Abstract
Morphological changes during development, tissue repair, and disease largely rely on coordinated cell movements and are controlled by the tissue environment. Epithelial cell sheets are often subjected to large-scale deformation during tissue formation. The active mechanical environment in which epithelial cells operate have the ability to promote collective oscillations, but how these cellular movements are generated and relate to collective migration remains unclear. Here, combining in vitro experiments and computational modeling, we describe a form of collective oscillations in confined epithelial tissues in which the oscillatory motion is the dominant contribution to the cellular movements. We show that epithelial cells exhibit large-scale coherent oscillations when constrained within micropatterns of varying shapes and sizes and that their period and amplitude are set by the smallest confinement dimension. Using molecular perturbations, we then demonstrate that force transmission at cell-cell junctions and its coupling to cell polarity are pivotal for the generation of these collective movements. We find that the resulting tissue deformations are sufficient to trigger osillatory mechanotransduction of YAP within cells, potentially affecting a wide range of cellular processes.
Significance
In vivo studies have underlined the crucial role played by the collective movements of epithelial cells in physiological or pathological conditions. Well-controlled in vitro systems are required to characterize the complex patterns of motion involved, as was done previously for collective swirls or pulsatile contractions. Here, we find evidence for a, to our knowledge, new mode of collective motion that endows cells in a confined tissue with a sense of its edges: the interplay of geometric confinement and large-scale coordination leads to the emergence of choreographed oscillations of the whole monolayer. This results in regular spatial and temporal patterns of deformations, which translate into molecular signals at the cell level and could thus be relevant to several shape-defining events during embryonic development.
Introduction
The formation of multicellular patterns of moving cells in living tissues is a hallmark of many developmental and pathological processes, including morphogenesis, tissue regeneration, and tumorigenesis (1, 2, 3). The ability of the cells to coordinate their motion over large scales (2, 4) enables the rapid transmission of mechanical information across the tissue, for example, during tissue invagination driven by actomyosin contractions (5), gastrulation (6), the propagation of velocity waves far from the free edge of migrating monolayers (7, 8), and the transmission of contact-guidance signals away from localized topographical cues (9). In addition, more local guidance of cellular clusters can be observed through the emergence of leader cells at the migrating front of epithelial sheets, dragging a small cluster of the followers along (10), or the formation of cryptic protrusions underneath the neighboring cells (11) in the bulk of epithelial tissues. These mechanisms may participate in the establishment of coordinated movements (12). It is now well established that physical constraints play a key role not only at the single-cell level (13, 14, 15) but also in the regulation of collective motion (16, 17, 18, 19, 20, 21), both in vitro and in vivo (22, 23, 24). Such collective behaviors are often accompanied by the emergence of oscillatory mechanisms such as calcium waves (25), actomyosin contractile activity (26), and cyclic activation of signaling pathways (e.g., Notch, Fgf, and Wnt) (27). These oscillatory behaviors occur at various timescales and play a major role in tissue reshaping (28).
Epithelial monolayers of Madin-Darby canine kidney (MDCK) cells in circular confinement have been shown to convert otherwise chaotic flow into one coherent rotation (29). This swirling behavior has been described for several cell types and relies on the interplay of confinement and local alignment, as shown both experimentally and theoretically (30, 31, 32, 33). Careful analysis further revealed regular pulsations in the radial direction that are uncoupled from these large movements (34). These pulsations were further investigated in (35), in which a coupling of the cellular polarization to local gradients of contractility was suggested as a potential mechanism. In anisotropic confinement, a work conducted in parallel to ours showed that the motion along the long axis of the confinement exhibits a transition from global to multinodal standing wave (36). These studies have been performed on a particular epithelial cell type, MDCK cells. However, the mechanical and rheological behaviors of epithelial tissues can exhibit various properties depending on their physiological origin (37, 38), which may lead to the emergence of various coordinated behaviors. Furthermore, the origin of these oscillatory movements remains unclear in terms of local cell polarity (10) versus large-scale communication through cell-cell junctions (17). Here, we describe a, to our knowledge, novel form of coherent oscillations in confined human keratinocytes (HaCaT) and enterocytes (Caco2). This is distinct from the previously reported observations on MDCK cells, which we were consistently able to reproduce in our system: in MDCK cells, the oscillations are radial, and they only constitute a low-amplitude component supplementing the dominant swirling motion; in HaCaT and Caco2 cells, all cells move in the same direction with no central symmetry, and only the collective direction of motion varies in time. These specific geometric properties have unanticipated consequences in anisotropic confinement and thus provide a potentially powerful mechanism for pattern formation. We find that the large-scale oscillations lead to alternating phases of tissue compression and extension that are sufficient to trigger a localized mechanosensitive signaling pathway as determined by the nucleocytoplasmic shuttling of the transcription factor, Yes-associated protein (YAP), which is an important regulator of tissue growth and regeneration and tumorigenesis (39, 40, 41). We demonstrate that the origin of this behavior lies in force transmission at cell-cell junctions, and we present a computational model coupling cell motility to intercellular forces directly, which is able to account in detail for our experimental observations.
Methods
Cell culture and reagents
HaCaT cells, MDCK cells, and CaCo2 cells were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin in an incubator at 37°C with 5% CO2 and humidification. Protein expression in HaCaT cells was depleted by retrovirus-mediated introduction of small hairpin RNA (shRNA) into cells, as described previously (37). The target sequences used were 5′-GACTTAGGAATCCAGTATA-3′ (for α-catenin) and 5′-GTGACC AACTTGTCCTCAA-3′ (for desmoplakin). As a control, shRNA with the nontargeting sequence 5′-ATAGTCACAGACATTAGGT-3′ was used. HaCaT cells stably expressing Life-Actin-GFP and histone2B-GFP were established by transfection of pCAG-mGFP-Actin plasmid (42) (gift from Ryohei Yasuda, Addgene plasmid # 21948; Watertown, MA) and pRRlsinPGK-H2BGFP-WPRE (gift from Beverly Torok-Storb, Addgene plasmid # 91788), respectively. For visualization of human YAP1 in live cells, wild-type HaCaT cells were transiently transfected with the pEGFP-C3-hYAP1 plasmid (43) (gift from M. Sudol, Addgene plasmid # 17843). For immunofluorescence, wild-type HaCaT cells were fixed with formaldehyde 4%, then labeled with an anti-human-YAP1 antibody (sc-101199; Santa Cruz Biotechnologies, Dallas, TX) or phalloidin-Alexa 568 (Life Technologies, Paisley, UK). For inhibition of contractility and actin polymerization experiments, drugs (blebbistatin 50 μM and CK-666 100 μM, respectively) were added at the beginning of the experiment, approximately 12–18 h after seeding the cells on the patterns.
Microcontact printing
Functionalization of substrates was done using microcontact printing techniques. Master silicon molds containing the patterns were made using SU-8 photoresist resin and were exposed for 2 h to silane gas before being used. Stamps for patterning were made using soft lithography techniques. Polydimethylsyloxane (PDMS; Sylgard 184; Dow Corning, Midland, MI) was mixed with curing agent at a ratio of 1:10, degassed, and poured over the wafer. PDMS was cured for 2 h at 80°C and removed from the mold before being cut. Plastic petri dishes and glass-bottomed petri dishes were first coated with a thin layer of PDMS before printing. A drop of 1:10 mixed and degassed PDMS was put on the center of the substrate. A spin-coater with a first cycle of 18 s at 500 rpm and a second cycle of 1 min at 5000 rpm was used to spread the PDMS over the substrate. The printing of the fibronectin was performed as previously described (44). Briefly, PDMS stamps were incubated for 45 min with a 50 μg/mL fibronectin solution partly conjugated with a Cy3 or Cy5 dye to control the quality of the transfer. The substrates were isolated for 10 min in an ultraviolet-ozone machine before printing. Stamps were rapidly soaked 1 s in distilled water and completely air dried before being applied for 1 min on the substrate. PDMS stamps can be sonicated in ethanol and washed in milli-Q water before being reused. The nonprinted surface of the substrate was passivated with a 2% pluronics F-127 solution for 2 h. After washing the substrate several times with phosphate-buffered saline, cells were seeded on the substrate at high concentration and washed after 10–15 min. The substrate was placed in the incubator overnight to let the cells spread and imaged the next morning. The patterns were chosen such that the cells were almost confluent at the beginning of the experiments.
Traction force microscopy
The substrates were prepared as described in a previous reference (37). Soft silicon substrates were made by mixing CyA and CyB at a ratio of 1:1 and directly poured on glass-bottomed petri dishes (FluoroDish, World Precision Instruments, Sarasota, FL) to obtain a 100-μm-thick layer. The substrate was cured at room temperature overnight on a flat surface. The surface was silanized using a solution of (3-aminopropyl)-triethoxysilane at 5% in absolute ethanol for 5 min. The substrate was then washed with absolute ethanol and dried at 80°C for 10 min. 200 nm carboxylated fluorescent beads (Invitrogen, Carlsbad, CA) were added in a deionized water solution at 1:500 for 5 min, washed with deionized water, and dried at 80°C for 10 min. For these substrates, we used a modified microcontact printing technique (23). The PDMS stamps were incubated with a fibronectin solution the same way as for the patterning on the PDMS. After being dried, the stamps were used to print a polyvinyl alcohol membrane. The printed piece of membrane was then deposited on the soft substrate for 30 min and then dissolved with 2% F-127 pluronics for 2 h. The cells were seeded at high concentration (1 million per petri dish) for 20–30 min and washed with phosphate-buffered saline when the desired concentration was reached. At the end of the experiment, cells were removed with 500 μL of 10% sodium dodecyl sulfate in the media. A stress-free image of the substrate was taken to be compared to the other images.
Live-cell imaging and analysis
Live imaging was performed with a 10× objective on a BioStation IM-Q (Nikon, Tokyo, Japan) at 37°C and 5% CO2 with humidification. One image was taken every 4 min for the experiments that did not need fluorescence. For the traction force microscopy (TFM) experiments, a phase-contrast image and a z-stack of the fluorescent beads were taken every 10 min. Images were first processed with ImageJ to obtain the best focus plane for each time point (Stack Focuser plugin) and stabilized (Image Stabilizer plugin), and background beads were removed. To analyze cellular movements, velocity fields were calculated by PIV analysis with MATPIV 1.6.1, a MATLAB (The MathWorks, Natick, MA) implemented script. An interrogation window of 32 (20.7 μm) or 64 pixels (41.4 μm) was selected for 4 or 10 min per frame respectively, with an overlap of 50–75%. Vectors higher than a speed threshold manually determined were removed, and a local median filter was applied. Gradient of the velocity field was calculated with a method adapted from (45). Briefly, based on the Green-Ostrogradsky theorem, we integrated the velocity vectors on a ring of radius 37.5 μm. This method has the advantage of reflecting the movement at the tissue scale more than local fluctuations and is less noise sensitive. We calculated the strain-rate tensor from the gradient of the velocity field as . Correlation length was calculated as previously described (46). Briefly, at a given time t, the (two-dimensional) spatial velocity autocorrelation function was computed in the Fourier space, then averaged azimuthally. The correlation length ξ(t) was obtained from a fit with a stretched exponential function C(Δr) = exp(−(Δr/ξ)v). In the text, we quote the range of typical values taken by this function in an unconstrained monolayer (HaCaT cells) or in a 500 μm square (Caco2 cells). For TFM experiments, the substrate displacements also were calculated with MATPIV 1.6.1. Interrogation windows were set to 24 pixels (15.5 μm) and 75% overlap. A median filter was applied to the displacement vector field. Traction force fields were computed with an FTTC plugin on ImageJ (47) with a regularisation factor set to 1 × 10−10. The stress within the tissue was calculated using Bayesian inversion stress microscopy (48) with a regularization parameter Λ = 10−6. For quantifications of the YAP nucleocytoplasmic (N/C) ratio (YAP-NCR), we proceeded as follows: first, the cytoplasm and the nucleus regions were manually defined in ImageJ, and the mean fluorescence intensity in the nucleus (IN) and in the cytoplasm (IC) were measured. The YAP-NCR was then defined as YAP-NCR = IN/IC.
Statistics
Differences between data were assessed using two-sample Kolmogorov-Smirnov tests implemented in MATLAB. On the plots, n.s., not significant, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
Computational model
We consider a two-dimensional tissue and describe each cell i independently by a phase field ϕi, where indicates the interior of the cell and its exterior. Each cell is described implicitly, and its boundary is defined to lie at the midpoint ϕi = 1/2. The phase fields satisfy an overdamped dynamics given by
| (1) |
where is the speed of the cell i, F is the free energy given below, α is the strength of the motility, ξ is a friction coefficient, and is the total force acting on one cell’s surface (including steric and viscous interactions, see below). The sole source of activity in the model is provided by α, which induces a propulsion in the direction of the polarity pi. For simplicity, we fix pi = (cosθi, sinθi) and define the following alignment dynamics for the angle
| (2) |
where Δθi ∈ [−π, π] is the angle between pi and and ηi is a Gaussian white noise. The angle θi is treated as an independent internal degree of freedom for each cell and denotes the direction at which the unit vector pi is pointing. Note that ϕi is not conserved because we require the cells to be compressible.
The role of the total free energy F is both to maintain the cell integrity as well as to define interactions between cells. It can be decomposed as follows: a Ginzburg-Landau free-energy term responsible for the stabilization of the diffuse interfaces, a quadratic soft constraint enforcing area conservation, and finally, two terms giving rise to repulsion forces between cells and with the confining walls. Following (49), these contributions are defined as
and we refer the reader to this reference for details. Note that because we only consider confluent tissues, we do not introduce an adhesive force between cells. This is justified because an imbalance of repulsion forces has the same effect as an adhesion force in the force-balance condition. Boundary conditions are implemented through the contribution Fwalls as a repulsion between a fixed phase field ϕw and the phase fields ϕi. An alternative approach that allows for a direct relation between cell-cell and cell-wall potential has been proposed by Marth and Voigt (50). The different parameters of the model are the following: cell stiffness (γ and ξ), compressibility (μ), repulsion strength (κ and κw), polarity dynamics (J and D), and activity (α). Within this model, the effects of microscopic contractility are accounted for in two emergent properties of the cells: first, the impact of the contractility on the movement of the cell is modeled by the active traction force that drives the cell motion; second, the effect of the contractility on increasing the cortical tension is accounted for by the cell elasticity (parameter γ in the model, which effectively minimizes deviations from a circular shape of the cell). Note that the interface width λ sets the basic length scale and is hence not considered as a parameter.
Total force at the cell-cell interface
We write the total force experienced by a cell as , where the steric, viscous, and wall forces are defined as
where is a direction parallel to the wall and μc and μw are dry-friction coefficients of the cells with other cells and the wall. Note that only cell interfaces (where ) contribute to the integrals. This form of the steric force is not unique but is well-motivated thermodynamically (49, 51). Note that the steric force is normal to the interface, whereas the viscous friction force is parallel to it. In practice, these two forces correspond to two different cellular mechanisms: the gradient of tension at cell-cell contacts and the forces exerted on focal adhesions during frictional cell movements.
Simulation details
We simulated Eq. 1 using a finite difference scheme on a square lattice with a predictor-corrector step. Throughout this article, we used the following numerical values for the simulation parameters: λ = R = 8, γ = 0.05, μ = 0.03, κ = 0.4, ξ = 1, α = 0.1. The phase field representing the walls is set to have an exponentially falling profile ϕw = exp(−2d), where d is the distance to the closest wall and κw = 2. We simulated square domains of edge length W = 50, 75, 100, 125, 150 lattice sites with N = 15, 34, 60, 94, 135 cells and set μw = 0 with D = 0.01, J = 0.03, μc = 0.05, and μw = 0. We simulated rectangular domains of length L = 600 and width W = 50, 75, 100, 125, 150 lattice sites with N = 180, 270, 360, 450, 540, 630, 720 cells. For both square and rectangular domains, we choose the ROI to be of the same shape as the domain but downscaled by a factor of 0.7 and centered. We introduced friction with the walls μw = 0.2 and set D = 0.2, J = 0.5, and μc = 0.5 to shorten the computation time. We have made sure that using these parameters in the square domains did not significantly affect our analysis. In particular, we also observe sustained oscillations in square confinement in this case.
Results
Confined human keratinocytes exhibit coordinated oscillations
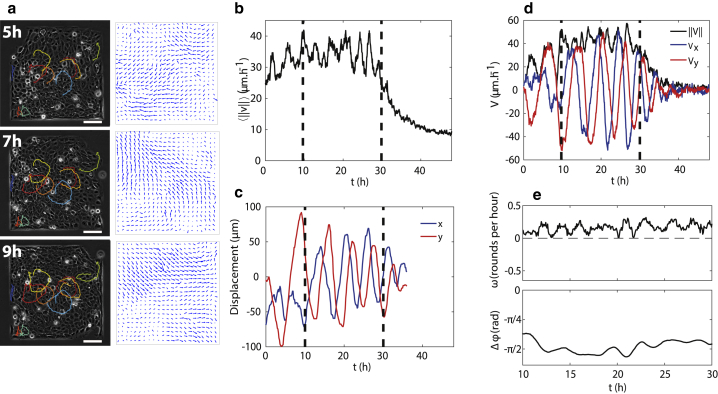
Human keratinocytes (HaCaT cells) were deposited on microcontact-printed square areas of fibronectin of size 500 × 500 μm surrounded by a nonadherent surface (see Methods). Cell displacement and velocity fields were analyzed using time-lapse microscopy and particle-image velocimetry (PIV) (Fig. 1 a). Cells were initially sparse but progressively expanded within the square through cell proliferation with a doubling time of about 24 h until the tissue occupied all the available space (confluence stage). The corresponding velocity magnitude averaged over the whole domain initially increased over time, then plateaued for almost 20 h, finally dropping to a low value (Fig. 1 b) in a way reminiscent of a “freezing” effect due to crowding (52, 53).
Figure 1.
Coordinated oscillations of a confined epithelium. (a) Left column shows snapshots of a confluent HaCaT layer on a square pattern at various times and representative trajectories of single cells within the pattern. Right shows velocity fields from PIV measurement at the corresponding times. The scale bar represents 100 μm. (b) Temporal evolution of the average velocity magnitude. The velocity increases slowly at first and then plateaus for almost 20 h until it finally decreases. Vertical lines denote the period of time chosen to best highlight the oscillations. (c) Displacement of a single cell in the center of the domain. (d) Evolution of the two projected components— and computed on a cropped area in the center of the square—and the norm of the velocity . (e) Angular velocity of the average direction of the velocity (top) and phase shift between Vx and Vy (bottom) within the time interval shown in (b)–(d). To see this figure in color, go online.
We then focused on the plateau stage, at which the cells were tightly packed but the movements still large. Individual cells moved along parallel elliptical trajectories with little net relative motion of cells with respect to each other, and no overall rotation of the tissue was observed (Figs. 1, a and c and S2). This led to the emergence of layer-scale coordinated movements in which all the cells moved together in a direction that rotated slowly with time (see Fig. 1 a; Video S1). As the cells moved collectively, they pushed each other against the domain boundary, leading to a decrease of their two-dimensional effective area and to a local increase of the density. The oscillatory nature of these collective movements was best revealed by considering the total velocity averaged over a region of interest (ROI) of 50 × 50 μm at the center of the pattern. Although the magnitude was almost constant and nonzero, the individual components and showed clear oscillations (Fig. 1 d) with a phase shift of π/2 (Fig. 1 e bottom), indicating that rotated at constant magnitude inside the ROI. The angular velocity of this rotation had a mean value of about 1 rad h−1, which translates to an oscillation period of ∼6 h. There was a slow increase of the frequency with time (Fig. 1 e top), which is likely due to a slow increase of the cell density. At later times, these movements gradually disappear (Fig. 1 d), although residual flows are still observed (see Fig. 1 b). Note that because the motion of cells is coordinated over the domain during the oscillation phase, the analysis was robust to changes in the size of the ROI (Fig. S1). We finally checked that the observed behavior also appeared in other epithelial cell types by repeating our experiments with Caco2 human intestinal cells and indeed observed marked oscillations in squares of 200 μm (Fig. S3).
A few cells have been tracked manually. Scale bar 100 μm.
The geometry of confinement controls the properties of the oscillations
We then studied how changing the geometry of confinement affected the properties of the oscillations by depositing HaCaT cells in squares of sizes 100, 200, 500, and 1000 μm. We characterized these changes by measuring the mean amplitude and period of the oscillations in and . The period was found to grow linearly with the domain size (Figs. 2 b and S4). Because the magnitude of is approximately constant across the center of the domain, this suggests that the reorientation cues come from the confinement boundaries, where the cells are most deformed (Fig. S2). The amplitude of the oscillations was found to be constant on a broad range of domain sizes but was reduced in smaller domains (Figs. 2 a and S4). We hypothesized that this reduction appeared for confinement sizes much smaller than the intrinsic length scale of the collective flows spontaneously generated by the epithelial cells. This was confirmed by measuring the velocity-velocity correlation length in unconstrained monolayers, which we found to be 600–1000 μm (Fig. S5). This suggests that there is a subtle interplay between the size of the confinement and the natural correlation length of the cells within a tissue: for the oscillations to appear, these two lengths must be of the same order for the confinement boundaries to provide restoring cues, allowing the direction of motion to slowly rotate. When the confinement size is much larger than the correlation length, those cues cannot be transmitted over the entire tissue, preventing the emergence of monolayer-scale movement. Conversely, when the domain is too small, large-scale collective flows of cells are screened. This is further seen in experiments using Caco2 cells: with a measured correlation length of 70 μm, oscillations appear in 200 μm but not in 500 μm squares.
Figure 2.
Geometry-dependent coordinated oscillations. (a and b) Amplitude A and period T of the oscillations as a function of the size of the square confinement. (c) A snapshot of rectangular confinement of size L × W = 3500 × 500 μm is shown. The frame represents the ROI used in (d), (e), and (h). The scale bar represents 200 μm. (d) A spatiotemporal map of ; velocity along the long axis x shows patches of correlated motion with a coherence size of about 500 μm. The velocity component Vx was averaged along the y direction (short axis) in the area outlined in orange in (c) and then plotted against time t and x (the long axis of the frame). (e) A spatiotemporal map of , velocity along the short axis, averaged over y and plotted against t and x, is given. (f and g) Amplitude A and period T of the oscillations in squares and rectangles as a function of the smallest dimension of the confinements. (h) Phase coherence length Lϕ as a function of the width of the rectangular pattern. Lϕ is defined as the distance over which Vx(x, t) reverses direction. All plots, mean (SD) from experiments. n.s., not significant, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 from a two-sample Kolmogorov-Smirnov test. To see this figure in color, go online.
To investigate this interplay further, we next considered anisotropic confinement and introduced high aspect ratio rectangular domains of width W = 200, 400, and 500 μm and length L = 2000–3000 μm (Fig. 2 c). Similar to square confinements, the cells exhibited large patches of collective motion. We then measured and over a rectangular ROI (shown in Fig. 2 c), averaging over the small dimension of the rectangle. The velocity component in the short direction, , showed long-range coordination along the whole x axis and oscillated between positive and negative values at each fixed position (Fig. 2 e). In contrast, the velocity component along the long axis, , was not correlated over the whole x axis but rather formed alternating patches of coordinated motion (Fig. 2 d). Such a velocity pattern corresponds to the cells moving in the rectangle in a fashion reminiscent of a standing wave along the short dimension of the channel W and a mix of multinodal standing wave and traveling wave along the long dimension of the confinement (Video S2).
Scale bar 100 μm.
Most strikingly, the properties of this motion were set by the width W of the confinement while being apparently independent of the length (36): the period and amplitude of the oscillations closely matched those measured in squares of size W (Fig. 2, f and g), and the typical size of the horizontal coordinated patches—as measured by the phase coherence length in Fig. 2 h—matched closely with W as well. Even a migrating cell sheet with a free edge, hence confined in a single dimension, exhibits the same kind of behavior, with clear coordinated oscillations in the direction of confinement and alternation of forward and backward moving zones in the perpendicular direction (Fig. S3). This suggests that the period and amplitude of the oscillations are in fact independent of the exact shape of the confinement and only depend on the smallest confinement size. As a test of this hypothesis, we used circular confinement patterns instead of squares and recovered the oscillating behavior with the same size-dependent properties (see Fig. S3). This implies that the smallest geometric constraint acting on the system is able to impose a specific length scale to the tissue and selects associated patterns of motion. This pattern-selection property might be of particular importance during development, which involves several shape formation and segmentation steps and during which collective oscillations have been shown to be crucial (54, 55, 56, 57, 58, 59).
Epithelial oscillations lead to density fluctuations and mechanotransduction
Next, we examined the impact of the oscillations on cell-density fluctuations by measuring local densities. Imaging histone-GFP HaCaT cells (Fig. 3 a) showed that the coordinated movements created large inhomogeneities in cell-density distribution as shown by density maps (Fig. 3 b). The relative density fluctuation magnitude Δρ/ρ = 18 ± 2% was consistent with the displacement-confinement relationship (where w is the characteristic cell size and Δx is the displacement amplitude; see Fig. S2). To further assess the spatiotemporal fluctuations of cell densities, we computed the divergence of the velocity field from the PIV analysis (Fig. 3 c). Positive divergence indicates local expansion of the tissue, whereas negative divergence indicates local contraction. Interestingly, we observed that the oscillations were accompanied by local density variations and created alternating phases of compression and extension at any given point of the tissue.
Figure 3.
Oscillation-induced cell contractions lead to YAP translocation and actin reorganization. (a) Example cells expressing histone-GFP followed in time by live imaging. Scale bar is 100 μm. (b) Corresponding cell-density map computed by counting the nuclei in 128 × 128 pixels windows overlapping by 87.5%. (c) A spatiotemporal kymograph of the divergence of the velocity in a square of width W = 500 μm, showing alternating phases of compression and expansion. (d) Immunostaining of YAP on a fixed sample of confined HaCaT cells with zoomed areas (marked with a square) illustrating cytoplasmic (left) and nuclear (right) YAP localization. (e) Temporal evolution of YAP-NCR (squares) and cell area (circles) for an example cell. The YAP-NCR was defined as the mean YAP-GFP fluorescence intensity in the nucleus divided by the mean YAP-GFP fluorescence intensity in the cytoplasm. The event of spreading at t ∼ 300 min corresponds to a sudden increase in YAP-NCR. (f) Time cross correlation of the single cell area and YAP-NCR, averaged over the cell population in three independent experiments. Standard error of the mean (SEM) is for n = 15 cells. (g) YAP-NCR as a function of the cell area in fixed samples of confined epithelia. (h) Confocal picture of actin staining in the basal plane of fixed HaCaT cells confined on a square of width W = 500 μm, overlaid with the local cell density. The scale bar represents 100 μm. Right displays zoomed areas showing different organizations of actin fibers with respect to the local density (1,3, low density; 2,4, high density). The scale bar represents 20 μm. The four pictures have the same scale and contrast. To see this figure in color, go online.
To investigate the potential mechanotransductive effects of such fluctuations on biomechanical signals, we analyzed the intracellular distribution of the transcription factor YAP, which is known to respond to compression (40, 60). Various mechanical factors have been shown to convert phosphorylated YAP (retained in the cytoplasm, where it is inactive) into its nonphosphorylated form (transcriptionally active and localized in the nucleus) (39, 40, 61). We transiently transfected YAP-GFP into HaCaT cells and then deposited them on 500 μm square patterns in a 1:10 mixture with nontransfected cells and observed a high activation of YAP in regions of low densities, whereas YAP appeared inactive in dense regions (Fig. 3 d bottom). We quantified the YAP-NCR with respect to cell area of individual cells and observed significant fluctuations of cell area, which were followed closely by variations in the YAP-NCR (Fig. 3 e; Video S3). The temporal cross correlation of both signals showed a maximum at Δt = 0 (Fig. 3 f), clearly indicating the direct relationship between cell area and YAP nuclear localization in our system. We could not determine a delay between the two signals, which could be due to the very fast, mechanically induced entry of YAP into the nucleus upon cell deformation (62). We then considered wild-type cells in the oscillatory phase and imaged YAP by immunostaining. This allowed us to observe the large inhomogeneities in cell density created by the oscillations at a given time (Fig. 3 d top) and to confirm the strong correlation between cell spreading and YAP nuclear translocation (Fig. 3 g). Taken together, these results show that alternating phases of extension and contraction resulting from epithelial oscillations induce a strong and spatially resolved regulation of YAP localization. Conversely, the inhibition of YAP transcriptional activity using verteporfin did not affect the oscillations (Fig. S6). It suggests that YAP translocation is only a molecular readout of the oscillations and might not retroact on them.
Scale bar 10 μm.
Those changes in local cell density were also accompanied by out-of-plane deformations: squeezed cells in high-density areas were taller, whereas at low density, the cells were flatter (Fig. S7). Finally, those variations of density and cell shape were also correlated to changes in the cytoskeleton organization: in the low-density areas, cells formed parallel, cell-scale actin fibers suggesting high tensional state, whereas in more compressed zones, F-actin was restrained to the cell cortex (Fig. 3 h). Hence, the mechanical state and active force production of the tissue were sensitive to the fluctuations in cell density, a clue of a potential feedback from and on cellular movements, which is necessary for the maintenance of the oscillations.
Local cell-cell forces, motion, and polarity are coupled to the collective dynamics
To gain further insights into the dynamics of the oscillations, we then used traction force microscopy (63, 64) to measure the forces exerted on the substrate by the cells. We observed that in our experiments, the traction forces were heterogeneous within the tissue, except at the edges of the pattern, where they were consistently large and directed inwards (Fig. 4 a). Intermittent patches of high force were observed as a result of lamellipodia transiently pulling on the substrate. This is consistent with a recent study of cell traction forces (65), which showed that above a critical length (four to five cells) the cells cannot contract as a unit, and intermittent patches of high force are expected. Combining traction force microscopy with Bayesian inference stress microscopy (48), we next measured the pressure P = −Tr(σ)/2 within the tissue and found it to be under homogeneous tension, except for a thin edge layer where the tension drops to zero (Fig. 4 b). Actin staining further showed that the tissue tension is supported by the actin cytoskeleton and transmitted across cell-cell junctions through small actin digitations (Fig. 4 c). As opposed to other types of cell-cell contacts in flat epithelia that are associated with parallel oriented F-actin bundles, radially oriented F-actin bundles are reminiscent of high tension at cell-cell contacts as previously described (66).
Figure 4.
Traction force and cell polarity are coupled to cell motion. (a) A map of the magnitude of the traction forces. The scale bar represents 100 μm. (b) A map of the pressure P = −Tr(σ)/2 within the confined tissue. The scale bar represents 100 μm. (c) Confocal images of actin staining in fixed HaCaT cells fixed during the oscillation phase. The arrows denote cell-cell junctions under tension. The scale bar represents 20 μm (same scale for three pictures). (d) The averages of vx and −Tx in a central square ROI, projected on the x axis, oscillate with similar period but are out of phase. (e) Normalized cross correlation between v and −T as a function of the time lag Δt. The correlation has a marked peak at Δt = 0.5 h for both W = 200 and 500 μm. Mean (SD) is of n = 11 and 8, respectively. (f) Top shows a schematic of the cell polarity: the orientation of the lamellipodium (red) with respect to the cell body (turquoise) defines the polarity (vector p), which can be in a different direction from the velocity v. The traction force T is a linear combination of the two. Bottom shows an example of a reorientation event (fluorescent cell embedded in a nonfluorescent confluent monolayer). F-actin in the basal plane (z = 0, red) and top of the cell (z = +7 and +8 μm, green and blue), visualized by confocal microscopy. The spatially shifted basal actin shows the protrusion orientation, used to define the cell polarity and denoted by a white arrow. The polarity, initially directed toward the bottom-left corner, realigns in the direction of motion (toward the upper-right corner) over time. The scale bar represents 20 μm.
It was recently argued (35) that the traction force and the velocity are not necessarily aligned. Indeed, we observed that the angle between these two directions followed a flat distribution when averaged over the whole experimental time (Fig. S10). However, at fixed times and averaging only over a centered ROI, we found that the traction forces and the velocity are correlated: traction forces follow velocities with a well-defined delay (Fig. 4 d). The cross correlation of the two signals indeed showed a clear peak at Δt = −0.5 ± 0.1 h both for 200 and 500 μm squares (Fig. 4 e). This suggests that cells adapt their motion to the surrounding tissue by polarizing in the direction where they are pulled by their neighbors, with a typical realignment time of the order of 30 min. Therefore, in contrast to the conclusions presented in (35), we find that the traction forces and the local velocity were not simply uncorrelated but rather showed alternating phases in which the traction force was aligned to the motion of the cells and phases in which it was pointing in the opposite direction.
We could visualize this phenomenon directly by mixing wild-type cells with a small proportion of HaCaT expressing lifeact-GFP and imaging them using a confocal spinning disk microscope. This allowed us to see clearly the lamellipodia created at the basal side of the marked cells and to observe several reorientation events in which a cell was pulled by the surrounding tissue in the direction opposite to its lamellipodium and then reoriented its polarity so as to align it with its direction of motion (Fig. 4 f).
Contributions of cellular-level mechanisms to the oscillations
Next, we asked which mechanisms at the cell level are able to modulate the oscillations. We began by studying the role of cell motility through drug inhibition of cytoskeletal activities. To this end, actomyosin contractility was targeted by the inhibition of myosin-II processivity using blebbistatin, and protrusion formation was suppressed by the inhibition of Arp2/3-mediated branched actin nucleation using CK-666.
Upon addition of blebbistatin at high concentration (50 μM), the cell speed fell rapidly to a very low value (Fig. S8). The oscillations, however, survived albeit with larger period and smaller amplitude (Fig. 5, a and b). The approximately constant product of period × amplitude ≈ 80 μm suggests that only the migration speed was affected by the drug. This implies that contrary to what was reported for the “breathing” oscillations (35), actomyosin contractility is not directly responsible for the observed oscillations even though it is necessary to drive the system out of equilibrium. Upon addition of CK-666 (100 μM), the speed first slightly dropped and then gradually decayed over more than 30 h (Fig. S8). Although none of the oscillations’ properties was affected immediately after the drug addition, the long-term slowdown was accompanied by a decrease of the total velocity and a fading out of the oscillatory behavior (Fig. S8). This is not surprising because the protrusive activity is necessary for the cells to direct their motion, so its suppression is expected to result in the loss of the directed collective motion of the cells. Finally, inhibition of Rac1 using the Z62954982 inhibitor led to a decrease of both the cell speed and the velocity coordination (Fig. S9). Together, these findings show that the most important contribution of cytoskeletal activities—contractility and protrusion formation—in the oscillations is just to fuel the cell motion itself. However, protrusion formation may have a more specific role in the coordination of cell velocities because it defines a particular direction of motion.
Figure 5.
Genetic and molecular perturbation experiments. (a and b) Amplitude A and period T of the oscillations in 500-μm-sized squares, for control cells and with blebbistatin at 50 μM and CK-666 at 100 μM. Mean (SD) is from n = 5 patterns. (c) A pressure map in α-catenin knocked-down cells. The scale bar represents 100 μm. (d–f) Kymographs of for wild-type (d), desmoplakin knocked-down (e), and α-catenin knocked-down (f) cells. n.s., not significant, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 from a two-sample Kolmogorov-Smirnov test. To see this figure in color, go online.
We then tested the contribution of cell-cell junctions to the oscillations by disturbing them using gene silencing via stably expressed shRNAs, targeting either desmoplakin or α-catenin. Desmoplakin is a critical component of desmosomes, which are cell-cell adhesion structures linked to the intermediate filament cytoskeleton (67, 68), whereas α-catenin is involved in the cadherin-actin linkage at the adherens junctions, where it is known to stabilize the junctions and participate in mechanochemical signal transduction (69, 70). Desmoplakin knocked-down HaCaT cells displayed oscillatory motion with period similar to that of control cells, but the velocity field showed more marked fluctuations and the amplitude of the movement was slightly decreased (Fig. 5, d and e). More strikingly, α-catenin knocked-down HaCaT cells exhibited completely disordered movements: cells were able to slip past each other as shown by the low value of the correlation length around 30 μm, which resulted in random trajectories and no measurable oscillation (Fig. 5 f; Video S4). This indicates that force transmission at adherens junctions is essential for the oscillations to emerge.
A few cells have been tracked manually. Scale bar 100 μm.
Disrupting the force transmission at the cell-cell junctions also impacted the internal stress σ of the tissue. In the wild-type cells, the pressure P = −Tr(σ)/2 is negative and homogeneous except in a thin edge layer (Fig. 4 b), showing that the tissue is always under tension. In α-catenin knocked-down HaCaT cells, P is still negative but three to four times lower on average, and its spatial distribution showed marked inhomogeneities (Fig. 5 c). This indicates that force transmission is strongly affected and supports the idea that the force transmission across cell-cell junctions is a critical factor in the appearance of oscillations.
A simple coupling between the interface forces and the cell polarity reproduces the experimental results in a generic model
We now show that a computational model that couples mechanical forces at the cell-cell interfaces to the individual cell polarity is capable of reproducing our experimental results. We describe individual cells as deformable and compressible active particles using a phase-field approach (49, 71) that models the interface of the individual cells explicitly and incorporates cell compressibility and intercellular forces directly (see Methods). Besides its deformable interface, each cell i has a velocity vi and is associated with a vector pi describing its polarity and defining the direction of the propulsive thrust it exerts on the substrate (72). We consider overdamped dynamics and write the force balance condition , where ξ is a friction coefficient, α is the strength of the motility, and is the total force acting on one cell’s boundary that has contributions from steric repulsion and friction with both neighboring cells and the walls; see Methods.
We now specify the dynamics of the polarity following our experimental observation that the cell velocity is correlated with the traction force with a well-defined delay. For simplicity, we fix pi = (cosθi, sinθi) and define the following alignment dynamics for the angle
| (3) |
where Δθi ∈ [−π, π] is the angle between pi and and ηi is a Gaussian white noise. The positive constants J and D are the strength of the alignment torque and the rotational diffusivity, respectively. Equation 2 describes the diffusive alignment of the polarity of the cells to the total force on their interfaces, and we show below that it induces the correct correlation of the velocity with the traction force. This formulation differs strongly from the model of Notbohm et al. (35), which crucially relies on prescribing an additional reaction-advection equation with a predefined relaxation time for an unknown chemical (such as phosphorylated myosins) controlling the strength of the isotropic active stress, resulting in an indirect coupling of the polarity direction to changes of local cell area. In contrast, our model does not require the introduction of an internal chemical and couples the polarity directly to the resultant pushing-pulling and tangential frictional forces exerted on the surface of the cell.
Simulations of confluent monolayers in square confinement show the emergence of sustained oscillations (Fig. 6, a and b; Video S5). As the cells move toward one of the edges, they start to compress each other, resulting in an increase of the steric repulsion force in the direction opposite to their motion, which then induces the reorientation of their polarity. We can pinpoint the crucial importance of the alignment mechanism in the generation of the oscillations by considering changes in the period of the oscillations as the coupling J is varied: for fixed box size, the period T scales as J−1 and diverges as J vanishes, showing that the oscillations effectively disappear when the alignment mechanism is absent (Fig. 6 d). This reflects our α-catenin knockdown experiments, where we found that force transmission through cell-cell junctions is crucial to the tissue oscillations Moreover, we finally check that our dynamics for the polarity induces the correct alignment between the velocity and the traction force. Indeed, the cross correlation between the cell velocity vi and the cell traction force −Ti = −αpi + ξvi = Ftot. shows a clear peak located at a nonzero time difference and whose location is independent of the box size W; see Fig. 6 f. This indicates that similarly to our experimental observations, the velocity follows the traction force with a well-defined delay in the simulations.
Figure 6.
Computational model of collective cell motion. (a) Snapshots of simulations showing an oscillating tissue at different times. The left-hand panels show the individual cells, where darker cells are more compressed, and the right-hand panels show the corresponding velocity field. (b) Velocity projected on the x and y axes for the same system. (c and d) Dependence of the period T on the system sizes W and alignment parameter J; see Eq. 2. The gray dashed lines are least-square fits of T ∝ W and T ∝ J−1. (e) Dependence of the amplitude over the active speed A/α for different system sizes W. (f) Cross correlation between v and −T for two system sizes. Mean (SD) is from n = 5 simulations. (g) A kymograph of averaged over the short direction for a rectangular box of size 600 × 100 with nonzero friction at the walls. (h) The period of the standing waves in rectangles compared to the period of oscillation in squares of size identical to the small dimension of the rectangle. The period of oscillation in a square of size 600 × 600, corresponding to the large dimension of the rectangles, is shown for comparison. Mean (SD) is from simulations. To see this figure in color, go online.
In agreement with the experimental results, the period of oscillations grows linearly with the system size, whereas the amplitude grows at first and then reaches a plateau (Fig. 6 e). The linear dependence of the period occurs because the speed of propagation of the velocity oscillations does not depend on the system size. Moreover, the amplitude is reduced in boxes smaller than the correlation length because the migration speed is decreased by the proximity of the walls, which hinders the motion of the cells. The amplitude of displacement also depends linearly on the system size (Fig. S11). Similarly, simulations of cells in rectangular confinements reproduce the experimental observation of standing waves along the short dimension of the channel (Fig. 6 g). The period of these oscillations is dictated by the smallest dimensions, as in the experiments, and corresponds to the period obtained in squares of similar dimension (Fig. 6 h). In conclusion, our model provides additional evidence that the interplay between force transmission at cell-cell interfaces and the reorientation of the cells lies at the origin of the oscillations.
It is important to note that the oscillations observed here should be contrasted to the oscillations of active particles with inelastic collisions (73, 74). The oscillations in the works by Grossman et al. (73), and Alaimo et al. (74) are observed only in anisotropic confinements with large aspect ratios (within ellipses) and have different dynamics: first, all particles align together, start moving as a coherent unit along the elongation direction, and upon collision of the coherent unit, with one of the boundaries the entire unit reflects back along the opposite direction. There is no oscillation reported by Grossman et al. in isotropic confinement, in which only coherent rotation is observed. Similarly, in the work of Alaimo et al., the initial, short-lived, oscillations are followed by a coherent circulation as soon as particles align as a coherent rotating unit in circular confinement. In contrast, the oscillations reported in our work are persistent in isotropic confinements. Moreover, a closer look at the trajectories of the cells (Fig. 1 a) reveals striking differences between the oscillations observed here and the ones reported in the aforementioned works: the collective motion observed here 1) shows the biggest displacement for cells at the center of the domain and 2) leads to centrifugal motion that creates local compression of the cells against the walls. It is worth noting that the observed oscillations in space can be fully characterized by a slow rotation of the velocity field of the cells, which we further characterize in the study.
The other important point to note here is the relation between the cell polarity and forces acting on the cells. Although previous studies, e.g., Notbohm et al. (35), reported no statistically significant alignment between the polarity and the force, we do observe such an alignment in our experiments and indeed find that it is necessary for the emergence of the waves. This conclusion is supported both by our molecular perturbation experiments and by our theoretical model, the dynamics of which explicitly incorporates such alignment and is able to capture the experimental results closely.
Discussion
In this work, we have provided evidence for a system-wide, coherent collective oscillations of epithelial cells under confinement. In isotropic geometries, oscillations that are coordinated over the whole confining domain emerge spontaneously. A striking feature of this phenomenon is that it does not rely on the phase synchronization of individual oscillators—as in most biological collective oscillations—but is an emergent property of the group. We identified a coupling of the cell polarity to the forces transmitted through adherens junctions as a key ingredient. Determining the molecular mechanisms underlying this coupling is an open challenge for future research: the polarity could reorient after signals induced by forces exerted on the cell-cell junctions, in a manner reminiscent of contact inhibition of locomotion (75). In contrast to previous studies (7, 17), we show that large-scale force transmission through cell-cell junctions is coupled to local polarity mechanisms at the single-cell level to produce efficient coordinated movements. In large unconstrained tissues, these active mechanisms lead to collective patterns of motion, driven by the coordination of velocities and feedback from resulting large cell-density fluctuations. Such spontaneous large-scale motions are critical during wound healing and epithelial gap closure (3, 37, 44). We indeed show that the emergence of oscillatory cell motions can favor the closure of nonadherent epithelial gaps in vitro (Video S6). Such observation could shed new light on the mechanisms at play during in vivo wound healing. The collective movements come with a broad distribution of time- and length scales (Fig. S5). Thus, the confinement here simply enhances a single wavelength among those. Interestingly, the different behaviors exhibited by various epithelial cell types may reflect more general rheological properties of the monolayers themselves. For instance, HaCaT skin cells exhibit coordinated movements on the millimeter scale, whereas this length scale is on the order of 100 μm for Caco2 intestinal cells. What is more, we can distinguish at least two very different types of collective epithelial motions, namely the previously described MDCK swirling and the coordinated oscillations we show here. As shown in Video S7, one or the other may arise in the same experimental conditions depending on the cell type. Interestingly, we have shown previously that those MDCK and HaCaT tissues exhibit different rheological properties (38). These facts appeal for an extensive examination of the differences in cellular properties leading to such changes in collective dynamics. This will be facilitated by the use of the model developed here, which allows for independently tuning single-cell mechanics as well as cell-cell passive (direct) or active—e.g., polarity coupling—force transmission. Along this line, our method used as a physiological rheometer (38) could help to investigate the different mechanical roles of intercellular junctional complexes, as previously shown for E- and P-cadherins (76).
The epithelium exhibits coordinated oscillations on a large scale, with a strong impact on the closure dynamics.
HaCaT cells (left) and MDCK cells (right) plated on 500 μm squares.
A noticeable aspect of the phenomenon is that because of the rotating character of the polarity axis, the two directions of movements are coupled. Hence, confinement in one single direction sets the length scale of the oscillations in the other direction, even in the presence of a freely moving edge with leader cells (Fig. S3). As a result, a highly anisotropic tissue exhibits very regular patterns of velocities along the long axis, whose scale is determined by the short axis. One could thus imagine such a mechanism to be relevant in segmentation processes of high aspect ratios, such as somite formation (27, 77).
Our molecular perturbation analyses clearly show the crucial role of adherens junctions in modulating the collective oscillations. They participate in the coordination of the cell polarities and also provide a route for the effective transmission of mechanical stresses to generate collective oscillations across the tissue. The observation of YAP translocation accompanying the oscillation-induced cell deformations further shows that the mechanical signals are sufficient to transduce mechano-chemical responses. Indeed, the deformations induced by these coordinated epithelial oscillations might provide a clock for the activation of cellular processes or define their location. In this regard, it has been shown previously that patterns of cell differentiation can follow patterns of mechanical signals (78). Beyond that, we anticipate that if such oscillations are able to emerge on sufficiently soft surfaces, they could also promote mechanical movements and tissue invagination in the third dimension, an important but poorly understood process during embryonic development.
Author Contributions
G.P., R.M., and J.d’A. contributed equally to this work. G.P., J.d’A., and B.L. designed the experiments. R.M., J.M.Y., and A.D. designed the model. G.P., J.d’A., and S.B. performed the experiments. G.P., J.d’A., and P.M. analyzed the experimental data. R.M. and A.D. performed the simulations and analyzed the simulation data. J.d’A., R.M., A.D., and B.L. wrote the manuscript. All authors read and commented on the manuscript.
Acknowledgments
Financial support from the European Research Council (ERC) under the European Union’s Seventh Framework Programme (FP7/2007-2013) / ERC grant agreement numbers 617233 (B.L.), Agence Nationale de la Recherche (ANR) (“POLCAM” [ANR-17- CE13-0013], CODECIDE [ANR-17-CE-13-0022], “MechanoAdipo” [ANR-17-CE13-0012]), the ANR "Labex Who Am I?" (ANR-11-LABX-0071), the Association pour la Recherche contre le Cancer, and the Ligue Contre le Cancer (Equipe labellisée) are gratefully acknowledged. We acknowledge the ImagoSeine core facility of the Institut Jacques Monod, member of IBiSA and France-BioImaging (ANR-10-INBS-04) infrastructures. We thank Sree Vaishnavi and Gianluca Grenci (micro fabrication core facility of Mechanobiology Institute, National University of Singapore) for microfabrication. G.P. is supported by the Human Frontier Science Programme (grant RGP0040/2012). R.M. is supported by grant P2EZP2 165261 of the Swiss National Science Foundation. A.D. is supported by a Royal Commission for the Exhibition of 1851 Research Fellowship.
Editor: Vivek Shenoy.
Footnotes
Grégoire Peyret, Romain Mueller, and Joseph d’Alessandro contributed equally to this work.
Supporting Material can be found online at https://doi.org/10.1016/j.bpj.2019.06.013.
Contributor Information
Amin Doostmohammadi, Email: amin.doostmohammadi@physics.ox.ac.uk.
Benoît Ladoux, Email: benoit.ladoux@ijm.fr.
Supporting Material
References
- 1.Friedl P., Gilmour D. Collective cell migration in morphogenesis, regeneration and cancer. Nat. Rev. Mol. Cell Biol. 2009;10:445–457. doi: 10.1038/nrm2720. [DOI] [PubMed] [Google Scholar]
- 2.Cetera M., Ramirez-San Juan G.R., Horne-Badovinac S. Epithelial rotation promotes the global alignment of contractile actin bundles during Drosophila egg chamber elongation. Nat. Commun. 2014;5:5511. doi: 10.1038/ncomms6511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park S., Gonzalez D.G., Greco V. Tissue-scale coordination of cellular behaviour promotes epidermal wound repair in live mice. Nat. Cell Biol. 2017;19:155–163. doi: 10.1038/ncb3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Montell D.J. Border-cell migration: the race is on. Nat. Rev. Mol. Cell Biol. 2003;4:13–24. doi: 10.1038/nrm1006. [DOI] [PubMed] [Google Scholar]
- 5.Martin A.C., Gelbart M., Wieschaus E.F. Integration of contractile forces during tissue invagination. J. Cell Biol. 2010;188:735–749. doi: 10.1083/jcb.200910099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roh-Johnson M., Shemer G., Goldstein B. Triggering a cell shape change by exploiting preexisting actomyosin contractions. Science. 2012;335:1232–1235. doi: 10.1126/science.1217869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Serra-Picamal X., Conte V., Trepat X. Mechanical waves during tissue expansion. Nat. Phys. 2012;8:628–634. [Google Scholar]
- 8.Tlili S., Gauquelin E., Graner F. Collective cell migration without proliferation: density determines cell velocity and wave velocity. R. Soc. Open Sci. 2018;5:172421. doi: 10.1098/rsos.172421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Londono C., Loureiro M.J., McGuigan A.P. Nonautonomous contact guidance signaling during collective cell migration. Proc. Natl. Acad. Sci. USA. 2014;111:1807–1812. doi: 10.1073/pnas.1321852111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reffay M., Parrini M.C., Silberzan P. Interplay of RhoA and mechanical forces in collective cell migration driven by leader cells. Nat. Cell Biol. 2014;16:217–223. doi: 10.1038/ncb2917. [DOI] [PubMed] [Google Scholar]
- 11.Farooqui R., Fenteany G. Multiple rows of cells behind an epithelial wound edge extend cryptic lamellipodia to collectively drive cell-sheet movement. J. Cell Sci. 2005;118:51–63. doi: 10.1242/jcs.01577. [DOI] [PubMed] [Google Scholar]
- 12.Ladoux B., Mège R.M. Mechanobiology of collective cell behaviours. Nat. Rev. Mol. Cell Biol. 2017;18:743–757. doi: 10.1038/nrm.2017.98. [DOI] [PubMed] [Google Scholar]
- 13.Mandal K., Wang I., Balland M. Cell dipole behaviour revealed by ECM sub-cellular geometry. Nat. Commun. 2014;5:5749. doi: 10.1038/ncomms6749. [DOI] [PubMed] [Google Scholar]
- 14.Liu Y.J., Le Berre M., Piel M. Confinement and low adhesion induce fast amoeboid migration of slow mesenchymal cells. Cell. 2015;160:659–672. doi: 10.1016/j.cell.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 15.Gupta M., Doss B.L., Ladoux B. Cell shape and substrate stiffness drive actin-based cell polarity. Phys. Rev. E. 2019;99:012412. doi: 10.1103/PhysRevE.99.012412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Woods M.L., Carmona-Fontaine C., Page K.M. Directional collective cell migration emerges as a property of cell interactions. PLoS One. 2014;9:e104969. doi: 10.1371/journal.pone.0104969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sunyer R., Conte V., Trepat X. Collective cell durotaxis emerges from long-range intercellular force transmission. Science. 2016;353:1157–1161. doi: 10.1126/science.aaf7119. [DOI] [PubMed] [Google Scholar]
- 18.Haigo S.L., Bilder D. Global tissue revolutions in a morphogenetic movement controlling elongation. Science. 2011;331:1071–1074. doi: 10.1126/science.1199424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Friedl P., Locker J., Segall J.E. Classifying collective cancer cell invasion. Nat. Cell Biol. 2012;14:777–783. doi: 10.1038/ncb2548. [DOI] [PubMed] [Google Scholar]
- 20.Wolf K., Te Lindert M., Friedl P. Physical limits of cell migration: control by ECM space and nuclear deformation and tuning by proteolysis and traction force. J. Cell Biol. 2013;201:1069–1084. doi: 10.1083/jcb.201210152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim J.H., Serra-Picamal X., Fredberg J.J. Propulsion and navigation within the advancing monolayer sheet. Nat. Mater. 2013;12:856–863. doi: 10.1038/nmat3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lecuit T., Lenne P.F. Cell surface mechanics and the control of cell shape, tissue patterns and morphogenesis. Nat. Rev. Mol. Cell Biol. 2007;8:633–644. doi: 10.1038/nrm2222. [DOI] [PubMed] [Google Scholar]
- 23.Vedula S.R., Leong M.C., Ladoux B. Emerging modes of collective cell migration induced by geometrical constraints. Proc. Natl. Acad. Sci. USA. 2012;109:12974–12979. doi: 10.1073/pnas.1119313109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Szabó A., Melchionda M., Mayor R. In vivo confinement promotes collective migration of neural crest cells. J. Cell Biol. 2016;213:543–555. doi: 10.1083/jcb.201602083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Balaji R., Bielmeier C., Classen A.K. Calcium spikes, waves and oscillations in a large, patterned epithelial tissue. Sci. Rep. 2017;7:42786. doi: 10.1038/srep42786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He L., Wang X., Montell D.J. Tissue elongation requires oscillating contractions of a basal actomyosin network. Nat. Cell Biol. 2010;12:1133–1142. doi: 10.1038/ncb2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hubaud A., Regev I., Pourquié O. Excitable dynamics and Yap-dependent mechanical cues drive the segmentation clock. Cell. 2017;171:668–682.e11. doi: 10.1016/j.cell.2017.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heisenberg C.P., Bellaïche Y. Forces in tissue morphogenesis and patterning. Cell. 2013;153:948–962. doi: 10.1016/j.cell.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 29.Doxzen K., Vedula S.R., Lim C.T. Guidance of collective cell migration by substrate geometry. Integr. Biol. 2013;5:1026–1035. doi: 10.1039/c3ib40054a. [DOI] [PubMed] [Google Scholar]
- 30.Rappel W.-J., Nicol A., Loomis W. Self-organized vortex state in two-dimensional Dictyostelium dynamics. Phys. Rev. Lett. 1999;83:1247–1250. [Google Scholar]
- 31.Huang S., Brangwynne C.P., Ingber D.E. Symmetry-breaking in mammalian cell cohort migration during tissue pattern formation: role of random-walk persistence. Cell Motil. Cytoskeleton. 2005;61:201–213. doi: 10.1002/cm.20077. [DOI] [PubMed] [Google Scholar]
- 32.Li B., Sun S.X. Coherent motions in confluent cell monolayer sheets. Biophys. J. 2014;107:1532–1541. doi: 10.1016/j.bpj.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin S.Z., Ye S., Feng X.Q. Dynamic migration modes of collective cells. Biophys. J. 2018;115:1826–1835. doi: 10.1016/j.bpj.2018.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deforet M., Hakim V., Silberzan P. Emergence of collective modes and tri-dimensional structures from epithelial confinement. Nat. Commun. 2014;5:3747. doi: 10.1038/ncomms4747. [DOI] [PubMed] [Google Scholar]
- 35.Notbohm J., Banerjee S., Marchetti M.C. Cellular contraction and polarization drive collective cellular motion. Biophys. J. 2016;110:2729–2738. doi: 10.1016/j.bpj.2016.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Petrolli V., Le Goff M., Balland M. Confinement-induced transition between wavelike collective cell migration modes. Phys. Rev. Lett. 2019;122:168101. doi: 10.1103/PhysRevLett.122.168101. [DOI] [PubMed] [Google Scholar]
- 37.Vedula S.R., Hirata H., Ladoux B. Epithelial bridges maintain tissue integrity during collective cell migration. Nat. Mater. 2014;13:87–96. doi: 10.1038/nmat3814. [DOI] [PubMed] [Google Scholar]
- 38.Nier V., Peyret G., Marcq P. Kalman inversion stress microscopy. Biophys. J. 2018;115:1808–1816. doi: 10.1016/j.bpj.2018.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dupont S., Morsut L., Piccolo S. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–183. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- 40.Aragona M., Panciera T., Piccolo S. A mechanical checkpoint controls multicellular growth through YAP/TAZ regulation by actin-processing factors. Cell. 2013;154:1047–1059. doi: 10.1016/j.cell.2013.07.042. [DOI] [PubMed] [Google Scholar]
- 41.Moroishi T., Hansen C.G., Guan K.L. The emerging roles of YAP and TAZ in cancer. Nat. Rev. Cancer. 2015;15:73–79. doi: 10.1038/nrc3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murakoshi H., Lee S.J., Yasuda R. Highly sensitive and quantitative FRET-FLIM imaging in single dendritic spines using improved non-radiative YFP. Brain Cell Biol. 2008;36:31–42. doi: 10.1007/s11068-008-9024-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Basu S., Totty N.F., Downward J. Akt phosphorylates the Yes-associated protein, YAP, to induce interaction with 14-3-3 and attenuation of p73-mediated apoptosis. Mol. Cell. 2003;11:11–23. doi: 10.1016/s1097-2765(02)00776-1. [DOI] [PubMed] [Google Scholar]
- 44.Vedula S.R., Peyret G., Ladoux B. Mechanics of epithelial closure over non-adherent environments. Nat. Commun. 2015;6:6111. doi: 10.1038/ncomms7111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zehnder S.M., Wiatt M.K., Angelini T.E. Multicellular density fluctuations in epithelial monolayers. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 2015;92:032729. doi: 10.1103/PhysRevE.92.032729. [DOI] [PubMed] [Google Scholar]
- 46.Malinverno C., Corallino S., Scita G. Endocytic reawakening of motility in jammed epithelia. Nat. Mater. 2017;16:587–596. doi: 10.1038/nmat4848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tsing Q. ImageJ plugins for traction force microscopy. 2012. https://sites.google.com/site/qingzongtseng/tfm
- 48.Nier V., Jain S., Marcq P. Inference of internal stress in a cell monolayer. Biophys. J. 2016;110:1625–1635. doi: 10.1016/j.bpj.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Palmieri B., Bresler Y., Grant M. Multiple scale model for cell migration in monolayers: elastic mismatch between cells enhances motility. Sci. Rep. 2015;5:11745. doi: 10.1038/srep11745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marth W., Voigt A. Collective migration under hydrodynamic interactions: a computational approach. Interface Focus. 2016;6:20160037. doi: 10.1098/rsfs.2016.0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bitbol A.F., Fournier J.B. Forces exerted by a correlated fluid on embedded inclusions. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 2011;83:061107. doi: 10.1103/PhysRevE.83.061107. [DOI] [PubMed] [Google Scholar]
- 52.Angelini T.E., Hannezo E., Weitz D.A. Glass-like dynamics of collective cell migration. Proc. Natl. Acad. Sci. USA. 2011;108:4714–4719. doi: 10.1073/pnas.1010059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Garcia S., Hannezo E., Gov N.S. Physics of active jamming during collective cellular motion in a monolayer. Proc. Natl. Acad. Sci. USA. 2015;112:15314–15319. doi: 10.1073/pnas.1510973112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fütterer C., Colombo C., Ott A. Morphogenetic oscillations during symmetry breaking of regenerating Hydra vulgaris cells. Europhys. Lett. 2003;64:137–143. [Google Scholar]
- 55.Lauschke V.M., Tsiairis C.D., Aulehla A. Scaling of embryonic patterning based on phase-gradient encoding. Nature. 2013;493:101–105. doi: 10.1038/nature11804. [DOI] [PubMed] [Google Scholar]
- 56.Shih N.P., François P., Amacher S.L. Dynamics of the slowing segmentation clock reveal alternating two-segment periodicity. Development. 2015;142:1785–1793. doi: 10.1242/dev.119057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mercker M., Köthe A., Marciniak-Czochra A. Mechanochemical symmetry breaking in Hydra aggregates. Biophys. J. 2015;108:2396–2407. doi: 10.1016/j.bpj.2015.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hillenbrand P., Gerland U., Tkačik G. Beyond the French flag model: exploiting spatial and gene regulatory interactions for positional information. PLoS One. 2016;11:e0163628. doi: 10.1371/journal.pone.0163628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Beaupeux M., François P. Positional information from oscillatory phase shifts : insights from in silico evolution. Phys. Biol. 2016;13:036009. doi: 10.1088/1478-3975/13/3/036009. [DOI] [PubMed] [Google Scholar]
- 60.Saw T.B., Doostmohammadi A., Ladoux B. Topological defects in epithelia govern cell death and extrusion. Nature. 2017;544:212–216. doi: 10.1038/nature21718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Benham-Pyle B.W., Pruitt B.L., Nelson W.J. Cell adhesion. Mechanical strain induces E-cadherin-dependent Yap1 and β-catenin activation to drive cell cycle entry. Science. 2015;348:1024–1027. doi: 10.1126/science.aaa4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Elosegui-Artola A., Andreu I., Roca-Cusachs P. Force triggers YAP nuclear entry by regulating transport across nuclear pores. Cell. 2017;171:1397–1410.e14. doi: 10.1016/j.cell.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 63.Dembo M., Wang Y.L. Stresses at the cell-to-substrate interface during locomotion of fibroblasts. Biophys. J. 1999;76:2307–2316. doi: 10.1016/S0006-3495(99)77386-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Trepat X., Wasserman M.R., Fredberg J.J. Physical forces during collective cell migration. Nat. Phys. 2009;5:426–430. [Google Scholar]
- 65.Li X., He S., Ji B. Cooperative contraction behaviors of a one-dimensional cell chain. Biophys. J. 2018;115:554–564. doi: 10.1016/j.bpj.2018.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Huveneers S., de Rooij J. Mechanosensitive systems at the cadherin-F-actin interface. J. Cell Sci. 2013;126:403–413. doi: 10.1242/jcs.109447. [DOI] [PubMed] [Google Scholar]
- 67.Broussard J.A., Yang R., Espinosa H.D. The desmoplakin-intermediate filament linkage regulates cell mechanics. Mol. Biol. Cell. 2017;28:3156–3164. doi: 10.1091/mbc.E16-07-0520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hatzfeld M., Keil R., Magin T.M. Desmosomes and intermediate filaments: their consequences for tissue mechanics. Cold Spring Harb. Perspect. Biol. 2017;9:a029157. doi: 10.1101/cshperspect.a029157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yonemura S., Wada Y., Shibata M. α-Catenin as a tension transducer that induces adherens junction development. Nat. Cell Biol. 2010;12:533–542. doi: 10.1038/ncb2055. [DOI] [PubMed] [Google Scholar]
- 70.Yao M., Qiu W., Yan J. Force-dependent conformational switch of α-catenin controls vinculin binding. Nat. Commun. 2014;5:4525. doi: 10.1038/ncomms5525. [DOI] [PubMed] [Google Scholar]
- 71.Aranson I.S. Springer International Publishing; Basel, Switzerland: 2015. Physical Models of Cell Motility. [Google Scholar]
- 72.Lee P., Wolgemuth C.W. Crawling cells can close wounds without purse strings or signaling. PLoS Comput. Biol. 2011;7:e1002007. doi: 10.1371/journal.pcbi.1002007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Grossman D., Aranson I.S., Ben Jacob E. Emergence of agent swarm migration and vortex formation through inelastic collisions. New J. Phys. 2008;10:023036. [Google Scholar]
- 74.Alaimo F., Simon P., Voigt A. A microscopic field theoretical approach for active systems. New J. Phys. 2018;18:083008. [Google Scholar]
- 75.Stramer B., Mayor R. Mechanisms and in vivo functions of contact inhibition of locomotion. Nat. Rev. Mol. Cell Biol. 2017;18:43–55. doi: 10.1038/nrm.2016.118. [DOI] [PubMed] [Google Scholar]
- 76.Bazellières E., Conte V., Trepat X. Control of cell-cell forces and collective cell dynamics by the intercellular adhesome. Nat. Cell Biol. 2015;17:409–420. doi: 10.1038/ncb3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hubaud A., Pourquié O. Signalling dynamics in vertebrate segmentation. Nat. Rev. Mol. Cell Biol. 2014;15:709–721. doi: 10.1038/nrm3891. [DOI] [PubMed] [Google Scholar]
- 78.Ruiz S.A., Chen C.S. Emergence of patterned stem cell differentiation within multicellular structures. Stem Cells. 2008;26:2921–2927. doi: 10.1634/stemcells.2008-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A few cells have been tracked manually. Scale bar 100 μm.
Scale bar 100 μm.
Scale bar 10 μm.
A few cells have been tracked manually. Scale bar 100 μm.
The epithelium exhibits coordinated oscillations on a large scale, with a strong impact on the closure dynamics.
HaCaT cells (left) and MDCK cells (right) plated on 500 μm squares.