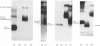Abstract
1. Rhnull human erythrocytes lack all of the antigens of the Rh and LW blood group systems and have abnormal shape and an increased osmotic fragility. In this paper two murine monoclonal antibodies raised against intact human erythrocytes were used to investigate further the abnormalities in these cells. BRIC 125 reacts weakly with Rhnull erythrocytes and BRIC 69 does not react at all. The results showed that BRIC 125 reacts with a component of Mr 47,000-52,000 which has a substantial content of N-glycans. In contrast, BRIC 69 reacted with a band of Mr 31,000 together with a very diffuse band of Mr 35,000-52,000. Treatment of BRIC 69 immunoprecipitates with endoglycosidase F/peptidyl-N-glycosidase F resulted in the loss of both BRIC 69 reactive components and the appearance of a new band of Mr similar to that of the Rh(D) polypeptide. 2. BRIC 125 had a broad reactivity with cells in peripheral blood, whereas the reactivity of BRIC 69 was confined to erythrocytes. BRIC 125, but not BRIC 69, reacted with human kidney tissue and bound to endothelium in peritubular capillaries, arteries and veins as well as the epithelial tissue of distal tubules. BRIC 125 stained haemopoietic cells, foetal hepatocytes and megakaryocytes in foetal liver and sinusoidal cells, hepatocytes and portal tracts in adult liver. In contrast, BRIC 69 reactivity was confined to haemopoietic cells in foetal liver. The BRIC 125 epitope has a wide tissue distribution, suggesting the occurrence of a related group of polypeptides which have a general functional role on cell surfaces. 3. Rhnull erythrocytes are deficient in at least four different membrane polypeptides.
Full text
PDF



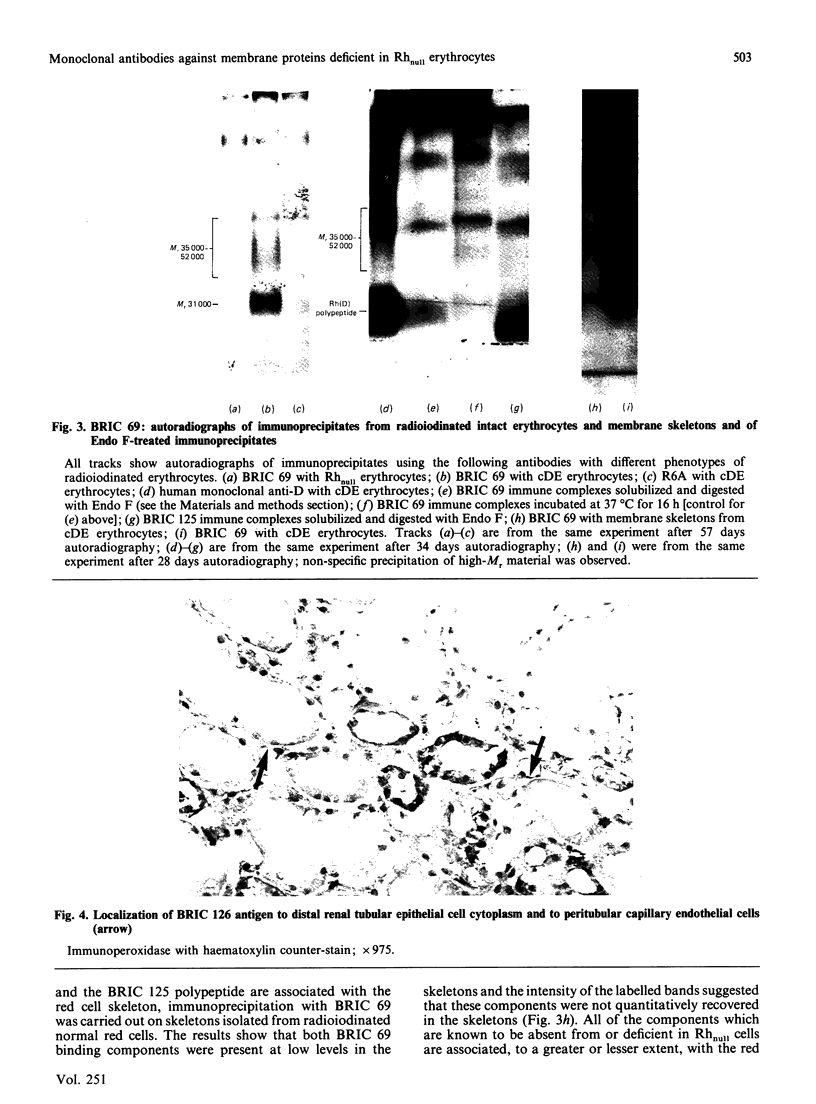


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anstee D. J., Edwards P. A. Monoclonal antibodies to human erythrocytes. Eur J Immunol. 1982 Mar;12(3):228–232. doi: 10.1002/eji.1830120311. [DOI] [PubMed] [Google Scholar]
- Anstee D. J., Mawby W. J., Tanner M. J. Abnormal blood-group-Ss-active sialoglycoproteins in the membrane of Miltenberger class III, IV and V human erythrocytes. Biochem J. 1979 Nov 1;183(2):193–203. doi: 10.1042/bj1830193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballas S. K., Clark M. R., Mohandas N., Colfer H. F., Caswell M. S., Bergren M. O., Perkins H. A., Shohet S. B. Red cell membrane and cation deficiency in Rh null syndrome. Blood. 1984 May;63(5):1046–1055. [PubMed] [Google Scholar]
- Doyle A., Jones T. J., Bidwell J. L., Bradley B. A. In vitro development of human monoclonal antibody-secreting plasmacytomas. Hum Immunol. 1985 Jul;13(3):199–209. doi: 10.1016/0198-8859(85)90012-6. [DOI] [PubMed] [Google Scholar]
- Gabra G. S., Bruce M., Watt A., Mitchell R. Anti-Rh 29 in a primigravida with rhesus null syndrome resulting in haemolytic disease of the newborn. Vox Sang. 1987;53(3):143–146. doi: 10.1111/j.1423-0410.1987.tb04938.x. [DOI] [PubMed] [Google Scholar]
- Gahmberg C. G., Karhi K. K. Association of Rho(D) polypeptides with the membrane skeleton in Rho(D)-positive human red cells. J Immunol. 1984 Jul;133(1):334–337. [PubMed] [Google Scholar]
- Gahmberg C. G. Molecular characterization of the human red cell Rho(D) antigen. EMBO J. 1983;2(2):223–227. doi: 10.1002/j.1460-2075.1983.tb01409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judson P. A., Anstee D. J. Comparative effect of trypsin and chymotrypsin on blood group antigens. Med Lab Sci. 1977 Jan;34(1):1–6. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mallinson G., Martin P. G., Anstee D. J., Tanner M. J., Merry A. H., Tills D., Sonneborn H. H. Identification and partial characterization of the human erythrocyte membrane component(s) that express the antigens of the LW blood-group system. Biochem J. 1986 Mar 15;234(3):649–652. doi: 10.1042/bj2340649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougal J. S., Browning S. W., Kennedy S., Moore D. D. Immunodot assay for determining the isotype and light chain type of murine monoclonal antibodies in unconcentrated hybridoma culture supernates. J Immunol Methods. 1983 Oct 28;63(3):281–290. doi: 10.1016/s0022-1759(83)80001-5. [DOI] [PubMed] [Google Scholar]
- Michot B., Bachellerie J. P., Raynal F., Renalier M. H. Homology of the 5'-terminal sequence of 28 S rRNA of mouse with yeast and Xenopus. Implication for the secondary structure of the 5.8 S--28 S RNA complex. FEBS Lett. 1982 Apr 19;140(2):193–197. doi: 10.1016/0014-5793(82)80892-2. [DOI] [PubMed] [Google Scholar]
- Moore S., Woodrow C. F., McClelland D. B. Isolation of membrane components associated with human red cell antigens Rh(D), (c), (E) and Fy. Nature. 1982 Feb 11;295(5849):529–531. doi: 10.1038/295529a0. [DOI] [PubMed] [Google Scholar]
- Parsons S. F., Judson P. A., Anstee D. J. BRIC 18: a monoclonal antibody with a specificity related to the kell blood group system. J Immunogenet. 1982 Dec;9(6):377–380. doi: 10.1111/j.1744-313x.1982.tb00998.x. [DOI] [PubMed] [Google Scholar]
- Parsons S. F., Mallinson G., Judson P. A., Anstee D. J., Tanner M. J., Daniels G. L. Evidence that the Lub blood group antigen is located on red cell membrane glycoproteins of 85 and 78 kd. Transfusion. 1987 Jan-Feb;27(1):61–63. doi: 10.1046/j.1537-2995.1987.27187121477.x. [DOI] [PubMed] [Google Scholar]
- Ridgwell K., Roberts S. J., Tanner M. J., Anstee D. J. Absence of two membrane proteins containing extracellular thiol groups in Rhnull human erythrocytes. Biochem J. 1983 Jul 1;213(1):267–269. doi: 10.1042/bj2130267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridgwell K., Tanner M. J., Anstee D. J. The Rhesus (D) polypeptide is linked to the human erythrocyte cytoskeleton. FEBS Lett. 1984 Aug 20;174(1):7–10. doi: 10.1016/0014-5793(84)81066-2. [DOI] [PubMed] [Google Scholar]
- Sturgeon P. Hematological observations on the anemia associated with blood type Rhnull. Blood. 1970 Sep;36(3):310–320. [PubMed] [Google Scholar]
- Sunderland C. A., Davies J. O., Stirrat G. M. Immunohistology of normal and ovarian cancer tissue with a monoclonal antibody to placental alkaline phosphatase. Cancer Res. 1984 Oct;44(10):4496–4502. [PubMed] [Google Scholar]