S, et al. Anomalous aortic origin of a coronary artery: a report from the Congenital Heart Surgeons Society Registry. World J Pediatr Congenit Heart Surg 2014;5:22–30. 10.1177/2150135113516984 [DOI] [PubMed] [Google Scholar]
- 4. Anantha Narayanan M, DeZorzi C, Akinapelli A, et al. Malignant course of anomalous left coronary artery causing sudden cardiac arrest: a case report and review of the literature. Case Rep Cardiol 2015;2015:1–4. 10.1155/2015/806291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Baek JH, Han SS, Lee DH. Native coronary artery and grafted artery spasm just after coronary artery bypass grafting: a case report. J Korean Med Sci 2010;25:641 10.3346/jkms.2010.25.4.641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Robaei D, Manasiev B, Jansz P, et al. Early graft failure complicating coronary artery bypass surgery for anomalous left coronary artery from the right coronary sinus. J Cardiol Cases 2013;7:e171–e172. 10.1016/j.jccase.2013.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mainwaring RD. “Cui periculum”—Who is at risk? J Thorac Cardiovasc Surg 2018;155:322–4. 10.1016/j.jtcvs.2017.10.027 [DOI] [PubMed] [Google Scholar]
- 8. Kowalówka AR, Malinowski M, Onyszczuk M, et al. Coronary artery spasm following on-pump coronary artery bypass grafting with 20 months follow-up. Kardiochir Torakochirurgia Pol 2016;13:361–5. 10.5114/kitp.2016.64883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lemmer JH, Kirsh MM. Coronary artery spasm following coronary artery surgery. Ann Thorac Surg 1988;46:108–15. 10.1016/S0003-4975(10)65869-9 [DOI] [PubMed] [Google Scholar]
- 10. Hung MJ, Hu P, Hung MY. Coronary artery spasm: review and update. Int J Med Sci 2014;11:1161–71. 10.7150/ijms.9623 [DOI] [PMC free article] [PubMed] [Google Scholar]